tumor cells
Síntese da 2',4,4'-trimetoxichalcona e estudo citotóxico in vitro em células
tumorais humanas
DOI:10.34117/bjdv5n10-255
Recebimento dos originais: 20/09/2019 Aceitação para publicação: 18/10/2019
Tamyrys Fernandes Vilar Bento
Graduanda em Biotecnologia pela Universidade Federal da Paraiba (UFPB). Universidade Federal da Paraiba
Endereço: R. Tab. Stanislau Eloy, 41-769 - Conj. Pres. Castelo Branco III, João Pessoa - PB, 58033-455
E-mail: tamyrysfvb@gmail.com
Luiz André Araujo Silva
Programa de Pós-Graduação Em Desenvolvimento e Inovação Tecnológica em Medicamentos pela Universidade Federal da Paraíba (UFPB)
Universidade Federal da Paraiba
Endereço: Campus I - Lot. Cidade Universitaria, PB, 58051-900 E-mail: luiz32@gmail.com
Flávio Valadares Pereira Borges
Programa de Pós-Graduação Em Produtos Naturais e Sintéticos Bioativos pela Universidade Federal da Paraíba (UFPB)
Universidade Federal da Paraiba
Endereço: R. Tab. Stanislau Eloy, 41-769 - Conj. Pres. Castelo Branco III, João Pessoa - PB, 58033-455
E-mail: flavinhovb@gmail.com
Bruno Hanrry Melo de Oliveira
Biomédico especialista em Biologia Molecular, mestrando em Biotecnologia pela Universidade Federal da Paraiba – UFPB
Universidade Federal da Paraiba
Endereço: R. Tab. Stanislau Eloy, 41-769 - Conj. Pres. Castelo Branco III, João Pessoa - PB, 58033-455
E-mail: hanrygb@hotmail.com
Fernando Ferreira Leite
Programa de Pós-Graduação Em Produtos Naturais e Sintéticos Bioativos pela Universidade Federal da Paraíba (UFPB)
Universidade Federal da Paraiba
Endereço: R. Tab. Stanislau Eloy, 41-769 - Conj. Pres. Castelo Branco III, João Pessoa - PB, 58033-455
Sâmia Sousa Duarte
Programa de Pós-Graduação Em Produtos Naturais e Sintéticos Bioativos pela Universidade Federal da Paraíba (UFPB)
Universidade Federal da Paraiba
Endereço: R. Tab. Stanislau Eloy, 41-769 - Conj. Pres. Castelo Branco III, João Pessoa - PB, 58033-455
E-mail: samiasduarte@gmail.com
Marianna Vieira Sobral
Professora da Universidade Federal da Paraiba – UFPB
Programa de Pós-Graduação Em Produtos Naturais e Sintéticos Bioativos pela Universidade Federal da Paraíba (UFPB)
Universidade Federal da Paraiba
Endereço: R. Tab. Stanislau Eloy, 41-769 - Conj. Pres. Castelo Branco III, João Pessoa - PB, 58033-455
E-mail: mariannavbs@gmail.com
Luis Cézar Rodrigues
Professor da Universidade Federal da Paraiba – UFPB
Programa de Pós-Graduação Em Desenvolvimento e Inovação Tecnológica em Medicamentos e Programa de Pós-Graduação em Biotecnologia pela Universidade Federal da Paraíba (UFPB)
Universidade Federal da Paraiba
Endereço: R. Tab. Stanislau Eloy, 41-769 - Conj. Pres. Castelo Branco III, João Pessoa - PB, 58033-455
E-mail: luiscezarodrigues@gmail.com
ABSTRACT
Chalcones are a class of natural compounds, aromatic ketones that forms central core for several important pharmacological activities reported such as anticarcinogenicity and antioxidation activities. Due to those important properties, the purpose of this work is the organic synthesis of 2’,4,4'-trimethoxychalcone and its cytotoxicity test against four human tumor cell lines: HL-60 (human promyelocytic leukemia), HCT-116 (human colorectal carcinoma), MCF-7 (human breast adenocarcinoma) and K562 (chronic myeloid leukemia) and also evaluated in normal cells L929 (murine fibroblast). The synthesis of 2', 4,4'-trimethoxychalcone was successful and in the viability cell assay (MTT assay), the HL-60 strain was found to have an inhibitory potential of 84.5% and an IC50 of approximately 20.84 µM.
Keywords: Chalcone, Organic Synthesis, Cytotoxicity, Aldol Condensation, HL-60. RESUMO
As chalconas são uma classe de compostos naturais, cetonas aromáticas que são o núcleo central de diversas atividades farmacológicas importantes relatadas tais quais anticarcinogenicidade e antioxidante. Devido a estas importantes propriedades, o objetivo deste trabalho é a síntese da 2',4,4'-trimetoxichalcona e seu teste de citotoxicidade em quatro linhagens de células tumorais humanas: HL-60 (leucemia promielocítica aguda), HCT-116 (carcinoma colorretal humano), MCF-7 (adenocarcinoma da mama humano) e K562 (leucemia mielóide crônica) em células normais L929 (fibroblasto murino). A síntese da 2',4,4'-trimetoxichalcona foi bem-sucedida e no ensaio de
potencial inibitório em 84,5% com uma IC50 de aproximadamente 20,84 µM.
Palavras-chave: Chalcona, Síntese Orgânica, Citotoxidade, Acilação, Condensação Aldólica,
HL-60.
1. INTRODUCTION
Cancer occur when cells in the human body frequently keep multiplying with the incapability to be controlled and thus forming tumors with the potential to metastasis (NALL 2018). Current treatments include chemotherapy, radiotherapy and chemically derived drugs. Treatments such as chemotherapy can put patients under a lot of pain and weakens their immune system. Therefore, there is a current focus on using alternative anticancer therapeutic treatments and their developments in the field.
Plants extract themselves have been used in medicine for their natural overall healing properties (GREENWELL 2015). Compounds that are characteristic to the plant kingdom have been studied for their properties to inhibit growth and initiate apoptosis of cancerous cells. Chalcones are secondary metabolites that can be considered as possible alternative sources of these medical treatments (MARTEL-FRACHET 2015).
Due to the great demand for new antitumor, antimicrobial and anti-inflammatory compounds (NEWMAN 2016) and the important biological activities of chalcones reported in the literature (DÍAZ-TIELAS 2016) and their activity against a variety of tumor cell lines this work focuses on the preparation of 2',4,4'-trimethoxychalcone. According to Sun (2016) most of cancer-related pathologies are drug resistant or develop resistance during treatment.
2. MATERIALS AND METHODS
2.1 PREPARATION OF 1,3-DIMETHOXYBENZENE
1,3-dimethoxybenzene – compound 1 (Figure 1). To a stirred solution of resorcinol (10g, 90mmol) and acetone (100mL) in a 500mL flat-bottomed flask in an ice bath was added K2CO3 (31g,
220mmol) and methyl iodide (13mL, 200mmol) drop wise over a period of 10min. After complete addition, the solution was stirred overnight at room temperature (rt). The reaction was monitored by thin layer chromatography (TLC), when reaction was finished acetone was evaporated under reduced pressure. The crude was diluted with distillated water and extracted two times with chloroform. The organic phase extracts were dried (Na2SO4) and evaporated. The title compound was obtained with
Figure 1 - Preparation of 1,3-dimethoxybenzene
2.2 PREPARATION OF ACETOPHENONE
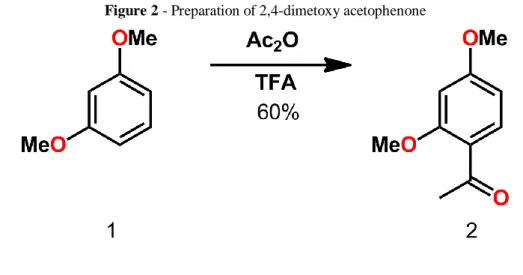
2,4-dimetoxy acetophenone – compound 2 (Figure 2). The product of last reaction, dimethoxy resorcinol (5g, 36mmol), acetic anhydride (7mL, 102mmol) and F3CCOOH (TFA) (37 mL) were
mixed in a flask, on magnetic stirrer at rt. After 1.5 hours an aqueous solution of NaHCO3 10%
(50mL) was added and stirred. TLC was performed (hexane/ethyl acetate 9:1), showing a more polar product (Rf: 0,5) once compared to dimethoxy resorcinol (Rf: 0,6). 2,4-dinitrophenylhydrazine (DNPH) revealed carbonyl groups on the acetophenone. The crude was diluted with water and extracted two times with ethyl acetate (50mL). The organic phase was dried (Na2SO4) and
concentrated, the product was purified by column chromatography using hexane as eluent (GUANGCHANG 2018). The product obtained was a brown liquid. Yield 4g (60%)
Rf 0.15 (in 2: 8 eluent AcOEt / hexane). IUPAC Nomenclature: 1- (2,4-dimethoxyphenyl) ethanone.
Figure 2 - Preparation of 2,4-dimetoxy acetophenone
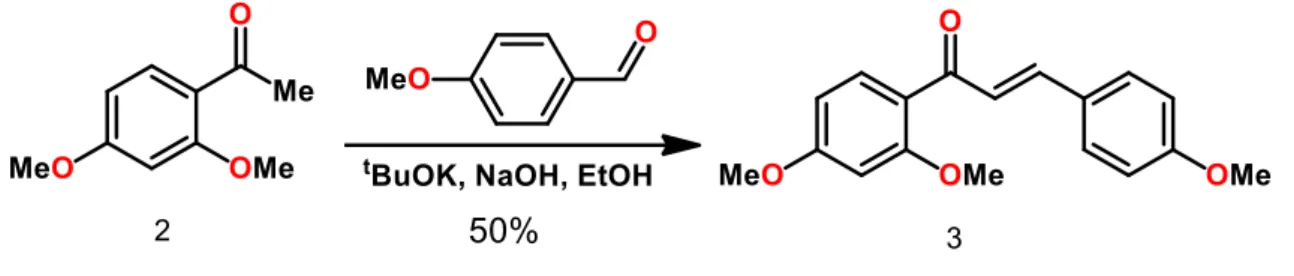
prepared a solution of the acetophenone (1g, 5mmol) in etanol (30mL), it was added p-anisaldehyde (755mg, 5mmol) and potassium tert-butoxide (622mg, 5mmol), NaOH in catalytic quantity. The solution was stirred for one week, the crude was diluted with water and extracted two times with ethyl acetate (50mL). The organic phase was dried (Na2SO4) and concentrated, the chalcone was purified
by column chromatography using hexane and ethyl acetate 9:1 as eluent. The product obtained was a yellow solid. Yield 0.82g (50%), 95ºC melting point Rf 0.5 (in AcOEt / Hexane 2: 8 eluent). IUPAC Nomenclature: 2-Propen-1-one, 1- (2,4-dimethoxyphenyl) -3- (4-methoxyphenyl).
Figure 3 - Preparation of 2',4,4'-trimethoxychalcone
3. ANTIPROLIFERATIVE ACTIVITY
The cytotoxicity of the compound was tested against four human tumor cell lines: HL-60 (human promyelocytic leukemia), HCT-116 (human colorectal carcinoma), MCF-7 (human breast adenocarcinoma) and K562 (chronic myeloid leukemia).
Cytotoxicity was also evaluated in normal cells L929 (murine fibroblast). Cells were cultured in RPMI-1640 medium (Sigma Aldrich), supplemented with 10% fetal bovine serum (Gibco), and 1% antibiotic solution (streptomycin and penicillin, Sigma Aldrich).
Cells were kept in an oven, at 37 °C, in a humid atmosphere enriched with 5% CO2. Stock
solutions of 20mM of the compound (dissolved in DMSO 100%) were prepared, and the test solution (50μM) diluted in RPMI-1640 medium, not exceeding the final concentration of 0,5% DMSO. For the cytotoxic assays, cells were seeded in 96-well plates (5 x 105 cells/mL for HL-60, and 3 x 105 cells/mL for the other cell lines) and incubated with 50 μM of the compound. DMSO (20%) was used as positive control.
Cell viability was determined by reduction of [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium] bromide (MTT) (MOSMANN 1983). After 72 h of incubation with the compound, the plates were centrifuged (500g, 5min, 25°C), the supernatant was removed, and MTT ( 5mg/mL) was added to each well (10µL). The plates were incubated for 3h, in a 5% CO2 oven, at
37 °C, for salt reduction and formation of formazan crystals. After the incubation period, 10% SDS (sodium dodecyl sulfate, HCl 0.01 N) was added for the solubilization of the formazan crystals. The
plates were left overnight on a shaker and the absorbance was measured at 570nm, in a microplate spectrophotometer (BioTekSynergy HT, USA).
The experiments were performed in triplicate and the percentage of inhibition was calculated in the GraphPad Prism 5.0 program.
4. RESULTS AND DISCUSSION
The 1H and 13C nuclear magnetic resonance data provided confirmation that the isolated compounds are the acetophenone and the expected chalcone, as shown in the literature.
Acetophenone (compound 2): 1H NMR (400 MHz, CDCl 3) δ 7.82 (d, J = 8.7 Hz, 1H), 6.50 (dd, J = 8.7, 2.3 Hz, 1H), 6.44 (d, J = 2.2 Hz, 1H), 3.87 (s, 3H), 3.84 (s, 3H), 2.56 (s, 3H).13C NMR (101 MHz, CDCl3) δ 198.22, 164.70, 161.22, 132.75, 120.98, 105.12, 98.30, 55.53, 55.45, 31.77. Chalcone (compound 3): 1H NMR (500 MHz, CDCl3) δ 7.73 (d, J = 8.6 Hz, 1H), 7.64 (d, J = 15.7 Hz, 1H), 7.54 (d, J = 8.7 Hz, 2H), 7.38 (d, J = 15.7 Hz, 1H), 6.90 (d, J = 8.7 Hz, 2H), 6.55 (dd, J = 8.6, 2.2 Hz, 1H), 6.49 (d, J = 2.2 Hz, 1H), 3.88 (s, 3H), 3.85 (s, 3H), 3.82 (s, 3H).13C NMR (126 MHz, CDCl3) δ 190.74, 164.05, 161.32, 160.34, 142.15, 132.78, 130.05, 128.22, 125.09, 122.52, 114.38, 105.22, 98.75, 55.83, 55.60, 55.44. Cytotoxic activity
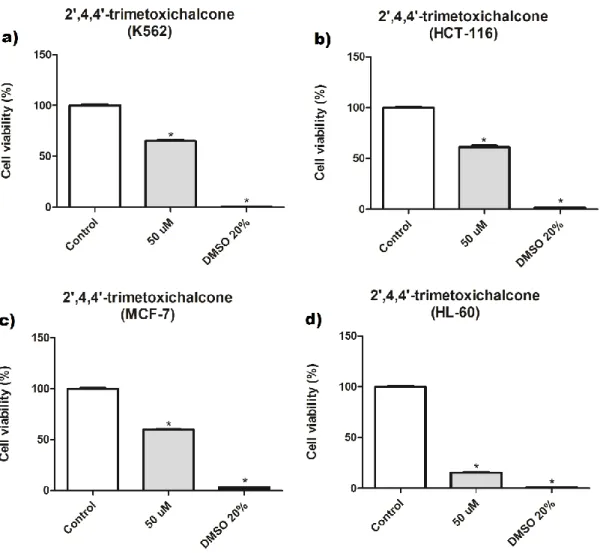
Samples were submitted to an analysis of variance (ANOVA) and the means were compared by the Tukey test at a 5% probability level. They were initially tested at 50 μM cytotoxicity, the effect of compound 3 on the viability of MCF-7, HL-60, K562, HCT-116 (tumor) and L929 (non-tumor) cells after 72 h of treatment can be seen in the graphs (Figure 4 and 5) and in the Table 1 below.
Figure 4 - The effect of compound 3 on the viability of K562, HCT-116, MCF-7 and HL-60, tumor cell lines.
Results are expressed as mean ± standard error of a quadruplicate independent experiment, tested at the concentration of 50 μM. Data were analyzed by ANOVA, followed by the Tukey post-test. * p <0.05 relative to the untreated control.
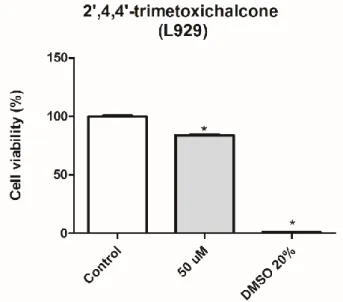
Figure 5- The effect of compound 3 on the viability of L929, a non-tumor cell line.
Results are expressed as mean ± standard error of a quadruplicate independent experiment, tested at the concentration of 50 μM. Data were analyzed by ANOVA, followed by the Tukey post-test. * p <0.05 relative to the untreated control.
Table 1 - Effect of compound 3 on the viability of MCF-7, HL-60, K562, HCT-116 (tumor) and L929 (non-tumor)
cells after 72 h of treatment
Results are expressed as mean ± standard error of a quadruplicate independent experiment, tested at the concentration of 50 μM. Data were analyzed by ANOVA, followed by the Tukey post-test. * p <0.05 relative to the untreated control.
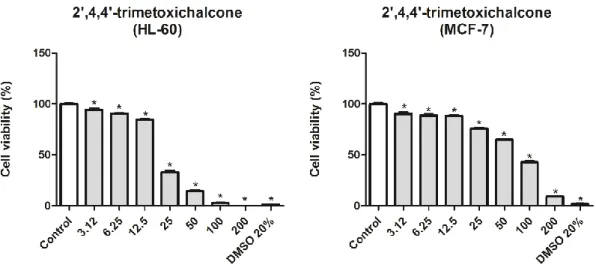
The compound 3 showed a great activity in the HL-60 line (84.5% inhibition), but in the other tumor lines (MCF-7, K562 and HCT) little activity was obtained. In non-tumor cell L929, compound 3 showed no activity. Afterwards, the half maximal inhibitory concentration (IC50) of this molecule
in the two tumor lines (MCF-7 and HL-60) was calculated because they presented the highest percentages of inhibition (Table 2), graphics can be seen below (Figure 6). For IC50 determination,
different concentrations of the substance (3,12, 6,25, 12,5, 25, 50, 100 and 200μM) were tested in quadruplicate. In conclusion, compound 3 was more cytotoxic for the HL-60 strain, with an IC50 of
approximately 20.84μM.
Substance Inhibition Percentage
MCF-7 HL-60 K562 HCT-116 L929
Compound 3
Results are expressed as mean ± standard error of a quadruplicate independent experiment, tested at the concentration of 50 μM. Data were analyzed by ANOVA, followed by the Tukey post-test. * p <0.05 relative to the untreated control.
Figure 6 – IC50 of the 2',4,4'-trimethoxychalcone on the viability of MCF-7 and HL-60 tumor cells after 72 h of
treatment
Results are expressed with ± the average error rate related to the singles experiments in quadruplicate tested at the concentration of 3.12 - 200 μM. Data were analyzed by ANOVA, followed by the Tukey post-test at a 5% probability level *p <0.05 compared to the control.
ACKNOWLEDGEMENTS
This work was a result of team work in Biotecnology Center (CBiotec) between Laboratory of OncoPharmacology (OncoFar) and Laboratory of Organic Synthesis. It was financially supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Postgraduate Program in Natural and Synthetic Bioactive Products (PgPNSB), Postgraduate Program in Biotechnology (PgBiotecM) and Laboratório Multiusuário de Caracterização e Análises (LMCA).
Substance IC50 (µM)
MCF-7 HL-60
REFERENCES
Díaz-Tielas C, Graña E, Reigosa MJ & Sánchez-Moreiras AM (2016) Biological Activities And Novel Applications Of Chalcones. Planta Daninha 34(3).
Greenwell M & Rahman PKSM (2015) Medicinal Plants: Their Use in Anticancer Treatment. Europe PMC Funders Group 6(10): 4103-4112.
Guangchang L & Xu, B (2018) Hydrogen bond donor solvents enabled metal and halogen-free Friedel–Crafts acylations with virtually no waste stream. Tetrahedrom Letters 59: 869-872.
Gudipudi, G, Sagurthi S & Perugu S (2014) Rational design and synthesis of novel 2- (substituted-2H-chromen-3-yl)-5-aryl-1 Himidazole Derivatives As An Anti-Angiogenesis And Anti-Cancer Agent. RSC Advances 4(99): 56489-56501.
Hossay A, Linsdall SM, Mamboury M, Rzepa HS & Spivey AC (2017) Total Synthesis of (+)-Lophirone H and Its Pentamethyl Ether Utilizing an Oxonium−Prins Cyclization. Organic Letters 19: 2486-2489.
Martel-Frachet V, Keramidas M, Nurisso A, DeBonis S, Rome C,5 Jean-Luc Coll, Boumendjel A, Skoufias DA, Ronot X (2015) IPP51, A Chalcone Acting As A Microtubule Inhibitor With In Vivoantitumor Activity Against Bladder Carcinoma. Oncotarget 6(16): 14669–14686.
Mosmann, T (1993) Rapid colorimetric assay for cellular growth and survival: application to to proliferation and cytotoxicity assays. J Immunological Methods 65 (1-2): 55–63.
Nall R (2018) What To Know About Cancer. Available at < https://www.medicalnewstoday.com/articles/323648.php> Acess on 29 May 2019.
Newman DJ & Cragg GM (2016) Natural Products as Sources of New Drugs from 1981 to 2014. Journal of Natural Products 79(3): 629-661.
Sun Y (2016) Tumor Microenvironment And Cancer Therapy Resistance. Cancer Letters 380(1): 205-215.