Bull Pan Am Health Organ 15(Z), 1981.
SEVERE
CUTANEOUS
ERUPTIONS
CAUSED
BY THIACETAZONE
USED TO TREAT
TUBERCULOSIS
IN RIO GRANDE
DO SUL, BRAZIL1
Public Health Dermatology
Team,* Tuberculosis
Team,3 and
Epidemiologic
Control
Unit,4 Rio Grande do Sul
State Department
of Health
The results of a Brazilian survq show that thkcetazone, a drug w&My used for tuberculosis treatment, can produce severe and occasionally fatal cutaneous reactions in some patients. These results have led health authorities in the stati where the survq was conducted to cons&r thkacetazonc unaccejtublefor use in routine treatment of tuberculosis,
Introduction
Drug-related cutaneous reactions are among the most common dermatoses of our time. These undesirable effects, the mecha- nisms of which are not always clear, may oc- cur even though great care has been taken to ensure that a given therapeutic agent is being properly administered. The skin eruptions, for the most part mild and transitory, general- ly occur alone but may be accompanied by systemic manifestations of varying intensity that are sometimes serious or even fatal. Drugs responsible for the reactions may be taken into the system via the most diverse routes-by ingestion, inhalation, inoculation, or absorption through the skin or mucosa; in
‘Also published in Portuguese in the Boleth a!e la Ojikina Sanitaria Prmatnen.caM 90(1):10-18, 1981.
25’ au erreira, supervisor; C&r F Duilio Varej5o Ber- nardi, team director; Antonio Carlos Gerbase, supervisor of venereal disease programs; Roberto Lopes Gervini, director of the internship unit; Mathias Kronfeld, intem- ist; Isabel Cristina Palma Kuhl, Paul0 Neves Figueiredo, Nei Alfonso Chassot, Julio Cesar Empinotti, and Edna Marie Paganella Marques, medical residents; Tank Cestari Ponzio, Humberto Antonio Salomon Ponzio, and Antonio Carlos Bastos Gomes, dermatologists; Gisela de Souza de1 Pino, pathologist; Zeila Gleci da Silveira Fmes, nurse; and Luiz Fernando Bopp Miiller, medical director of the outpatient clinic.
mother’s milk; or across the placenta (3). The responsible drug can be hard to identify; for while some drugs customarily provoke the same type of readily recognizable reaction, others produce extremely varied manifesta- tions.
Today, 1 to 5 per cent of all patients in general hospitals suffer undesirable effects from drugs (2); the frequency of these reac- tions, among them the cutaneous reactions, is alarming. In fact, according to some authors, nearly everybody in the world today has at some time showed signs of intolerance to one kind of medication or another. The increasing number of drugs in use, the possible side effects that are unknown in many cases, multi- ple drug administration, and long-term treat- , ment are all factors that contribute to the
rising incidence of cutaneous drug reactions
I (4.
Among the most serious cutaneous reac- tions are those involved with the Stevens- Johnson syndrome and Lyell’s disease-syn-
dromes which are often fatal because of the ex- tensive mucocutaneous and visceral involve- ment. The tissue loss caused by bullous lesions and/or epidermal necrolysis gives rise to clinical pictures resembling those of severe burns.
3Werner Paul Ott, head; Marlow Kwitko, team super-
visor; and Leo Romor Vargas, director of the Hos@af These two syndromes, produced by a wide Sanathio Partenon. variety of sometimes hard-to-trace causes, are *Cl&is Heitor Tigm, director. usually triggered by such agents as barbitu-
114 PAHO RI JT.T.ETIN . ld 1.5. no. 2. 19Al
rates, penicillin, phenolphthalein, phenylhy- dantoin and related drugs, pyrazolon and its derivatives, and sulfa drugs. Other reported causes include allopurinol, benzothiadiazides, codeine, nitrofurantoin, phenacetin, phenyl- carbazine, propranolol, tetracyclines, and thiacetazone (12).
Thiacetazone has been widely used in recent years for antituberculosis treatment. In fact, the eighth report of the WHO Expert Committee on Tuberculosis, published in
1964 (13), recommended thiacetazone as a cheap, effective drug for routine treatment of new tuberculosis cases; however, the commit- tee also stressed the need for further toxicity studies employing a representative sample of cases. The committee’s ninth report, pub- lished in 1974 (II), reaffirmed the drug’s ad- vantages-efficacy and low cost-and indi- cated that its toxicity level had been found ac- ceptable in many communities. However, serious side effects were reported in studies from Recife, Brazil (9), Morocco (5), and the island of Trinidad (8) around this time; and, as a result of the two latter studies, the drug was not introduced into Morocco or Trinidad and Tobago.
Methodology
Rio Grande do Sul, Brazil’s southernmost state, has an area of approximately 282,000 km2 and a population of about 8000,000 in- habitants. The incidence of tuberculosis in Rio Grande do Sul has declined in recent years, there being 7,223 cases (94.6 per
100,000 inhabitants) in 1976, and 6,110 cases (78.3 per 100,000) in 1977 (II). The State Health Department’s Tuberculosis Unit is ex- clusively responsible for the area’s control program.
A new therapeutic regimen, adopted for treatment of the disease in Rio Grande do Sul on 1 June 1975, called for 150 mg per day of thiacetazone and 420 mg per day of isoniazid for 12 months, plus 1 g per day of streptomy- cin, administered intramuscularly, during the first month. This “IST” regimen replaced the
previous isoniazid, streptomycin, and PAS regimen in both inpatient and outpatient treatment.
Nevertheless, in view of the reports on thi- acetazone’s toxic effects-particularly ery- throderma (2) and the Stevens-Johnson syn- drome (12)-a prospective survey was con- ducted to assess the frequency of these effects. For this purpose cases were observed at the
Hospital Sanatdrio Partenon, a tuberculosis hospital, taking advantage of a specialized dermatology unit attached to the hospital. The following patients were included in the study:
1) Those who began IST treatment after admission to the hospital.
2) Those who first received IST ambulato- ry treatment and who were free of skin erup- tions or other signs of toxic drug reaction upon subsequent admission to the hospital.
Results
The survey, concluded on 31 August 1977, included 1,890 patients, distributed by sex and age as shown in Table 1. The total number of patients admitted to the Hospital Sanatdrio Partenon and treated with IST from 1 June 1975 to 31 August 1977 was 1,926, but
36 of these were excluded from the study because the reason for their admission was serious drug intolerance during ambulatory treatment. (Thirty-four of these 36 patients
Table 1. Patients treated at the Hospitnl Sanatdrio Po+inon during the period 1 June 1975- 31 August 1977 who were included in the study,
by age group and sex. AIF group
(in years)
o-9 -
10-19 91 20-29 243 30-39 283 40-49 288 50-59 200 60-69 118 2 70 48 Total 1,271
- -
85 176 210 453 126 409 81 369 50 250 41 159
26 74
Dcnnatolou Team
lREACTIONS
TO THIACETAZONE
115
J.A.R.,
male, 63 years of age. This patient was treated with IST at Hospital SQnutbriO
Partmon beginning
on 9 October 1975. Lyell’s
disease was diagnosed on 3 November.
Though hydrocortisone
therapy improved
regression of skin lesions,
116 PAHO BULLETIN . vol. 15, no. 2, 1981
had skin eruptions and the other two had hepatitis symptoms.) These cases were not in- cluded in our calculations because they were really part of the population receiving ambu- latory treatment. However, they were regis- tered and included in the projections for the entire IST-treated population in the state.
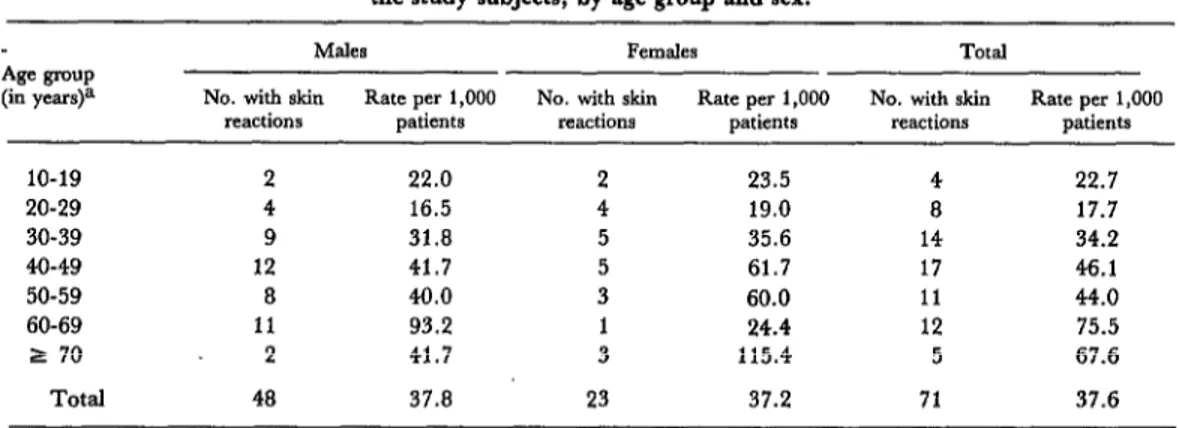
Seventy-one cases of drug-related cutane- , ous reactions were found in the 1,890 subjects studied, yielding a coefficient of 37.6 cases per 1,000 subjects. There were 48 male cases and 23 female cases; but because more male sub- jects were studied, the incidence coefficients
(37.8 cases per 1,000 males, 37.2 cases per 1,000 females) were very similar. With respect to age, although the coefficients were high for all groups, they appeared highest among the older patients (see Table 2).
All the observed skin reactions appeared between two and 92 days after initiation of treatment (the average was 27.3 days); 52 cases (73.2 per cent) occurred during the first 30 days of treatment.
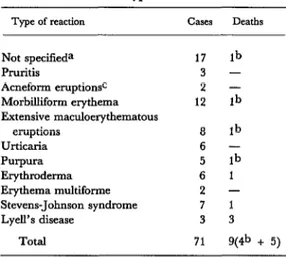
The various skin eruptions were classified by severity and type (see Table 3) as mild (uncomplicated pruritis, acneform eruption, morbilliform erythema, and suspected but unspecified reactions); moderate (extensive maculoerythematous eruption, urticaria, and purpura); or severe (erythroderma, erythema
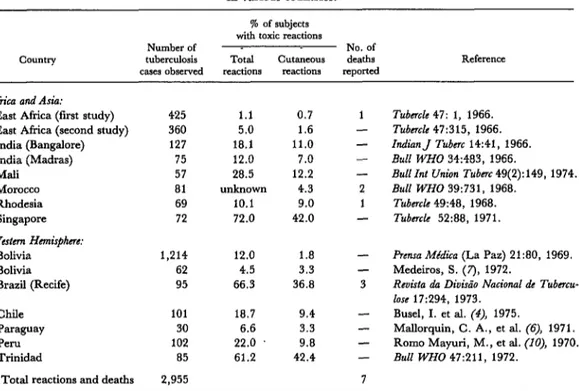
multiforme, Stevens-Johnson syndrome, and Lyell’s disease). In terms of severity, the 71 cases were distributed as follows: mild, 34 (18.0 cases per 1,000); moderate, 19 (10.1 cases per 1,000); and severe, 18 (9.5 cases per 1,000). These reactions were deemed respon- sible for five deaths (deaths from uncertain causes being excluded), so that overall mortal- ity from these reactions within the sample was 2.6 deaths per 1,000 subjects. This mortality is very similar to the overall mortality ob- served among subjects in other IST studies (see Table 4); these latter studies included 2,955 subjects treated with IST and reported a total of seven deaths; so even though the reported mortality varied considerably from study to study, overall mortality came to 2.4 deaths per 1,000 subjects.
Projections and Observed Reactions in Rio Grande do Sul
A total of 12,434 tuberculous patients re- ceived the IST regimen in Rio Grande do Sul between 1 June 1975 and 31 August 1977. If cutaneous reactions and reaction-related deaths occurred at the same frequency among these patients as among the patients studied at
the Hospital Sanathio Partenon, then the
numbers of mild, moderate, and severe cases
Table 2. The number and rate per 1,000 of drug-related skin eruptions among the study subjects, by age group and sex.
Age group (in yea+
M&S Females Total
No. with skin Rate per 1,000 No. with skin Rate per 1,000 No. with skin Rate per 1,000 reactions patients reactions patients reactions patients
10-19 2
20-29 4
30-39 9
40-49 12
50-59 8
60-69 11
2 70 2
Total 48
22.0 16.5 31.8 41.7 40.0 93.2 41.7 37.0
23.5 19.0 35.6 61.7 60.0 24.4 115.4
4 22.7
8 17.7
14 34.2 17 46.1 11 44.0 12 75.5
5 67.6
23 37.2 71 37.6
Denmtologv Team l REACTIONS TO THIACETAZONE 117
Table 3. Types of skin reactions observed among study subjects, showing the number of reactions of each we and the number of deaths among patients
with each type of reaction.
Type of reaction cases De&S Not specifieda 17 lb
Pruritis 3 -
Acneform eruptionsc 2 - Morbilhform erythema 12 lb Extensive maculoerythematous
eruptions a lb
Urticaria 6 -
PWpura 5 lb
Erythroderma 6 1
Erythema muhiforme 2 - Stevens-Johnson syndrome 7 1 Lyell’s disease 3 3
Total 71 y& + 5) aPatients with residual lesions who were not seen by a dermatologist until remission; the reactions involved were generally mild.
bcause of death uncertain. These deaths befell patients who had other serious complications preceding the cutaneous reaction-such as severe cardiopathy or severe cardiac insufficiency; these deaths were not included in the total mortality figure for this reason.
cProbably caused by isoniazid.
and deaths in Rio Grande do Sul State during this period would have been as follows:
Cutaneous reactions:
Mild . . . 224 cases Moderate . . . 126 cases Severe . . . 118 cases Total . . . 468 cases Deaths . . .,. . . . _ 32
A retrospective survey based on informa- tion supplied by health units throughout the state and by regional tuberculosis program supervisors revealed 128 cases of drug-related eruptions in patients receiving ambulatory treatment during the period. These cases in- cluded the 34 previously mentioned that were admitted to the Hospital Sanathio Partenon.
Overall, 37 of the 128 cases were classified as mild, 17 as moderate, and 74 as severe. Seven
deaths also occurred, five of them among the patients admitted to the Hospital Sanathio Partenon. However, these mortality figures are incomplete, follow-up not having been possi- ble in most of the reported cases (see Table 5). Since notification of ambulatory cases was in&mplete (many health units not having reported drug-related eruptions), the disparity between the relatively large number of severe cases and the relatively small numbers of mild and moderate ones is understandable. Severe cases being more evident than mild or moder- ate ones, they are more easily observed and reported. This suggests that the figure for severe skin eruptions (74) should be the most accurate of the three. Overall, the 74 severe cases found among ambulatory patients and the 18 found among Hospital Sanatdrio Partenon
inpatients yield a total of 92 severe cases state- wide.
This amounts to 26 fewer than the 118 pro- jected on the basis of the hospital study. That difference can be accounted for not only by the incompleteness of the ambulatory case data, but also by the fact that no data were available on 8 per cent of the 12,434 ambula- tory patients who failed to terminate the course of treatment. In this regard, it is logical to suppose that in some cases severe drug- related reactions were responsible for prema- ture termination of treatment.
All of this suggests that the incidence coeffr- cients derived from the hospital study were probably quite close to the actual statewide coefficients. This conclusion would appear to justify considering the IST combination’s
toxicity level to be unacceptably high for routine treatment of tuberculosis. It also ap- pears that the observed reactions were attrib- utable to thiacetazone, since no similar toxic
ii8 PAHO BULLETIN l vol. 15, no. 2, 1981
Table 4. Toxic reactions and deaths found in other studies of thiacetazone treatment in various countries.
Country
% of subjects with toxic reactions
Number of No. of
tuberculosis Total cutB.neous deaths Reference cases observed reactions reactions reported
Africa and Asia: East Africa (first study) East Africa (second study) India (Bangalore) India (Madras) Mali
Morocco Rhodesia Singapore
425 1.1 0.7 1 Tuber& 47: 1, 1966. 360 5.0 1.6 - Tubnclc 47:315, 1966. 127 18.1 11.0 - Indian J Tubnc 14:41, 1966.
75 12.0 7.0 - Bull WHO 34:483, 1966.
57 28.5 12.2 - Bull Int Union Tubcrc 49(2):149, 1974. 81 unknown 4.3 2 Bull WHO 39:731, 1968.
69 10.1 9.0 1 Tuber& 49:48, 1968. 72 72.0 42.0 - Tub&e 52:88, 1971. Western Hcmisfhm:
Bolivia Bolivia Brazil (Recife)
1,214 12.0 1.8 -
62 4.5 3.3 -
95 66.3 36.8 3
Prensa MldiGa (La Paz) 21:80, 1969. Medeiros, S. (7), 1972.
Revista da Diukio National de Tubncu- lose 17:294, 1973.
Chile 101 18.7 9.4 - Busel, I. et al. (4), 1975.
Paraguay 30 6.6 3.3 - Mallorquin, C. A., et al. (6), 1971. Peru 102 22.0 9.8 - Romo Mayuri, M., et ai. (lo), 1970. Trinidad 85 61.2 42.4 - Bull WHO 47:211, 1972.
Total reactions and deaths 2,955 7 Source: Antonio Pio, Pan American Health Organization, personal communication.
Table 5. Drug-related cutaneous reactions and drug-related deaths reported among ambulatory tubercuIosis patients treated with IST in the state of Rio Grande do SuI
between 1 June 1975 and 31 August 1977.
Type of reaction
Ambulatory Ambulatory
cases admitted cases reported Total
to the Hospital but not referred ambulatory KnOWI .%~t6rio Parknon to the hospital cases reported deathsa Not specilied
Pmritis
Acneform eruptions Morbilliform erythema Extensive maculoerythematous
eruptions Urticaria Purpura Erythroderma Erythema multiforme Stevens-Johnson syndrome Lyeii’s disease
Total
5 1 6
- 19 19
- 1 1
4 9 13
2 - - 7 1 11 4 34
- 2
8 8
5 5
30 37
9 10
7 18
5 9
94 128
- - -
1
- - -
1
3b
Dermatology Team l REACTIONS TO THIACETAZONE 119
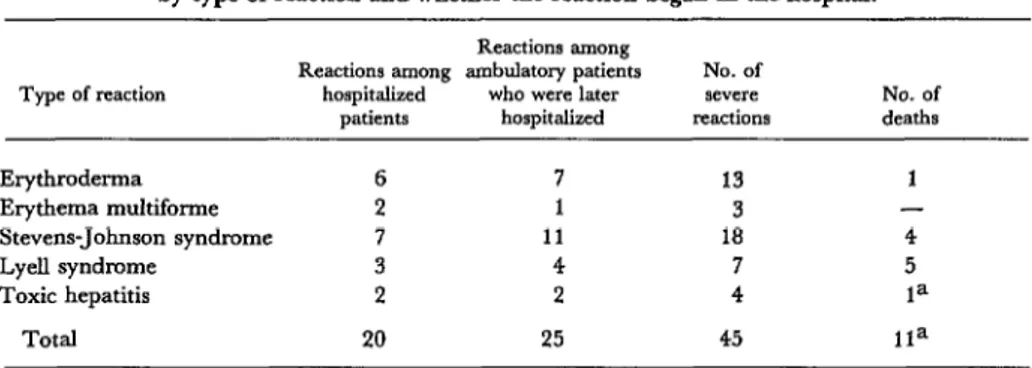
Table 6. Severe reactions to IST treatment observed at the Iios~ital Snnati Pat-tenon by type of reaction and whether the reaction began in the hospital.
Type of reaction
Erythroderma Erythema multiforme Stevens-Johnson syndrome LyeIl syndrome
Toxic hepatitis Total
Reactions among
Reactions among ambulatory patients No. of
hospitalized who were later severe No. of patients hospitalized reactions deaths
6 7 13 1
2 1 3 -
7 11 18 4
3 4 7 5
2 2 4 la
20 25 45 11a
aThe death from toxic hepatitis was excluded from the hospital study’s drug-related mortality figures because the patient’s general condition was already very poor when hepatitis symptoms appeared.
Other Observed Signs of Toxicity
Besides skin eruptions, other toxic symp- toms (especially of the digestive tract) were observed among the inpatients treated with IST. Specifically, three cases of gastric intoler- ance (severe nausea and vomiting) and one case of “uncontrollable dizziness” among the
1,890 Hospital Sanatdrio Partenon patients made it necessary to substitute second-line drugs. In addition, two of the patients developed hepati- tis (probably toxipathic hepatitis) and one died. This death was excluded from the hospital study’s mortality data because the pa- tient’s general condition was very poor to begin with, and so death could not be definite- ly attributed to thiacetazone-prompted toxi- pathic hepatitis. Overall, treatment had to be
discontinued for 69 (3.65 per cent) of the 1,890 study subjects because of gastric intoler- ance (in three subjects), toxic hepatitis (in two), uncontrollable dizziness (in one), and skin eruptions (in 63).
Information about other toxic effects among ambulatory patients is incomplete, but 26 cases were reported in which treatment had to be discontinued because of gastric intoler- ance. In addition, two patients with symptoms of toxic hepatitis were referred to the Hospital Sanathio Partenon, and one of these subse- quently died.
Counting both direct admissions and refer- rals from the outpatient service, a total of 45 patients with severe toxic reactions were seen at the Hospital Sanatbrio Partenon (Table S), and 11 patients in this group died.
SUMMARY
The State Health Department of Rio Grande do acneform lesions had been detected during the Sul, Brazil’s southernmost state, began treating state’s previous extensive experience with isoniazid tuberculosis patients with a regimen of isoniazid, and streptomycin.
streptomycin, and thiacetazone (IST) on 1 June Although outpatient data are incomplete, there 1975. However, reports of thiacetazone’s possible are grounds for believing that the incidence of toxic toxic effects prompted a long-term study of 1,890 effects among all 12,424 patients receiving the IST patients who received this regimen at the state’s regimen was simiiar to that observed among the
120 PAHO BULLETIN l vol. 15, no. 2, 1981
REFERENCES
(I) Amos, H. Allergic Drug Reactions. Edward Arnold, London, 1976.
(2) Baer, R., and B. Levine. Adverse Cutane- ous Reactions to Drugs. In T. Fitzpatrick, K. Amdt, W. Clark, Jr., et al. (eds.). Dermatology in General Medicine. McGraw-Hill, New York, 1971, pp. 1281-1314.
(3) Bruinsma, W. A Gu& to Drug Eruptions. Ex- cerpta Medica, Amsterdam, 1973.
(4) Busel, I., et al. Evaluaci6n operational de 2 esquemas de tratamiento en pacientes portadores de tuberculosis pulmonar, Koch direct0 positivo, mmca antes tratados. Mimeographed document,
1975.
(5) Chicou, F. J., G. H&rick, M. Huet, et al. Essai comparatif de deux types de traitement oral de la tuberculose pulmonaire. Bull WHO 39:731- 769, 1968.
(6’ Mallorquin, C. A., et al. Tratamiento de la tuberculosis con diateben. In Actas, XVII Con- greso Panamericano de Tuberculosis y Enfermeda- des Respiratorias, Asunci6n, Paraguay, 20-24 September 1971, p. 581.
(7) Medeiros, S. Experiencia con tiacetazona en el tratamiento initial de la tuberculosis pulmonar. In Actas, III Simposio Intemacional QuimioterApi- co de la Tuberculosis, Bogotg, Colombia, 4-9 September 1972, p. 95.
(8) Miller, A. B. , A. J. Nunn, D. K. Robinson, et al. A second international cooperative investiga- tion into thiacetazone side effects. Bull WHO 47: 211-227, 1972.
(9) Rizzo, A. Tiacetazona em associaclo trfplice em tuberculosos virgens de tratamento. Rev&a da Divkio Nackal & Tube&use (Brazil) 17:294-306,
1973.
(10) Romo Mayuri, M., et al. Tratamiento am- bulatorio de la tuberculosis pulmonar con Diate- ben. In Actas, IX Congreso National de Tubercu- loses y Enfermedades Respirator& Chiclayo, Peru, 1970, p. 6.
(II) Secretaria da SaGde do Estado do Rio Grande do Sul. Informe Epidemioldgico. Port0 Alegre, l-5 April, 1978.
(12) Weinstein, L. Antimicrobial Agents. In: L. Goodman and A. Gilman. The Pharmacological Basis of Therapeutics (5th ed.). MacMillan, New York, 1975, pp. 1201-1247.
(13) World Health Organization. WHO i&pen Committee (M Tuberculosis: Eighth R.$oti. WHO Tech- nical Report Series No. 290. Geneva, 1964.
(14) World Health Organization. WHO Expert Committee on Tuberculosis: Ninth Report. WHO Tech- nical Report Series No. 552. Geneva, 1974.
PREVENTION OF NEONATAL. TETANUS
1
Tetanus claims at least a million human lives a year around the world, about half being those of newborn infants; and in many countries such neonatal tetanus kills about 85 per cent of those afflicted. Infants typically contract the disease at birth, when delivered in non-aseptic conditions- especially when the umbilical cord is cut with unclean instruments (ritual knives, pieces of glass, and so forth) or when the umbilical stump is dressed with ashes, soil, or cow dung.
Tetanus is best prevented by immunization. The immunity resulting from two injections of tetanus toxoid is highly effective and lasts for several years. Although newborns cannot themselves be immunized in time to prevent tetanus acquired at birth, immunity lasting several weeks can be transferred to the baby if the mother is immunized during pregnancy or if she already has a high level of immunity at the time she becomes pregnant.