Seed priming of bread wheat with Iron and Zinc: effects in
germination, mitosis, nucleolar activity and grain yield
Dissertação de Mestrado em Genética Molecular Comparativa e Tecnológica
Sara Alexandra Domingos Reis
Orientador: Professor Doutor José Eduardo Lima-Brito
Co-Orientador: Doutora Ana Isabel Ferreira de Carvalho
i
Universidade de Trás-os-Montes e Alto Douro
Seed priming of bread wheat with Iron and Zinc: effects in
germination, mitosis, nucleolar activity and grain yield
Dissertação de Mestrado em Genética Molecular Comparativa e Tecnológica
Sara Alexandra Domingos Reis
Orientador: Professor Doutor José Eduardo Lima-Brito
Co-Orientador: Doutora Ana Isabel Ferreira de Carvalho
Composição do Júri:
______________________________________________ ______________________________________________ ______________________________________________
iii
This original research was developed to achieve the Master Degree in Molecular Comparative and Technological Genetics “DR, 2ª série – Nº 133 – Regulamento nº 658/2016 de 13 de julho de 2016”.
v
This dissertation is dedicated to my parents and brother
vii
Acknowledgements
This work was developed in the Laboratory of Plant Cytogenomics at the University of Trás-os-Montes e Alto Douro (UTAD).
I would like to thank to the several people that contributed to the realization of my work.
To the Rector of the University of Trás-os-Montes e Alto Douro, Professor António Fontainhas Fernandes, Ph.D., I thank the possibility of the realization of my dissertation at this university.
To the Direction of the 2nd Cycle of Studies (Master Degree) in Molecular, Comparative and Technological Genetics of UTAD, I thank for accepting this dissertation. Particularlly, I would like to thank Professor Paula Martins-Lopes, Ph.D., and Professor Raquel Chaves, Ph.D., for their availability.
To Professor José Eduardo Lima-Brito, Ph.D., my supervisor, in charge for the Laboratory of Plant Cytogenomics of UTAD, I would like to thank for giving me the opportunity to join his team, conduct my research, friendship, transmission of knowledge, critical review of the work and understanding, concerning the encouragement to overcome any difficulties.
To Ana Isabel Ferreira de Carvalho, Ph.D., my co-supervisor, I thank for all the care, time spent, knowledge and disposal and availability throughout this work. I also would like to thank for all the supporting words and moments of distraction and friendship in and out the laboratory.
To all my teachers, among these years, I thank for all the taught knowledge which gave me the bases for the performance and interpretation of this work.
To my lab companions, thank you for all the fellowship and the good work environment that made every day easier and funnier.
To Ivo Pavia, MSc., I would like to thank for all the help and collaboration in this work.
To my dear friends, that have been by my side through all this years and stages of my life, I would like to thank you for your true and genuine friendship, for all the support, understanding and for caring so much about me!
viii and, of course, for the moments of distraction.
To Fábio Sigre, I would like to thank you for all the love, care, support and understanding in the good and bad days, for all the words and all the laughs. Thank you for never letting me give up of my dreams and objectives.
To my beautiful family, for being my foundation, for all the love that makes me the person I am, for being present, supportive, kind and bringing me so much happiness.
To my dear Father, I thank you for being an example and inspiration, both scientifically and personally, for all the help and tireless support, for never doubting me, for the care and love that you give me. Thank you for always being proud of me!
To my dear Mother, for being the most extraordinary human being I know, for all the things you taught me, the kindness and humility that you incused in me all these years, for being patient, indefatigable and trying to make me a better human being every day!
To my favorite person, my little brother, thank you for being so dear to me and despite of your young age, thank you for being so reasonable, understanding and supportive. Thank you for showing me an inspiring smile every day and bring so much color to my life.
To all not mentioned here but they were always by my side throughout this work, thank you so much for everything.
ix
Seed priming of bread wheat with Iron and Zinc: effects in germination,
mitosis, nucleolar activity and grain yield
Abstract
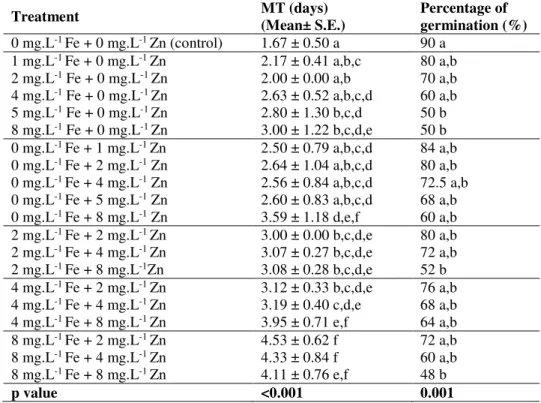
Iron (Fe) and Zinc (Zn) are important micronutrients in plant metabolism and development. However, in some types of soils, the availability of soluble Fe and Zn can be reduced, and the exogenous application of these micronutrients is required. The suitable dosage of each micronutrient to be applied for biofortification purposes in bread wheat and other plant species requires previous studies at different levels. This study intends to evaluate the effects of seed priming with different dosages (1 mg.L-1 to 8 mg.L-1) of Fe and/or Zn in germination, mitosis, nucleolar activity and yield-related characters of the Portuguese bread wheat cv. ‘Jordão’. Concentrations of Fe and/or Zn higher than 4 mg.L-1 significantly decreased the germination rate and increased the mean time to germination relative to control (0 mg.L-1 Fe + 0 mg.L-1 Zn). Fe and/or Zn concentrations higher than 2 mg.L-1 significantly decreased the mitotic index (MI) (p < 0.001) and increased the percentage of dividing cells with irregularities relative to control. Additionally, in seed priming with 2 mg.L-1 Fe + 2 mg.L-1 Zn, the average values of tillering and other yield-related components were higher than in control. Seed priming treatments performed with 8 mg.L-1 Fe and/or 8 mg.L-1 Zn were considered toxic to bread wheat, causing negative effects in germination, mitosis and yield-related characters. At the nucleolar level, the control showed a maximum of four nucleoli per nucleus whereas the remaining treatments showed five or six nucleoli per interphase cell. Anomalies such as irregular nucleolar shapes, dispersion of silver stained particles in the nucleus and nucleolar disruption were found. The highest frequency of irregularities was found in treatment 8 mg.L-1 Fe + 8 mg.L-1 Zn. The average nucleolar area diminished
significantly (p ˂ 0.001) with the increase of nucleoli number in all treatments being lower in the treatments 4 mg.L-1 Fe + 4 mg.L-1 Zn and 8 mg.L-1 Fe + 8 mg.L-1 Zn. Hence, the nucleolar
area was statistically affected by the effects treatment (p < 0.001), nucleoli number (p < 0.001) and by the interaction treatment x nucleoli number (p < 0.001). Seed priming is a cost-effective method for agronomic biofortification of bread wheat, and the present work revealed that dosages of 2 mg.L-1 Fe + 2 mg.L-1 Zn were non-cytotoxic, ensured a high rate of germination (80%) and normal dividing cells (90%), and enhanced the evaluated yield-related
x related components of bread wheat.
As far as we know, the effects of seed priming with different dosages of Fe and/or Zn in germination, mitosis, nucleolar activity and yield-related characters have never been studied in the bread wheat cultivar ‘Jordão’. Thus, the present work may contribute in the future for its improvement at the nutritional and production levels.
Keywords: Germination; micronutrients; mitosis; nucleolar activity; wheat seed priming; yield-related characters.
xi
“Seed priming” de trigo mole com Ferro e Zinco: efeitos na germinação,
mitose, atividade nucleolar e produção
Resumo
O Ferro (Fe) e o Zinco (Zn) são micronutrientes importantes no metabolismo e desenvolvimento das plantas. No entanto, devido à reduzida disponibilidade de Fe e Zn solúveis em alguns tipos de solos, a aplicação exógena destes micronutrientes torna-se necessária. A concentração adequada de cada micronutriente para aplicação no âmbito da biofortificação em trigo mole e noutras espécies vegetais requer estudos prévios a diferentes níveis. Este estudo visa a avaliação dos efeitos de priming de sementes com diferentes concentrações (1 mg.L-1 a 8 mg.L-1) de Fe e /ou Zn na germinação, mitose, atividade nucleolar e em características relacionadas com a produção do trigo português cv. ‘Jordão’. As concentrações de Fe e/ou Zn superiores a 4 mg.L-1 diminuíram significativamente a taxa
de germinação e aumentaram o tempo médio de germinação em relação ao controlo (0 mg.L-1
Fe + 0 mg.L-1 Zn). As concentrações de Fe e/ou Zn superiores a 2 mg.L-1 diminuíram
significativamente o índice mitótico (IM) (p < 0,001) e aumentaram a percentagem de células em divisão com anomalias relativamente ao controlo. Para além disso, no priming de sementes com 2 mg.L-1 Fe + 2 mg.L-1 Zn verificaram-se valores médios de afilhamento e de outros componentes de produção mais elevados que no controlo. Os tratamentos com 8 mg.L
-1 Fe e/ou 8 mg.L-1 Zn foram considerados tóxicos pois afetaram negativamente a germinação,
a mitose e as componentes de produção avaliadas. Ao nível nucleolar, o controlo mostrou um máximo de quatro nucléolos por núcleo, enquanto os restantes tratamentos apresentaram cinco ou seis nucléolos por célula interfásica. Foram encontradas anomalias tais como formas irregulares do nucléolo, partículas coradas com prata dispersas no núcleo e ruptura nucleolar. A maior frequência de anomalias foi encontrada no tratamento com 8 mg.L-1 Fe + 8 mg.L-1 Zn. A área nucleolar média diminuiu significativamente (p < 0,001) com o aumento do número de nucléolos em todos os tratamentos, tendo sido menor nos tratamentos 4 mg.L-1 Fe + 4 mg.L-1 Zn e 8 mg.L-1 Fe + 8 mg.L-1 Zn. Assim, a área nucleolar foi estatisticamente afetada pelos efeitos tratamento (p < 0,001), número de nucléolos (p < 0,001) e pela interação tratamento x número de nucléolos (p < 0,001). O priming de sementes é um método económico para a biofortificação agronómica de trigo mole, e o presente trabalho, revelou que
xii
outras componentes relacionadas com a produção de grão. Por outro lado, os tratamentos de 4 mg.L-1 Fe + 4 mg.L-1 Zn e 8 mg.L-1 Fe + 8 mg.L-1 Zn foram os mais prejudiciais para a germinação, mitose, atividade nucleolar e características relacionadas com o rendimento de grão no trigo “Jordão”.
Ao melhor do conhecimento, nunca foram estudados os efeitos do priming de sementes com diferentes concentrações de Fe e/ou Zn na germinação, mitose, atividade nucleolar e componentes relacionadas com a produção, na cultivar de trigo mole 'Jordão'. Assim, o presente trabalho pode contribuir para a melhoria nos níveis nutricionais e de produção deste cereal no futuro.
Palavras-chave: Actividade nucleolar; germinação; micronutrientes; mitose; priming de sementes de trigo; características relacionadas com a produção.
xiii
Index
Acknowledgements ... vii
Seed priming of bread wheat with Iron and Zinc: effects in germination, mitosis, nucleolar activity and grain yield ... ix
Abstract ... ix
“Seed priming” de trigo mole com Ferro e Zinco: efeitos na germinação, mitose, atividade nucleolar e produção ... xi
Resumo ... xi
Index of Figures ... xv
Index of Tables ... xvii
List of Abbreviations ... xix
1. General literature review ... 1
1.1 Introduction ... 1
1.2 The origin, phylogeny and taxonomy of wheat ... 1
1.3 The economical and nutritional importance of wheat ... 4
1.4 Micronutrients ... 6
1.4.1 Iron and Zinc – The importance to plants ... 7
1.4.2 Deficiency and toxicity effects of Fe and Zn ... 8
1.5 Exogenous application of micronutrients ... 10
1.5.1 Seed priming ... 12
1.5.2 Improvement of germination and yield-related components after seed priming 14 1.6 Cytogenetic and cytotoxic studies related to Fe and Zn ... 15
1.7 Influence of abiotic stress in nucleolar activity ... 16
1.8 Objectives ... 18
2. Materials and methods ... 21
2.1 Plant material ... 21
2.2 Seed priming ... 21
2.3 Germination ... 22
2.4 Preparation of mitotic chromosome spreads ... 23
2.5 Score of mitotic phases and irregularities ... 23
2.6 Count of nucleoli number and interphase irregularities and measurement of nucleolar area ... 24
2.7 Characterization of yield-related components ... 25
xiv
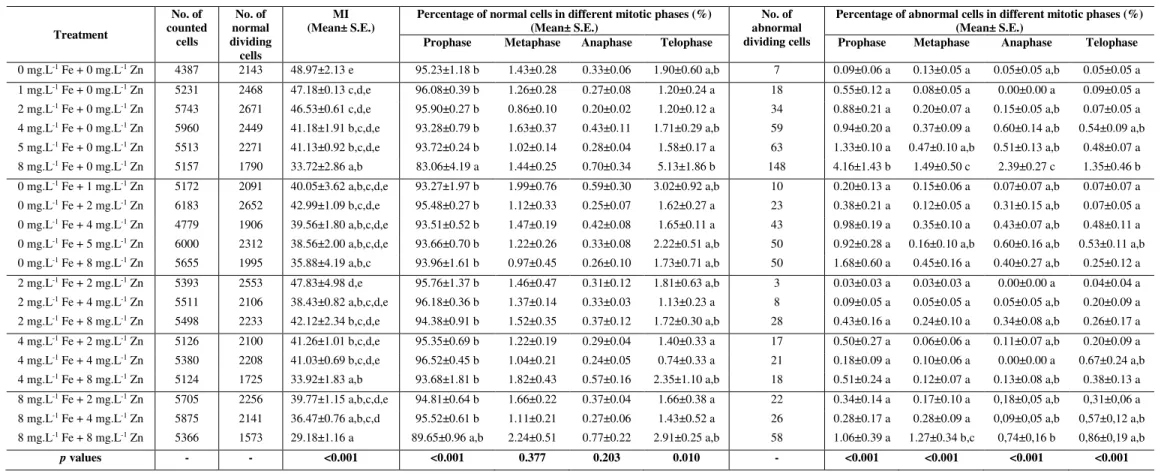
3.2 Mitotic index and percentage of total normal and abnormal mitotic phases ... 28
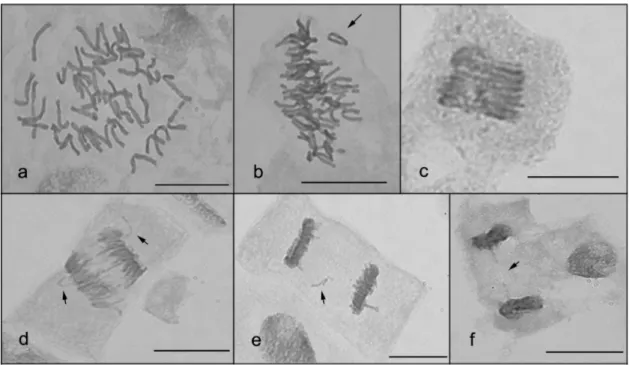
3.3 Types and frequency of mitotic irregularities ... 32
3.4 Number of nucleoli and irregularities in interphase cells ... 36
3.5 Nucleolar area ... 39
3.6 Characterization of yield-related components ... 41
4. Discussion ... 45
4.1 Influence of Fe and/or Zn priming in bread wheat germination ... 46
4.2 Effects of Fe and/or Zn priming in mitosis ... 48
4.2.1 Abnormal mitotic phases, MI and %DCA ... 48
4.2.2 Irregularities in mitotic cells ... 50
4.3 Consequences of Fe and/or Zn priming in nucleolar activity ... 51
4.3.1 Nucleoli number and irregularities in interphase nuclei ... 51
4.3.2 Nucleolar area variation ... 54
4.4 Impact of Fe and/or Zn priming in yield-related characters ... 55
5. Conclusions ... 57
xv
Index of Figures
Figure 1. Map of the ancient Middle East showing the Fertile Crescent region. ...2 Figure 2. Phylogeny of domesticated species of the Triticum spp., including einkorn, emmer, spelt,
durum, and common wheat. ...3
Figure 3. World and Europe cereal production in the period 1960-2014.Erro! Marcador não definido. Figure 4. Production and harvested area of wheat in Portugal in 2000’s. ...5 Figure 5. Global distribution of regions with Zn deficiency. ...10 Figure 6. Mitotic spindle and chromosomal irregularities observed in dividing root-tip cells of bread
wheat ‘Jordão’ stained with aceto-carmine. ...32
Figure 7. Root-tips cells stained with silver nitrate. ...37 Figure 8. Interphase root-tip cells of bread wheat seeds treated with Fe and/or Zn and stained with
xvii
Index of Tables
Table 1. Single and double micronutrient seed priming treatments performed with Fe and/or Zn in
variable concentrations and with distilled water. ...22
Table 2. Solutions with Fe and/or Zn in variable concentrations used for seed priming. ...24 Table 3. Mean time to germination (MT) ± S.E. and percentage of germination determined per
treatment. ...27
Table 4. Average values ±S.E. of mitotic index and percentage of normal and abnormal mitotic cells
in different phases ...29
Table 5. Average number of dividing root-tip cells of bread wheat seeds primed with variable
concentrations of Fe and/or Zn showing different irregularities, and average percentage of dividing cells with anomalies (DCA) per treatment. ...33
Table 6. Number of interphase cells with variable number of nucleoli scored in the different
treatments. ...37
Table 7. Number of interphase cells with different types of irregularities detected per treatment. ...39 Table 8. Average nucleolar area (µm2) ± S.E. determined in bread wheat silver stained interphase root-tip cells with one to four nucleoli with regular shapes. ...40
Table 9. Characterization of yield components in bread wheat ‘Jordão’ plants whose seeds were
xix
List of Abbreviations
% - Percentage
%DCA - Average percentage of dividing cells with anomalies per treatment
CACl2 – Calcium chloride
cv. - Cultivar
DNA – Deoxyribonucleic acid EU – European Union
FAO – Food and Agriculture Organization of the United Nations
FAOSTAT - Statistics Division of Food and Agriculture Organization of the United Nations Fe - Iron
g, mg, μg - gram, miligram, microgram GE – Genetic engennering
h - Hours
IGS- Intergenic spacer
INIAV – Elvas – Instituto Nacional de Investigação Agrária e Veterinária - Elvas
K3PO4 – Potassium phosphate
KCl – Potassium chloride
KH2PO4 – Monopotassium phosphate
KNO3 - Potassium nitrate
mg.L-1 – milligram per litter
MgSO4 – Magnesium sulfate
MI- Mitotic Index
mM, M – milimolar, molarity mm – milimeters
MT- Mean time for germination NaCl – Sodium chloride
NOR- Nucleolar Organizer Region
NSkMS- Number of spikelets of the main spike NSMS- Number of seeds of the main spike NSperSk - Number of seeds per spikelet ºC – Celsius degrees
P- Phosphorus Pb – Lead
Pb(NO3)2 – Lead nitrate
PEG - Polyethylene glycol rDNA – ribosomal DNA RNA – Ribonucleic acid RNP - ribonucleoproteins rRNA – ribosomal RNA S.E. – standard error
subsp. – subspecies UK – United Kingdom
UTAD – University of Trás-os-Montes e Alto Douro v/v – Volume per volume
1
1.
General literature review
1.1
Introduction
The global climate changes and the increase of human population worldwide call for the improvement of crop production.
Wheat constitutes one of the most important crops at the economic and nutritional levels being highly used in the human diet. Since some diseases are associated to micronutrients deficit in humans, some genetic breeding programs aimed to select wheat genotypes enriched in Iron (Fe) and Zinc (Zn) for further production of varieties highly enriched in these micronutrients. Improved varieties can be fortified with micronutrients using different approaches such as seed priming. However, the exogenous application of micronutrients should be cautiously performed since when used in excessive amounts they may be toxic to plants. Therefore, in the last decade, a high number of studies focused on testing the exogenous application of variable concentrations of micronutrients, in order to evaluate their phytotoxic effects and to define the best strategies for biofortification of different plant species, including bread wheat.
The following literature review will focus on: the origin, phylogeny and taxonomy of wheat, the economic and nutritional importance of wheat; the crucial roles of the micronutrients Fe and Zn in plant growth, development and metabolism; approaches for their exogenous application to plants in order to improve production and nutritional value without being phytotoxic, an overview of cytogenetic studies related to micronutrients excess and other abiotic stresses, their consequences in germination, mitosis, nucleolar activity, and yield-related characters, that were performed in wheat and other species.
1.2
The origin, phylogeny and taxonomy of wheat
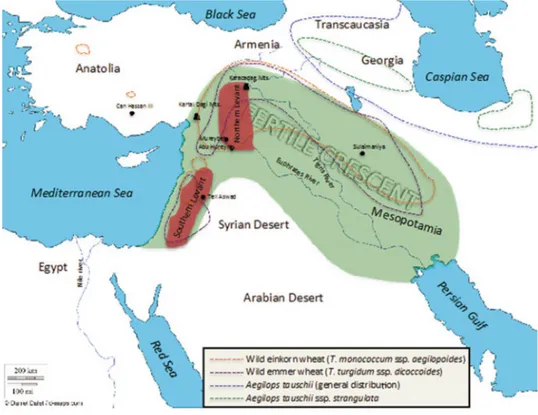
Ancient cultivated wheats (einkorn, emmer, and spelt) were originated 10,000 years ago in the Fertile Crescent, an area in the Middle East corresponding to the actual territories of Israel, Jordan, Lebanon, Turkey, Syria, Iran and Iraq (Arzani and Ashraf, 2017) (Fig. 1), where their wild ancestors are still found (Harlan and Zohary, 1966).
2
Wheat was introduced 5,000 years ago in India, China and Europe (Brammer, 2000; Moraes-Fernandes et al., 2000), and nowadays, it is widely dispersed and adapted to different soils and climatic conditions due to its genetic improvement, being cultivated in semi-arid regions of the Middle East and Africa and in high rainfall regions such as India and China (Brammer, 2000; Moraes-Fernandes et al., 2000; FAO, 2017).
Figure 1. Map of the ancient Middle East showing the Fertile Crescent region (green) (Faris 2014).
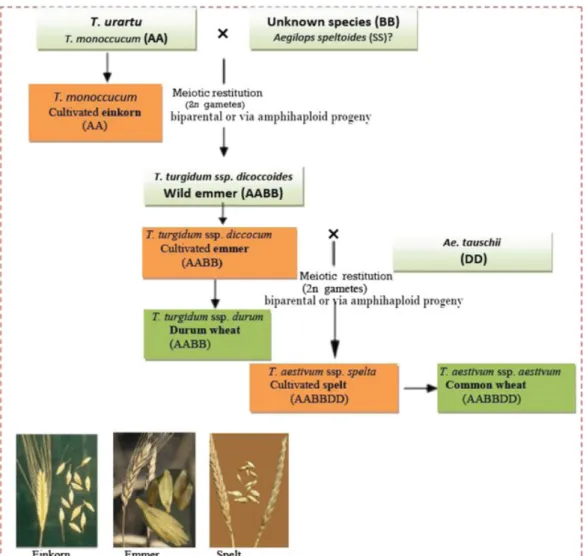
The earliest cultivated einkorn, emmer and spelt are hulled (glumed) wheats and comprise three polyploidy levels: diploid (2x), tetraploid (4x) and hexaploid (6x), (basic chromosome number x = 7). Einkorn (Triticum monococcum L.; AA; 2n = 2x = 14) is nowadays cultivated in restricted areas of the Globe (Arzani 2011). Emmer (Triticum turgidum L. subsp. dicoccum Schrank ex Schübler; AABB; 2n = 4x = 28) is a domesticated form of Triticum turgidum subsp. dicoccoides (wild emmer wheat) and Triticum turgidum subsp. durum (Desf) Husn. (durum wheat) was originated from the domesticated emmer (Fig. 2). Spelt (Triticum aestivum subsp. spelta; AABBDD; 2n = 6x = 42) is referred as the ancestor of the free-threshing common wheat. Thus, einkorn, emmer, and spelt represent the three cultivated species of hulled wheat which include a bridging species between the cultivated (bread wheat and durum wheat) and wild wheats (Arzani and Ashraf, 2017; Fig. 2).
3
Figure 2. Phylogeny of domesticated species of the Triticum spp., including einkorn, emmer, spelt, durum, and common wheat (Arzani and Ashraf, 2017).
Alloploidization, the fusion of genomes from different species, accelerated the genome evolution of wheat as this process results in changes like gene loss, gene silencing, gene activation and duplications. Also, the allopolyploid condition of wheat facilitates sporadic genomic changes (evolutionary changes) that are not attainable at the diploid level (Levy and Feldman, 2002; Sabot et al., 2005). Along with the genetic characteristics, the thousands of years of cultivation and cross-fertilization made modern wheat a very different plant from their ancestors.
Regarding the taxonomic classification, wheat belongs to the tribe Triticeae, family Poaceae and genus Triticum. The tribe Triticeae includes approximately 500 annual and perennial species with different ploidy levels (Löve, 1984; Wang and Lu, 2014). This tribe includes cereals with high economic importance, such as bread and durum wheat (genus
4
Triticum), barley (genus Hordeum) and rye (genus Secale), as well as other genera (e.g. Agropyron, Aegilops, Haynaldia, Elymus, Psathyrostachys, Brachypodium and Leymus) (Miller, 1987; Barkworth and von Bothmer, 2009).
1.3
The economical and nutritional importance of wheat
Maize, rice and wheat, are the most economically important cereals, followed by barley, sorghum, oats and rye (Vasal, 2002).
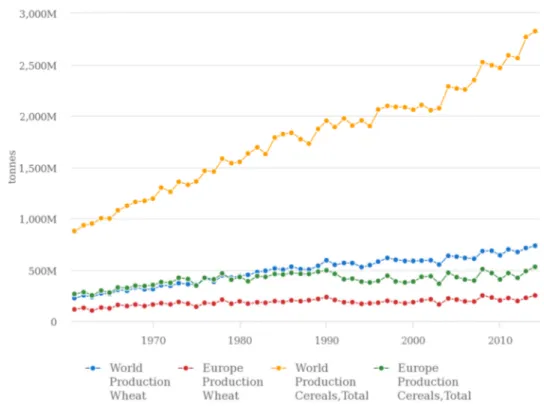
World production of cereals has been increasing since 1960’s but in Europe, the production remained stable (Fig. 3). In 2014, the cereal production was 2,823 million of tonnes in the World and reached 527 millions of tonnes in Europe. Regarding the wheat production, it also increased in World but it was less evident in Europe (Fig. 3). In 2014, the World wheat production (733 million of tonnes) corresponded to 26% of the total production of cereals (FAOSTAT, 2017).
Figure 3. Production of cereals (million of tones) in the World and Europe in the period 1960 to 2014 (FAOSTAT, 2017).
5
China followed by the United States, India, Canada, France, Russia, Australia, Pakistan, Ukraine and Turkey top the list of the World's top ten wheat producers.
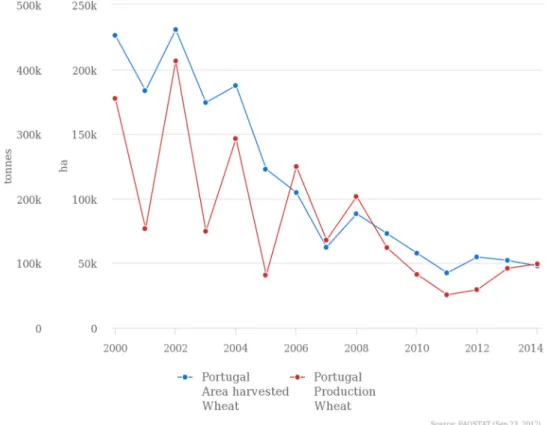
In Portugal, wheat constitutes the third most produced cereal after maize and rice. However, the wheat harvested area and production have been declining over the 2000’s (Fig. 4). In 2014, the production was only 98,794 tonnes and the harvested area was 47,826 ha (FAOSTAT, 2017). In addition to the reduction of harvested area, the environmental changes and lack of water, especially in the south regions where wheat is produced, may also explain the low production of wheat in Portugal.
Figure 3. Production and harvested area of wheat in Portugal between 2000 and 2014 (FAOSTAT, 2017).
In EU the cereals occupy 32.3% of arable land showing their importance (EUROSTAT, 2017). France constitutes the top cereal producer and it is the country with the highest area harvested (EUROSTAT, 2017).
Bread wheat is widely cultivated worldwide and comprises more than 95% of the available wheat cultivars (Arzani and Ashraf, 2017). Bread wheat is used as a major staple food in many countries, constituting the most abundant source of calories and protein in the human diet, supplying nearly 20% of the total dietary protein worldwide (Braun et al., 2010).
6
Durum or macaroni wheat (T. turgidum L. subsp. durum) is widely used for the production of pasta.
The global distribution and utilization of wheat is a consequence of its unique dough rheological properties and the bread-baking quality of its flour; pasta quality also largely depends on the gluten protein composition and content in durum wheat grains (Arzani and Ashraf, 2017).
Several billion people around the World depend on wheat for protein, starch, fibre, and various essential micronutrients (Shewry, 2009). In Portugal, wheat represents more than 75 % of the human consumption of cereals (EUROSTAT, 2017).
Each whole grain cereal constitutes a source of many essential vitamins, minerals and phytochemicals and it is composed by three major fractions: (1) bran - outer layer of the grain (rich in fibre, omega-3 fatty acids, vitamins and dietary minerals); (2) endosperm - main part of the grain (rich in starch); (3) germ - smallest part of the grain (containing vitamin E, folic acid, thiamine, phosphorus, magnesium) (Sarwar et al., 2013). However, when wheat and other cereals are refined by the removal of bran and germ, the remaining endosperm is mostly carbohydrate and lacks the majority of the other nutrients (Sarwar et al., 2013). Hence, most of the cereal cultivars do not contain sufficient Fe and Zn in the grains to meet the daily dietary recommendations for these micronutrients (Zhao et al., 2009; Chasapis et al., 2012).
1.4
Micronutrients
Fe and Zn integrates the list of essential micronutrients along with Boron (B), Chlorine (Cl), Copper (Cu), Manganese (Mn), Molybdenum (Mo) and Nickel (Ni), that are required by plants at reduced concentrations for adequate growth, nutrition, development, and reproduction (Barker and Pielbeam 2007, Lohry 2007). Kirkby and Romheld (2007) reported the roles of some micronutrients as follows: components of cell walls (B) and cell membranes (B, Zn); constituents of enzymes (Fe, Mn, Cu, Ni); cofactors (Mn, Zn) and involved in photosynthesis (Fe, Cu, Mn, Cl).
Plant nutrition specialists and agronomists have been shown increasing interest in micronutrients because of their importance for crop production. Inadequate micronutrient content in crops is limiting to its growth and development and, also reducing the efficiency of the use of fertilizers containing N-P-K macronutrients (Kirkby and Romheld, 2007). In
7
addition, the micronutrients Cu, Mn, Zn and B are particularly involved in the reproductive phase of plants, which is determinant for their productivity. The micronutrients Mn, Zn and Mo also confer resistance to biotic and abiotic stresses (Mengel and Kirkby, 2001). Crops in farmers' fields require optimum micronutrient contents to fulfill the specific needs of the plants and guarantee the product quality, resistance to stress and an adequate level of micronutrients in the plant and soil (Kirkby and Romheld, 2007). In the field, under normal conditions, the water soluble fractions of micronutrients present in the soil are absorbed by plants via rhizosphere (Epstein and Bloom, 2004).
In addition, there is an increasing awareness of the importance of micronutrients to the human health. Evidence shows that the proportion of the global human population facing malnutrition due to inadequate micronutrients has risen over the past several decades (Miller and Welch, 2013; Kumar et al., 2016). Malnutrition, or nutritional deficiency, is one of the main reasons underlying the high morbidity and mortality rates especially in poor households that, compared with those with higher incomes, are more likely to eat more starch than protein from meat or other sources (Arzani and Ashraf, 2017).
1.4.1 Iron and Zinc – The importance to plants
As referred before, micronutrients such as Fe and Zn are essential to plants, once they are required for key metabolic processes and biological functions (Singh et al., 2017). Although excessive amounts of Fe and Zn generated phytotoxicity, their accumulation in plants is tightly regulated (Palmgren et al., 2008). The molecular mechanisms of micronutrients homeostasis began to be unravelled in the last decade (Alexandre et al., 2012; Rout and Sahoo, 2015). Therefore, micronutrients have been applied for bioremediation of contaminated sites and biofortification to overcome nutritional deficiencies (Palmgren et al., 2008).
Fe is one of the most abundant metals in the earth's crust but its availability to plants, mainly through the rhizosphere, may be reduced accordingly the soil redox potential and pH (Morrisey and Guerinot, 2009; Rout and Sahoo, 2015). According to these authors, in aerobic soils or with neutral pH, Fe is readily oxidized, and is present predominately in the form of insoluble ferric oxides. At lower pH, the ferric Fe is freed from the oxide, and becomes more available for uptake by roots (Morrisey and Guerinot, 2009; Rout and Sahoo, 2015).
8
Fe plays crucial roles in DNA synthesis, cell division, photosynthesis, respiration, nitrogen (N2) fixation and plant growth, being particularly involved in the formation of
chlorophyll, acting as oxygen carrier and activating enzymes of different metabolic processes (Morrisey and Guerinot, 2009; Rout and Sahoo, 2015). Besides, Fe is a major component of plant redox systems, having a structural role in enzymes such as cytochrome oxidase, xanthine oxidase, catalases and peroxidases, which are constituents of chloroplasts and mitochondria (Guerinot and Yi, 1994; Marschner, 1995; Marenco and Lopes, 2009; Rout and Sahoo, 2015).
Zn is a component of over 300 plant enzymes and vital proteins like Zn-finger DNA binding proteins (Vallee and Falchuk, 1993; Laity et al., 2001). In plant cells, Zn is involved in major biochemical functions such as protein folding, catalytic and regulatory functions in enzymes (Broadley et al., 2007; Hafeez et al., 2013). Zn is also involved in stabilization of plasma membrane and protection of its integrity, and is also a cofactor in many essential antioxidant enzymes (Zhao and McGrath, 2009).
1.4.2 Deficiency and toxicity effects of Fe and Zn
Both, deficiency and excess of micronutrients like Fe and Zn, leads to survivor problems in plants and humans (Welch and Graham, 2004; Rout and Sahoo, 2015).
Plant responses to Fe deficiency include changes in root morphology and up-regulation of genes involved in Fe uptake (Morrissey and Guerinot, 2009).
The Zn deficiency in plants can reduce growth, development, grain yield, nutritional quality, protein synthesis, resistance to diseases, and generates chlorosis and necrosis in the apical root meristem (Broadley et al., 2007; Marenco and Lopes, 2009; Alexandre et al., 2012; Rout and Sahoo, 2015).
Since 30% of the World's cropland is too alkaline for optimal plant growth, and because some staple crops, like rice, are especially susceptible to Fe deficiency, much research has focused on how plants cope with Fe limitation (Morrissey and Guerinot, 2009). Besides, in situations of Fe deficiency, the concentration of Zn in soil raises due to their competition for the same uptake mechanisms but the inverse circumstances also occur.
At high concentrations in plants, Fe and Zn reduce the production of dry matter (Li et al., 2002), induce foliar and radicular necrosis, interfere with seed germination, lead to
9
seedling death, growth inhibition, produce deformed and chlorotic leaves, reduce plant weight and overall biomass (Carneiro et al., 2002; Rout and Sahoo, 2015).
Fe is an essential element for almost all living organisms as it participates in a wide variety of metabolic processes, including oxygen transport, DNA synthesis, and electron transport (Abbas et al., 2014). Nowadays, although low iron intake and/or bioavailability are responsible for most anemia in industrialized countries, they account for only about half of the anemia in developing countries (Allen et al., 2006) where infectious and inflammatory diseases (especially malaria), blood loss from parasitic infections, and other nutrient deficiencies (vitamin A, riboflavin, folic acid, and vitamin B12) are also important causes (Brabin et al., 2011)
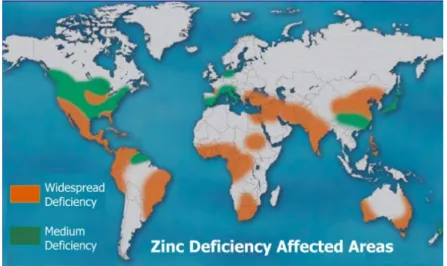
Zn deficiency is responsible for many severe health complications, including impairments of physical growth, immune system and learning ability, combined with the increased risk of infections and cancer development (Hotz and Brown, 2004; Gibson, 2006; Prasad, 2007; Manwaring et al., 2016). Zn deficiency in soils and plants is a common problem reported in several countries due to the low solubility of Zn in soils (Sillanpaa, 1982; Cakmak et al., 1999; Alloway, 2004; Hotz and Brown, 2004; Cakmak, 2008) (Fig. 5).
Probably, the first soil contamination by Zn was originated by the old metallurgy of Mediterranean, and later by the Indian metallurgy (Chaney, 1993). Currently, toxicity comes from mining and foundry activities in soils treated with sewage sludge, especially in pH soils where metals are better absorbed (Broadley et al., 2007), anthropogenic activities occurring in industrial sites or as result of buildings construction, and environmental corrosion of zinc surfaces (Kabata-Pendias, 2000). Hence, factors such as industrial residuals or acid rains can greatly enhance the Zn availability in soils to be used by plants (Blake and Goulding, 2002).
Even though the soils are vastly contaminated with Zn and other minerals, on a global scale, over three billion people suffer from the deficiency of vitamins and essential micronutrients such as Fe and Zn (Hotz and Brown 2004; Chasapis et al., 2012; Bailey et al., 2015).
Some studies demonstrated that the combination of Fe and Zn in reduced concentrations can be beneficial to plants since one can alleviate the toxicity symptoms induced by the other, promoting the increase of the plant nutritional value and/or disease resistance due to their metabolic interplay (Rosen et al., 1977; Li et al., 2001; Cakmak, 2008). In this sense, some authors verified that inhibited plant growth due to Zn toxicity was reversed by Fe addition (Burleson and Page, 1967). A positive and highly significant
10
correlation between Fe and Zn has been demonstrated extensively in pearl millet, which allowed increased levels of both micronutrients without compromising its yield (Manwaring et al., 2016).
Figure 4. Global distribution of regions with Zn deficiency (Cakmak, 2008).
Micronutrient deficiency is increasing in cereals mostly due to intensive cropping systems, use of modern high-yielding cultivars, loss of topsoil organic-matter content by erosion, burning of crop residues and acid soils (Fageria et al., 2006). Besides, crops like wheat often contain suboptimal quantities of Fe and Zn because most of their content is removed by milling (Borrill et al., 2014).
Due to the existence of problems of Fe- and Zn-deficiency, farmers and breeders started to administrate such essential micronutrients in the soil or directly in the plants.
1.5
Exogenous application of micronutrients
Depending on the physical-chemical properties of each soil, some micronutrients may be below the required amounts by plants and can be exogenously applied through different approaches.
The three recommended approaches to overcome micronutrient deficiencies include food supplementation, food fortification and biofortification.
Food supplementation and fortification are being widely applied in some countries (Cakmak, 2008). However, these approaches are expensive and not easily accessible by those
11
living in developing countries (Bouis, 2003; Stein et al., 2007; Pfeiffer and McClafferty, 2007).
Biofortification seems to be a good alternative to overcome those problems. Genetic biofortification is a strategy that uses plant breeding techniques to produce staple food crops with higher micronutrient levels, reducing levels of anti-nutrients and increasing the levels of substances that promote nutrient absorption (Bouis, 2003). It offers a sustainable solution to malnutrition problems by exploring natural genetic variation to develop mineral-dense crop varieties (Pfeiffer and McClafferty, 2007). Plant breeders screen existing accessions in global germplasm banks to determine whether sufficient genetic variation exists to breed for a particular trait. It is expected that adoption of micronutrient-dense wheat varieties will be driven by their improved agronomic properties, higher yield potential, resistance to new strains of rusts, and tolerance to climate change induced heat and drought stresses (Cakmak, 2012; Velu et al., 2014). Genetic biofortification appears to be a most sustainable and cost-effective approach useful in improving micronutrients concentrations in grain (Cakmak, 2008). However, the breeding approach is a long-term process requiring substantial efforts and resources.
Transgenic approaches could also be a further option in improving food crops with micronutrients (Vasconcelos et al., 2003; Christou and Twyman, 2004; Zhu et al., 2007; Farré et al., 2011; Yuan et al., 2011; Borg et al., 2012; Masuda et al., 2013; Singh et al., 2017, among others), but major constraints that hinder the adoption of GE technology are expected (Pérez-Massot et al., 2013).
Alternatively, agronomic biofortification, the application of fertilizers, offers a rapid, effective and practical solution to the problem, and represents a useful complementary approach to on-going breeding programs (Cakmak, 2008).
Within the agronomic biofortification approach, micronutrients may be supplied to plants by soil application, foliar spraying and seed priming (Gibson, 2006; Cakmak, 2008). However, soil micronutrient applications are less effective in increasing micronutrients in cereal grains, when compared with foliar applications (Cakmak et al., 2010a, b). Knowledge of the different forms of micronutrient fertilizers and timing of foliar micronutrient application is crucial for enhancing grain micronutrients. The most effective method is the soil plus foliar application method, which may result in an about 3-fold increase in grain micronutrients concentration (Cakmak et al., 2010a).
12
The HarvestZinc (www.harvestzinc.org) initiative has been investigating different fertilizer strategies and the most efficient Zn application method for promoting Zn uptake and maximizing grain Zn accumulation (Cakmak, 2012; Velu et al., 2014).
Seed priming with micronutrient solutions is an alternative way to increase seed micronutrients prior to sowing.
1.5.1 Seed priming
Seed priming is a physiological method that improves seed performance, germination, initial plant growth, shoot weight, height and root length (Sivritepe and Dourado, 1995; Ajouri et al., 2004; Burguieres et al., 2007; Sarlach et al., 2013; Hussian et al., 2014; Sharma et al., 2014). These features arose from the impact of seed priming on enzymatic, metabolic and biological functions of seeds, enabling the plants to better respond to water stress (Ahmed et al., 2016).
Seed priming can improve tolerance to drought, salinity, chilling, heavy metals as well as nanoparticles stress by regulating gene expression and protein abundance of multiple stress-responsive pathways (Jisha et al., 2013; Ibrahim, 2016; Wojtyla et al., 2016).
Seed priming is performed before sowing during a defined time period and under controlled environmental conditions (Sharma et al., 2014). Recently, Lutts et al. (2016) reviewed the different types of seed priming as follows : (i) hydropriming – performed with distilled water (Afzal et al., 2002); (ii) osmopriming – done with osmotic solutions (Rouhi et al., 2011); (iii) solid matrix priming developed with solid carriers (Sharma et al., 2014), (iv) biopriming done with bacterial inoculation of seeds (Callan et al., 1990), (v) hormopriming performed with plant growth regulators or (vi) nutripriming using solutions containing the limiting nutrients instead of pure water (Lutts et al., 2016).
Hydropriming is a low-cost, environmentally friendly and constitutes the simplest method of seed priming. The method relies on seed soaking in pure water and re-drying to original moisture content prior to sowing (Afzal et al., 2002). No additional chemical substances are used as a priming agent. The main disadvantage of hydropriming is uncontrolled water uptake by seeds (Lutts et al., 2016). Despite that, many reports indicated beneficial effect of hydropriming in various crop plants, such as wheat (Basra et al, 2002), Indian mustard (Srivastava et al., 2002), sunflower (Kaya et al., 2006), mung bean (Posmyk
13
et al., 2007), canola (Omidi et al., 2009), chickpea and maize (Rahman et al., 2011), capsicum (Patade et al., 2012), durum wheat (Fercha et al., 2013), and rice (Goswami et al., 2013).
Instead of pure water, osmopriming involves soaking seeds in osmotic solution with low water potential (Rouhi et al., 2011). Polyethylene glycol (PEG), mannitol, sorbitol, glycerol, and inorganic salts, such as NaCl, KCl, KNO3, K3PO4, KH2PO4, MgSO4, and CaCl2,
are compounds usually used in osmopriming (Yacoubi et al., 2013). Osmopriming has been used with success in spinach, sorghum, Brassica napus L., alfalfa and rice, under unfavorable conditions, such as salt, water, chilling, and nano-ZnO stresses (Chen and Arora, 2011;
Yacoubi et al., 2013; Kubala et al., 2015; Salah et al., 2015; Zhang et al., 2015)
Solid matrix priming is performed with solid carriers (Sharma et al., 2014) like vermiculite, peat moss, charcoal, sand, clay, and some commercially offered substrate (Di Girolamo et al., 2002; Paparella et al., 2015) and the water uptake by seeds is controlled (Paparella et al., 2015). The procedure has been used in hot pepper (IIyas et al., 2002), soybean (Mercado and Fernandez, 2002), onion (Kępczyńska et al., 2003), maize (Zhang et al., 2007) and carrot (Singh et al., 2015).
Biopriming is carried out by bacterial inoculation of seeds (Callan et al., 1990) and has been used with success in pearl millet and radish (Raj et al., 2004; Kaymak et al., 2009)
In hormopriming, seeds imbibition occurs in the presence of plant growth regulators (abscisic acid, auxins, gibberellins, kinetin, ethylene, polyamines, and salicylic acid) (Nawaz et al., 2011) that can have direct impact on seed metabolism. Hormopriming has been used in wheat (Afzal et al., 2006; Iqbal et al., 2006; Iqbal and Ashraf, 2013), rice (Farooq et al., 2009; Zheng et al., 2016) and white clover (Galhaut et al., 2014).
Seed priming performed with micronutrient solutions or nutripriming, accordingly the classification of Lutts et al., (2016), may increase the micronutrient concentrations in seeds (Cakmak, 2008), ensure good root growth and contribute to better protection against soil borne pathogens (Cakmak, 2012). However, for each plant species, the suitable dosage of each micronutrient to be exogenously applied must be defined. The excess of micronutrients can be toxic (Palmer and Guerinot, 2009), compromising plant development, inducing cell cycle and chromosomal irregularities (Rosen et al., 1977), and affecting the plant fertility due to the formation of unviable gametes (Liu et al., 1996). Regarding some of these consequences, the testing of different Fe and Zn dosages exogenously applied to wheat and
14
other plant species should be monitored not only at the physiological and agronomic levels but also under the cytogenetic scope.
1.5.2 Improvement of germination and yield-related components after seed priming
Seed priming with macro- and micronutrients has been reported to result in vigorous early seedling growth, better stand establishment, increased germination rate and greater germination uniformity (Arif et al., 2005; Ali et al., 2007; Abbas et al., 2014; Lutts et al., 2016). Also, plants resultant from primed seeds often exhibit a faster growth than those produced from unprimed ones (Lutts et al., 2016).
Seed priming is a suitable improving method of seed germination and seedling emergence in different plant species (Harris et al., 1999; Wattanakulpakin et al., 2012; Wojtyla et al., 2016). The beneficial impact of priming on plant growth may be due to an improved nutrient use efficiency allowing a higher relative growth rate and to an improved regulation of the plant water status (Lutts et al., 2016).
It was proven that nutripriming enables the seed to rapidly imbibe, reviving the seed metabolism and resulting in a high rate and homogeneity of germination (Rowse, 1995).
Harris et al. (1999) showed that nutrient seed priming can increase drought tolerance, reduce pest damage and ultimately increase crop yield. Later, Harris et al. (2000) reported higher grain yield in plants treated with nutrient seed priming relative to untreated (control) plants. Ali et al. (2008) showed an increase in the number of plants, tillers and grains per spike, grain weight and yield in wheat and maize plants whose seeds were primed with Zn and P relative to the control plants. All of the tested nutrient management practices produced a significant increase in crop yield (Ali et al., 2008).
The treatment of seeds with micronutrients potentially provides a simple inexpensive method for improving micronutrient plant nutrition, decreasing the time between sowing and emergence and increasing seedlings vigour (Parera and Cantliffe, 1994; Harris, 1996).
Priming seeds in solutions of macro- or micronutrients has been shown to improve yield of rice (Peeran and Natanasabapathy, 1980; Abbas et al., 2014), wheat (Khalid and Malik, 1982; Marcar and Graham, 1986; Wilhelm et al., 1988) and forage legumes (Sherrell, 1984). However, priming with high nutrient concentrations can damage the seed and inhibit germination (Roberts, 1948).
15
According to Ali et al, (2008), organic sources, nutrient seed priming, soil application and foliar application of nutrients, resulted in better crop yield and yield-related components. It is important to create an integrated approach with these techniques so it would be more economical and environmental friendly to achieve higher yield and thus food security (Ali et al., 2008).
1.6
Cytogenetic and cytotoxic studies related to Fe and Zn
Several environmental factors such as pollution and contamination of the soil with heavy metals can cause anomalies in plants, such as: DNA damage; irregularities in cell cycle and chromosomes (e.g. anaphases bridges, stickiness, vagrant, laggard and fragmented chromosomes); reduced mitotic index (MI) and increased frequencies of abnormal dividing cells; nucleolar irregularities (e.g. irregular shaped nucleoli and nucleolar disruption), among others (Rosen et al., 1977; Liu et al., 1996; Connolly and Guerinot 2002; Berry et al., 2003; Naidoo and Chirkoot 2004; Fageria et al., 2008; Hafeez et al., 2013; Bouain et al., 2014). The accumulation of interphasic, mitotic and chromosomal irregularities compromises the cell division, formation and viability of gametes, and plant fertility (Liu et al., 1996).
In wheat, when the occurrence of the irregularities is genetically determined, the instability of the cultivar becomes recurrent (Moraes-Fernandes, 1982).
Jensen (1965) suggested that faults in the plant's reproductive system, mutations, and natural crosses would be responsible for the occurrence of aberrant morphological types (off-types), making it difficult to maintain the purity and homogeneity of the variety. In different bread wheat varieties was found that the non-orientation of chromosomes in meiosis, the occurrence of univalents at metaphase, and chromatin loss in the parent plants were associated with the variability of their progeny in terms of seeds weight per plant, height of plant and percentage of fertility (Powers, 1932). These results indicated that in some cases meiotic instability is an important character for breeding programs.
Several cygenetic and cytotoxic studies have been performed by numerous researchers in order to study the effects of different micronutrient concentrations and other abiotic stresses in different plant species including wheat.
Liu et al. (1996) studied the effects of different Zn concentrations in root growth, cell division and nucleoli of onion by observing the chromosomal morphology in chromosome
16
spreads prepared with root-tips fixed in Carnoy's reagent and stained with Carbol Fuchsin solution.
Rout and Das (2009) elucidated the cytotoxic effects of Zn in plants. These authors described that the major changes were seen in the nucleus of the root-tip cells where the chromatin material was highly condensed, some of the cortical cells were disrupted and the nuclear membrane was dilated in the presence of 7.5 mM of Zn; the cytoplasm became structureless, occurred disintegration of cell organelles and the development of vacuoles were also observed.
El-Ghamery and Basuoni (2015) studied the cytotoxic effects of aqueous cinnamon in root-tips of Allium cepa L. and Vicia faba L. stained with Feulgen for further determination of the MI, frequency of cells in the various mitotic phases (normal and irregular), and identification of chromosomal anomalies.
Pekol et al., (2016) studied the cytotoxicity caused by drought and salt stress in wheat root-tips stained with aceto-carmine for further assessment of MI, proportion of mitotic phases and identification of chromosomal irregularities.
Other techniques, like silver nitrate staining, have been used to determine the effect of abiotic stress and excess and/or deficiency of different micronutrients in nucleolar activity.
1.7
Influence of abiotic stress in nucleolar activity
The eukaryotic nucleus is a complex, highly organized organelle, containing a range of domains and nuclear bodies involved in DNA replication and gene expression (Lamond and Earnshaw, 1998). The most prominent nuclear sub-compartment is the nucleolus that was first observed more than 200 years ago (Boisvert et al., 2007).
The nucleolus plays key roles in different cellular processes: cell cycle; cell growth, aging and apoptosis; telomerase production and activity; RNA processing; ribosomal biogenesis; maturation, assembly and export of ribonucleoproteins (RNPs); and monitor responses to cellular stress (e.g. nucleolar disruption) (Lamond and Earnshaw, 1998; Bardin et al., 2000; Carmo-Fonseca et al., 2000; Rubbi and Milner, 2003; Horn and Vousden 2004; Boulon et al., 2010; Jiang et al., 2014; Zhang et al. 2014).
Nucleoli have a dynamic nature and their composition can vary dramatically under different cellular conditions, which may explain the importance of study the structure and
17
function of subcellular organelles under a range of growth conditions and cell cycle stages in order to evaluate their biological roles fully (Boisvert et al., 2007).
The ribosomal DNA (rDNA) transcribes for the ribosomal RNA (rRNA) genes that are organized in repetitive units, repeated in tandem, within chromosomal loci named Nucleolar Organizer Regions (NORs) or secondary constrictions (McClintock, 1934). In the case of wheat, each repetitive unit includes the 18S, 5.8S and 26S rRNA genes as well as the intergenic spacer (IGS) (see Barker et al., 1988; reviewed in Pikaard, 2000).
The NORs that were able to form nucleoli (transcriptionally active) retain in metaphase non-histone proteins or phosphoproteins involved in the transcription of the rRNA genes (Roussel et al., 1996). These proteins contain carboxyl (Goodpasture and Bloom, 1975; Howell et al., 1975) or hydroxyl residues in the aminoacid tyrosin (Stack et al., 1991) that reduce silver under acid conditions providing the differential staining of the NORs that were transcribed during the preceding interphase (McClintock, 1934).
The ability of silver nitrate to stain the nucleoli in interphase and NORs in metaphase cells, enabled the exhaustive study of rRNA genes expression, nucleolar activity and/or NOR methylation patterns in cereals (Crosby, 1957; Bhowal, 1972; Darvey and Driscoll, 1972; Flavell and O’Dell, 1979; Flavell et al., 1988; Martini and Flavell, 1985; Jiang and Gill, 1994; Mukai et al., 1991; Sardana et al., 1993; Morais-Cecílio et al., 2000; Carvalho et al., 2010, among many others). Therefore, this technique enables the evaluation of the consequences of abiotic stress in the transcriptional activity of the rDNA.
The size, morphology, and number of silver stained nucleoli have been highly correlated with the nucleolar activity and rate of protein synthesis in the cells (Pekol et al., 2009; Boulon et al., 2010). Although the cell itself responds quickly to stress situations by affecting its growth, cell cycle, and metabolism, the nucleolus has been considered as a central hub for coordination of stress responses (Boulon et al., 2010; Sengupta et al., 2010).
Recently, some authors used silver nitrate staining and/or indirect immunofluorescent microscopy to determine the effects of abiotic stress in the activity and morphology of nucleolus (Teerarak et al., 2009; Jiang et al., 2014; Zhang et al., 2014; Pekol et al., 2016). Teerarak et al. (2009) studied the influence of salt in the number of nucleoli per nucleus in Allium cepa L. plants. Jiang et al. (2014) analysed the effects of lead (Pb) on the morphology and structure of the nucleolus in the root-tips of Allium cepa L.. Zhang et al. (2014) verified toxic effects in root-tips of Pinus massoniana exposed to Al ions, particularly affecting cell
18
division and the nucleolar cycle and proteins. Pekol et al. (2016) evaluated the genotoxic and cytotoxic effects of drought and salt stress in wheat species with different ploidy levels.
The effects of seed priming with Fe and/or Zn solutions in the nucleolar activity, cell cycle and/or chromosomes of bread wheat was not yet described in the literature.
1.8 Objectives
With this work, we intended to evaluate the effects of seed priming with different concentrations of Fe and/or Zn in the germination, cell cycle, nucleolar activity, and yield-related characters of the Portuguese bread wheat cv. ‘Jordão’.
Is also aimed to determine, based on statistical analyses, which concentration of Fe and/or Zn that would be more suitable for wheat biofortification.
21
2.
Materials and methods
2.1
Plant material
In this work, seeds of the Portuguese bread wheat (T. aestivum; AABBDD; 2n = 6x = 42) cv. ‘Jordão’, kindly given by José Coutinho (INIAV-Elvas) were used. This cultivar belongs to the Portuguese Catalogue of Varieties (CNV, 2017) and have semi-precocious vegetative cycle, excellent adaptation to Mediterranean conditions, great tillering capacity, high productive performance, high baking potential, and high resistance to Septoria tritici (leaf blotch), Puccinia recondita (brown rust), Puccinia hordei (leaf rust) and to other wheat diseases.
The produced plantlets were installed in pots containing soil and peat and their development was followed in order to ensure the self-fertilization of the main spikes and tillers of each plant, and to obtain adult plants for the characterization of yield-related components after the harvest.
2.2
Seed priming
The seeds were primed with aqueous solutions of Fe [Iron (II) sulphate heptahydrate, FeSO4.7H2O] and/or aqueous solutions of Zn [Zinc sulphate heptahydrate, ZnSO4.7H2O]
(Table 1). The concentrations presented in Table 1 correspond to the amounts of Fe and/or Zn in each priming solution of FeSO4.7H2O and/or ZnSO4.7H2O, respectively. The negative
control (later refereed only as control) corresponds to seeds primed with distilled water also named as hydropriming (Table 1). Hence, a total of 20 treatments of seed priming (including the control) were performed (Table 1).
22
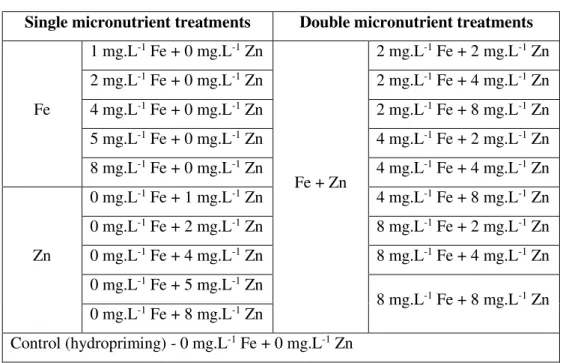
Table 1. Single and double micronutrient seed priming treatments performed with Fe and/or Zn in variable concentrations (mg.L-1) and with distilled water (control).
Single micronutrient treatments Double micronutrient treatments
Fe 1 mg.L-1 Fe + 0 mg.L-1 Zn Fe + Zn 2 mg.L-1 Fe + 2 mg.L-1 Zn 2 mg.L-1 Fe + 0 mg.L-1 Zn 2 mg.L-1 Fe + 4 mg.L-1 Zn 4 mg.L-1 Fe + 0 mg.L-1 Zn 2 mg.L-1 Fe + 8 mg.L-1 Zn 5 mg.L-1 Fe + 0 mg.L-1 Zn 4 mg.L-1 Fe + 2 mg.L-1 Zn 8 mg.L-1 Fe + 0 mg.L-1 Zn 4 mg.L-1 Fe + 4 mg.L-1 Zn Zn 0 mg.L-1 Fe + 1 mg.L-1 Zn 4 mg.L-1 Fe + 8 mg.L-1 Zn 0 mg.L-1 Fe + 2 mg.L-1 Zn 8 mg.L-1 Fe + 2 mg.L-1 Zn 0 mg.L-1 Fe + 4 mg.L-1 Zn 8 mg.L-1 Fe + 4 mg.L-1 Zn 0 mg.L-1 Fe + 5 mg.L-1 Zn 8 mg.L-1 Fe + 8 mg.L-1 Zn 0 mg.L-1 Fe + 8 mg.L-1 Zn Control (hydropriming) - 0 mg.L-1 Fe + 0 mg.L-1 Zn
Per treatment, 50 seeds were directly primed in distilled water (control) and in different concentrations of Fe and/or Zn (Table 1) during 8 h at 25 ºC, in the dark.
2.3
Germination
At the end of the treatments, the primed seeds were washed thoroughly with distilled water, air dried and transferred to Petri dishes containing filter paper moistened in distilled water, and allowed to germinate at 25 ºC, in the dark.
Germination was monitored daily over a period of 11 days. The seeds were considered to be germinated when the radicle presented about 2 mm. The germinated seeds were counted for determination of the germination percentage. The mean time to germination (MT) (in days) per treatment was also calculated.
23
2.4
Preparation of mitotic chromosome spreads
Root-tips with around 1 cm length were collected and immediately fixed in a solution composed by absolute ethanol: glacial acetic acid in the proportion 3:1 (v/v) freshly prepared. The fixed root-tips used for mitotic phases and irregularities analyses (section 2.5), were stained with 2% aceto-carmine solution during 48 h at 25 ºC.
The preparation of both mitotic chromosome and interphase nuclei spreads followed the squashing protocol described by Lima-Brito et al. (1996).
After air dried, the slides of interphase nuclei for nucleolar activity analyses (section 2.6) were stained with silver nitrate using the salt-nylon technique described by Stack et al. (1991).
The glass coverslip was removed with liquid nitrogen. The slides were air dried and then mounted with a drop of VectaShield® mounting medium (Vector Laboratories, Peterborough, UK) and a 24x50 mm glass coverslip.
The images were captured on a fluorescence microscope Olympus BX41 (Olympus America, Inc., Hauppage, NY, USA) using a XC10 charge-coupled device (CCD) digital camera (Olympus America, Inc., Hauppage, NY, USA) and the software cellSens (Olympus Soft Imaging Solutions GmbH, Münster, Germany).
The images were prepared for presentation with the software Adobe® Photoshop® version 6.0.1 (Adobe Systems, San Jose, California).
2.5
Score of mitotic phases and irregularities
Five to six mounted slides of each seed priming treatment performed (Table 1) were observed and scored. In each slide a total of 50 fields were recorded. For each field, the total number of cells (interphase nuclei, normal and abnormal dividing cells) was scored. In all dividing cells the normal and abnormal mitotic phases were identified and scored.
Based on these data, we calculated the mitotic index (MI) [number of dividing cells/ number of counted cells x 100], where the counted cells correspond to the sum of interphase and mitotic cells. We also calculated the percentage of dividing cells with anomalies (%DCA)
24
[number of dividing cells with anomalies/ total number of dividing cells x 100%] per treatment.
2.6
Count of nucleoli number and interphase irregularities and
measurement of nucleolar area
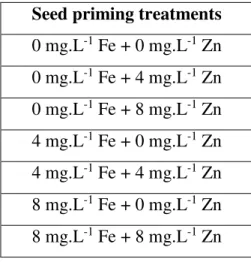
After analyzing the results achieved in mitotic phases and irregularities (section 2.4), only seven seed priming Fe and/or Zn treatements were selected from the 20 treatments performed initially (see section 2.1) in order to score the nucleoli number and the interphase irregularities for further nucleolar activity analyses.
The seven seed priming treatments selected were: the control (0 mg.L-1 Fe + 0 mg.L-1 Zn), the intermediate (4 mg.L-1) and the maximum (8 mg.L-1) concentrations of Fe and/or Zn (Table 2).
Table 2. Single and double micronutrient seed priming treatments performed with Fe and/or Zn in variable concentrations (mg.L-1) and with distilled water (control) selected for the study of nucleolar activity.
Seed priming treatments 0 mg.L-1 Fe + 0 mg.L-1 Zn 0 mg.L-1 Fe + 4 mg.L-1 Zn 0 mg.L-1 Fe + 8 mg.L-1 Zn 4 mg.L-1 Fe + 0 mg.L-1 Zn 4 mg.L-1 Fe + 4 mg.L-1 Zn 8 mg.L-1 Fe + 0 mg.L-1 Zn 8 mg.L-1 Fe + 8 mg.L-1 Zn
Three to six silver nitrate stained slides from each seed priming treatment were observed and scored. Per slide, 50 observation fields were counted. Per observation field, interphase nuclei with a variable number of nucleoli were scored. Additionally, it was also registered interphase irregularities such as: silver stained particles dispersed in the nucleus; silver stained particles exuded from the nucleolus to the nucleus evidencing nucleolar disruption; and nucleoli with irregular shapes.
25
The nucleolar area (μm2) = πr2, was measured in a variable number of interphase nuclei per treatment, using the Digimizer Image Analysis Software (Medcalc, Ostend, Belgium), a magnification of 400x and a scale bar of 50 μm.
The nucleolar area was only determined in interphase cells presenting 1 to 4 nucleoli with regular shapes, due to the reduced frequency of interphse cells with 5 and 6 nucleoli.
2.7
Characterization of yield-related components
Seven yield-related components were characterised in a total of 135 adult plants that resulted from the 20 seed priming treatments performed with Fe and/or Zn (Table 1). Therefore, after harvesting, the following yield-related components were characterized: number of spikelets of the main spike (NSkMS), number of seeds of the main spike (NSMS), number of seeds per spikelet (NSperSk), weight of seeds of the main spike (WSMS), tiller number (TN), number of seeds of the secondary spikes (NSSS) and weight of seeds of the secondary spikes (WSSS).
2.8
Statistical analyses
Most of the results are presented as mean values ± S.E. per treatment. The results of the germination, mitosis and the yield-related characters were statistically analysed. For the statistical analysis regarding the cell counting, each slide was considered a repetition and five to six slides were scored per seed priming treatment.
The statistical Analyses of Variance (ANOVA) and post-hoc Tukey tests were performed with the software IBM SPSS Statistics v23.
The p-value significance, due to the different effects and their interaction, was established for probabilities lower than 5% (p < 0.05).