w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Clostridium
difficile
infection
in
Chilean
patients
submitted
to
hematopoietic
stem
cell
transplantation
Javier
Pilcante,
Patricio
Rojas,
Daniel
Ernst,
Mauricio
Sarmiento,
Mauricio
Ocqueteau,
Pablo
Bertin,
Maria
García,
Maria
Rodriguez,
Veronica
Jara,
Maria
Ajenjo,
Pablo
Ramirez
∗PontificiaUniversidadCatólica,Santiago,Chile
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received27April2015 Accepted27July2015
Availableonline19August2015
Keywords: Clostridiumdifficile
Infection
Hematopoieticstemcell transplantation Outcomes
a
b
s
t
r
a
c
t
Introduction:Patients submitted to hematopoietic stem cell transplantation have an increasedriskofClostridiumdifficileinfectionandmultipleriskfactorshavebeen identi-fied.Publishedreportshaveindicatedanincidencefrom9%to30%oftransplantpatients howevertodatethereisnoinformationaboutinfectioninthesepatientsinChile.
Methods:AretrospectiveanalysiswasperformedofpatientswhodevelopedC.difficile infec-tionafterhematopoieticstemcelltransplantationsfrom2000to2013.Statisticalanalysis usedtheStatisticalPackagefortheSocialSciencessoftware.
Results:Twohundredandfiftypatientswerestudied(meanage:39years;range:17–69), with147(59%)receivingallogeneictransplantsand103(41%)receivingautologous trans-plants.Onehundredandninety-two(77%)patientshaddiarrhea,with25(10%)casesofC. difficileinfectionbeingconfirmed.Twentyinfectedpatientshadundergoneallogeneic trans-plants,ofwhichtenhadacutelymphoblasticleukemia,threehadacutemyeloidleukemia andsevenhadotherdiseases(myelodysplasticsyndrome,chronicmyeloidleukemia,severe aplasticanemia).Intheautologoustransplantgroup,fivepatientshadC.difficileinfection; twohadmultiplemyeloma,onehadamyloidosis,onehadacutemyeloidleukemiaandone hadgerminalcarcinoma.TheoverallincidenceofC.difficileinfectionwas4%withinthefirst week,6.4%inthefirstmonthand10%inoneyear,withnodifferenceinoverallsurvival betweeninfectedandnon-infectedgroups(72.0%vs.67.6%,respectively;p-value=0.56). Patientsinfectedafterallogeneictransplantshadaslowertimetoneutrophilengraftment comparedtonon-infectedpatients(17.5vs.14.9days,respectively;p-value=0.008).Inthe autologoustransplantgrouptherewasnosignificantdifferenceintheneutrophil engraft-menttimebetweeninfectedandnon-infectedpatients(12.5daysvs.11.8days,respectively;
p-value=0.71).Intheallogeneictransplantgroup,themediantimetoacute graft-versus-hostdiseasewassimilarbetweenthetwogroups(p-value=0.08),aswastheincidenceof grades1–4acutegraft-versus-hostdisease(40%vs.48%;p-value>0.05).
∗ Correspondingauthorat:HematologyOncologyDepartment,PUniversidadCatólicadeChile,Lira85,Piso4,Santiago,Chile.
E-mailaddress:pramirez@med.puc.cl(P.Ramirez).
http://dx.doi.org/10.1016/j.bjhh.2015.07.010
Conclusion: TheincidenceofC.difficileinfectionafterhematopoieticstemcell transplan-tationwaslow,withasignificantnumberofcasesoccurringshortlyaftertransplantation. Allogeneictransplantshadathree-timehigherriskofinfectioncomparedtoautologous transplants,butthiswasnotassociatedwithincreasedmortality,decreasedoverallsurvival orhigherriskofacutegraft-versus-hostdisease.
©2015Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Publishedby ElsevierEditoraLtda.Allrightsreserved.
Introduction
Clostridiumdifficile(CD)isananaerobicGram-positive spore-forming bacillus, responsible for nearly 20% of antibiotic-associated diarrhea in developed countries. The clinical presentation is varied but may include mild to moderate diarrhea,toxicmegacolonwithperforationordeath.1C.
dif-ficile infection (CDI) has an impact on length of hospital stay, costs, morbidity and mortality in adult patients and children,2 and it is a major concern for patients who are
immunosuppressed,especiallyafterhematopoieticstemcell transplantation(HSCT).3–5Theemergenceanddissemination
ofahighvirulentstrain(NAP-1/027)haschangedthe epidemi-ologyofCDI,withhighmortalityandmorbidityaswellahigh riskofrecurrence, and potentialimpact on certainpatient subgroupsincludingHSCTrecipients.6–8
ThedayssurroundingHSCTareusuallyaccompaniedby gastrointestinal(GI)tractdisordersmainlyduetomucositis, withelevated predisposition todiarrhea and CDI.9 Clinical
manifestationsofCDIinthissettingmayvaryfrom asymp-tomaticcolonizationtofulminant colitis.10,11 Thehighrisk
of CDI in HSCT patients is due to prolonged hospitaliza-tion,chemotherapy-relateddamageof theenteric mucosal barriers,12 use of broad spectrum antibiotics
(prophylac-tic and therapeutic),13 use of proton-pump inhibitors,14,15
and in some cases due to infection prior to the HSCT hospitalization.16
The incidence of CDI in cancer patients receiving chemotherapyis3–7%,withapproximately8%developinga moresevere disease.17 There are data thatshow the rates
ofCDI in HSCTare nine-fold higher than generalpatients (24.0vs.2.6per10,000patients-days,respectively),and1.4-fold highercomparedwithotheroncologypatients(24.0vs.16.8 per10.000patients-days,respectively).18Largeepidemiologic
studiesshowanoverallincidenceofCDIof9.2%atoneyear aftertransplant.19 InpatientssubmittedtoallogeneicHSCT
(allo-HSCT),theincidencevariesbetween15%and30%,18,20
andhasbeenassociatedwiththedevelopmentofacute graft-versus-host-disease(aGVHD)oftheGItractandnon-relapse mortality.21
InChilethereare fewreportsofthis infectionin hospi-talizedpatients,mainlyduetolackofsurveillanceinmany centers,withonlyoneretrospectivestudyshowinganoverall incidenceofCDIof5.3cases/1000dischargesperyear,mainly inkidneydiseasepatients,22withnocancerorHSCT
recipi-entpatientsincluded.SincenoCDIdatainHSCTrecipients isavailableinChile,theaimofthisstudyistodescribethe features ofCDI in HSCTrecipients compared to published
international data,inordertoimprove all themeasuresto preventthiscomplicationinaresource-limitedsetting.
Methods
Patients
A retrospective analysis was performed ofall the patients submitted to HSCTfrom January 2000to June 2013at the HospitalClínicoUniversidadCatólica,inSantiago,Chile.This institutionisa468-bedteachinghospitalwitha24-bed hema-tologyunit, andHematologyand InfectiousDiseasesteams dedicatedtopatientcare.DatawereobtainedfromtheHSCT database and from electronic and paper patient records. The data collected included demographics, diagnosis,type oftransplantand conditioning regimen,presenceandtype ofaGVHDinpatientswithCDI,timetoCDI,antibioticsand proton-pumpinhibitor prescriptionpriortoand duringthe HSCTadmission.ThestudywasapprovedbytheEthics Com-mitteeandbytheMedicalInvestigationCenterCommitteeat theHospital.
Transplantationprocedure
The patients were divided in two groups, allo-HSCT and auto-HSCT.Theconditioningregimensutilizedinallo-HSCT were myeloablative, including cyclophosphamide and total bodyirradiation(TBI),busulfanpluscyclophosphamide,and reduced intensity conditioning (RIC), including fludarabine pluscyclophosphamide,fludarabineplusbusulfanand busul-fanpluscyclophosphamide.Patientssubmittedtoauto-HSCT were conditioned using Melphalan 140mg/m2, Melphalan
200mg/m2 and ifosfamide,carboplatinand etoposide(ICE).
All patientswerehospitalizedinprivaterooms witha pro-tective environment (double door rooms with a positive air pressure system and high efficiency particle arresting filters). Prophylaxis for standard infectious diseases using levofloxacin, fluconazoleand acyclovirwas started onDay
−1and continueduntildischarge.Omeprazolewasusedin
neutrophil engraftment, defined as the first of three con-secutivedaysofneutrophilcountwith0.5×109 cells/Lwas
obtainedforallpatientsaswastimetoplateletengraftment, definedasthe firstoffiveconsecutive dayswithcountsof 20×109cells/Lwithouttransfusionsupport.Thepresenceand
typeofaGVHDwasdiagnosedandgradedaccordingtothe GlucksbergcriteriaandtheInternationalBoneMarrow Trans-plantRegistryDatabase(IBMTR)severityIndex.23,24AllaGVHD
wasdocumentedforinfectedpatients,iftheinformationwas available,withdayofdiagnosisanditscorrelationwithCDI.
C.difficileinfection
ThedefinitionofCDIinHSCTpatientswasanepisodeof diar-rheawithapositivetestforC.difficiletoxinatanytimefromthe startoftheconditioningregimenuntiloneyearafterthe trans-plantation(Day−7untilDay+365).DocumentationofCDIwas
performedusingtwodiagnosticmethodsavailableinthe insti-tution:EnzymeImmunoassay(EIA)forC.difficiletoxinsAand Bfrom January2000toFebruary 2012andthenpolymerase chainreaction(PCR)toxinassay(X-pertTMC.difficile,
GeneX-pertTechnology,CepheidCompany,Sunnyvale,CA,USA)until theendofthestudy.Sampleswereobtainedatthebedside and sentdirectly to thelaboratory forimmediateanalysis. Theseverityofthediseasewasgradedbasedonthe consen-susoftheChileanSocietyofInfectiousDiseaseandChilean Society ofGastroenterology.Classificationincluded mild to moderatedisease(non-severeCDI),complicatedCDI(change inmentalstatus,megacolon,shock,increasedlevelsoflactic acid>2.2mmol/L)andsevereCDI(leukocytes>20×109cells/L,
hypoalbuminemia<3g/dL,creatinine>2.2mg/dLorincreased morethan50%ofbaselinevalue).PatientswithCDIconfirmed byapositivetoxintestwereputincontactisolation,managed withhandhygieneusingsoapand water,avoiding alcohol-basedhandsanitizersandtreatedwithmetronidazole(500mg q8PO)orvancomycin(125–250mgq6PO),dependingonthe severityofthedisease.AntibiotictherapyforCDIwas contin-ueduntilresolvingthediarrhealepisodeandtheneutropenia. Inmildtomoderatecases,metronidazolewaspreferredover vancomycinasthefirst-linetreatment.Incasesofintolerance tometronidazole,oralvancomycinwasusedasthesecond linetherapy.Inpatientswithmoresymptomsattributableto infection,butnotsufficienttofulfillmoreseverecriteria,or incasesofrecurrentCDI,vancomycinwaspreferredas first-linetreatment.Dataforknownriskfactorswasdocumented forpatientswithCDI,includingtheuseofprophylacticand therapeuticantibioticsinthepreviousthreemonths,useof proton-pumpinhibitors(omeprazole),useofenteral or par-enteralnutritionandpreviousepisodesofCDI.
Statisticalanalysis
ThestatisticalanalysiswasperformedusingtheIBM Statisti-calPackagefortheSocialSciencessoftwareversion21(IBM Company, Armonk, NewYork, USA). Variablesare reported asnumbersandpercentages.Survivalcurveswereobtained usingtheKaplan–Meiermethodandwerecomparedwiththe Log-RankTest,thettestwasusedforindependentsamples
Table1–Patientcharacteristics.
Allo-HSCT Auto-HSCT Total
n 147 103 250
Age-years(range) 36(17–61) 45(18–69) 39(17–69)
Gender(male–%) 60% 64% 60%
Conditioning-n(%)
MA 132(90%) 102(99%) 234(94%)
RIC 15(10%) 1(1%) 16(6%)
Mainindicationfortransplant-n(%)
AML 48(33%) 8(8%) 56(22%)
ALL 47(32%) 1(1%) 48(19%)
MM 0(0%) 36(35%) 36(14%)
HL/NHL 6(4%) 43(42%) 49(20%)
Others 43(29%) 18(17%) 61(24%)
Allo-HSCT: allogeneic hematopoietic stem cell transplantation; Auto-HSCT: autologoushematopoieticstemcelltransplantation; MA: myeloablative; RIC: reduced intensity conditioning; AML: acutemyeloidleukemia;ALL:acutelymphoblasticleukemia;MM: multiplemyeloma;HL:Hodgkin’slymphoma;NHL:non-Hodgkin lymphoma;CDI:Clostridiumdifficileinfection.
andFisherexacttestforanalysisbetweengroupsintermsof CDIandtypeoftransplantationandconditioning.
Results
Patientcharacteristics
Duringthestudyperiod,250HSCTwereperformed, includ-ing147allo-HSCTand103auto-HSCT.Patientcharacteristics aresummarizedinTable1.Themedianageofpatients sub-mittedtoallo-HSCTwas36years(range:17–61years)andin theauto-HSCTgroup,itwas45years(range:18–69).Themain indicationsforHSCTwereacuteleukemia(n=104;42%), lym-phoma(n=49;20%)andmultiplemyeloma(n=36;14%);93% (n=234)ofpatientsreceivedmyeloablativeconditioning(MA). Ofthe250patientsstudied,diarrheawasseenand docu-mentedin192(77%)cases,ofwhich25(10%)hadCDI,20(80%) intheallo-HSCTgroup(Table2).Alloftheinfectedpatients hadmildtomoderatedisease,andnodeathswereattributable tothisinfection.IntheCDIgroup,onlythree(12%)patients receivedvancomycinasfirst-linetherapy,withthemajority of the patients (22 patients; 88%) receiving metronidazole, withamediantimetotreatmentoftwoweeks(range:7–24 days).Apartfromonepatientwhoreceivedintravenous van-comycinfortheempiricmanagementoffebrileneutropenia, all patientsweremanaged withoralmetronidazoleor van-comycinuntilresolutionoftheinfection.Nosideeffectsof CDItherapywerereported.
IncidenceofC.difficileinfection
Table2–Clostridiumdifficileinfectionineachsubgroup ofpatients.
Allo-HSCT (n=147)
Auto-HSCT (n=103)
Total (n=250)
Totalinfection 20(14) 5(5) 25(10)
AML 3(15) 1(20) 4(16)
ALL 10(50) 0 10(40)
MM – 2(40) 2(8)
HL/NHL 0 0 0
CML 4(20) 0 4(16)
MDS 1(5) 0 1(4)
SAA 2(10) 0 2(8)
Others 0 2(40) 2(8)
Datapresentedasn(%).
Allo-HSCT: allogeneic hematopoietic stem cell transplantation; Auto-HSCT: autologous hematopoietic stem cell transplanta-tion; AML: acute myeloid leukemia; ALL: acute lymphoblastic leukemia;MM:multiplemyeloma;HL:Hodgkin’slymphoma;NHL: non-Hodgkinlymphoma;CML:chronicmyeloid leukemia;MDS: myelodysplasticsyndrome;SAA:severeaplasticanemia.
Othersincludedonecaseofamyloidosisandonecaseofgerminal cancer.
0 60 120 180 240 300 360 0
10 20
All patients Auto-HCT Allo-HCT
Days after HCT
% accumulated cases CD (+)
Figure1–One-yearcumulativeincidenceofClostridium difficileinfectioninpatientssubmittedtohematopoietic stemcelltransplantation.
18days).Afterauto-HSCT,thecumulativeincidencesofCDI inthefirstweek,monthandyearwere1.9%,2.9%and4.9%, respectively(mediantimetoinfection21days)(Figure1).Forty percentofcasesoccurredbeforeDay+7aftertransplantation, andtheremainingcasesoccurredduringthefollow-up,until Day+365.Twenty-twopatientswithCDItookoral metronida-zole(500mgt.i.d.).Threepatientsreceivedoralvancomycinas first-linetherapy.OnlyfourpatientsintheCDIgroup(16%)had asecondepisodeofCDIafterthetransplant(threebetween Day+40and Day+60and oneafterDay+200),all ofwhom weresuccessfullytreatedwithoralmetronidazole.Therewere nodifferencesinincidenceofotherinfectionsinpatientswith andwithoutCDI.
RiskfactorsforC.difficileinfection
In the risk factors analysis, all infected patients had receivedproton-pumpinhibitors(omeprazole20mgperday) and all received standard infectious disease prophylaxis (levofloxacin,sulfamethoxazoletrimethoprim,acyclovirand
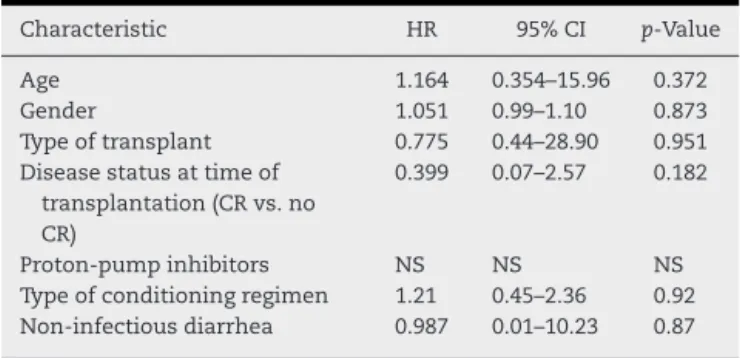
Table3–Multivariateanalysisofriskfactorsfor Clostridiumdifficileinfection.
Characteristic HR 95%CI p-Value
Age 1.164 0.354–15.96 0.372
Gender 1.051 0.99–1.10 0.873
Typeoftransplant 0.775 0.44–28.90 0.951
Diseasestatusattimeof transplantation(CRvs.no CR)
0.399 0.07–2.57 0.182
Proton-pumpinhibitors NS NS NS
Typeofconditioningregimen 1.21 0.45–2.36 0.92
Non-infectiousdiarrhea 0.987 0.01–10.23 0.87
CR:completeremission;HR:hazardratio;CI:confidenceinterval.
fluconazole). Eighty-one percent of the infected patients (n=21) used antibiotics other than prophylaxis during the three months before transplantation, mainly for febrile neutropenia, and pulmonary, urinary and central venous catheter-relatedinfections,andother relatedinfections.No informationabout antibioticuse before thetransplant was availableinthemajorityofpatientswithoutCDI.The antibi-oticsused primarilyas treatmentoftheseconditionswere cephalosporins, amikacin, vancomycin and carbapenems. Noneoftheinfectedpatientsreceivedenteralorparenteral nutrition before the CDI. Patients transplanted for acute lymphoblastic leukemia (ALL)and acute myeloid leukemia (AML) represented40%(10/25)and 16%(4/25) ofCDI cases, respectively(p-value=0.041).Multivariateanalysisfoundno correlationbetweenCDIwiththeanalyzedriskfactors, includ-ing age, gender, disease status at transplantation, type of transplant, typeof conditioningregimen, presenceof non-infectious diarrhea and the use ofproton-pump inhibitors (Table3).
C.difficileinfectionandacutegraft-versus-hostdisease
Incidenceofgrades1–4aGVHDwassimilarbetweenthetwo groups;40%ofpatientswithCDIand48%ofpatients with-outCDI.Grades3–4aGVHDwasalsosimilarbetweenthetwo groups(22%).Themediantimeuntilthiscomplicationwas35 daysininfectedpatientsand30daysinnon-infectedpatients (p-value=0.08).
C.difficileinfectionandtimetoneutrophilandplatelet engraftment
Inpatientssubmittedtoallo-HSCT,themediantimeto neu-trophilengraftmentofpatientswithCDIwaslongerthanin non-infectedpatients (17.5days vs.14.9 days, respectively;
Table4–Mediantimetoneutrophilandplateletengraftment.
Engraftment Allo-HSCT Auto-HSCT
CDI(+) CDI(−) p-Value CDI(+) CDI(−) p-Value
Neutrophils(days) 17.5 14.9 0.008 12.5 11.8 0.71
Platelets(days) 32.2 25.7 0.48 31.5 28.1 0.91
Allo-HSCT:allogeneichematopoieticstemcelltransplantation;Auto-HSCT:autologoushematopoieticstemcelltransplantation;CDI(+): Clostrid-iumdifficileinfection;CDI(−):noClostridiumdifficileinfection.
0 100 200 300 400
0.00 0.25 0.50 0.75 1.00
CD (+) CD (–)
P=0.56
Days after HCT
Ov
e
ra
ll su
rvi
va
l
Figure2–Overallsurvivalatoneyearinpatientssubmitted tohematopoieticstemcelltransplantationwithand withoutClostridiumdifficileinfection.
C.difficileinfectionandsurvival
There was no statistically significant difference in overall survival(OS)betweenthegroupwithCDIand non-infected patientsatoneyearafterthetransplant(72%vs.67.6%, respec-tively;p-value=0.56)(Figure2).
Intheauto-HSCTgroup,1-yearOS was77%forpatients withCDIand60%forpatientswithoutCDI(p-value=0.34).In theallo-HSCTgroup,1-yearOSwas75%forpatientswithCDI and61%forpatientswithoutCDI(p-value=0.19).
Discussion
ThisisthefirststudyreportingCDIoutcomesinaHSCT popu-lationinChile;theoverallincidencewassimilartopreviously publisheddata.16–18 TherewasahigherincidenceofCDIin
patientssubmittedtoallo-HSCTcomparedtopatients sub-mitted to auto-HSCT, a difference that was seen in other studies.19,25–27 No correlation was found between CDI and
increasedfrequencyofotherinfectionsduringthe transplan-tationperiod,andthisstudywasnotabletodemonstrateany correlationbetweentheseverityoftheCDIand knownrisk factors,whichmaybeduetotheubiquityofthesetraditional measuresinpatientssubmittedtoHSCT.28,29
Inother studies,someclinicalcharacteristics are associ-atedwithincreasedriskofCDI,suchasthe disease status attransplantation,age,genderorconditioningregimen.28,30
However, this study did not demonstrate any correlation withthesecharacteristics,possiblyduetothesmallsample sizeofpatientswithCDI.AllthepatientswithCDIreceived at least two types of antibiotic families (cephalosporins,
broad-spectrum penicillin, carbapenems, vancomycin and quinolones) withinthethreemonthsbeforethe procedure, butwithnofurtherriskforthedevelopmentofthediseasein comparisontonon-infectedpatients.
In terms of the transplantation procedure, CDI only occurred in patientssubmitted tomyeloablative condition-ingregimens,afindingthatwasconcordantwithstudiesthat showthatthesetypeofregimensaremoretoxic,increasing endothelialdamage,affectingtheintegrityoftheintestinal mucosa31 andincreasingimmunodeficiency.12,17 Theuseof
TBIis,accordingtosomeauthors,themainriskfactorforthe developmentofCDIduringthefirstweekofconditioning.27
However, in this study, the conditioning regimens did not affecttheCDIrate,probablybecauseonly16outof240patients receivedRIC(p-value=0.24).
ThepeakofCDIinthecurrentstudyoccurredduringthe firstweekaftertransplantation(40%ofcaseswasdiagnosed beforeDay+7),whichcouldberelatedtotheintestinal tox-icitypeakafterTBI.AftertheinitialpeakofCDIinthefirst weekaftertransplant,thecumulativeincidenceremained sta-bleovertimeespeciallyafterauto-HSCT.Interestinglyitseems thattheincidenceofCDIafterallo-HSCTcontinuestoincrease almosttenmonthsaftertransplantationandthenstabilizes, probablyduetolongerimmunosuppressioninthesepatients secondarytoGVHDanddelayedimmunereconstitution com-paredtoauto-HSCT.
PreviousstudieshaveshownthatCDIisariskfactorfor thedevelopmentofGVHD, withavariableincidenceofCDI withaGVHDindifferentstudies,andincreasedfrequencyand severityofepisodesandnewonsetofGIaGVHDassociated withCDIuntilDay 180aftertransplant.19,21,32–34 Thisstudy,
however,didnotdemonstratethisassociation,withno differ-enceintermsofaGVHDandCDI,possiblybecausethenumber ofinfectedpatientswithaGVHDwassmall.
ThisstudyalsofoundthatCDIwasstatisticallymore fre-quentinpatientstransplantedforALLcomparedtoAML,a findingthatcouldbeexplainedbythefactthatthe underly-ingdiseaserequiresmoreintensiveandlongerchemotherapy, priortotheHSCT.Otherpossibleexplanationsforthisfinding arethegreaterimmunodeficiencyinALLpatients,higherrates of antibioticuse for frequentfebrile neutropenia episodes, withmorehospitalizationsforcomplicationsrelatedto ther-apyorotherunknownfactors.
previousevidencethatthisantibioticisthebestalternative as first-line therapy for mild to moderate CDI.35,36 In this
study,onlythreepatientsreceivedvancomycinasafirst-line therapy,twopatientsreceivingoralvancomycinforGI intol-eranceofmetronidazoleandonepatientduetoconcomitant treatmentoffebrileneutropenia,withagoodresponseinall cases.
In this patient population, there were no deaths asso-ciatedwith CDI, a result that is compatible with previous reports.27Whileotherstudieshavefoundincreasedmortality
afterCDIinallo-HSCTpatients,thishasbeenassociatedwith thediagnosisofthemoresevereformsofthedisease.19,34,37
Onlyfour patients (16%) had a second episode ofCDI, all ofwhichoccurred afterDay+40,were ofmildtomoderate severityandrespondedtometronidazole,suggestingthatthis therapy remains efficient even in relapsed cases of CDI.27
TherecurrencerateofCDIobservedinthispopulationwas lowerthanthatobservedinpreviousreportsthatdescribeda meanincidenceofrecurrenceof20%inpatientstreatedwith metronidazoleorvancomycin.38Nospecificreasonsforthis
lowrelapserateofCDIcanbeconcludedfromthisstudybut possiblyitcouldberelatedtofactorssuchasthedifferentdiet andintestinalfloracomparedtodevelopedcountries,lower antibioticuseinthepost-transplantsetting,lessrestrictive dietaftertransplant,specificcharacteristicsoftheClostridium speciesorlocalpracticesinthehospital.Ontheotherhand, CDIinfectionresultedinastatisticallysignificantdelayin neu-trophilengraftmentinallo-HSCTpatients.Thisobservationis concordantwiththeliteraturethatshowsthatcertain infec-tionscancomplicatethe procedureand causeengraftment failure, but untilnow, the maininfectious diseases associ-atedwithengraftment failureareviral infections39 suchas
CMV,40–42 and human herpes virus 6 (HHV-6).43,44 To date,
thereisnoavailabledataindicatinganassociationbetween engraftmentdelayorfailureanddiarrheaassociatedwithCDI, especiallyofmildtomoderateseverity.
ThepresenceofCDIdidnothaveanimpactinOSduring thefirstyearaftertransplant,afindingthatisinagreement withotherreportsfoundintheliterature.27,34Possible
expla-nationsforthisfindingincludetherapididentificationofthe infectionduetothehighindexofsuspicion,absenceofsevere cases,goodsensitivityofC.difficilestrainstometronidazole andvancomycin,goodperformancestatusatthemomentof theinfectionandotherfactorsthatwerenotelucidatedinthe presentstudy.
Themainlimitationsofthisstudyincludeitsretrospective natureandthelackofdataonsomepatientsespeciallythose withoutCDI,butdespitethis,theresultscomparefavorablyto whathasbeenpreviouslypublishedfromdevelopedcountries.
Conclusions
Thesefindingsemphasizetheimportanceofmeasuresto pre-ventCDI,including early suspicion, the appropriateuse of antibiotics,useofcontactprecautionsandtreatment accord-ingtotheseverityofthedisease.Itisimportanttoassessthe roleoftheenvironmentandcleaningprotocolsoftheunit,as thehighestincidenceofCDIoccurswithinthefirstweekafter transplantation.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.McFarlandLV,BauwensJE,MelcherSA,SurawiczCM, GreenbergRN,ElmerGW.Ciprofloxacin-associatedClostridium difficiledisease.Lancet.1995;346(8980):977–8.
2.DubberkeER,CarlingP,CarricoR,DonskeyCJ,LooVG, McDonaldLC,etal.StrategiestopreventClostridiumdifficile
infectionsinacutecarehospitals:2014update.InfectControl HospEpidemiol.2014;35Suppl2:S48–65.
3.BobakD,ArfonsLM,CregerRJ,LazarusHM.Clostridium difficile-associateddiseaseinhumanstemcelltransplant recipients:comingepidemicorfalsealarm.BoneMarrow Transplant.2008;42:705–13.
4.McDonaldLC,OwingsM,JerniganDB.Clostridiumdifficile
infectioninpatientsdischargedfromUSshort-stayhospitals, 1996–2003.EmergInfectDis.2006;12(3):409–15.
5.RedelingsMD,SorvilloF,MascolaL.IncreaseinClostridium difficile-relatedmortalityrates,UnitedStates,1999–2004. EmergInfectDis.2007;13(9):1417–9.
6.KuijperEJ,vandenBergRJ,DebastS,VisserCE,VeenendaalD, TroelstraA,etal.Clostridiumdifficileribotype027,toxinotype III,theNetherlands.EmergInfectDis.2006;12(5):827–30.
7.BadgerVO,LedeboerNA,GrahamMB,EdmistonCEJr.
Clostridiumdifficile:epidemiology,pathogenesis,management, andpreventionofarecalcitranthealthcare-associated pathogen.JPENJParenterEnteralNutr.2012;36(6):645–62.
8.FreemanJ,BauerMP,BainesSD,CorverJ,FawleyWN, GoorhuisB,etal.ThechangingepidemiologyofClostridium difficileinfections.ClinMicrobiolRev.2010;23(3):529–49.
9.TrifilioSM,PiJ,MehtaJ.ChangingepidemiologyofClostridium difficile-associateddiseaseduringstemcelltransplantation. BiolBloodMarrowTransplant.2013;19(3):405–9.
10.BartlettJG.Clinicalpractice.Antibiotic-associateddiarrhea.N EnglJMed.2002;346(5):334–9.
11.KellyCP,PothoulakisC,LaMontJT.Clostridiumdifficilecolitis.N EnglJMed.1994;330:257–62.
12.AnandA,GlattAE.Clostridiumdifficileinfectionassociated withantineoplasticchemotherapy:areview.ClinInfectDis. 1993;17(1):109–13.
13.ZarFA,BakkanagariSR,MoorthiKM,DavisMB.Acomparison ofvancomycinandmetronidazoleforthetreatmentof
Clostridiumdifficile-associateddiarrhea,stratifiedbydisease severity.ClinInfectDis.2007;45:302–7.
14.DubberkeER,ReskeKA,YanY,OlsenMA,McDonaldLC, FraserVJ.Clostridiumdifficile-associateddiseaseinasettingof endemicity:identificationofnovelriskfactors.ClinInfectDis. 2007;45(12):1543–9.
15.PakyzAL,JawaharR,WangQ,HarpeSE.Medicationrisk factorsassociatedwithhealthcare-associatedClostridium difficileinfection:amultilevelmodelcase–controlstudy among64USacademicmedicalcentres.JAntimicrob Chemother.2014;69(4):1127–31.
16.KinnebrewMA,LeeYJ,JenqRR,LipumaL,LittmannER, GobourneA,etal.EarlyClostridiumdifficileinfectionduring allogeneichematopoieticstemcelltransplantation.PLOS ONE.2014;9(3):e90158.
17.GorschlüterM,GlasmacherA,HahnC,SchakowskiF,ZiskeC, MolitorE,etal.Clostridiumdifficileinfectioninpatientswith neutropenia.ClinInfectDis.2001;33(6):786–91.
infectionduringhematopoieticstemcelltransplantation. ClinTransplant.2011;25(1):E82–7.
19.AlonsoCD,TreadwaySB,HannaDB,HuffCA,NeofytosD, CarrollKC,etal.EpidemiologyandoutcomesofClostridium difficileinfectionsinhematopoieticstemcelltransplant recipients.ClinInfectDis.2012;54(8):1053–63.
20.LeungS,MetzgerBS,CurrieBP.IncidenceofClostridiumdifficile
infectioninpatientswithacuteleukemiaandlymphoma afterallogeneichematopoieticstemcelltransplantation. InfectControlHospEpidemiol.2010;31(3):313–5.
21.ChakrabartiS,LeesA,JonesSG,MilliganDW.Clostridium difficileinfectioninallogeneicstemcelltransplantrecipients isassociatedwithseveregraft-versus-hostdiseaseand non-relapsemortality.BoneMarrowTransplant. 2000;26(8):871–6.
22.HerreraP,CoteraA,FicaA,GaldoT,AlvoM.Highincidence andcomplicationsofClostridiumdifficilediarrheaamong patientswithrenaldiseases.RevMedChil.
2003;131(4):397–403.
23.GlucksbergH,StorbR,FeferA,BucknerCD,NeimanPE,Clift RA,etal.Clinicalmanifestationsofgraft-versus-hostdisease inhumanrecipientsofmarrowfromHL-A-matchedsibling donors.Transplantation.1974;18(4):295–304.
24.RowlingsPA,PrzepiorkaD,KleinJP,GaleRP,PasswegJR, Henslee-DowneyPJ,etal.IBMTRSeverityIndexforgrading acutegraft-versus-hostdisease:retrospectivecomparison withGlucksberggrade.BrJHaematol.1997;97(4):855–64.
25.BilgramiS,FeingoldJM,DorskyD,EdwardsRL,BonaRD,Khan AM,etal.IncidenceandoutcomeofClostridiumdifficile
infectionfollowingautologousperipheralbloodstemcell transplantation.BoneMarrowTransplant.
1999;23(10):1039–42.
26.AveryR,PohlmanB,AdalK,BolwellB,GoldmanM,Kalaycio M,etal.Highprevalenceofdiarrheabutinfrequencyof documentedClostridiumdifficileinautologousperipheral bloodprogenitorcelltransplantrecipients.BoneMarrow Transplant.2000;25(1):67–9.
27.WillemsL,PorcherR,LafaurieM,CasinI,RobinM,XhaardA, etal.Clostridiumdifficileinfectionafterallogeneic
hematopoieticstemcelltransplantation:incidence,risk factors,andoutcome.BiolBloodMarrowTransplant. 2012;18(8):1295–301.
28.AnanthakrishnanAN.Clostridiumdifficileinfection: epidemiology,riskfactorsandmanagement.NatRev GastroenterolHepatol.2011;8(1):17–26.
29.HowellMD,NovackV,GrgurichP,SoulliardD,NovackL, PencinaM,etal.Iatrogenicgastricacidsuppressionandthe riskofnosocomialClostridiumdifficileinfection.ArchIntern Med.2010;170(9):784–90.
30.GerdingDN,JohnsonS,PetersonLR,MulliganME,SilvaJJr.
Clostridiumdifficile-associateddiarrheaandcolitis.Infect ControlHospEpidemiol.1995;16(8):459–77.
31.IssaM,AnanthakrishnanAN,BinionDG.Clostridiumdifficile
andinflammatoryboweldisease.InflammBowelDis. 2008;14(10):1432–42.
32.vanKraaijMG,DekkerAW,VerdonckLF,vanLoonAM,VinjéJ, KoopmansMP,etal.Infectiousgastro-enteritis:an
uncommoncauseofdiarrhoeainadultallogeneicand autologousstemcelltransplantrecipients.BoneMarrow Transplant.2000;26(3):299–303.
33.TomblynM,GordonL,SinghalS,TallmanM,WilliamsS, WinterJ,etal.RarityoftoxigenicClostridiumdifficileinfections afterhematopoieticstemcelltransplantation:implications forsymptomaticmanagementofdiarrhea.BoneMarrow Transplant.2002;30(8):517–9.
34.DubberkeER,ReskeKA,SrivastavaA,SadhuJ,GattiR,Young RM,etal.Clostridiumdifficile-associateddiseaseinallogeneic hematopoieticstem-celltransplantrecipients:risk
associations,protectiveassociations,andoutcomes.Clin Transplant.2010;24(2):192–8.
35.WenischC,ParschalkB,HasenhundlM,HirschlAM,Graninger W.Comparisonofvancomycin,teicoplanin,metronidazole, andfusidicacidforthetreatmentofClostridium
difficile-associateddiarrhea.ClinInfectDis.1996;22(5):813–8.
36.TeasleyDG,GerdingDN,OlsonMM,PetersonLR,GebhardRL, SchwartzMJ,etal.Prospectiverandomisedtrialof
metronidazoleversusvancomycinfor
Clostridium-difficile-associateddiarrhoeaandcolitis.Lancet. 1983;2(8358):1043–6.
37.SpadãoF,GerhardtJ,GuimarãesT,DulleyF,AlmeidaJunior JN,BatistaMV,etal.IncidenceofdiarrheabyClostridium difficileinhematologicpatientsandhematopoieticstemcell transplantationpatients:riskfactorsforsevereformsand death.RevInstMedTropSaoPaulo.2014;56(4):325–31.
38.FeketyR,McFarlandLV,SurawiczCM,GreenbergRN,Elmer GW,MulliganME.RecurrentClostridiumdifficilediarrhea: characteristicsofandriskfactorsforpatientsenrolledina prospective,randomized,double-blindedtrial.ClinInfectDis. 1997;24(3):324–33.
39.LocatelliF,LucarelliB,MerliP.Currentandfutureapproaches totreatgraftfailureafterallogeneichematopoieticstemcell transplantation.ExpertOpinPharmacother.2014;15(1):23–36.
40.EnrightH,HaakeR,WeisdorfD,RamsayN,McGlaveP,Kersey J,etal.Cytomegaloviruspneumoniaafterbonemarrow transplantation.Riskfactorsandresponsetotherapy. Transplantation.1993;55(6):1339–46.
41.MeyersJD,LjungmanP,FisherLD.Cytomegalovirusexcretion asapredictorofcytomegalovirusdiseaseaftermarrow transplantation:importanceofcytomegalovirusviremia.J InfectDis.1990;162(2):373–80.
42.GoodrichJM,BoeckhM,BowdenR.Strategiesforthe preventionofcytomegalovirusdiseaseaftermarrow transplantation.ClinInfectDis.1994;19(2):287–98.
43.ZerrDM,CoreyL,KimHW,HuangML,NguyL,BoeckhM. Clinicaloutcomesofhumanherpesvirus6reactivationafter hematopoieticstemcelltransplantation.ClinInfectDis. 2005;40(7):932–40.