w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
The
essential
oil
of
Artemisia
capillaris
protects
against
CCl
4
-induced
liver
injury
in
vivo
Qinghan
Gao
a,b,1,
Xin
Zhao
a,1,
Lei
Yin
a,1,
Yuanbin
Zhang
a,
Bing
Wang
a,
Xiuli
Wu
a,
Xinhui
Zhang
a,
Xueyan
Fu
a,c,∗,
Weihong
Sun
d,∗aSchoolofPharmacy,NingxiaMedicalUniversity,Yinchuan,China bSchoolofPublicHealth,NingxiaMedicalUniversity,Yinchuan,China
cKeyLaboratoryofHuiEthnicMedicineModernization,MinistryofEducation(NingxiaMedicalUniversity),Yinchuan,China dGeneralHospitalofNingxiaMedicalUniversity,Yinchuan,China
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received13July2015 Accepted11January2016 Availableonline28January2016
Keywords: Essentialoil Carbontetrachloride Hepatoprotectiveeffects Invivo
H&E GC–MS
a
b
s
t
r
a
c
t
TostudythehepatoprotectiveeffectoftheessentialoilofArtemisiacapillarisThunb.,Asteraceae,on CCl4-inducedliverinjuryinmice,thelevelsofserumaspartateaminotransferaseandalanine amino-transferase,hepaticlevelsofreducedglutathione,activityofglutathioneperoxidase,andtheactivitiesof superoxidedismutaseandmalondialdehydewereassayed.AdministrationoftheessentialoilofA. capil-larisat100and50mg/kgtomicepriortoCCl4injectionwasshowntoconferstrongerinvivoprotective effectsandcouldobservablyantagonizetheCCl4-inducedincreaseintheserumalanineaminotransferase andaspartateaminotransferaseactivitiesandmalondialdehydelevelsaswellaspreventCCl4-induced decreaseintheantioxidantsuperoxidedismutaseactivity,glutathionelevelandglutathioneperoxidase activity(p<0.01).Theoilmainlycontained-citronellol,1,8-cineole,camphor,linalool,␣-pinene, -pinene,thymolandmyrcene.ThisfindingdemonstratesthattheessentialoilofA.capillariscanprotect hepaticfunctionagainstCCl4-inducedliverinjuryinmice.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Liver disease, a commondisorder caused by viral hepatitis, alcoholism, liver-toxic chemicals, unhealthy dietary habits and environmentalpollution,isaglobalconcern(Papayetal.,2009). However, medical treatment for this disease is often hard to administerandhasaconfinedeffect.TraditionalChineseherbal medicines,whichunderlienumerousprescriptionsusedtotreat liverdiseases,arestillwidelyusedbytheChinese(Zhaoetal.,2014).
Artemisia capillaris Thunb., Asteraceae,according to theBencao Gangmu,themostfamousrecordsofChineseTraditionalMedicine, hasbeenwidelyusedasamedicinetoclearheat,promotediuresis andremovejaundiceandhasalsobeenusedasaflavorin bever-ages,vegetables,andpastriesbecauseofitsparticularfragrance.A. capillarishasbeenregardedasatypeofChinesefolkmedicineand foodbyagrowingnumberofpeople.Therefore,therehavebeen
∗ Correspondingauthors.
E-mails:xueyanfu2661@163.com(X.Fu),tungbuo@163.com(W.Sun).
1Theseauthorsequallycontributedtothismanuscript;theyshouldbeconsidered
firstauthor.
considerableeffortstodevelopusefulherbalmedicines,suchasA. capillaris,forthetreatmentofliverdisease.
Inrecentyears,herbalmedicineshavegainedmoreattention andpopularityforthetreatmentofliverdiseasebecauseoftheir safetyandefficacy(Dingetal.,2012).A.capillarishasbeenproven topossessgoodhepatoprotectiveactivitybasedonmodern phar-macological methods (Han et al.,2006).It is alsoanimportant medicinalmaterialinChinaand isa popularanti-inflammatory
(Chaetal.,2009a),choleretic(YoonandKim,2011),andanti-tumor
(Fengetal.,2013)herbalremedy.
Phytochemicalstudieshaverevealedanumberofvolatile essen-tialoils, coumarins, and flavonol glycosides aswell asa group ofunidentifiedaglyconesfromA.capillaris(Komiyaetal.,1976;
Yamaharaetal.,1989).TheessentialoilofA.capillaris(AEO)isone
ofthemainpharmacologicalactivecompoundsandconfers anti-inflammatory(Chaetal.,2009a)andanti-apoptoticproperties(Cha
etal.,2009b).However,asAEOisoneofthemaincompoundsof
A.capillaris,thepotentialhepatoprotectiveactivitiesofthemajor constituentsfromA.capillarisshouldbeexplored.
In this study, the protective effect of AEO on carbon tetrachloride(CCl4)-inducedhepatotoxicitywasevaluatedby
bio-chemical methods, such as hepatic reduced glutathione (GSH),
http://dx.doi.org/10.1016/j.bjp.2016.01.001
malondialdehyde(MDA)levels,superoxidedismutase(SOD),and glutathioneperoxidase(GSH-Px)activity,aswellastheactivities ofaspartateaminotransferase(AST)andalanineaminotransferase (ALT)intheserum.TheextentofCCl4-inducedliverinjurywasalso
analyzed through histopathological observations, accompanied withphytochemicalanalysisbyGC–MStoidentifytheconstituents ofAEO.
Materialsandmethods
Drugsandchemicals
The essential oil of Artemisia capillaris Thunb., Asteraceae, wasobtainedfromKangshenNaturaloilsCo.,Ltd(Jishui,Jiangxi Province,China).CCl4waspurchasedformDamaoChemical
Com-pany (Tianjin, China). Bifendate tablets were purchased from Yunpeng,ShanxiPharmaceuticalCo.,Ltd.(no.A130602).The diag-nostickitsusedforthedeterminationofALT,AST,SOD,MDA,GSH andGSH-PxwereobtainedfromtheNanjingJianchengInstituteof Biotechnology(Nanjing,China).
AEOanalysis
AEOwasanalyzedby gaschromatography–mass spectrome-try(GC/MS)usingaShimadzuQP2010plusGCwithRxi-5SilMS (30m×0.25mm; 0.25mfilm thickness). The GC/MS was run under thefollowing conditions: a fused-silica capillary column with heliumat 1.60ml/min and an injector at 250◦C. The GC oventemperaturewasinitiallyheldat40◦Cfor5min.Thereafter, thetemperaturewasraisedwithagradientof3◦Cmin−1untilit
reached230◦C.Thistemperaturewasheldfor3min.Finally,the temperaturewasthenraisedwitha gradientof1◦Cmin−1 until
260◦Candagainheldfor10min.Massspectraweretakenat70eV from33to500Da.Identificationofthecompoundswasbasedon thecomparisonofthemassspectraldataonacomputermatched withNIST(similarityindex>85%)andthosedescribedinthe lit-erature(Sylvestre etal., 2006; Chenget al., 2008; Ait-Ouazzou
etal.,2012;ArgyropoulouandSkaltsa,2012; ´Cavaretal.,2012;Ben
Mansouretal.,2013;Chenetal.,2013;GouveiaandCastilho,2013;
MuruganandMallavarapu,2013;Singhetal.,2013;Bagherietal.,
2014;DaSilvaetal.,2014;Desaietal.,2014;Pandeyetal.,2014;
Qietal.,2014;Ratheretal.,2014;Sadgroveetal.,2014;Sereshti
etal.,2014;Taoetal.,2014;Tianetal.,2014).Theidentification
ofcompoundswasperformedaccordingtotheirretentionindices relativetoC8–C24n-alkanesandMS.
Animals
MaleICRmiceat6–8weeksofage(18–22g)werepurchased fromtheMedicineExperimentAnimalCenterofNingxiaMedicine University.Theanimalswereallowedtoacclimatizefortwodays toanimalroomconditionsandweremaintainedonastandard pel-letdietandwateradlibitum.Thefoodwaswithdrawnontheday beforetheexperiment,buttheanimalswereallowedfreeaccess towater.AllanimalswerecaredforincompliancewiththeGuide fortheCareandUseofLaboratoryAnimals(1996).The experimen-talprocedureswereapprovedbyourinstitutionalanimalresearch ethicscommittee(approvalnumber:SYXK(NING)2011-0001).
Rodentmodeldesign
Theanimalswererandomlydividedintofivegroupsoffifteen miceeach.Thecontrolgroupand modelgroupmicewere gav-agedwithsesameoilfor6days.Positivecontrolgroupmicewere gavagedwithbifendatetablets(BT,10mg/kg)for6days.The exper-imentalgroupsweretreatedwith100mg/kgand50mg/kgAEO
dissolvedinsesameoilfor6days.Onday6,thecontrolgroupwas treatedwithsesameoil,andalloftheothergroupsweretreated withasingledoseof0.2%CCl4insesameoil(10ml/kg)by
intraperi-tonealinjection.Themicewerethenfastedfreeofwater,andblood samples werecollected from the retrobulbarvessels; collected bloodwascentrifugedat3000×gfor10mintoseparatetheserum. Cervicaldislocationwasperformedimmediatelyafterwithdrawal ofblood,andliversampleswerepromptlyremoved.Onepartof theliversamplewasimmediatelystoredat−20◦Cuntilanalysis, andanotherpartwasexcisedandfixedina10%formalinsolution; theremainingtissueswerestoredat−80◦Cforhistopathological analysis(Wangetal.,2008;Hsuetal.,2009;Nieetal.,2015).
Measurementofthebiochemicalparametersintheserum
Liverinjurywasassessedbyestimatingtheenzymaticactivities ofserumALTand ASTusingthecorrespondingcommercialkits accordingtotheinstructionsforthekits(Nanjing,JiangsuProvince, China).Theenzymaticactivitieswereexpressedasunitsperliter (U/l).
MeasurementofMDA,SOD,GSHandGSH-Pxinliverhomogenates
Livertissueswerehomogenizedwithcoldphysiologicalsaline ata1:9ratio(w/v,liver:saline).Thehomogenateswerecentrifuged (2500×gfor 10min) tocollectthe supernatantsfor the subse-quentdeterminations.Liverdamagewasassessedaccordingtothe hepaticmeasurementsoftheMDAandGSHlevelsaswellasthe SODandGSH-Pxactivities.Alloftheseweredetermined follow-ingtheinstructionsonthekit(Nanjing,JiangsuProvince,China). TheresultsforMDAandGSHwereexpressedasnmolpermg pro-tein(nmol/mgprot),andtheactivitiesofSODandGSH-Pxwere expressedasUpermgprotein(U/mgprot).
Histopathologicalanalysis
Portionsoffreshlyobtainedliverwerefixedina10%buffered paraformaldehyde phosphate solution. The sample was then embeddedin paraffin,slicedinto3–5msections, stainedwith hematoxylinandeosin(H&E)accordingtoastandardprocedure, andfinallyanalyzedbylightmicroscopy(Tianetal.,2012).
Statisticalanalysis
Theresultswereexpressedasthemean±standarddeviation (SD).TheresultswereanalyzedusingthestatisticalprogramSPSS Statistics,version19.0.Thedataweresubjectedtoananalysisof variance(ANOVA, p<0.05)followed byDunnett’stest and Dun-nett’sT3testtodeterminethestatisticallysignificantdifferences
betweenthevaluesofvariousexperimentalgroups.Asignificant differencewasconsideredatalevelofp<0.05.
Resultsanddiscussion
ConstituentsofAEO
Table1
ChemicalcompositionofessentialoilfromArtemisiacapillarisThun.(AEO).
Compound Rta RIcalcb RIlitc %d
␣-Pinene 11.133 928 933 7.21
Camphene 11.340 943 946 1.52
Sabinene 13.269 968 969 1.09
-Pinene 13.640 975 974 3.99
Myrcene 14.313 987 988 2.02
Iso-cineole 15.612 1012 1012 1.27
p-Cymene 16.025 1020 1020 2.28
Limonene 16.273 1025 1024 2.00
1,8-Cineole 16.424 1028 1026 13.09
Artemisiaketone 17.965 1057 1056 1.44
Terpinolene 19.226 1081 1086 1.30
Linalool 20.112 1098 1095 11.33
trans-Pinocarveol 17.965 1135 1135 1.44
Camphor 22.215 1140 1141 12.59
Terpinen-4-ol 23.998 1176 1174 1.21
␣-Terpineol 24.744 1191 1186 1.89
Dodecane 25.173 1199 1200 2.44
-Citronellol 26.407 1226 1211 16.23
Thymol 29.356 1288 1289 3.22
Longipinene 34.330 1401 1402 2.16
(E)-Caryophyllene 34.813 1413 1417 1.73
aRt–retentiontime(min)formalineartemperatureprogram. bRIcalc–retentionindexcalculatedforeachcompound. c RIlit–retentionindexobtainfrompublishedliteratures. d %–relativeabundancesfromthepeakareaintegration.
(7.21%),-pinene(3.99%),thymol(3.22%), andmyrcene(2.02%). Thevariationinthechemicalcompositionmayberelatedtothe environmentalconditionsthattheplantwasexposedto,suchas mineralwater,sunlight,thestageofdevelopmentandnutrition.
Aspreviouslysuggested,fewstudieshavereportedthe hepato-protectiveeffectsof someofthesemajorcompounds.However, therearefindingsthatindicatethatmajorcompoundspossessan anti-inflammatoryeffect,suchasthymol(Bragaetal.,2006), euca-lyptol,limonene,andlinalool(KuandLin,2013).-Myrceneand 1,8-cineolewerealsofoundtoeliminateTCDD-inducedoxidative stressinrats(Ciftcietal.,2011).
Measurementofthebiochemicalparametersintheserum
CCl4-inducedhepaticinjury isa commonlyused
experimen-talanimalmodel(JohnstonandKroening,1998).Apreviousstudy reportedthatCCl4administrationisassociatedwithactivationby
thecytochromesystem(e.g.,CYP2E1)toformtrichloromethyl
radi-cals(CCl3•).ItisgenerallythoughtthatCCl4 toxicityisduetoa
reactiveintermediatethatisgeneratedbyitsreductivemetabolism. Thishighlyreactiveintermediateisknowntoinducelipid peroxida-tion,oxidativestress,hepaticnecrosisandapoptosis(Weberetal.,
2003).
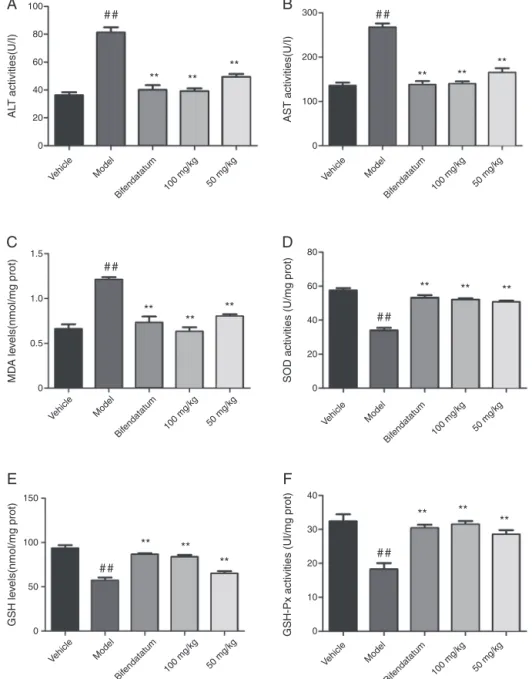
Inthisassay,theresultsofthehepatoprotectiveeffectsofAEOon theenzymaticactivitiesofserumALTandASTareshowninFig.1A andB,respectively.AnintraperitonealinjectionofCCl4 induced
anotableincreaseinALTandASTactivitiesincomparison with untreatedcontrolmice(p<0.01);theincreasedserumactivitiesof ALTandASTenzymesinCCl4treatedmiceconfirmedthehepatic
damage.Conversely,pretreatmentwithAEOatdosesof100mg/kg and50mg/kg,aswellasbifendatetablets,obviouslyreversedthe toxin-inducedincreaseintheALTandASTlevelscomparedwith toxinmodelgroup2(p<0.01).Whenthedosereached100mg/kg, theresultswereasgoodasBT.
ALTandASTareimportantmetabolicenzymesoftheliver.After CCl4administration,theserumALTandASTactivitiesinmicewere
evaluated.These enzymesnormallyexist in thecytoplasm, but upon liverinjury, theycanenter thecirculatory systemdueto
toxicity-mediatedalteredpermeabilityofthecellularmembrane
(WillsandAsha,2006).Inapreviousstudy,itwasreportedthat
administrationofCCl4inmicecausedincreasedALTandAST
activ-ities.
OurdatashowedthattheliverdamageinducedbyCCl4
ele-vateslivermarkerenzymes.Elevatedactivitiesofserumenzymes, ALTandASTareindicativeofcellularleakageandthelossofthe functionalintegrityofthecellmembraneintheliver,while pre-treatmentwithAEOeffectivelydecreasedtheamountofASTand ALTleakage.
MeasurementofMDA,SOD,GSHandGSH-Pxinliverhomogenates
ThelevelsofMDAsuggestenhancedperoxidationleadingto tissuedamageandfailureoftheantioxidant-defensemechanisms topreventtheformationofexcessivefreeradicals(Chengetal.,
2013; Pareeket al., 2013).However, AEO at 50 and 100mg/kg
couldmarkedlypreventtheincreaseinMDAformation(Fig.1C), whichclearlydemonstratedtheabilityofAEOtorelievelipid per-oxidation.MDAiswellknowntobethemostabundantindividual aldehyderesultingfromlipidperoxidationandiscommonlyused asanindicatoroflivertissuedamageinvolvingaseriesof oxida-tivechainreactions(Huangetal.,2012).Inthepresentresearch, micetreatedwithCCl4showedastrikingincreaseinMDAlevels
compared tountreatednormal mice(p<0.01).SODand GSH-Px
arethemajorenzymesthatplayanimportantroleinthe elim-ination of toxic metabolites, which are the major cause of the liver pathology caused by CCl4 (Cengiz et al., 2013; Xia et al.,
2013).Here,administrationofCCl4tomicesharplydecreasedthe
SODandGSH-Pxactivitiesinmouselivertissues,asevidencedby theinhibitionoftheirenzymaticactivities.However,thedecrease in theseenzymatic activities wassignificantlyelevated by pre-treatmentwithAEO,suggestingthattheycouldprotectthethree antioxidantenzymesinCCl4-damagedlivers.Theprotectiveeffect
of AEO waspotent in terms of the SOD and GSH-Px activities, whichismostlikelyrelatedtotheeliminationoftoxic metabo-lites(Fig.1DandF).SODisamanganese-containingenzymeinthe mitochondriaandconvertsthedismutationofsuperoxideanions into hydrogen peroxide (H2O2) (Reiter et al., 2000).GSH-Px is
animportant enzymethat catalyzesthereduction ofH2O2 and
hydroperoxidesandterminatesthechainreactionoflipid peroxi-dationbyremovinglipidhydroperoxidesfromthecellmembrane (Aietal.,2013).Similarly,GSHisanimportantwater-phase antiox-idantandanessentialcofactorforantioxidantenzymes;itprotects themitochondriaagainstendogenousoxygenradicals(Hanetal., 2006).TheendogenousantioxidantGSHperoxidaseinhibits oxida-tive stressby quickly removing superoxideradicals (Chaudiere et al.,1999) and playsanimportant role inclearing intracellu-lar hydrogenperoxide and lipid peroxides. Therefore, thelevel ofGSHinthebodyisconsideredtobeanimportantindicatorof antioxidativecapacity(Campoetal.,2001;Iwamotoetal.,2002).
Fig.1EshowedthatCCl4treatmentinducedasignificantdecrease
inthelevelofGSHinliverhomogenatescomparedtocontrollivers. ThetreatmentofmicewithAEOat50and100mg/kgsignificantly increasedthehepaticGSHcontentcomparedwiththeCCl4-treated
group.Thisfindingindicatedthatthefreeradicalsreleasedinthe liverwereeffectivelyscavengedduringAEOtreatment.Allofthese data suggest that thehepatoprotective effects of AEO onCCl4
-inducedliverdamageinmiceareassociatedwithitscapacityfor freeradicalscavengingandantioxidation.
Histopathologicalexaminationofmouselivers
100
A
B
C
D
E
F
300
200
100
0 80
60
40
20
** ** **
** ** **
## ##
** **
**
** ** ** ##
##
** ** **
** ** **
##
##
AL
T activities(U/I)
AST activities(U/I)
MD
A le
ve
ls(nmol/mg prot)
GSH le
vels(nmol/mg prot)
GSH-Px activities (Ul/mg prot)
SOD activities (U/mg prot)
0
80
60
40
20
0
40
30
20
10
0 1.5
1.0
0.5
0
150
100
50
0
Vehicle Model
Bifendatatum 100 mg/kg
50 mg/kg
Vehicle Model
Bifendatatum 100 mg/kg
50 mg/kg
Vehicle Model
Bifendatatum 100 mg/kg
50 mg/kg
Vehicle Model
Bifendatatum 100 mg/kg
50 mg/kg
Vehicle Model
Bifendatatum 100 mg/kg
50 mg/kg
Vehicle Model
Bifendatatum 100 mg/kg
50 mg/kg
Fig.1.EffectsoforalpretreatmentwithAEOonCCl4-inducedhepaticinjury.Theresultsofthebiochemicalparameters(ALT,AST)andhepaticlevels(MDA,SOD,GSH
andGSH-Px)arerepresentedasthemean±SD(n=15).ThemicewerepretreatedwithAEO(100,50mg/kg)oncedailyfor6consecutivedays.Thecontrolmiceweregiven sesameoil.DifferencesbetweenthegroupsweredeterminedbyANOVAfollowedbyDunnett’stest.*p<0.05,**p<0.01comparedtothemodelgroup,and#p<0.05,##p<0.01
comparedtothecontrolgroup.
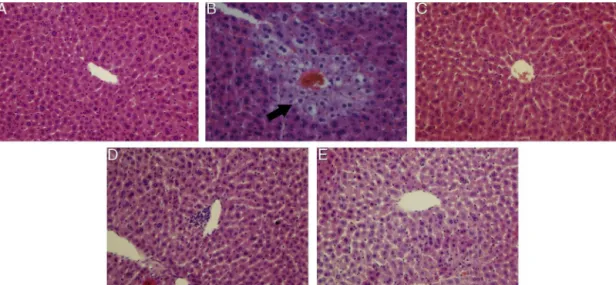
hepaticcellswithawell-preservedcytoplasmandlegiblenucleus, hepatocytesthatwereradiallyarrangedaroundthecentralvein, andawell-definedsinusoidalline(Fig.2A).CCl4causeddamageto
thehepaticarchitectureandproducedhistologicalchanges,suchas severehepatocellulardegenerationandnecrosisaroundthecentral vein,sinusoidaldilatation,lossofcellularboundaries, inflamma-torycell infiltration, and cytoplasmic vacuolation,all of which confirmedthesuccessfulestablishment ofliverinjury (Fig.2B). In contrast, CCl4-intoxicated mice pretreated with BT showed
nearnormalization of liver tissues withno significant changes inhepatocytes(Fig.2C).The liverdamagewasnoteworthyand dose-dependentlyreducedbypretreatmentwithAEOatdifferent doses,asindicatedbythesignificantreductioninthenumberof ballooning-degeneratedhepatocytes and significantlydecreased
necrotic area. In the grouppretreated with a low doseof AEO (Fig.2E),theliversectionsshowedmoderatehypertrophyof hepa-tocyteswitharelativelyintactcentralvein,ashrunkensinusoidal areaandreducedinflammatorycells.Theadministrationofahigh doseofAEO(Fig.2D)inducedanear-normalappearance, suggest-ingthatAEOatadoseof100mg/kgwasmoreeffectivecompared tothedoseof50mg/kgandthatAEOcouldprotecttheliverfrom acuteCCl4-inducedhepaticdamage.Thiswasingoodagreement
withtheresultsfromtheserumbiochemicalmarkers.
Insummary,theresultsfromthisstudyclearlydemonstratethat AEOiseffectiveforthepreventionofCCl4-inducedhepaticinjury
inmice.Thisstudyindicatesthepotentialforthedevelopmentof AEOasapotentialhepatoprotectiveagentincasesofCCl4induced
Fig.2.EffectsofAEOonthehistologicalchangesoftheliverafterCCl4treatmentinmice(originalmagnification400×):(A)controlgroup;(B)CCl4-intoxicatedgroup(model
group);(C)bifendatatum(10mg/kg)+CCl4;(D)AEO(100mg/kg)+CCl4;(E)AEO(50mg/kg)+CCl4.
Authorcontributions
QG,LYandXZcontributedtoperformingthelaboratorywork. YZandXZcontributedtotheestimationofthechemical compo-sition.BWcontributedtotheanalysisofthedata.LYwrotethe manuscript.QGandXWcontributedtothecriticalreadingofthe manuscript.XFandWSdesignedthestudy,supervisedthe labora-toryworkandcontributedtothecriticalreadingofthemanuscript. Alloftheauthorshavereadthefinalmanuscriptandapprovedits submission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (Project No. 2013BAI05B01).
References
Ai,G.,Liu,Q.,Hua,W.,Huang,Z.,Wang,D.,2013.Hepatoprotectiveevaluationof thetotalflavonoidsextractedfromflowersofAbelmoschusmanihot(L.)Medic:
invitroandinvivostudies.J.Ethnopharmacol.146,794–802.
Ait-Ouazzou,A.,Lorán,S.,Arakrak,A.,Laglaoui,A.,Rota,C.,Herrera,A.,Pagán,R., Conchello,P.,2012.Evaluationofthechemicalcompositionandantimicrobial activityofMenthapulegium,Juniperusphoenicea,andCyperuslongusessential oilsfromMorocco.FoodRes.Int.45,313–319.
Argyropoulou,C.,Skaltsa,H.,2012.Identificationofessentialoilcomponentsof Mar-rubiumthessalumBoiss.&Heldr.growingwildinGreece.Nat.Prod.Rep.26, 593–599.
Bagheri,H.,AbdulManap,M.Y.B.,Solati,Z.,2014.Responsesurfacemethodology appliedtosupercriticalcarbondioxideextractionofPipernigrumL.essential oil.LWT–FoodSci.Technol.57,149–155.
BenMansour,M.,Balti,R.,Rabaoui,L.,Bougatef,A.,Guerfel,M.,2013.Chemical compositionangiotensinI-convertingenzyme(ACE)inhibitory,antioxidantand antimicrobialactivitiesoftheessentialoilfromsouthTunisianAjugapseudoiva
Rob.Lamiaceae.ProcessBiochem.48,723–729.
Braga,P.C.,DalSasso,M.,Culici,M.,Bianchi,T.,Bordoni,L.,Marabini,L.,2006. Anti-inflammatoryactivityofthymol:inhibitoryeffectonthereleaseofhuman neutrophilelastase.Pharmacology77,130–136.
´Cavar,S.,Maksimovi ´c,M.,Vidic,D.,Pari ´c,A.,2012.Chemicalcompositionand antiox-idantandantimicrobialactivityofessentialoilofArtemisiaannuaL.fromBosnia. Ind.CropsProd.37,479–485.
Cengiz,N.,Kavak,S.,Guzel,A.,Ozbek,H.,Bektas,H.,Him,A.,Erdo˘gan,E.,Balahoro˘glu, R.,2013.InvestigationofthehepatoprotectiveeffectsofSesame(Sesamum indicumL.)incarbontetrachloride-inducedlivertoxicity.J.Membr.Biol.246, 1–6.
Cha,J.D.,Moon,S.E.,Kim,H.Y.,Lee,J.C.,Lee,K.Y.,2009a.Theessentialoilisolated fromArtemisiacapillarispreventsLPS-inducedproductionofNOandPGE(2)by inhibitingMAPK-mediatedpathwaysinRAW2647macrophages.Immunol. Invest.38,483–497.
Cha,J.D.,Moon,S.E.,Kim,H.Y.,Cha,I.H.,Lee,K.Y.,2009b.EssentialoilofArtemisia capillarisinducesapoptosisinKBcellsviamitochondrialstressandcaspase activationmediatedbyMAPK-stimulatedsignalingpathway.J.FoodSci.74, T75–T81.
Chen,Y.,Zhou,C.,Ge,Z.,Liu,Y.,Liu,Y.,Feng,W.,Li,S.,Chen,G.,Wei,T.,2013. Compo-sitionandpotentialanticanceractivitiesofessentialoilsobtainedfrommyrrh andfrankincense.Oncol.Lett.6,1140–1146.
Cheng,N.,Ren,N.,Gao,H.,Lei,X.,Zheng,J.,Cao,W.,2013.Antioxidantand hepato-protectiveeffectsofSchisandrachinensispollenextractonCCl4-inducedacute
liverdamageinmice.FoodChem.Toxicol.55,234–240.
Cheng,S.S.,Liu,J.Y.,Lin,C.Y.,Hsui,Y.R.,Lu,M.C.,Wu,W.J.,Chang,S.T.,2008. Ter-minatingredimportedfireantsusingCinnamomumosmophloeumleafessential oil.Bioresour.Technol.99,889–893.
Ciftci,O.,Ozdemir,I.,Tanyildizi,S.,Yildiz,S.,Oguzturk,H.,2011.Antioxidativeeffects ofcurcumin,beta-myrceneand1,8-cineoleagainst 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicol. Ind. Health 27, 447–453.
Da Silva,J.K.R.,Pinto, L.C.,Burbano,R.M.R., Montenegro,R.C., Guimarães,E.F., Andrade,E.H.A.,Maia,J.G.,2014.EssentialoilsofAmazonPiperspeciesandtheir cytotoxicantifungal,antioxidantandanti-cholinesteraseactivities.Ind.Crop. Prod.58,55–60.
Desai,M.A.,Parikh,J.,De,A.K.,2014.Modellingandoptimizationstudieson extrac-tionoflemongrassoilfromCymbopogonflexuosus(Steud.).Wats.Chem.Eng. Res.Des92,793–803.
Ding,R.B.,Tian,K.,Huang,L.L.,He,C.W.,Jiang,Y.,Wang,Y.T.,Wan,J.B.,2012.Herbal medicinesforthepreventionofalcoholicliverdisease:areview.J. Ethnophar-macol.144,457–465.
Feng,G.,Wang,X.,You,C.,Cheng,X.,Han,Z.,Zong,L.,Zhou,C.,Zhang,M.,2013. AntiproliferativepotentialofArtemisiacapillarispolysaccharideagainsthuman nasopharyngealcarcinomacells.Carbohydr.Polym.92,1040–1045.
Gouveia,S.C.,Castilho,P.C.,2013.ArtemisiaannuaL.:essentialoilandacetoneextract compositionandantioxidantcapacity.Ind.Crop.Prod.45,170–181. Guo,F.Q.,Liang,Y.Z.,Xu,C.J.,Li,X.N.,Huang,L.F.,2004.Analyzingofthevolatile
chemicalconstituentsinArtemisiacapillarisherbabyGC–MSandcorrelative chemometricresolutionmethods.J.Pharm.Biomed.Anal.35,469–478. Han,K.H.,Jeon,Y.J.,Athukorala,Y.,Choi,K.D.,Kim,C.J.,Cho,J.K.,Sekikawa,M.,
Fukushima,M.,Lee,C.H.,2006.AwaterextractofArtemisiacapillarisprevents 2,2′-azobis(2-amidinopropane)dihydrochloride-inducedliverdamageinrats.J.
Med.Food9,342–347.
Hsu,Y.W.,Tsai,C.F.,Chen,W.K.,Lu,F.J.,2009.Protectiveeffectsofseabuckthorn (HippophaerhamnoidesL.)seedoilagainstcarbontetrachloride-induced hepa-totoxicityinmice.FoodChem.Toxicol.47,2281–2288.
Huang,Q.,Zhang,S.,Zheng,L.,He,M.,Huang,R.,Lin,X.,2012.Hepatoprotective effectsoftotalsaponinsisolatedfromTaraphochlamysaffinisagainstcarbon tetrachlorideinducedliverinjuryinrats.FoodChem.Toxicol.50,713–718. Johnston,D.E.,Kroening,C.,1998.Mechanismofearlycarbontetrachloridetoxicity
inculturedrathepatocytes.Pharmacol.Toxicol.83,231–239.
Komiya,T.,Naruse,Y.,Oshio,H.,1976.StudiesonInchinkoII.Studiesonthe com-poundsrelatedtocapillarisinandflavonoids.YakugakuZasshi96,855–862. Ku,C.M.,Lin,J.Y.,2013.Anti-inflammatoryeffectsof27selectedterpenoid
com-poundstestedthroughmodulatingTh1/Th2cytokinesecretionprofilesusing murineprimarysplenocytes.FoodChem.141,1104–1113.
Nie,Y.,Ren,D.,Lu,X.,Sun,Y.,Yang,X.,2015.Differentialprotectiveeffectsof polyphenolextractsfromapplepeelsandfleshesagainstacuteCCl(4)-induced liverdamageinmice.FoodFunct.6,513–524.
Pandey,V.,Verma,R.S.,Chauhan,A.,Tiwari,R.,2014.Compositionalvariationin theleafflowerandstemessentialoilsofHyssop(HyssopusofficinalisL.)from Western-Himalaya.J.Herb.Med.4,89–95.
Papay,J.I.,Clines,D.,Rafi,R.,Yuen,N.,Britt,S.D.,Walsh,J.S.,Hunt,C.M.,2009. Drug-inducedliverinjuryfollowingpositivedrugrechallenge.Regul.Toxicol.Pharm. 54,84–90.
Pareek,A.,Godavarthi,A.,Issarani,R.,Nagori,B.P.,2013.Antioxidantand hepato-protectiveactivityofFagoniaschweinfurthii(Hadidi)Hadidiextractincarbon tetrachlorideinducedhepatotoxicityinHepG2celllineandrats.J. Ethnophar-macol.150,973–981.
Qi,X.-L.,Li,T.-T.,Wei,Z.-F.,Guo,N.,Luo,M.,Wang,W.,Zu,Y.-G.,Fu,Y.-J.,Peng,X., 2014.Solvent-freemicrowaveextractionofessentialoilfrompigeonpealeaves [Cajanuscajan(L.)Millsp.]andevaluationofitsantimicrobialactivity.Ind.Crop Prod.58,322–328.
Rather,M.A.,Dar,B.A.,Shah,W.A.,Prabhakar,A.,Bindu,K.,Banday,J.A.,Qurishi,M.A., 2014.ComprehensiveGC–FIDGC–MSandFT-IRspectroscopicanalysisofthe volatilearomaconstituentsofArtemisiaindicaandArtemisiavestitaessential oils.Arab.J.Chem.,http://dx.doi.org/10.1016/j.arabjc.2014.05.017.
Reiter,R.,Tan,D.-X.,Osuna,C.,Gitto,E.,2000.Actionsofmelatonininthereduction ofoxidativestress.J.Biomed.Sci.7,444–458.
Sadgrove, N.J.,Goncalves-Martins, M.,Jones, G.L.,2014. Chemogeography and antimicrobialactivityofessentialoilsfromGeijeraparvifloraandGeijera sali-cifolia(Rutaceae):twotraditionalAustralianmedicinalplants.Phytochemistry 104,60–71.
Sereshti,H.,Heidari,R.,Samadi,S.,2014.Determinationofvolatilecomponents ofsaffronbyoptimisedultrasound-assistedextractionintandemwith dis-persiveliquid–liquidmicroextractionfollowedbygaschromatography–mass spectrometry.FoodChem.143,499–505.
Singh,S.,Kapoor,I.P.S.,Singh,G.,Schuff,C.,DeLampasona,M.P.,Catalan,C.A.N., 2013.Chemistryantioxidantandantimicrobialpotentialsofwhitepepper(Piper nigrumL.)essentialoilandoleoresins.Proc.Natl.Acad.Sci.IndiaB83,357–366.
Sylvestre,M.,Pichette,A.,Longtin,A.,Nagau,F.,Legault,J.,2006.Essentialoilanalysis andanticanceractivityofleafessentialoilofCrotonflavensL.fromGuadeloupe. J.Ethnopharmacol.103,99–102.
Tao,N.,Jia,L.,Zhou,H.,2014.Anti-fungalactivityofCitrusreticulataBlanco essen-tialoilagainstPenicilliumitalicumandPenicilliumdigitatum.FoodChem.153, 265–271.
Tian,J.,Zeng,X.,Zhang,S.,Wang,Y.,Zhang,P.,Lü,A.,Peng,X.,2014.Regional varia-tionincomponentsandantioxidantandantifungalactivitiesofPerillafrutescens
essentialoilsinChina.Ind.CropProd.59,69–79.
Tian,L.,Shi,X.,Yu,L.,Zhu,J.,Ma,R.,Yang,X.,2012.Chemicalcompositionand hepatoprotectiveeffectsofpolyphenol-richextractfromHouttuyniacordatatea. J.Agric.FoodChem.60,4641–4648.
Wang,N.,Li,P.,Wang,Y.,Peng,W.,Wu,Z.,Tan,S.,Liang,S.,Shen,X.,Su,W., 2008.HepatoprotectiveeffectofHypericumjaponicumextractanditsfractions. J.Ethnopharmacol.116,1–6.
Weber,L.W.,Boll,M.,Stampfl,A.,2003.Hepatotoxicityandmechanismofactionof haloalkanes:carbontetrachlorideasatoxicologicalmodel.Crit.Rev.Toxicol.33, 105–136.
Wills,P.J.,Asha,V.V.,2006.ProtectiveeffectofLygodiumflexuosum(L)Sw.extract againstcarbontetrachloride-inducedacuteliverinjuryinrats.J. Ethnopharma-col.108,320–326.
Xia,D.Z.,Zhang,P.H.,Fu,Y.,Yu,W.F.,Ju,M.T.,2013.Hepatoprotectiveactivityof puerarinagainstcarbontetrachloride-inducedinjuriesinrats:arandomized controlledtrial.FoodChem.Toxicol.59,90–95.
Yamahara, J., Kobayashi, G., Matsuda, H., Katayama, T., Fujimura, H., 1989. Theeffect ofscoparone a coumarinderivative isolated from theChinese crudedrug Artemisiaecapillarisflos,ontheheart.Chem.Pharm. Bull.37, 1297–1299.
Yoon,M.,Kim,M.Y.,2011.Theanti-angiogenicherbalcompositionOb-XfromMorus albaMelissaofficinalis,andArtemisiacapillarisregulatesobesityingenetically obeseob/obmice.Pharm.Biol.49,614–619.