Resear
ch
Ethical-legal evaluation of clinical records in Brazilian
dentistry courses
Sueli de Souza Costa 1, Flávia Martão Flório 2
Abstract
This study analyzes the dental records used in the Brazilian dentistry courses, considering their suitability regarding the ethical guidelines of the legislation in force in the country. All the coordinators of the 220 graduation courses registered on the Federal Council of Dentistry’s website were invited, and 96 (43.6%) accepted to participate in our research. For the collection and analysis of data, we used a structured questionnaire with ethical and legislative questions. Of the total sample, 53.2% presented all the necessary minimum documents, but none of them met all the requirements of patient identification, anamnesis, informed consent form, and odontograms. Moreover, 17.8% fulfilled all the items for planning, and 61.5% had the authorization for the use of data and images. We concluded that these records do not conform to the current legislation and must be updated in order to obtain an improvement in the quality of the information, avoiding administrative, moral and legal problems.
Keywords: Ethics, dental. Forensic dentistry. Liability, legal. Legislation as topic. Forms and records control. Resumo
Análise ético-legal de prontuários clínicos de cursos de odontologia brasileiros
Este estudo objetiva analisar prontuários odontológicos utilizados na graduação em odontologia no Brasil, considerando sua adequação à legislação e diretrizes éticas em vigor. Os coordenadores dos 220 cursos cadastrados na página eletrônica do Conselho Federal de Odontologia foram convidados, e 96 deles aceitaram participar da pesquisa. Para coletar e analisar os dados, utilizou-se roteiro estruturado com questões éticas e legais. Do total da amostra, 53,2% dos prontuários apresentaram todos os documentos mínimos necessários, mas nenhum cumpriu todos os requisitos de identificação do paciente, anamnese, termo de consentimento livre e esclarecido e odontograma. Além disso, 17,8% cumpriram todos os itens relativos a planejamento e 61,5% atenderam às exigências de autorização para uso de dados e imagens. Conclui-se que os prontuários não se adequam à legislação atual, devendo ser revistos a fim de melhorar a qualidade da informação e evitar problemas administrativos, morais e jurídicos.
Palavras-chave: Ética odontológica. Odontologia legal. Responsabilidade legal. Legislação como assunto. Controle de formulários e registros.
Resumen
Análisis ético-legal de registros clínicos de cursos de grado en odontología en Brasil
Este estudio objetivó analizar los registros odontológicos utilizados en cursos de grado en odontología en Brasil, verificando su adecuación a la legislación y directrices en vigor. Se invitaron a todos los coordinadores de 220 cursos registrados en la página electrónica del Consejo Federal de Odontología, y 96 aceptaron participar. Para la recolección y análisis de datos, se utilizó un guion estructurado abordando cuestiones éticas y de legislación. Del total, el 53,2% de los registros clínicos presentaron los documentos mínimos requeridos; ninguno cumplió todos los requisitos de identificación del paciente, anamnesis, formulario de consentimiento informado y odontograma; el 17,8% cumplió todos los ítems de planificación; y el 61,5% atendió a los ítems de autorización del uso de datos y imágenes. Se concluye que estos registros no se adecuan a la legislación vigente y deben ser actualizados para mejorar la calidad de las informaciones, evitando problemas de orden administrativo, moral y legal.
Palabras clave: Ética odontológica. Odontología forense. Responsabilidad legal. Legislación como asunto. Control de formularios y registros.
Approval CEP-Centro de Pesquisas Odontológicas São Leopoldo Mandic CAAE 50721215.9.0000.5374
1. PhD scsueli@gmail.com – Faculdade São Leopoldo Mandic 2. PhD flavia.florio@slmandic.edu.br – Faculdade São Leopoldo Mandic, Campinas/SP, Brasil.
Correspondence
Flávia Martão Flório – Rua José Rocha Junqueira, 13, Ponte Preta CEP 13045-755. Campinas/SP, Brasil. The authors declare no conflict of interest.
When knowledge is democratized, the act of learning engages students and teachers and contributes to the transformation of society 1.
As a teaching object, ethics cannot be limited to theoretical concepts, especially in the area of health, in which practical learning is essential 2. The practice
cements theory, and future professionals need to know the reality that they will soon face.
Most graduates rely on documents studied in undergraduate courses 1-8, which generally highlight
technical aspects of the profession. However, the practice is not limited to technical aspects, covering ethical, legal and administrative issues. The set of these dimensions guides the relationship between professional s and patients, for which the records are essential 9,10.
The Brazilian Code of Medical Ethics (CEM) in article 87 forbids, for example, physicians from not elaborating legible medical records with the clinical data necessary for the good conduct of the case 11.
The code reinforces that inadequate registration omits information, disrespecting the user’s legal rights [and] denying their autonomy to maintain the medical history preserved and documented 12.
In dentistry, records are also a fundamental part of the patient-physician relationship 9,10. In
this sense, higher education institutions (HEI) are legally responsible for the treatment given by students to patients. It is up to HEIs to assume the requirements related to records and establish corrective strategies when necessary, letting the documentation to be prepared based on current ethical and legal foundations 7,13-15.
To qualify records, it is necessary the interaction between administration, information management, and health professionals 7,13-15.
Without this checking, there may be incomplete clinical records, documents in disagreement with the rules of the Brazilian Federal Council of Dentistry (CFO), lack of signatures and insufficient knowledge from a clinical, administrative and legal perspective 14-20. These failures are serious because,
in the case of a lawsuit, well-prepared records are the most important means of defense 7,13.
The concern with records increases as patients became more aware of their rights 21. The Brazilian
Federal Constitution 22 guarantees inviolability of
the personal life, right to health and confidentiality and respect for individuality. And both the Brazilian Civil Code 23 (and thePenal Code 24 address the same
issues, in addition to malpractice, misconduct and neglect, omission, damage, and legal redress.
The Brazilian Consumer Protection Code (CPC) 25 focuses on service provision and is reinforced
by the Code of Dental Ethics (CDE) 26, which makes
clear the reversal of the burden of proof in judicial or administrative demand, because the institution or the dentist must keep the record. Both CEM 11 and
CDE 26 explain that the professional conduct should
concern to the health of the human being, and that the acquired knowledge should be used for the benefit of the patient.
The CDE 26 also presents a specific chapter
on dental documentation, and the correct filling of records is widely debated and based on the literature. Documentation must contain identification data, two odontograms (pre- and post-treatment), space for describing radiographic findings, notes on pre-existing oral conditions, treatment planning, completed procedures and prescribed medications. The documents must also have notes on care for oral and maxillofacial lesions, copies of prescriptions and health certificates, models, radiographs, photographs, computed tomography (CT) scans, referrals, payment receipts and information about treatment abandonment, as well as clinical, administrative, legal and ethical documents generated by the physician-patient relationship. The same is valid for electronic records 16-21,26-30.
The informed consent form (ICF) 31 must
also be attached. In this document, the patient attests that he or she was informed about the risks of the treatment, attending to prescriptions in article 6, item III, of the CPC 25 and the CDE 26.
HEIs must ensure that this document is signed and that a copy is provided to the patient, in accordance with the resolution of the National Health Council (CNS) 466/2012 31.
Keeping this in mind, we did not find in the literature analyses of clinical records of Brazilian HEIs. To fill this gap, this research evaluates the records used in dentistry undergraduate courses in Brazil and thus verify their adequacy to ethical and legal norms in force.
Method
This cross-sectional descriptive study was conducted between August 2016 and September 2017, after approval by the research ethics committee of the Dental Research Center São Leopoldo Mandic. In 2015, through a search on the CFO website 32,we obtained registration information
Resear
from all the 220 dentistry undergraduate courses in Brazil. Their coordinators or directors were invited to participate in the research. The HEIs that accepted the invitation sent files with dental records, and the data were compiled by the researchers.
For record analysis a questionnaire was prepared with ethical and legal questions, which divided into seven tables. The instrument considered the minimum legal requirements and recommendations from the CFO, grouping them in a standard form (initial data, mandatory in all records) and specificity according to the main guidelines and laws (Constitution 22; Civil Code 23 23; Penal Code 24;
CPC 25; CDE 26; and CNS Resolution 466/2012 31).
The first table lists the minimum necessary documents (excluding those that cannot be verified and representing the limitations of this study) that must be included in all records to guarantee the rights and duties of dentists and patients. We considered the following information: patient’s identification; notes on pre-existing clinical conditions (anamnesis) and treatment planning; authorization to use images and data from records; informed consent form (ICF); and odontograms.
The second table contains mandatory items for the patient’s identification: name, home and professional address, telephone number, affiliation, birthday, sex, marital status, nationality and/or naturalness, identity card number and tax-payer’s identification number, referral, how the dentist was selected, identification of the legal guardian with date and signature, and a third party telephone number for contact.
The other table contains anamnesis data (as suggested by the CFO) subdivided into topics, register of the main complaint and the evolution of the disease. The medical history is the first topic and refers to: concomitant treatment, doctor’s name and telephone number, medicines, allergies and illnesses reports, previous interventions, hemorrhage in surgeries, and current or past diet. The second topic addresses oral-dental history and concerns to the last visit to a dentist and to oral hygiene issues. Finally, the third topic accounts (habits) for the patient’s practices, the statement that the information is true and is endorsed by signature and date, physical examination (extra- and intraoral radiographic examination) and initial odontogram with clinical conditions record.
The fourth table has items about treatment planning (options and patient’s choice, date of the act, and signature), while the fifth refers to the
authorization to use images and data from records (clarification, date of the act and signature.) In the sixth table, we find the items that must be included in the ICF: declaration of knowledge about the treatment, material used in the procedure, possible risks, benefits, costs, alternatives, patient’s decision, option to stop the treatment, clarification that the document is printed in two copies and signatures.
Finally, in the seventh table, we presented the items that must be included in the odontogram: notes on the type of procedure, date and time of the intervention, patient’s signature at each appointment and name, signature and registration number of the dentist at the Regional Council Dentistry (CRO).
One point was assigned to each item in the medical record. After counting the scores, we analyzed the data obtained. The limitations of this study are related to the fact that there is no way to measure them, given the rules that guide the legislation and various codes.
Results
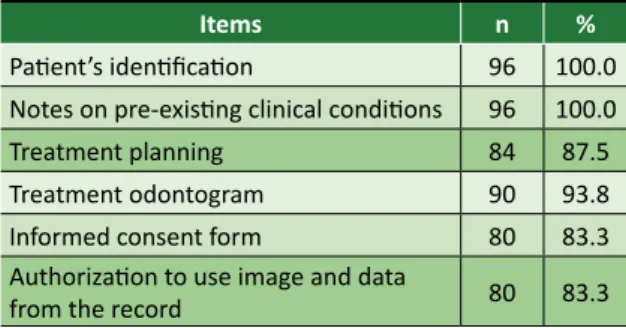
Of the HEIs registered on the CFO website, 43.6% agreed to participate in the survey. From the total of public institutions registered on the website, 27.2% agreed to participate in the research, and from the total of private institutions, 60%. We analyzed 96 records – all physical, since no digital documents were sent to the researchers – and found that 53.2% of them had all the necessary documents, even if incomplete. All the records had patient’s identification and notes on pre-existing clinical conditions (Table 1).
Table 1. Items related to the initial documentation in the analyzed records
Items n %
Patient’s identification 96 100.0 Notes on pre-existing clinical conditions 96 100.0 Treatment planning 84 87.5 Treatment odontogram 90 93.8 Informed consent form 80 83.3 Authorization to use image and data
from the record 80 83.3
Regarding patient’s identification, none of the documents analyzed met all requirements. “Patient’s name,” “home address” and “birthday” appeared on all records. On the other side, “the indication” (21%)
Resear
and “date and signature of legal guardian” (27.1%) are the least frequent items.
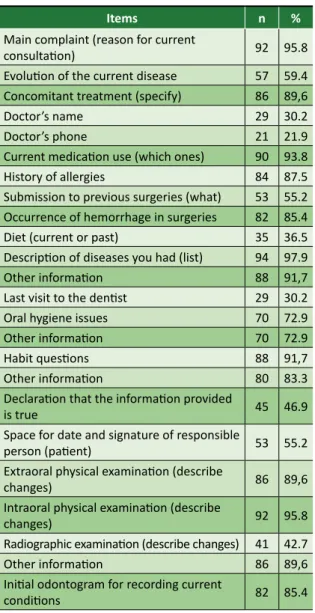
There was a variation from 21.9% to 97.9% in the meeting of anamnesis (Table 2) and none of the records fulfilled all the requirements. Almost half of the analyzed documents bear the “declaration that the information is true” (46.9%), and 55.2% reserve “space for the date and signature of the person responsible for the patient”.
Table 2. Items related to anamnesis in the analyzed records
Items n %
Main complaint (reason for current
consultation) 92 95.8
Evolution of the current disease 57 59.4 Concomitant treatment (specify) 86 89,6
Doctor’s name 29 30.2
Doctor’s phone 21 21.9
Current medication use (which ones) 90 93.8 History of allergies 84 87.5 Submission to previous surgeries (what) 53 55.2 Occurrence of hemorrhage in surgeries 82 85.4 Diet (current or past) 35 36.5 Description of diseases you had (list) 94 97.9 Other information 88 91,7 Last visit to the dentist 29 30.2 Oral hygiene issues 70 72.9 Other information 70 72.9
Habit questions 88 91,7
Other information 80 83.3 Declaration that the information provided
is true 45 46.9
Space for date and signature of responsible
person (patient) 53 55.2 Extraoral physical examination (describe
changes) 86 89,6
Intraoral physical examination (describe
changes) 92 95.8
Radiographic examination (describe changes) 41 42.7 Other information 86 89,6 Initial odontogram for recording current
conditions 82 85.4
Only 17.8% of the records fulfilled all items related to treatment planning. The lowest frequencies were found for “treatment choice” (19.8%), “treatment options” (27.1%) and “space for the signature of the person in charge” (31.3%). Table 3 shows the fulfillment of items related to the ICF. Again, none of the records met all requirements.
Table 3. Items related to the informed consent form in the analyzed records
Items n %
Declaration of knowledge of the
treatment to be carried out 11 11.5 Material to be used in the procedure 0 0.0 Possible risks of the procedure 8 8.3
Treatment costs 2 2.1
Knowledge of treatment alternatives 8 8.3 Patient’s decision about treatment
(agreement) 31 32.3
Option to stop treatment 4 4.2 Clarification that the form has two
copies 0 0.0
Other information 70 72.9 Space for place and date of signature 73 76.0 Signature space (patient) 77 80.2 Space for dentist’s signature 23 24.0
In the odontogram chart the items less fulfilled were “time of the procedure” (0%) and “space for signature (of the patient) at each consultation” (57.3%). “Notes on the type of procedure” and “notes on the date of the procedure” (both with 95.9%) were the most frequent. None of the records covered all items. Finally, in the seventh chart we found a variation from 70.9% (clarification of the use of images) to 83.4% (space for the responsible person’s signature), and 61.5% of the records answered all items.
Discussion
None of the records met all the established criteria, which can generate legal, administrative, and ethical conflicts. Even HEIs records based on CFO models need adaptation, as the legislation is constantly evolving, and the changes of the 2012 CDE 26 and CNS
Resolution 466/2012 31 have not yet been incorporated
by these institutions. This can reflect on the students’ professional lives, as they usually use the document presented in the undergraduate course as a model in their clinics/offices.
The percentage of essential documents found in the records ranged from 83.3% to 100%. HEIs that do not present complete documentation may have difficulties in defending themselves in eventual ethical and legal processes. Moreover, the CDE 26
informs that dentists and institutions are obliged to
Resear
conserve their records in their own file and the Law determines the burden of proof.
The lack of essential documents became clear especially in relation to treatment planning, ICF and authorization for the use of images and data. This absence compromises vigilance and brings moral and legal consequences. For example, from a legal point of view the absence of ICF assumes that the patient did not authorize the procedure, once the CPC 25 and the CDE 26 establish the need
for specific documentation.
Another important point is to preserve the individual’s image (photos, radiographs, tomographies, etc.) used for legal identification and directly linked to personality 9,13,17,22. The
absence of authorization damages the patient’s autonomy and freedom to decide 22,23,26,31, violating
the right to privacy guaranteed by the Brazilian Constitution 22 (article 5, item X), by the Civil
Code 23 (articles 20 and 21), and by the CDE 26
(article 14, item III), predicting compensation for moral and/or material damages. And the same is valid for personal data, which can also be used solely with the consent of the holder or in cases specified by Law 22,23,26.
The authorization to use images and data is present in almost all records, but in eight of them there is no space for signature of the holder. Thus, in this case HEIs cannot use patients’ information in scientific publications or even in lectures and classes 22, even with academic or teaching purposes.
Some doubts remain regarding the validity of the documents even in the most complete records, because as they do not fully comply with ethical and legal standards, imprudence, malpractice, or negligence can be characterized 23,24, and this
would require compensation for damages 23.
The absence of field research to identify the legal guardian, for example, omits fundamental information. Without these data, the legal accuracy of any information is invalidated.
Concerning anamnesis, the records register the reasons for the consultation and diseases of the patient, as well as the use of medications, allergies, and extra- and intraoral examinations. This knowledge is necessary to help professionals in their decisions, avoiding negligence, malpractice, and imprudence. However, the absence of a declaration about the veracity of information and space for the date and signature of the responsible or patient invalidate the document according to the Law.
In addition, some records do not have an initial odontogram, which makes it impossible to plan the treatment and subsequently verify its effectiveness. In a court, the HEI or the dentist will not be able to present evidence. Considering this, alternatives to the defined procedure should be registered, demonstrating that the patient can participate in the decision-making process 25,26.
According to the article 17 of the CDE 26, the
odontogram is essential to make comparisons with the initial situation, and thus demonstrate the results of the intervention. In almost all records there are dates and notes related to the type of procedure. Concerning the moment of the appointment, some HEIs use digital control (for example, with electronic turnstiles that record the time of entry and exit of the patient). However, this control should meet the standards of physical records with the patient’s digital signature, since the simple record of his or her presence does not guarantee that the designated procedure has been carried out.
The records show the concern to comply with Resolution CNS 466/2012 31 and the CDE 26 regarding
the ICF. The signature of the patient/legal guardian is present in 80.2% of the documents analyzed. However, none of the records reported that the term is printed in two copies or described the material used in the procedure, and only 2.1% addressed treatment costs.
The ICF is a mandatory document for doing experiments with humans or handling data 31, being
illegal to publish results or disclose information about people when legal requirements are not met. The rules for preparing this document should be followed by HEIs, considering their social role determined by the Constitution 22. Moreover, the
CPC 25 guarantees in article 6, item III, as a basic
consumer right, adequate and clear information about the different products and services, with the correct specification of quantity, characteristics, composition, quality, incident taxes, and price, as well as the risks involved in procedures, which is in line with the ICF required by the CDE 26.
However, although the patient’s statement showing knowledge about the treatment is necessary, only 11.5% of the records presented it.
Given to the fact there are no reports in the literature on the analysis of clinical records of undergraduate dentistry courses in Brazil, it is impossible to compare the data of this study. On the other hand, it is a consensus that HEIs should serve as a model for legal documentation related
Resear
to the medical activity, always respecting the law. However, by not adapting their records to current regulations, HEIs are not only against the Law, but also disrespect the patient’s right and ethical guidelines. The lack of information violates the principles of autonomy, justice, beneficence, non-maleficence, and equity.
HEIs are legally responsible for the treatment offered by the student and is co-responsible for the record. These institutions and professors should establish pedagogical strategies to guarantee the regulation of documents in accordance with the current legislation and ethical principles. Thus, they can combine high quality technical treatment and respect for the patient, avoiding problems with the Law, the CRO, and society.
Final considerations
None of the records analyzed was fully adequate to the ethical and legal standards in force, which demonstrates the need for the HEIs and teachers to update them to improve the quality of information and encourage students to correctly complete this type of document. This simple and indispensable improvement can avoid administrative, ethical, and legal problems. In addition, the complete and easily accessible documentation enables comparative studies to verify the true dimension of the oral health of the Brazilian population. Therefore, records are essential instruments to formulate and adapt public policies related to the right to health.
Article based on the doctoral thesis defended by Sueli de Souza Costa, and supervised by professor Flávia Martão Flório.
References
1. Freire P. Educação como prática da liberdade. 19ª ed. Rio de Janeiro: Paz e Terra; 1989.
2. Gerber VKQ, Zagonel IPS. A ética no ensino superior na área da saúde: uma revisão integrativa. Rev. bioét. (Impr.) [Internet]. 2013 [acesso 8 ago 2018];21(1):168-78. DOI: 10.1590/S1983-80422013000100020
3. Skinner BF. Tecnologia do ensino. São Paulo: Herder; 1972. 4. Piaget J. Seis estudos de psicologia. Rio de Janeiro: Forense; 1967.
5. Bordenave JD, Pereira AM. Estratégias de ensino-aprendizagem. 8ª ed. Petrópolis: Vozes; 1986. 6. Abreu MC, Masetto MT. O professor universitário em sala de aula: prática e princípios teóricos.
São Paulo: MG; 1996.
7. Kliemann A, Osmari D, Machado RR, Braun KO. A responsabilidade dos docentes no ensino sobre o prontuário odontológico. In: Anais do XVIII Encontro do Grupo Brasileiro de Professores de Dentística; 14-17 jan 2009; Foz do Iguaçu. Ponta Grossa: Universidade Estadual de Ponta Grossa; 2009. 8. Bruhn AM, Newcomb TL, Sheth-Chandra M. Assessment of mass fatality preparedness and
response content in dental hygiene education. J Dent Educ [Internet]. 2016 [acesso 8 ago 2018];80(5):605-11. DOI: 10.1002/j.0022-0337.2016.80.5.tb06121.x
9. Vieira JB. Análise de prontuários e da legalidade da criação do banco de imagens dos pacientes portadores de câncer atendidos na Faculdade de Odontologia de Araçatuba – Unesp [dissertação] [Internet]. Araçatuba: Universidade Estadual Paulista; 2009 [acesso 8 ago 2018]. Disponível: https://bit.ly/2UKqWHI
10. Oliveira DL, Yarid SD. Prontuário odontológico sob a ótica de discentes de odontologia. Rev Odontol Unesp [Internet]. 2014 [acesso 6 abr 2020];43(3):158-64. DOI: 10.1590/rou.2014.031 11. Conselho Federal de Medicina. Código de Ética Médica: Resolução CFM nº 2.217, de 27 de
setembro de 2018, modificada pelas Resoluções CFM nº 2.222/2018 e 2.226/2019 [Internet]. Brasília: CFM; 2019 [acesso 6 abr 2020]. Disponível: https://bit.ly/3dZu9L5
12. Sampaio AC, Silva MRF. Prontuários médicos: reflexo das relações médico-paciente. Rev. bioét. (Impr.) [Internet]. 2010 [acesso 8 ago 2018];18(2):451-68. p. 454. Disponível: https://bit.ly/2JKRfqQ 13. Ditterich RG, Portero PP, Grau P, Rodrigues CK, Wambier DS. A importância do prontuário
odontológico na clínica de graduação em odontologia e a responsabilidade ética pela sua guarda. Rev Inst Ciênc Saúde [Internet]. 2008 [acesso 8 ago 2018];26(1):120-4. Disponível: https://bit.ly/2Xgz0l8 14. Németh G, Paula LM, Varella MA, Angeletti P. Prontuário odontológico na clínica de cursos de odontologia. Rev Abeno [Internet]. 2001 [acesso 3 set 2018];1(1):77-81. Disponível: https://bit.ly/3fNkQNW
15. Farzandipour M, Meidani Z, Rangraz Jeddi F, Gilasi H, Shokrizadeh Arani L, Fakharian E, Saddik B. A pilot study of the impact of an educational intervention aimed at improving medical record documentation. J R Coll Physicians Edinb [Internet]. 2013 [acesso 8 ago 2018];43(1):29-34. DOI: 10.4997/JRCPE.2013.106
16. Almeida CAP, Zimmermann RD, Cerveira JGV, Julivaldo FSN. Prontuário odontológico: uma orientação para o cumprimento da exigência contida no inciso VIII do art. 5º do Código de Ética Odontológica
Resear
[Internet]. Rio de Janeiro: Conselho Federal de Odontologia; 2004 [acesso 26 abr 2017]. Disponível: https://bit.ly/2xaf2Od
17. Silva RF, Portilho CDM, Reges RV, Leles CR, Freitas GC, Daruge E Jr. Importância pericial dos registros odontológicos decorrentes de tratamento restaurador. Rev Dental Press Estét [Internet]. 2007 [acesso 26 abr 2017];4(4):32-8. Disponível: https://bit.ly/34OHoMi
18. Costa SM, Braga SL, Abreu MHNG, Bonan PRF. Avaliação da comprovação de documentos emitidos durante o atendimento odontológico e do arquivamento das radiografias nos prontuários de saúde da Unimontes, Montes Claros, Brasil. Pesq Bras Odontoped Clin Integr [Internet]. 2008 [acesso 8 ago 2018];8(2):209-13. Disponível: https://bit.ly/39IItEo
19. Tsuchiya MJ, Gomes EM, Abe DM, Oliveira FVN, Massaoka C, Oliveira RN. Human identification through the analysis of dental records registered in the context of a dental institution. Rev Gaúch Odontol [Internet]. 2013 [acesso 8 ago 2018];61(3):389-93. Disponível: https://bit.ly/2X9TAng 20. Costa SS, Silva AM. Prontuário em odontologia do trabalho: um modelo para uso na especialidade. Rev
Bras Med Trab [Internet]. 2014 [acesso 8 ago 2018];12(2):85-95. Disponível: https://bit.ly/3bTHXF5 21. Serra MC. Confecção e guarda da documentação odontológica: prevenção de problemas legais. J
Assessor Odontol. 1999;3(17):29-34.
22. Brasil. Constituição da República Federativa do Brasil de 1988. Diário Oficial da União [Internet]. Brasília, 5 out 1988 [acesso 23 abr 2018]. Disponível: https://bit.ly/2JLT2Mh
23. Brasil. Lei nº 10.406, de 10 de janeiro de 2002. Institui o código civil. Diário Oficial da União [Internet]. Brasília, 11 jan 2002 [acesso 23 abr 2018]. Disponível: https://bit.ly/3aOLV1x 24. Brasil. Decreto-Lei nº 2.848, de 7 de dezembro de 1940. Código penal. Diário Oficial da União
[Internet]. Brasília, 31 dez 1940 [acesso 23 abr 2018]. Disponível: https://bit.ly/2Rh2rjn 25. Brasil. Lei nº 8.078, de 11 de setembro de 1990. Dispõe sobre a proteção do consumidor e dá
outras providências. Diário Oficial da União [Internet]. Brasília, 12 set 1990 [acesso 23 abr 2018]. Disponível: https://bit.ly/2JLT3Ql
26. Conselho Federal de Odontologia. Resolução CFO nº 118, de 11 de maio de 2012. Revoga o Código de Ética Odontológica aprovado pela Resolução CFO nº 42/2003 e aprova outro em substituição. Diário Oficial da União [Internet]. Brasília, 14 jun 2012 [acesso 23 abr 2018]. Disponível: https://bit.ly/3e2UJCW
27. Marques GH, Martins KPH. Responsabilidade médica e suas implicações na prática clínica. Rev. bioét. (Impr.) [Internet]. 2015 [acesso 8 ago 2018];23(1):51-60. DOI: 10.1590/1983-80422015231045 28. Silva M. Documentação em odontologia e sua importância jurídica. Odontol Soc [Internet]. 1999
[acesso 23 abr 2018];1(1/2):2-4. Disponível: https://bit.ly/2RjTLIV
29. Benedicto EN, Lages LHR, Oliveira OF, Silva RHA, Paranhos LR. A importância da correta elaboração do prontuário odontológico. Odonto [Internet]. 2010 [acesso 7 abr 2020];18(36):41-50. DOI: 10.15603/2176-1000/odonto.v18n36p41-50
30. Amos KJ, Bearman M, Palermo C. Evidence regarding teaching and assessment of record-keeping skills in training of dental students. J Dent Educ [Internet]. 2015 [acesso 8 ago 2018];79(10):1222-9. DOI: 10.1002/j.0022-0337.2015.79.10.tb06016.x
31. Conselho Nacional de Saúde. Resolução CNS nº 466, de 12 de dezembro de 2012. Aprova diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União [Internet]. Brasília, 13 jun 2013 [acesso 23 abr 2018]. Disponível: https://bit.ly/2xWGwa1 32. Conselho Federal de Odontologia [Internet]. c2016 [acesso 1º ago 2016]. Disponível:
https://bit.ly/3dmyVRD
Participation of the authors
Both authors developed this article. Sueli de Souza Costa
0000-0003-4127-7324 Flávia Martão Flório
0000-0001-7742-0255 Received: 2.13.2019 Revised: 1.21.2020 Approved: 1.28.2020