FACULDADE DE ENGENHARIA DA UNIVERSIDADE
DO PORTO
Bonelike
®
Associated to Stem Cells
for the Regeneration of the Bone
Tissue
Ana Cláudia da Costa Lopes
MEB 2008/2010
25 de Outubro de 2010
2
Resumo
A engenharia de tecidos é uma ciência em evolução com o objectivo de restaurar danos em tecidos ou órgãos que não sejam capazes de uma regeneração rápida, completa e bem sucedida. Os substitutos ósseos fazem parte deste objective, porém por vezes apresentam dificuldades difíceis de ultrapassar como a rejeição imunológica, a necessidade de mais que uma cirurgia, a morbilidade associada ao local de colheita, a transferência de doenças infecciosas entre outras. Todas estas desvantagens estão associadas às diferentes origens dos enxertos ósseos.
Os enxertos ósseos devem por isso eliminar a possibilidade das desvantagens referidas se realizarem bem como possuir propriedades que aumentem o ritmo do processo de regeneração integrando as células da forma mais semelhante possível ao organismo in vivo.
São várias as linhagens celulares que estão envolvidas no processo de regeneração in vivo, porém as mais importantes são sem dúvida as células estaminais mesenquimais, presentes na medula óssea, que podem originar tecido ósseo, cartilagíneo e adiposo. Estas células estaminais mesenquimais originam as células que intervêm nos processos de remodelação óssea.
O objectivo deste trabalho é avaliar a resposta biológica das células presentes no aspirado da medula óssea com diferentes formatos de Bonelike®, substituto ósseo sintético. Tendo estes dois formatos, Bonelike® Pellets e Bonelike® Poro, características diferentes com diferentes propósitos clínicos, irá ser avaliada a aderência, crescimento e migração das células nestes materiais e verificar a diferenciação das células estaminais mesenquimais em células da linhagem osteoblástica.
Os resultados mostraram que ambos os formatos de Bonelike®, permitem o crescimento, a proliferação e induzem a diferenciação das células mesenquimais em direcção a uma linhagem osteoblástica.
3
Abstract
Tissue engineering is a science still in evolution with the goal of repair damaged tissues and organs which are not capable of a quick, complete and well succeed regeneration. Bone substitutes are part of that goal, although sometimes they present some hard difficulties to pass through like immunological rejection, the need of an extra surgery, morbidity associated to the donor site, transmission of infectious diseases, among others. All these disadvantages are associated to the different origins of the bone grafts.
The perfect bone grafts should eliminate the possibility of some of those disadvantage occur as well as possess special properties that improve the rhythm of the regeneration process integrating the cells the most similar way as in the organism in vivo.
Several cellular lineages are involved in the regeneration process in vivo, however the most important group are the mesenchymal stem cells, presents in bone marrow, that can originate osteogenic, chondrogenic and adipogenic cells. These mesenchymal cells can differentiate into all the cells that interfere in the bone remodeling process.
The aim of the present work was to evaluate the biological response of the cells presents in bone marrow aspirate with different formulations of Bonelike®, a synthetic bone graft. Bonelike® Pelletes and Bonelike® Poro have different properties and so different clinical applications in which adherence, growth and migration of cells were tested as well as the differentiation of mesenchymal stem cells into a osteoblastic lineage.
Results showed that both formulations of Bonelike®, allow the growth, proliferation and induce the differentiation of mesenchymal cells towards to an osteoblastic commitment.
4
Acknowledgements
I am grateful to my supervisor Professor José Domingos Santos and Professora Maria Ascensão Lopes.
My specials thanks to Professora Helena Fernandes and to all the members of Laboratório de Farmacologia da Faculdade de Medicina Dentária da Universidade do Porto, specially Raquel Almeida.
I would like to thank my family for the encouragement and support given during these two years.
5
Contents
Resumo ... 1 Abstract ... 3 Acknowledgements ... 4 Chapter 1 ... 9 General introduction ... 9 1. Bone Physiology ... 9 1.1 Morphology ... 9 1.2 Bone Cells ... 11 1.2.1 Osteoblasts ... 11 1.2.2 Osteocysts ... 12 1.2.3 Osteoclasts ... 14 1.3 Bone remodeling ... 14 1.3.1 Healing process ... 15 2. Bone marrow... 17 2.1 Stem cells ... 17 2.1.1 Origin ... 17 2.1.2 Differentiation ... 20 2.1.3 Lineage Cells ... 21 2.1.4 Identification ... 25 3. Bone grafts ... 29 3.1 Autografts ... 29 3.2 Allografts ... 29 3.3 Xenografts ... 29 3.4 Synthetic... 30 3.4.1 Bonelike® ... 31 3.4.1.1 Composition ... 313.4.1.2 Structure and Properties ... 32
4. Tissue Engineering ... 34
Chapter 2 ... 37
Experimental Procedures ... 37
Preparation of Bonelike® ... 37
In vitro biological studies ... 38
Results and Discussion ... 41
Bonelike® characterization... 41
In vitro biological studies ... 44
Conclusion ... 59
6
List of Figures and Tables
Figure 1 -Bone structure. In this squeme is represented the Haversian system
and the osteocytes[6]. ... 11
Figure 2 -Proliferation vs Differentiation in osteoblastic lineage [3]. ... 12
Figure 3 -Schematic representation of the influence of the osteocytes in the remodelating process. O –osteocyst; L –cellular lineage; B -osteoblast; C –osteoclast [3]. ... 13
Figure 4 -Plasticity of a Hematopoietic stem cell [22]. ... 20
Figure 5 -Different lineages that a HPSC can originate [17]. ... 22
Figure 6 -Different lineages that a MSC can originate [3]. ... 24
Figure 7 -Process of identification of surface markers using fluorescent tags [17]. ... 26
Figure 8 -FACS method, separation of SC by their fluorescence [17]. ... 27
Figure 9 -CD34 expression in the HPSC that will originate platelets [35]. ... 28
Figure 10 -Bonelike® Pellets structure. SEM analysis. ... 32
Figure 11 -Bonelike® Poro 2000-6000µm. SEM analysis. ... 33
Figure 12 –Principal Intervenients in the tissue regeneration process in tissue engineering. ... 35
Figure 13 -Microstructures of bone substitutes. SEM analysis. ... 35
Figure 14 –Cell separator from the Kit with the three distinct parts. From the top, cell poor plasma, nucleated cell concentrate and on the bottom the red blood cells. ... 38
Figure 15 –Bonelike® Pellets. a) pellets with some irregular details (arrows) in surface, b) microporosity present in the pellets surface (arrows). SEM analysis. ... 42
Figure 16 –Bonelike® Poro samples. a) high level of porosity, pores with different sizes, b) irregular surface with microposity (arrow). SEM analysis. ... 43
Figure 17 -Bonelike® Pellets seeded with the bone marrow concentrate, at 1 day of culture, a),b) showing some erythrocytes (arrows). SEM analysis. ... 45
Figure 18 -Bonelike® Pellets colonized with bone marrow cells, at 4 to 23 days. a) presence of defects through the surface; b), c), d), f) cells take advantage of microposity to attache to surface material (arrows); e), g) cluster of cells on the material surface (arrows). SEM analysis. .... 47
Figure 19 -Bonelike® Pellets colonized with bone marrow cells. a) cluster of cells at day 23, b) evidence of mineralized deposits associated with the cell layer within the cell clusters (arrow). SEM analysis. ... 48
Figure 20 -Bonelike® Pellets colonized with bone marrow cells, at 23 days. Representative spectrum of the mineralized deposits. X-ray spectrum. ... 48
Figure 21 -Bonelike® Pellets colonized with bone marrow cells on day 23. a) cluster of cells, and cells growing on defects present on the surface(see arrows); b) control culture; 20x.. ... 49
Figure 22 -Cell growth rate over colonized Bonelike ® Pellets and control cultures. MTT assay. *significantly different from control. ... 50
7 Figure 23 -ALP activity measured over colonized Bonelike® Pellets and control
cultures. * significantly different from control. ... 51 Figure 24 -Bonelike® Poro, at 1 day of culture. a), b) erythrocytes on the porous
surface (arrows). SEM analysis. ... 52 Figure 25 -Bonelike® Poro colonized with bone marrow cells, at 4 to 23 days. a),
b) cells taking advantage of microporosity to attach to the material (arrows); c) cells colonizing the interior of pores; d), e), f) bridges made by cells through some irregularities of the material (arrows); g), h) several layers of cells adapted to the topological morphology of the material’s surface(arrows). SEM analysis. ... 53 Figure 26 -Bonelike® Poro colonized with bone marrow cells, at 23 days. a), b),
c) and d) abundant mineralized deposits closely associated with the cell layer (see arrows). SEM analysis. ... 55 Figure 27 -Bonelike® Poro colonized with bone marrow cells, at 23 days. X-ray
spectrum of the mineralized deposits. ... 56 Figure 28 -Bonelike® Poro colonized with bone marrow cells at day 23. a)
exuberant proliferation through the entire scaffold; b) control culture; 20x. ... 56 Figure 29 –Cell growth rate over colonized Bonelike® Poro and control cultures.
MTT assay. *significantly different from control. ... 57 Figure 30 -ALP activity measured over colonized Bonelike® Poro and control
cultures. * significantly different from control. ... 58 Table 1 -Embryonic germ layers from which differentiated tissues develop,
adapted from Stem Cells: Scientific Progress and Future Research Directions [17]. ... 18
8
Chapter 1
9
Chapter 1
General introduction
1. Bone Physiology
Bone is structurally complex, live and mineralized. It shows some rigidity but it keeps a certain degree of elasticity that allows him to absorb energy and when under stress and tension it can deform reversibly [1-3].
Bone plays important functions in our body, among the most important ones are: mechanical support, connection to muscles which allows locomotion [4] , protection of the vital organs and bone marrow (BM), they also act like ions reserve, calcium and phosphorus are the principal, so this way bone became the primary responsible for the internal homeostasis, and for the main place for production of blood cells (hematopoiesis ) [2].
During the life period of human body, bone mass suffers constantly remodeling process, that include formation of new bone and reabsorption, these mechanisms are integrating part of the skeleton renewal, that include loss and gain of bone mass (mineral homeostasis) [2, 4]. When bone is required its deposition is induced on the other and as soon as it stops the lack of activity translates into reabsorption. The majority of bone matrix is composed by collagen type I (90%), and all the molecules are organized in fibril. Proteins and peptidoglycans are also a part of this matrix [2]. The mineral phase is composed by ions such as calcium and phosphate with a similar composition to hydroxyapatite (HA) [Ca10(PO 4)6(OH)2].
1.1 Morphology
In our body exists three main types of bone: the long bones, like tibia and femur, flat bones such as skull and jaw, and short bones like carpal and tarsal.
10
It can even be found in bone structure two anatomically different zones: epiphysis and diaphysis. These two parts are separated by a layer of cartilage, which is composed by proliferative cells, it’s responsible for the longitudinal growth of the bone. Diaphysis is the middle region that allows mechanical stability [5]. BM can be found in this area.
Morphologically bone is divided in: cortical bone and spongy or trabecular bone. The external part is made of a thin layer, 80-90% of that layer is mineralized. The compact bone is denser that the spongy one, so it has two functions: mechanical and protection [2]. For having BM spongy bone has functions mostly related to metabolic process.
Cortical bone is composed by a thin layer of collagen fibers with parallel disposition or concentrically around canal with vases – the Haversian channel. These systems have in their central axis, the Havers channel, this channel have concentrically lamellas with fibers around [5]. These channels communicate each other, and with marrow cavity and the external surface of the bone trough transversal channels called Volkman channels, this can be distinguished from the Havers because they don’t show this concentrically lamellas.
The Haversian system (Figure 1) is a structural unit of the cortical bone, and they can be found in the region of the diaphysis.
These systems also have a central channel with blood vessels (one arteriole and one venule) and these vessels are also surrounded by lamellas. Between the lamellas may occur many lacunas which communicate with the central channel. In the lacunas several osteocyctes are presents, arranged in circles around the Havers channel. Havers system or osteon are isolated and separated from the others osteon with cement.
Spongy bone is formed by a thin layer of trabeculae, and in their gaps can found the BM.
11 Figure 1 -Bone structure. In this squeme is represented the Haversian system and the
osteocytes[6].
1.2 Bone Cells
The three main types of cells that are responsible for the formation, management and the reabsorption of the bone are the osteoblasts, osteocysts and the osteoclasts respectively. The progenitor cells are originated from the mesenchymal ones, during the post-natal period they localized mostly in the periosteum and endeosteum, which are the layers that cover the inner and outer surface of the bone, respectively.
1.2.1 Osteoblasts
These are the cells responsible for the bone formation, they have their origin in mesenchymal stem cells (MSC), these stem cells (SC) are locally stimulated by growth factors like fibroblast grow factor, bone morphogenic proteins and transcription factors, such as proteins that bound themselves to DNA of the eurcariotic cells to allow a connection between the RNA-polimerase enzim and the DNA, to make possible transcription and translation, such as Runx2 and Osterix [3, 7].
12
The MSC suffer a division, and then differentiate in a pre-osteoblast cell, as can be seen in Figure 2. Along this process of differentiation there are a number of markers that permit the identification of the stage of maturation the cells are [7]. Beside these morphological characteristics, the analysis of the expression of certain molecules allows the osteoblast identification. The Cbfa-1 (core-binding factor A1) is an osteoblastic transcription factor express in a differentiation stage [8], the Osteopontin is especially detected during the proliferation stage of these cells, and the alkaline phosphatase is an indicator of the level of the osteoblastic differentiation, the bone sialoprotein is expressed is the stage of maturation of osteoblasts [3, 7]. The recently formed bone matrix, together with the active osteoblasts that are not yet calcified is called osteoid.
Figure 2 -Proliferation vs Differentiation in osteoblastic lineage [3].
Another substance is also sintethized by the osteoblasts, the osteoprotogerin is an inhibitor of the differentiation of monocytes and macrophages into osteoclasts [3].
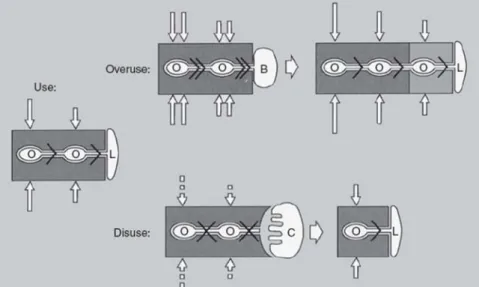
1.2.2 Osteocysts
Osteoblasts may be arrested in the calcified matrix and then they differentiated in osteocyctes. The osteocyctes function in a grid, every osteon is a major structure that plays an important role in the management and metabolic activity in the bone tissue (Figure 3) forming this way a three dimensional and sensitive structure like a specialized communication system.
13 This structure offers two advantages:
1) An enormous contact surface bone-cells, twice superior to the contact area that osteoblasts have;
2) An extensive communication system, intra and extra cellular between the inner places and the bone surface.
It is thought that osteocytes can be involved in the calcium diffusion process from the inside to the outside of the bone and vice-versa.
Figure 3 -Schematic representation of the influence of the osteocytes in the remodelating process. O –osteocyst; L –cellular lineage; B -osteoblast; C –osteoclast [3].
In the initial stage (USE) the normal mechanical strength provides a regular flux of interstitial fluid through the lacunas. This flux keeps the osteocysts viable and promotes their activation, so the osteoblastic activity is suppressed as well as osteoclastic attack. However in a situation where the amount of flux increased (Overuse) the osteocysts are activated and start to produce signals for osteoblastic recruitment. A subsequent bone formation reduces the activity of osteoblasts until the initial and regular pressures can be achieved. When a decreased (Disuse) of the flux provided by the mechanical strength happens, the osteocysts are inactivated by lack of that same flux. This inactivation leads to a release of
14
recruitment signal for the osteoclasts. The bone reabsorption caused by the osteoclastic activity leads to a reestablishment of the normal bone mechanical use.
1.2.3 Osteoclasts
The bone reabsorption is essential to skeleton maturation, including the remodeling processes and bone growth [1], as for example the moving process of tooth.
Osteoclasts are large cells, originated thought the fusion of several cells from the monocyto-macrophage system which have their origin in cells of BM [3, 4]. The BM cells, when under the effect of growth factors synthesized by osteoblasts (like the Macrophage Colony Stimulating Factor), promote the differentiation and multiplication of monocytes and macrophages as well as their fusion into osteoclasts [3, 4].
The regulation of osteoclastic activity is also dependent on the presence or absence of some hormones. The paratiroide hormone acts directly on osteoblasts, which secrete interleukin 11 that stimulate the osteoclasts. The calcitonin also has a direct effect on osteoclasts, promote the decrease of bone reabsorption [3].
1.3 Bone remodeling
To be such a dynamic tissue, after growing is completed, bone undergoes constants remodeling processes in structure. These processes function as an adaptation to the needs and effort developed by the organism [9]. For instance a professional tennis player has different needs in his superior members, so he probably will have different structure at bone level. The bone size, his shape and bone mass distribution will be different in his superior members [3].
It has already been seen, that histologically, exist two types of bone, the cortical and the spongy bone. The cortical bone is reshaped by the removal and the filling of osteons (or Havers systems). The active osteoclasts reabsorb the damaged bone forming this way a lacuna. So if a certain depth is reached in the lacuna, starts a new stage and the osteoclasts reabsorbed all the osteon. In the next phase osteoblasts start to produce and depositing bone. In the process of formation of a
15 new osteon, the osteoblasts can be trapped in the matrix, if this happens they transform into osteocysts.
Due to the different structure (absence of Havers systems) that spongy bone has, it does not follow the same path of cortical bone and the osteoblasts do not get arrested in the matrix during the remodeling process. The osteoclasts can be activated and start to reabsorb bone, if osteoclasts receive signals to stop their activity, the osteoblasts can begin to do their function and restore the missing bone [3].
The sequence of events in bone remodeling can be described as in the following order:
1) Osteocysts connected between them;
2) Small fracture that leads to the osteocysts apoptosis;
3) The site where osteocysts undergoes apoptosis provides the information to the activated osteoclasts about where the damage occurs;
4) Osteoclasts reabsorbed the damaged bone and remove all the dead cells by phagocytosis;
5) Reversal process happens, formation of cement; 6) Osteoblasts start depositing the new bone;
7) And as a consequence some of them can be trapped in the matrix transforming themselves into osteocysts, rebuilding that way the grid of osteocysts.
1.3.1 Healing process
The healing process involves the same cells that the bone remodeling process needs [10].
The bone shows an incredible ability to regenerate most part of the damage through the proliferation of the osteoprogenitors cells [1]. After a fracture occur the following sequence of steps happens:
16
i) The blood vessel broke so as consequence a hematoma is formed, which leads to an afflux of inflammatory cells and the substances secreted by them, like growth factors activate the osteoblastic progenitor cells;
ii) The hematoma is transformed in a fibrous tissue that some way provides some support to the boundaries of the damaged site but does not have enough toughness to support the surrounding tissues.
iii) The osteoblasts begin to deposit the new formed and still immature bone. This type of primary bone tissue is characterized by an irregular disposition of collagen;
iv) The MSC give origin to the hyaline cartilage that involves the entire fractured site;
v) The new bone in the fractured site mineralize;
vi) In the end all the primary bone that has been deposited is remodel and transformed into secondary bone with regular structure and lamellar shape with layers of collagen [11, 12].
The age has an important role in the healing process. The rate of a fracture healing is largely superior in children compared to adults and may be related to vascularization as well as the periosteum cells that have a less effective cellular response in relation with the growth factors in people with advantage age [9, 13]. The nutrition is especially important, the calcium and phosphorus have to be in good levels to a good healing process [9].
17
2. Bone marrow
BM is one of the places in the human body where is possible to find SC.
SC originate all the bone forming cells, those responsible for reabsorption as well as all the cells in blood and immunological system. It is by definition the hematopoietic organ. From BM also come progenitor cells of cartilage and adipogenic tissue. The blood vessels surrounding the BM constitute by their own a physical barrier to the immature blood cells stopping them to leave the BM before their complete maturation.
2.1 Stem cells
A SC is an undifferentiated cell capable of self-renewal for indefinites periods of time [14-16]. SC undergoes an asymmetric division and the result is a cell equal to the first and other cell called progenitor cell. So in the end of the division process we will have one SC that originates two different cells. Every time a division happens a SC is perpetuating his population. The progenitor cell will follow is differentiation path and raise eventually cells more specialize and committed with a specific function, forming this way a cell lineage.
In a regular embryo the cells cross many stages, in the beginning they are undifferentiated, then committed and at last differentiated. The zygote for instance although his totipotency is not considered a SC because when it divides it will not generate cells equal to itself.
SC are now being study as possible carriers of genes to specific tissues in human body, these investigations have been made mostly for therapy cancer [17]. It is thought that in the future these cells will probably be able to correct cromossomic anomalies in the first’s stages of development [17].
2.1.1 Origin
Which make these cells so special is mostly: 1) Being undifferentiated cells and not specialize;
18
2) Their capacity to self-renewing and division for long periods; 3) Being able to differentiate into distinct cell lineages.
A totipotent cell, the zygote, has potential to originate all kinds of cells and tissues. The zygote passes through divisions and then differentiates in a developed organism, so it has an unlimited potential of differentiation.
The pluripotent SC are capable of give rise cells from the three embrionary layers (Table 1) mesoderm, endoderm and ectoderm. All together can originate any kind of tissue in an adult organism except the placenta and support tissues of uterus. The only sources of pluripotent SC are those who have been isolated and cultivated from human embryos.
Embryonic Germ Layer Differentiated Tissue
Endoderm Thymus
Thyroid, parathyroid glands Larynx, trachea, lung
Gastrointestinal (GI) organs(liver,pancreas) Lining of the respiratory tract
Mesoderm Bone marrow (blood)
Lymphatic tissue
Skeletal, smooth, and cardiac muscle Connective tissues(bone, cartilage) Heart and blood vessels (vascular system)
Ectoderm Skin
Neural tissue (neuroectoderm) Adrenal medulla
Connective tissue of the head and face Eyes, ears
Table 1 -Embryonic germ layers from which differentiated tissues develop, adapted from Stem Cells: Scientific Progress and Future Research Directions [17].
The unipotent SC are usually adult SC meaning that they have the capacity of only differentiates in one cell lineage. However if a tissue had suffered any damage and some types of cells are needed the pluripotent SC are activated.
19 The SC can have two possible origins: the embryo and the adult organism. There are two major types of SC: adult and embrionary stem cells (ESC). ESC are descendents from an internal cellular mass present in blastocyst (4-5 day) [14, 18]. Until 2001 the scientific community has discovered 30 cellular lineages of pluripotent human cells which can differentiate from the blastocyst cells [17].
By now scientists already have identified adult SC in brain, BM, peripheral blood, blood vessels, skin epithelium, digestive system, cornea, retina, dental pulp, liver, pancreas, hair follicle and in oral mucosa. In another words it have been founded adult SC in organs descendent from the three embrionary layers.
This kind of SC in an adult organism may proliferate for long periods (as definition of adult SC for all the life of the organism) [14]. Adult SC are rare, difficult to isolate and purify in laboratory which makes it very hard to get for research. Their insufficient number is definitely a limiting factor with respect to using them to repopulate a damaged area. However it’s possible to expand small populations with a bioreactor, studies have been done in order to make this situation a reality [19-21].
Besides their origin one of the main differences between ESC and adult SC are their different capacity of differentiation. ESC are clearly pluripotents and adults SC are only unipotent [16]. But could adult SC be removed from is natural environment and being induced or manipulated so they can differentiate into types of cells that initially they would not become? There are not any definitive answers yet.
ESC can be achieved in great numbers in vitro and they can stay in the undifferentiation stage for many generations although their tendency to form tumors are still a problem [14]. If the difficulties associated to the adult SC were considered the ideal conditions in laboratory for these cells to proliferate without differentiating has not yet been discovered. The problem is particularly more severe with the isolated hematopoietic progenitor stem cells (HPSC) from the blood or from the BM. Despite proliferation may occur in case of transplant to a human or to an animal when in in vitro conditions they stop proliferating or do it in a limited way.
20
2.1.2 Differentiation
Differentiation is the cellular process when a cell unspecialized became committed to a certain cellular lineage. For differentiation to occur in a SC or in a progenitor cells it is necessary that biological conditioning happens. The signals that modulate this procedure in specialize cells are until now unknown.
When a SC suffers its first division gives rise to a cell exactly like the first one and other called progenitor cell. The progenitor cell enters in a division process and originates two cells, one is a progenitor cell and the other could be a cell with a higher level of specialization. These cells are responsible for replacing all the tissues in damaged areas and maintaining the organs functionality.
Some SC are gifted with plasticity. Plasticity is a recent termination that is applied every time that an adult SC from a specific tissue as the ability to generate a specialize cell from another kind of tissue (Figure 4). Unfortunately this process is not well understood yet. A recent example of this termination is that under specific experimental conditions the adult SC from de BM may originate cells that look like neurons [17]. Evidences suggest that giving to an adult SC the proper environment some will be capable of a genetic reprogramming which leads to cells with characteristic from other kind of tissues [17].
Figure 4 -Plasticity of a Hematopoietic stem cell [22].
Some intrinsic, inherent to the cell, and extrinsic factors like inductors interfere in the differentiation process of SC and progenitor cells. Niche is the termination that includes the surrounding environment and these factors. The surrounding cells in a niche are those who keeps the SC in a quiescent stage or in a slowly rate of division. The environmental factors shape the surrounding cells which will induce
21 the SC to divide. So we can assume that cells fate is controlled and defined by environmental and inductors factors [17].
2.1.3 Lineage Cells
The human body has as many cells lineages as types of tissue and specialize cells. In this situation is interesting look deeper in two types of SC in the BM, the HPSC and the MSC. In Figure 5 all lineages that one single HPSC may originate are represented as well as their functions.
All blood cells including the cells from the immunological system have their origin in a HPSC which are a pluripotent cell [23]. With the intervention of several environmental factors like cytokines these progenitors cells highly undifferentiated (derived from hematopoietic stem cell) may originate all cells existing in blood [24]. One of the lineages that a HPSC could give rise is a myeloid lineage. The myeloid progenitors cells might differentiate itself into: megakaryocytes (multinucleated cells which form blood platelets), erythroblasts (multiply and differentiate in erythrocytes), myeloblasts (originate neutrophils, eosinophils and basophils), monoblasts (monocytes precursors) and denditric cells. In response to mediators, chemokines, leukocytes migrate from blood to the tissues where they repair the injured tissue and remove bacteria, parasites and dead cells which can lead to a serious infection. After their migration into some tissues the monocytes from the blood differentiate into macrophages [18].
Cells with greater relevance in the immunological system are lymphocytes which are originated from a progenitor cell from the BM. We can distinguish two types of lymphocytes: the T lymphocytes responsible for the immune response and the B lymphocytes that as a major task of producing the antibodies and the natural killer cells which are also related to the T lymphocytes. In case of transplant is only necessary that one single HPSC is present in the transplanted cells for a total reposition of all cells of the immune system and the blood [25].
22
Figure 5 -Different lineages that a HPSC can originate [17].
One of the difficulties scientists face is that HPSC behave like white blood cells in culture which make it more difficult to identify simply by morphology. Still exists many difficulties relating to culture and expansion of this type of SC without the differentiation process occurs. If it was possible keep these cells in culture without their differentiation scientist probably would have the chance to study deeper and looking for more knowledge about their molecular activity, their regulators and substances that intervene in the self-renewal process allowing higher rates of success in transplants with HPSC.
The HPSC may be found in the BM, being the BM their most classical source, as well as in peripheral blood, in the umbilical cord blood (UCB) and in the hematopoietic system of the fetus. The SC existing in the BM can be coerced to migrate into the blood stream through the injection of a cytokine, like the stimulate factor of granulocytes. One day before the harvest this cytokine is injected in the patient. To the harvesting a catheter is inserted intravenously, so the blood passes through a filter system that separates the white blood cells and the CD34+, so that the erythrocytes can be returned to the patient. This method has been chosen by several doctors for being less invasive then a lumbar puncture.
23 About the MSC, another group of cells that come from the BM [26], their biologic and clinic interest increase dramatically in the last decades evidence of that is the huge number of investigation groups that devote their time to these group of cells. The MSC has also the ability of self-renewing and they can differentiate at least in three cell lineages. They support the HPSC expansion through the cytokines expression and through the reconstruction of the hematopoietic environment [14, 24].
The different possibilities of in vitro differentiation are connected with environmental factors, usually combinations of chemical inductors, growth factors and cytokines. There are many regulators like Leptin (peptide hormone discovered in 1994) which is an important factor of mesenchymal differentiation [15]. Has a significant effect in promoting osteogenesis and in inhibition of adipogenesis in MSC form the BM [15].
Characteristics of MSC do not generate consensus among the scientists, many laboratories use different techniques to isolate and expand MSC.
In an attempt to solve this problem The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy proposed a set of characteristics that cells must have in order to be classified as MSC [27, 28]:
• Adherence to plastic;
• Specific surface antigen expression; • Multipotent differentiation potential.
First the MSC should be adherent to plastic culture surface [23, 29], second 95% of MSC must express the protein molecules CD105, CD73 and CD90 being that expression verified by flow cytometry [23, 28]. Additionally these cells mustn’t express CD45, CD34 and CD14 [29] (classical hematopoietic markers) [28]. CD45 is a typically specific marker of the surface of the progenitor cells of white blood cell. New markers that may be discovered in the future could change some of these criteria. MSC have to finally be able to differentiate into osteoblasts, adipocytes and chondroblasts under controlled in vitro conditions (Figure 6).
24
Figure 6 -Different lineages that a MSC can originate [3].
Cells that are plastic-adherent but do not differentiate into lineage cells originated by MSC are considered to be fibroblasts, which have a similar morphology [26]. The best place to harvest MSC is in the BM, but the rate of proliferation and differentiation of this organ decrease with age. Harvest MSC from the BM is an invasive method that may have some risks to the patient. So in the last few years some researchers have been trying to find another source of MSC [15, 30], the umbilical cord is one of them and has the advantage of not causing any damage neither to the mother neither to the child, cells from umbilical cord can be cryopreserved.
The umbilical cord has two arteries and one vein, protected by a connective tissue called Wharton’s jelly. Inside the abundant extracellular matrix of Wharton’s jelly it can be found the recent described population of stem cells named Human Umbilical Cord Perivascular Cells (HUCPVC) [31].
HUCPVC have showed high potential of proliferation and capacity to differentiate into osteogenic phenotype [31]. They demonstrate even a higher potential of proliferation than MSC from the BM and they are also capable of differentiation into osteoblasts, adipocytes and chondrocytes [31].
It is also proved that differentiation in an osteoblastic lineage by the human umbilical cord perivascular cells happened in a faster rhythm compared to the MSC from the BM. In an attempt to isolate and characterize MSC from the BM and from
25 the UCB, in the same conditions, only those harvested from the BM could be successfully isolated [32].
The UCB is also a source of HPSC, but they do not have mesenchymal progenitors in it [33]. However the MSC circulate in the blood of premature fetus and may be isolated with success. Romanov et al [33] tried to obtain MSC from the subendothelial layer of human umbilical cord vein. They reached the conclusion that a population with characteristics of the MSC showed a morphologic resemblance with fibroblasts, expressing mesenchymal surface markers and are capable of differentiation at least in an osteoblastic and adipogenic lineage.
They discovered that a lower percentage of MSC can be found in UCB and a higher percentage in the matrix. The cells from the blood and from the matrix of the umbilical cord multiply faster than the cells from the BM, showing less differentiation [33].
2.1.4 Identification
The most common way to identify any SC types is through molecules existing on the surface of those cells (markers), specific for every cellular lineage. Some markers are only expressed in some phases of their cellular cycle, with allows us to know in which phase of the cycle that cell is in [27].
Flow cytometry allow the evaluation of cellular viability, that have an important role in medical diagnosis in cases of diseases from immunological system, detection of quantities and cellular types presents in blood.
26
Flow cytometry uses different light emissions (different wave lengths) that match to different specific dyes for different molecules. Usually the fluorescent dyes are connected to protein molecules.
For identification of every cellular lineage is necessary using a complementary molecule in the cell surface (Figure 7), this molecule has aggregate a fluorescent dye and his color will be detected by flow cytometry.
Figure 7 -Process of identification of surface markers using fluorescent tags [17].
Another method that besides giving us the identification of different molecules in a heterogeneous population in suspension, can also allow their separation is named Fluorescent Activated Cell Sorting (FACS). This method is showed in Figure 8. Cells in suspension are crossed by a laser beam that detects their fluorescence. Fluorescent cells stay negatively charged, while the non-fluorescent stays positively charged. The charge differences permit the separation of SC from the others cells present in suspension. Using this method is possible to have an entire population of cells that express all the same markers and can be later expanded in vitro.
27 Figure 8 -FACS method, separation of SC by their fluorescence [17].
The HPSC expresses mostly in their progenitor phase the CD34 molecule , and further it advances in their cycle his expression decrease until is not present.
In the Figure 9 it can been confirmed that a HPSC expresses the CD34 molecule before his maturation process, in the differentiation phase [34]. However this protein is not by itself an exclusive indicator of the presence of HPSC [24]. The myeloid lineage expresses mostly the CD15 and the CD33, while the lymphoid lineage the CD3 and CD19 molecules.
28
Figure 9 -CD34 expression in the HPSC that will originate platelets [35]. .
The greater the information available about the different specific cell markers much better work could be done and a higher quality of laboratory techniques could be achieved. Ethical questions will have to be taken under concern and legislation would have to be made in order to prevent future controversies related to the use and origin of some cells, like cells from the fetus in name of the scientific progress.
29
3. Bone grafts
Bone grafts are materials used to substitute bone, their main goal is promote osteoregeneration in injured areas.
3.1 Autografts
Autograft is an autologous graft and still is until today the gold standard graft, is basically the harvest of bone from one site to another site of transplantation in the same patient [36]. The major limitations of this type of grafts are their amount of availability and the nature of the harvest procedure, it is also usually associated to these grafts high morbidity to donor site [36]. However they do not possess the risk of infection and immunological rejection.
3.2 Allografts
These are allogenic grafts and have their origin in post mortem human bone tissue. With these grafts it is easier to solve some problems characteristic of the autografts such as elimination of the second surgical procedure and quantity of tissue available once it is possible use bone banks. On the other hand the risk of infectious disease transmission to the patient is the biggest problem [36]. Among these diseases some contaminations of HIV and Hepatitis B and C and bacterial infection has already been reported [37]. Another major concern regarding this type of grafts is that the processes used to their preparation, preservation and sterilization could modify their physical-chemical properties affecting this way their biological behavior.
3.3 Xenografts
Xenografts are grafts with an animal origin, usually bovine. The advantages of xenografts include their relative abundant supply, ease of use and potentially favorable clinical performance. The major concern regarding to these procedures is
30
the risk of disease transmission such a bacterial, viral and prion transmission. Immune response is also a problem related to the safety of this material [38]. The manufacturing process of some of these bone substitutes includes heating for several hours at temperatures above 1000ºC transforming the material into ceramics [39]. So products may lose their unique microporosity from native bone and therefore their osteoconductive capacity could be reduced. The resorption rate may also be affected because ceramics cannot be such well degraded as nonsintered materials [39].
3.4 Synthetic
For many years the synthetic bone grafts have had a great development. Ideally these are supposed to promote the cellular adhesion, differentiation and proliferation of cells. So is particularly important understand the behavior and structure of the extracellular matrix. These materials may have similar chemical properties and biological structure to human bone. From clinical point of view these grafts eliminate the disease transmission risk as well as the need for a second surgical procedure [2].
There are a huge number of factors that can influence the cellular growth which can interfere with the necessary mineralization of the osteoblasts that have an important role in the bone regeneration [40]. And there are some essential properties that a synthetic graft should have like osteoinduction, osteoconductivity and promote osteogenesis which is the cellular process of new bone formation. Osteoinduction include recruitment, proliferation and differentiation of osteoprogenitors cells into osteoblasts, and osteoconductivity is related to the structural framework and environment requested to cellular migration, attachment and growth [2].
The most commonly used materials are Hydroxyapatite (HA) Ca10(PO4)6(OH)2,
Tricalcium phosphatase Ca3(PO4)2 in a α and β forms and bioglasses [37]. Calcitite
31 Bonelike® from MedMat Innovation-Biosckin are examples of some of these products available in market.
Biocompatibility of HA as material to bone replacement was tested by flow cytometry, concluding that it was compatibility with cellular growth [41, 42].
3.4.1 Bonelike
®Bonelike® is a synthetic bone graft, developed by J. D. Santos and co-authors (Patent WO0068164), based on glass-reinforced hydroxyapatite [2].
It has the ideal environment for bone cell adhesion, proliferation and differentiation. Due to its chemical composition is reabsorbed in a slow and controlled way and interfere in the bone’s natural process of remodeling [2]. For being a synthetic bone graft is completely safe regarding to disease transmission.
Bonelike® can be used in different forms: granular form, dense blocks or in a 3D macroporous structure depending on the clinical purpose.
Many clinic trials in orthopedic had already been made using Bonelike® granules implanted in bone defects in tibia [43], macroporous cylinder blocks implanted in patient’s tibia [44] and wedge in treatment of medial compartment osteoarthritis [45]. In all of them it has been showed that Bonelike® promote osteoprogenitors cell differentiation into osteoblasts and has a positive effect during the formation of a mineralized matrix [2, 46-48]. Clinical tests in maxillofacial surgery have demonstrated that granules of Bonelike® have been successfully used in the treatment bone defects caused by cyst removal [49, 50], as well as used in coating titanium dental implants in order to promote osteointegration [51].
3.4.1.1 Composition
Bonelike® is prepared with the incorporation of a CaO-P2O5 based glass in the HA
matrix by means of a liquid phase sintering process in order to increase the mechanical properties of HA and to introduce ions commonly found in bone tissue. The incorporation of glass instigate the decomposition of the HA part to a secondary phase, β-TCP. At higher temperatures this β- phase changes to α-TCP.
32
Once these phases have higher solubility than hydroxyapatite, Bonelike® releases to the medium ions such as calcium, phosphorous, sodium and fluoride which induce osteoblastic differentiation leading to a strong connection between bone and the bone graft [2].
3.4.1.2 Structure and Properties
This synthetic bone graft has two important characteristics. Firstly Bonelike® enhance bioactivity by reproducing the inorganic phase of HA in bone which contains several ionic substitution which modulates its biological behavior and secondly improve mechanical properties by using CaO-P2O5 based glasses that
act as liquid phase sintering process of HA [2, 52].
Different forms of Bonelike® have different porosities, densities and in consequence their superficial areas are different to. These properties are fundamental so the proper type of Bonelike® could be chosen to the correct clinical situation.
Pores of Bonelike® by allow diffusion of proteins and essential growth factors present in the patient’s blood into Bonelike® interstitial spaces, promote osteoconductivity enhancement.
Microstructure of Bonelike® Pellets (Figure 10) is positive for surface adsorption of native proteins in patient’s blood allowing the scaffold to be recognized by the organism.
33 Bonelike® Poro has higher interconnective porosity that facilitates the blood vessel and osteoblastic cells migration, through the interior of the bone graft increasing the rate of formation of new bone. Porous materials permit the growth of a capillary vascular network and the establishment of cell-cell contact between the bone graft and osteoprogenitors cells that are absolutely essential for growth and cellular organization.
Figure 11 -Bonelike® Poro 2000-6000µm. SEM analysis.
Different types of porosity present in Bonelike® Poro, micro meso and macroporosity (Figure 11) together with the degree of interconnectivity improve the nutrients and gases diffusion in the physiological fluids as well as the removal of metabolic waste.
34
4. Tissue Engineering
Tissue engineering is a term which includes concepts from life sciences, such as biology and engineering, with surgical procedures to develop strategies for the regeneration of tissue.
The procedures used in tissue engineering are now being used in a large number to optimize medical treatments and develop new methods. The main goal to achieve is speeding up the healing and damaged tissues. The application of allografts and total joint replacement are the most successful options in bone regeneration. On the other hand some problems are still happening due to their mechanical and biological properties [53].
In tissue engineering these procedures are part of routine: cell harvesting, signaling molecules and a support matrix (scaffold). A scaffold is a polymeric support where the cells will be seeded together with growth factors for a posterior implantation in human body to promote bone regeneration in the damaged area. This technique seeks to create a faster growth of the cells as much as is possible, so they can differentiate and proliferate like they were in is natural environment replacing the injured tissues by functional ones.
Cells from adult organs can be used as sources for cultured cells [54]. Cells are dissociated from tissue by the use of enzymes or mechanical forces to disrupt the extracellular matrix. Cells in culture can be maintained as single cells, as small explants of intact tissue, or as whole segments of organs.
Cells are the most important factor in any regenerative process [55]. Sometimes is necessary to expand cell population so that an effective process of regeneration may occur, that is accomplished with bioreactors [19-21].
For a successful regeneration of the injured tissues the cells, signals and the scaffold has to establish a connection so they can interact and restore the damaged area forming a complete regenerated and functional tissue (Figure 12). Scaffolds can be formed by many materials like membranes, gels, ceramics, cells also can have different origins, stem cells, adult organs, and signals have the task of inducing cells to form an extracellular matrix.
35 Cells must react to their surrounding and be able to integrate the new environment and synthesizing extracellular matrix [16, 56].
Figure 12 –Principal intervenients in the tissue regeneration process in tissue engineering.
Concerning the case of bone tissue is essential de capability of supporting additional loads, so scaffolds must be able to do this while the cells reconstruct the biomatrix (Figure 13) [57].
Figure 13 -Microstructures of bone substitutes. SEM analysis.
Knowledge at the molecular level of cell interaction with synthetic materials has advanced rapidly in the last decade so the designs of new materials for tissue regeneration tend to be designed more carefully.
36
Chapter 2
37
Chapter 2
Experimental Procedures
Preparation of Bonelike
®Bonelike
®Pellets
Pellets were fabricated according to the Patent WO0068164. Preparation of pellets begins with the mixing of Hydroxyapatite and bioglass with microcrystalline cellulose, followed by extrusion and spheronization. Pellets were then dried at 60ºC, after the drying the thermal treatment was made. The thermal treatment has two steps, in the first one heating at a temperature of 600ºC causes the combustion of the microcrystalline cellulose, followed by a temperature increase to 1300ºC for 1h, for sintering the pellets follow by natural cooling inside the furnace. The obtained pellets show a particle size range of 1000-4000µm.
Bonelike
®Poro
Bonelike® Poro is the result of a suspension composed of hydroxyapatite, bioglass, microcrystalline cellulose and polyvinyl alcohol (PVA) and subsequent combustion of the microcrystalline cellulose and sintering at 1300ºC. PVA and microcrystalline cellulose both act as agents of formation of porosity and PVA, being an organic ligand, allows keeping all the solid components together in the suspension avoiding their deposition. Bonelike® Poro show a particle size range of 2000-6000µm.
In both materials surface area was achieved by BET method and porosity was determined by Mercury Porosimetry. Bonelike® Pellets and Poro were autoclaved before used in the biological studies.
38
In vitro biological studies
Bone Marrow Concentrate
Human bone marrow was collected during orthopedic surgical procedure, with patient informed consent. The Biomet Kit MarrowStimTM Concentration System was used to achieve a rapid preparation of autologous concentrate of nucleated cells from a sample of bone marrow aspirate (Figure 14).
The needle was coated with heparin before the extraction of the bone marrow. After the extraction of the bone marrow aspirate the cell separator was loaded with the aspirate and centrifuged at 3200RPM for 15 min. After removing the plasma from the cell separator, the portion of nucleated cell concentrate was extracted. The concentrate of nucleated cells obtained was about 3mL.
Figure 14 –Cell separator from the Kit with the three distinct parts. From the top, cell poor plasma, nucleated cell concentrate and on the bottom the red blood cells.
Flow Cytometry
Flow cytometry showed that the bone marrow concentrate contained 6x107 nucleated cells and 2,5x105 hematopoietic cells. This concentrate was diluted with minimum essential medium (α-MEM) and the resulting cell suspension was seeded at 3x106 cell/cm2 over 0.10g/cm2 of Bonelike® (Pellets or Poro) placed in 24-well plates, and cultured for 30 days. Cultures performed in standard tissue culture plates (absence of materials) were used as control.
39
Cell Culture
Cultures – seeded Bonelike® and control - were performed in α-MEM containing 15% fetal bovine serum (FBS), 100µg/mL penicillin, 10 IU/mL streptomycin, 2.5µg/ml fungizone and supplemented with 50µg/mL ascorbic acid and 10mM of β -glycerophosphate. Incubation was carried out in a humidified atmosphere of 95% air and 5% CO2 at 37ºC. Culture medium was changed twice a week.
Previous studies showed that Bonelike® pre-incubation was not necessary to enhance the performance of the material.
MTT
Cell viability/proliferation of the seeded Bonelike® samples and control was determined by MTT assay, that relies on the ability of viable cells to oxidize MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to an insoluble blue formazan product. Cultures were incubated with 0.5mg/mL-1 of MTT for the last 4h of the culture period tested. Then, the materials were transferred to a new plate, the formed formazan salt was dissolved with dimethylsulphoxide, and the absorbance was determined at 600nm. MTT assay was performed at days 1, 4, 9, 17, 23 and 30 of culture time. .
Total protein and ALP activity
Total protein (TP) content regarding seeded Bonelike® and control was determined in 0.1M NaOH cell lysates according to the method of Lowry using bovine serum albumin as standard.
ALP activity was assayed in cell lysates (obtained by the treatment of the seeded material with 0.1% triton), by the hydrolyses of p-nitrophenol phosphate in alkaline buffer solution, pH 10.3, for 30 min, and colorimetric determination of p-nitrophenol at 405 nm. Both ALP and Total protein content assays were performed at days 9, 17, 23 and 30 of culture time. ALP activity was normalized by total protein content,
40
and was expressed in nanomoles of p-nitrophenol produced per min per µg of protein (nmolmin-1/µgprotein).
SEM
For SEM observation, cells were fixed with 1.5% glutaraldehyde in 0.14M sodium cacodylate. Samples were dehydrated in graded series of alcohols and further dried with Hexamethyldisilazane. Samples were mounted onto aluminium supports, super-coated with gold, and observed in a Joel JSM 35C scanning electron microscope equipped with an X-ray energy dispersive spectroscopy voyager XRMA System, Noran Instruments.
Statistical analysis
Three replicas were performed for each assay. Results of ALP activity and MTT are presented as mean ± standard deviation (SD). Comparisons between seeded Bonelike® samples and the control were performed by the Student’s t-test. Differences were considered statistically significant at p< 0.05.
41
Results and Discussion
Bonelike
®characterization
Bonelike® samples were prepared in the Laboratory of Medmat Innovation, Biosckin SA, and were examined by SEM (Figure 15). Bonelike® is a mixture of hydroxyapatite and bioglass, which result in a triphasic mixture of hydroxyapatite,
α- and β- tricalcium phosphate (α-TCP and β–TCP) [2, 47, 52], under the shape of spherical granules named Bonelike® Pellets, and bigger scaffolds with a heterogeneous shape, but with macroporous structure named Bonelike® Poro.
β-TCP is resorbed in the body at a very fast rhythm, however the α-TCP does not have the same resorb rate as the β-TCP. α-TCP when in contact with human plasma hydrolyzes itself. So, in vivo as the β-TCP is resorb from the beginning, the scaffold will not just dissolve away, because the α–TCP which have a slower rate of resorption maintain the structure of macroporous scaffold which will be later resorbed by the osteoclasts cells [58-60] .
The α-TCP and β- TCP phases are much more soluble than the hydroxyapatite, so as Bonelike® is implanted in the organism, releases calcium and phosphorous that induce differentiation of osteoblastic cells [2, 47, 52]. Livingston et al showed that hydroxyapatite combined with β-TCP stimulate the osteogenic differentiation of mesenchymal stem cells [61, 62]. This material besides stimulating osteoblastic differentiation without adding growth factors also enhance the osteoblast-specific gene expression [63, 64].
Muller et al consider that surface chemistry has a bigger role in the differentiation process than the topography [63], however Zhang et al showed that is the surface structure of the material that interfere more in cells differentiation [64] and Kasten et al even say that pore distribution and size as well the surface structure are the essential features for osteogenic differentiation [65].
Bonelike® Pellets showed a particle size range of 1000-4000µm. Pellets have a microporosity determined by mercury porosimetry of 25.3% and a surface area of 0.0171m2/g as determined by BET method. These pellets present microposity and
42
their surface have some rugosity that allow cells to attach and migrates through the scaffold, as it may be seen in Figure 15.
Figure 15 –Bonelike®
Pellets. a) pellets with some irregular details (arrows) in surface, b) microporosity present in the pellets surface (arrows). SEM analysis.
In terms of clinical applications, Bonelike® Pellets are suitable for fill small bone defects and their size and spherical shape permit the injectability through a syringe if it is necessary, so this material can been seen as an injectable bone graft substitute.
Scaffolds of Bonelike® Poro showed a particle size range of 2000-6000µm, (see Figure 16), with macro (pore diameter range 224.62 µm), meso (pore diameter range 19.097 µm) and microporosity (pore diameter range <2.0 µm) which results in an interconnective porosity presenting 70% of total porosity determined by mercury porosimetry. This characteristic influence the type of topography of the surface with 0.1152m²/g of surface area as determined by BET method, being much more heterogeneous compared to that of the Bonelike® Pellets.
Concluding both materials have the same composition but differ in terms of shape, micro and macroporosity distribution as well as total porosity.
Microposity allows the attachment of cells, meso and macroporosity permit the migration of cells through the interior of scaffold with the possibility of colonization
43 of a much bigger area. Bonelike® Poro have interconnective porosity which facilitate the growth of blood vessels, vascularization, through the interior of scaffold allowing bone growth through the inside [66]. Formation of new blood vessels and the establishment of a capillary network induce the interactions cell-cell which are essential for cell-cellular organization [17, 67, 68]. Studies have proved the importance of cell-cell contact in stem cell differentiation [69, 70], and those interaction can be controlled by a cell-material interaction with particular attention to the design of the surface material [69]. Bonelike® Poro show a high percentage of porosity, this particular structure is achieved with the use of microcrystalline cellulose and polyvinyl alcohol [71-73].
Figure 16 –Bonelike®
Poro samples. a) high level of porosity, pores with different sizes, b) irregular surface with microposity (arrow). SEM analysis.
From the clinical point of view, the different shape of Bonelike® Poro scaffolds allows the surgeon to handle separately every scaffold with tweezers, which sometimes is useful in surgery, their irregular shape can also permit a better fixation at the bone defect decreasing this way the movement of the material in the damaged area. Bonelike® Poro is intended to be used in large bone defects in orthopaedic applications.
Topological structure of the surface material could influence cell behavior as well as morphology, proliferation and growth activity [74], so for both formulation of
44
Bonelike® Pellets and Poro, differences in result are expected due to their different shape, structure and porosity.
In vitro biological studies
A concentrate of mononuclear cells prepared from a human bone marrow aspirate was seeded over Bonelike® Pellets and Bonelike® Poro, in order to evaluate the cell response to the material regarding attachment, growth and osteoblastic differentiation.
Flow cytometry (data not shown) revealed that prepared bone marrow concentrate contained 6x107 nucleated cells and 2.5x105 hematopoietic stem cells. Some studies show that the percentage of MSC in the nucleated cells harvested from the BM is only 0.01 to 0.001% of the total nucleated cells [23, 75]. MSC present in the nucleated component of the bone marrow concentrate, originate the osteoblastic lineage cells, so they are the most important cells in terms of bone reconstitution [23, 31]. Osteoclasts originated from MSC are also very important in the regeneration process that involves calcium phosphate (Ca-P) scaffolds, they contribute to the Ca-P degradation and also stimulate osteoblastic activity [64, 76, 77]. However, in vivo hematopoietic progenitor stem cells (HPSC) are also important, as these cells are responsible for the new vascularization of the damaged area, with constant supply of nutrients and essential growth factors, as well as the immunological behavior [17, 24]. In vivo MSC and HPSC are both essential to a successful bone regeneration process [24, 27].
Colonized materials were cultured for 30 days in experimental conditions known to favor the development of the osteoblast phenotype, namely in the presence of ascorbic acid and beta-glycerophosphate (bGP) [7]. Ascorbic acid plays an important role in the production of the collagenous bone extracellular matrix, some studies showed that the presence of various concentrations of this compound results in a dose-dependent synthesis of collagen and suggested that the resulting increase in the accumulation of the extracellular matrix is associated with a higher
45 alkaline phosphatase (ALP) activity and ability to form a mineralized matrix [78, 79]. bGP is routinely added to bone cell cultures to induce osteogenesis and promote calcium phosphate deposition [80-82]. The mechanism by which bGP induces mineralization is closely linked to the high ALP activity of bone cell cultures, this compound is rapidly hydrolyzed by ALP to produce high levels of local phosphate ions providing the chemical conditions for mineral deposition [80]. ALP is an enzyme expressed by MSC during the osteogenesis and is considered a marker for their differentiation [63, 83, 84].
Bonelike
®Pellets
Bonelike® Pellets were cultured with a concentrate of nucleated cells prepared from a bone marrow aspirate, as described in the section “Experimental procedures”. Cultures were maintained for 30 days. SEM appearance of the colonized material at early culture time (1 day) is presented in Figure 17. The concentrate of BM contained some erythrocytes, which were removed during subsequent changes of the culture medium (see arrows).
Figure 17 -Bonelike® Pellets seeded with the bone marrow concentrate, at 1 day of culture, a),b)
46
SEM images regarding culture times from 4 to 23 days (see Figure 18) showed that MSC were able to attach and spread over the surface of Bonelike®, taking advantage of the surface microporosity and topological structure. At day 4, cells showed an elongated fibroblastic morphology that changed progressively to a more extended and polygonal appearance at days 9 and 17. Cells used the topographic irregularities on the surface to adhere, which is clearly visible at days 9 and 17 (see arrows). Cells proliferated with culture time, and by days 17 and 23, abundant cell clusters were visible over the material surface. These clusters appeared to be associated with particular surface features, namely small defects scattered over the surface, where it is easier to cells to create niches. Within the cell clusters/niches, cell layer appeared well organized with established cell-to-cell communication (days 17 and 23) abundant cytoplasmic extensions and a perfect adaptation to the underlying material microporosity and topography at days 9, 17 and 23 of culture. By day 23 of culture, the cell clusters present evidences of matrix mineralization, Figure 19 (arrow). Mineralized deposits were identified in close association with the cell layer.
Mesenchymal stem cells are able to differentiate in an osteogenic lineage [26, 31, 33]. Studies concerning the development of the osteoblast phenotype suggest a temporal sequence of differentiation involving the adhesion of the mesenchymal stem cells to a biological (pre-existing bone tissue) or artificial (biomaterial scaffold) substratum, active cell proliferation accompanied by the production of a collagenous extracellular matrix and expression of osteoblast-related markers, like ALP (seen in Figure 23) and extracellular proteins, and, ultimately, matrix mineralization (Figure 20) [3, 7, 47, 85].
Figure 20 shows a representative spectrum only of the mineralized deposits signaled in Figure 19 b), displaying the presence of Calcium and Phosphorous peaks.
47
Figure 18 -Bonelike® Pellets colonized with bone marrow cells, at 4 to 23 days. a) presence of
defects through the surface; b), c), d), f) cells take advantage of microposity to attache to surface material (arrows); e), g) cluster of cells on the material surface (arrows). SEM analysis.
Day 4
Day 9
Day 17
![Figure 1 -Bone structure. In this squeme is represented the Haversian system and the osteocytes[6]](https://thumb-eu.123doks.com/thumbv2/123dok_br/19290727.992232/11.892.268.640.106.443/figure-bone-structure-squeme-represented-haversian-osteocytes.webp)
![Figure 2 -Proliferation vs Differentiation in osteoblastic lineage [3].](https://thumb-eu.123doks.com/thumbv2/123dok_br/19290727.992232/12.892.193.720.532.701/figure-proliferation-vs-differentiation-in-osteoblastic-lineage.webp)
![Table 1 -Embryonic germ layers from which differentiated tissues develop, adapted from Stem Cells: Scientific Progress and Future Research Directions [17]](https://thumb-eu.123doks.com/thumbv2/123dok_br/19290727.992232/18.892.103.760.483.865/embryonic-differentiated-tissues-develop-scientific-progress-research-directions.webp)
![Figure 4 -Plasticity of a Hematopoietic stem cell [22].](https://thumb-eu.123doks.com/thumbv2/123dok_br/19290727.992232/20.892.245.666.738.839/figure-plasticity-hematopoietic-stem-cell.webp)
![Figure 5 -Different lineages that a HPSC can originate [17].](https://thumb-eu.123doks.com/thumbv2/123dok_br/19290727.992232/22.892.128.764.117.496/figure-different-lineages-hpsc-originate.webp)
![Figure 6 -Different lineages that a MSC can originate [3].](https://thumb-eu.123doks.com/thumbv2/123dok_br/19290727.992232/24.892.179.715.94.354/figure-different-lineages-msc-originate.webp)
![Figure 7 -Process of identification of surface markers using fluorescent tags [17].](https://thumb-eu.123doks.com/thumbv2/123dok_br/19290727.992232/26.892.351.547.394.603/figure-process-identification-surface-markers-using-fluorescent-tags.webp)
![Figure 8 -FACS method, separation of SC by their fluorescence [17].](https://thumb-eu.123doks.com/thumbv2/123dok_br/19290727.992232/27.892.283.618.103.549/figure-facs-method-separation-sc-fluorescence.webp)
![Figure 9 -CD34 expression in the HPSC that will originate platelets [35].](https://thumb-eu.123doks.com/thumbv2/123dok_br/19290727.992232/28.892.231.697.121.337/figure-cd-expression-hpsc-originate-platelets.webp)