ABSTRACT
www.scielo.br/jaos
Decreased phagocytic function in neutrophils and
monocytes from peripheral blood in periodontal
disease
Valéria Martins Araújo CARNEIRO1, Ana Cristina Barreto BEZERRA2, Maria do Carmo Machado GUIMARÃES3, Maria
Imaculada MUNIZ-JUNQUEIRA4
1- DDS, MSc, PhD, Adjunct Professor of Periodontics, Periodontics Division, University of Brasilia, Brasilia, DF, Brazil.
2- DDS, MSc, PhD, Associate Professor of Pediatric Dentistry. Post-Graduation Program Advisor, Health Science Center, University of Brasília, Brasília, DF, Brazil. 3- DDS, MSc, PhD, Adjunct Professor of Periodontics, Periodontics Division, University of Brasilia, Brasilia, DF, Brazil.
4- MD, MSc, PhD, Associate Professor, Laboratory of Cellular Immunology, Faculty of Medicine, University of Brasilia, Brasilia, DF, Brazil.
Corresponding address: Valéria Martins Araújo Carneiro - Universidade de Brasília/UnB - Departamento de Odontologia - Campus Universitário Darcy Ribeiro - 70910-900 - Brasília - DF - Brasil - Phone:+55 61 3307 2514 - e-mail: valeriamartins@unb.br
Received: December 02, 2010 - Modiication: June 12, 2011 - Accepted: July 08, 2011
P
hagocytosis by neutrophils and monocytes constitutes the main defense mechanism against bacterial challenges in periodontitis. Phagocytosis by neutrophils has already been evaluated, whereas phagocytic function of monocytes has hardly been addressed so far. Objectives: The aim of this study was to assess phagocytosis by neutrophils and monocytes in periodontitis. Material and Methods: The sample included 30 subjects with severe periodontitis and 27 control subjects without periodontal disease. The phagocytic index (PhI) was calculated as the mean number of adhered/ingested Saccharomyces cerevisiae per phagocytozing monocyte or neutrophil multiplied by the percentage ofphagocytes involved in phagocytosis. Results: A signiicant reduction in phagocyte functions
was observed in individuals with periodontitis. The median of PhI of neutrophils using non-sensitized S. cerevisiae was 3 for the control group, and 1.5 for the periodontitis group (p=0.01, Mann-Whitney test). The median of PhI of monocytes with non-sensitized S. cerevisiae was 26.13 for the control group, and 13.23 for the periodontitis group (p=0.03, Mann Whitney test). The median of PhI of monocytes assessed with sensitized S. cerevisiae
was 97.92 for the control group and 60.1 for the periodontitis group (p=0.005, t-test). Conclusion: The data demonstrated a reduction in the function of phagocytes, suggesting a decrease in immune defenses in periodontitis.
Key words: Periodontitis. Neutrophils. Monocytes. Phagocytosis.
INTRODUCTION
It has been considered that periodontal infection or periodontitis results from the imbalance between the direct and indirect effects of pathogenic bacteria and the host immune response7. Data support
the microbial etiology of periodontal disease and
the role played by speciic pathogenic species5,25.
Polymorphonuclear (PMN) neutrophils constitute
the irst line of defense against these pathogens
in the subgingival area. In the presence of plaque, an inflammatory infiltrate is frequently in the gingival tissues, showing mononuclear phagocytes, lymphocytes, and polymorphonuclear leukocytes16,22. Additionally, inlammatory response changes the
microenvironment of the bioilm and selects for
specific organisms28. The interaction between
lipopolysaccharide (LPS) and these predominant
cell types in the inlammatory iniltrate stimulates
the production and elevation of prostanoids, mainly prostaglandin e2 in the gingival luid of sites that present attachment loss30. In addition to the
local effects, the bacterial virulence factors and
inlammatory mediators arising from this
parasite-host interaction may create and sustain a chronic
systemic inlammatory process in the bloodstream17.
responsible for altering the neutrophil function1.
In addition, new perspectives have indicated that periodontitis occurs as a hyperactive immune/
inlammatory response to the speciic bacteria of
plaque in predisposed individuals, involving the excessive generation of oxygen radicals and the release of proteases14. However, despite this new
paradigm that PMN is not “hypofunctional” or
“deicient”, but “hyperfunctional”14, phagocytosis in
this context is not yet elucidated.
Data on phagocytosis by neutrophils from peripheral blood in individuals with periodontitis are controversial. There are reports both of reduction2,4,12,29 and increase of phagocytosis by
neutrophils13,21. Studies addressing the phagocytosis
by monocytes in these patients are still scarce. Therefore, it still has to be determined whether phagocytosis by monocytes in individuals with periodontitis is different compared to individuals without periodontal disease. For this reason, it is important to investigate the immune function represented by phagocytosis in periodontitis to gain insight into the defense against pathogens involved in disease conditions and the pathophysiological mechanism involved, including those of infectious nature, which are present during the course of the disease. Therefore, this study evaluated the phagocytic function of monocytes and neutrophils in periodontal disease, in comparison with control individuals without periodontal disease.
MATERIAL AND METhODS
Subjects and study groups
This study was approved by the Institutional Review Board of the Health Sciences Faculty – UnB – University of Brasilia (045/2008). The subjects were evaluated and selected for inclusion in the present study at the periodontal clinic of the University Hospital of Brasilia, Brazil. The sample included 30 subjects with periodontal disease and 27 subjects with healthy periodontium, all otherwise healthy and non-smokers. The periodontitis group consisted of 20 women and 10 men (age range 21-45 years, mean age 34.5 years) with at least 18 present teeth. The severe periodontitis was diagnosed according to the following inclusion criteria23: radiographic
evidence of bone loss extending to >30% of the root length in multiple teeth, age >18 years, presence of >2 teeth/quadrant with a pocket depth of >6 mm and concomitant attachment loss of >3 mm. The control group consisted of 18 women and 9 men (age range 21-44 years, mean age 34 years) with
clinical probing depths (PD) ≤3 mm and clinical attachment level (CAL) ≤3 mm, ≤10% sites with
bleeding on probing and no radiographic evidence of bone loss. The following exclusion criteria were considered: previous mechanical periodontal therapy
and antimicrobial therapy for systemic or topical oral use in the last 12 months, pregnant or lactating women, diabetes, morbid obesity, autoimmune, infectious, allergic, and gastrointestinal diseases, malnutrition, renal alterations, cancer or any other clinical situation that might alter the function of the immune system, use of medications that could alter
the level of inlammatory mediators, and smoking.
Clinical examination
The initial periodontal evaluation of each patient included periapical radiographic documentation by the parallelism technique. The clinical examinations were performed by an experienced examiner and included visible plaque accumulation (PI) without the use of any disclosing agent, bleeding on probing (BOP), probing depth (PD) and clinical attachment level (CAL). The measurements were assessed at four sites around each tooth, namely buccal, lingual and proximal sites (the greatest depth was recorded for each proximal surface) using a manual probe (Michigan O probe with Williams markings), excluding third molars. Analyses of degrees of mobility and furcation involvement were recorded.
Phagocytosis test
Phagocytosis of Saccharomyces cerevisiae was adapted from a technique previously described19.
Briefly, samples of 40 µL per marked area of heparinized whole peripheral blood obtained by venipuncture from each subject were placed on clean glass slides containing 8 marked areas with 7-mm diameter each, in duplicate preparations, and incubated in a wet chamber for 45 min at 37°C. The slides were then rinsed with 0.15 M phosphate-buffered saline (PBS) pH 7.2 at 37°C to remove non-adherent cells. After washing, neutrophils and monocytes remained adhered onto the slide approximately in the same proportion as they were in the whole blood. Adherent cells (12,534±5,050 cells/marked area; 5.63±0.85% monocytes and 93.5±1.08% neutrophils) were incubated with a suspension of 2.5x105S. cerevisiae in 20 μL Hanks-tris (Sigma Co., St Louis, MO,
USA) pH 7.2, with 10% heat-inactivated fetal calf serum (FCS) (Gibco/Invitrogen, Grand Island, NY, USA) for 30 min in a wet chamber at 37°C. To
evaluate the inluence of complement molecules
on phagocytosis in periodontitis the S. cerevisiae were incubated at 37°C for 30 min with 10% fresh
serum from the donor in Hanks-Tris solution. Slides were then rinsed with 0.15 M PBS at 37°C to eliminate nonphagocytosed S. cerevisiae and the
inal washing was done with 30% FCS in Hanks-tris.
assessed by light microscopy. Microscopic ields distributed throughout the slide were randomly selected and all monocytes or neutrophils in
each particular ield were examined. The PhI was calculated as the mean number of phagocytozed S. cerevisiae per phagocytosing monocytes or neutrophils, multiplied by the percentage of these cells engaged in phagocytosis19.
Baking yeast (S. cerevisiae) was prepared according to a technique previously described20.
Statistical analysis
Statistical analysis was performed using the Prism® software, 2005 (Graphpad, San Diego, CA,
USA). Beforehand the variables in the samples
were previously veriied for normality, using the
Skewness and Kurtosis and Kolmogorov-Smirnov tests. The t-test was used for comparison between two variables with normal distribution, and the Mann-Whitney test was used for those that did not present normal distribution. The differences between variables were considered statistically
signiicant when the bi-caudal probability of their
occurrence due to chance (error type I) was lower than 5% (p<0.05). As several data showed non-normal distribution, for homogeneity, all data were graphically expressed as median, quartiles and extremes.
RESULTS
Clinical and demographic characteristics
The clinical and demographic characteristics of the two groups are summarized in Table 1. Distribution of age and gender was similar between control and periodontitis groups. Subjects
with periodontitis had signiicantly higher body
mass index than control (p=0.001). Statistically
signiicant differences were also observed for all
periodontal parameters. Subjects with periodontitis showed severe destructive periodontal disease when observed by the percentage of sites with PD <4 mm to >7 mm and CAL <4 mm to >7 mm. The hematological characteristics of the groups
are listed in Table 2. No statistically signiicant
difference was observed between groups, except for C reactive protein (CRP). The mean of serum levels of CRP was 0.21±0.25 for the control group and 0.51±0.62 for the periodontitis group (p=0.01).
Phagocytosis test
PhI of neutrophils and monocytes for non-opsonized S. cerevisiae
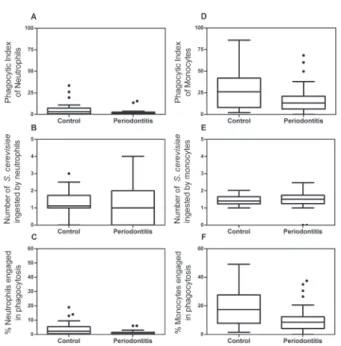
The median of the PhI of neutrophils from
individuals with periodontitis (1.5) was signiicantly
lower than that of normal controls (3.0), (p=0.01, Mann-Whitney test) (Figure 1A). This occurred
Characteristics/ Parameters
Control (n=27)
Periodontitis (n=30)
(p) Test
Age (years; mean+SD) 33.2±6.4 33.5±6.8 0.8441**
Gender (males/females: n) 9/18 10/20 1.0000***
Numbers of teeth (mean+SD) 28±1.3 27.5±4.8 0.3545*
BMI 20.3±1.28 26.53±5.49 0.0001*
SBP(mmHg) 120.9 ± 4.8 122.4±14.8 0.9188*
DBP (mmHg) 80.5±2.5 80.9±11.6 0.0744*
PI (%; mean+SD) 4.8±2.1 61.37±33.59 0.0001*
BOP (%; mean+SD) 2.7±1.2 41.83±30.04 0.0001*
PD (mm; mean+SD)
≤4 mm 100% 3.8±3.97
5-6 mm 0% 16.1±9.33
≥7 mm 0% 9.8±8.8
CAL (mm; mean+SD)
≤4 mm 100% 5.8±4.5
5-6 mm 0% 17.9±9.2
≥7 mm 0% 13.0±11.4
Table 1- Demographic characteristics and full-mouth clinical parameters
* Mann-Whitney test ** t-test
because there was a reduction in the percentage of neutrophils involved in phagocytosis in the periodontitis group ((0.50%) when compared to the normal control group (2.25%) (p=0.006, Mann-Whitney test) (Figure 1C), whereas no statistically
signiicant difference was observed between groups
for the mean number of yeasts adhered to/ingested by neutrophils (p=0.50, Mann-Whitney test) (Figure 1B).
T h e s a m e s i t u a t i o n wa s ve r i f i e d w h e n phagocytosis by monocytes was tested. The median of the PhI of monocytes from individuals with
periodontitis (13.23) was signiicantly lower than
that of the normal controls (26.13) (p=0.02, Mann-Whitney test) (Figure 1D), because a statistically
signiicant reduction in the percentage of monocytes
involved in phagocytosis was observed in the periodontitis group (8.50%) when compared to the control group (17.25%) (p=0.01, Mann Whitney test) (Figure 1F). Conversely, no difference was observed between groups for the mean number of yeasts adhered to/ingested by monocytes (p=0.71, t test) (Figure 1e).
PhI of neutrophils and monocytes for opsonized S. cerevisiae
A different situation occurred when phagocytosis was evaluated using sensitized S. cerevisae. No statistical difference was observed in the PhI of neutrophils between individuals with periodontitis (117.9) and normal control individuals (157.0) (p=0.14, t-test) (Figure 2A). Periodontitis had no influence on the mean number of yeasts adhered/ingested by neutrophils (p=0.15, Mann-Whitney test) as illustrated in Figure 2B. There was no statistical difference in the involvement of neutrophils in phagocytosis between the two study groups (p=0.16, Mann-Whitney test) (Figure 2C).
Characteristics/ Parameters
Control (n=27)
Periodontitis (n=30)
(p) Test
Triglycerides (mg/dL) 86.1±35.4 98.9±46.2 0.2675*
Total cholesterol (mg/dL) 172±31.4 173.9±3.3 0.8222**
HDL-cholesterol (mg/dL) 48.2±12 42.5±12.3 0.0747*
LDL-cholesterol (mg/dL) 106±24.4 111.5±27.3 0.4348**
Glucose (mg/dL) 85.3±6.7 90.6±14.1 0.1528*
Eosinophils 146.1±103.3 222.9±175.8 0.0646*
Basophils 14.7±31.8 8.1±21.3 0.627*
Lymphocytes 2,239±530.7 2,137±499.5 0.4607**
Monocytes 422.6±140.9 388.5±160.1 0.2369*
Total leukocytes 6,093±1,216 6,308±1.473 0.5111*
C-Reactive Protein 0.21±0.25 0.51±0.62 0.0105*
Table 2- Biochemical and hematological characteristics
* Mann-Whitney test ** t-test
Figure 1-In vitro evaluation of the phagocytic capacity of neutrophils (left) or monocytes (right) in individuals with periodontal disease (P) and normal control individuals, using 2.5x105 non sensitized yeast per well. A: Reduction
The median proportion of neutrophils involved in phagocytosis in the periodontitis group was 65.50%, compared to 71.50% in the control group.
However, a different result was veriied when
phagocytosis was tested by monocytes, where the median of PhI of monocytes from individuals with
periodontitis (60.10) was signiicantly lower than
that from the normal controls (97.92) (p=0.005, t-test) (Figure 2D). This decrease was caused by a lower percentage of monocytes involved in phagocytosis, namely 36.25% for the periodontitis group and 51.75% for the control group (p= 0.0016, t-test) (Figure 2F). The medians of the mean number of yeasts adhered to/ingested by monocytes were statistically similar between both groups, as illustrated in Figure 2e.
DISCUSSION
This is the first description of decreased phagocytic capacity of monocytes in human periodontal diseases. In the present study phagocytosis by monocytes and neutrophils
were compared between individuals with healthy periodontium and those with periodontitis. In this work, killed Saccharomyces cerevisiae was used because receptors involved in their uptake are involved in phagocytosis by neutrophils and monocytes of pathogenic bacteria present in periodontal disease27. When using live bacteria, their virulence factors may inluence phagocytosis.
Thus, when investigating the phagocytosis, two
lines of reasoning can be deined: the direct action
of bacteria in phagocytosis and the effects of host-parasite interactions in phagocytosis. As our aim was to evaluate the effects of this interaction in the
host, this justiies another stimulus provided to the
cell, including yeasts. The use of different stimuli is reported in the literature: N-formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP)29, Staphylococcus
aureus5, Candida albicans2,25, Aggregatibacter
a c t i n o m y c e t e m c o m i t a n s , P o r p h y r o m o n a s gingivalis13,21.
The present study showed a signiicant reduction
in the phagocytic capacity of monocytes, both using opsonized and non-opsonized S. cerevisiae (Figure 1D and 2D). However, for neutrophils, the disease decreased phagocytosis only when it was assessed using non-opsonized yeasts (Figure1A). This lower phagocytic capacity of neutrophils in periodontitis has also been reported by other researchers2,4,12,29.
In this study, lower phagocytosis using non-opsonizedS. cerevisiae was observed in the group of individuals with periodontitis, both by neutrophils (Figure 1A) and monocytes (Figure 1D). These data suggest that the phagocytic function of both neutrophils and monocytes was intrinsically affected because, in the absence of serum components, the level of phagocytosis was lower in the periodontitis group. Regarding the monocytes, for both analyses, the PhI was lower in individuals with periodontal disease (Figure 1D and 2D). A reduced number of monocytes involved in phagocytosis in this group was also evident (Figure 1F and 2F).
The mechanisms for the deficient response of phagocytes have not been explained yet, however some hypotheses can be suggested. It is known that many periodontal pathogens develop particular strategies for subverting the mechanisms of phagocytosis27. Among the mechanisms of
immunosuppression, the role of LPS has been considered. Studies have indicated that the LPS of P. gingivalis appears to be antagonists of the toll-like receptor-4, thus competing with LPS of other species to couple with that receptor. This is
a possible mechanism of deiciency of the innate
immune system of the host, since recognition of
the bacterial pathogens identiied by the toll-like
receptor-4 would be blocked6.The recruitment of
neutrophils and macrophages in mice infected with Aa and lacking the toll-like receptor-2 led to the Figure 2-In vitro evaluation of the phagocytic capacity of
neutrophils (left) or monocytes (right) in individuals with periodontal disease (P) and normal control individuals, using 2.5x105 sensitized yeast per well. A: Phagocytic
reduction in the inlux of these cells in the peritoneal
cavity. Infection with Aa in this experimental model
caused a signiicant decrease in the cytokine and
chemokine levels and reduction in the phagocytic capacity of neutrophils and monocytes, in addition to alveolar bone loss11.
Another possibility that has been described is the direct toxicity of Aa to neutrophils and monocytes by the production of leukotoxin26. Strains of Aa have
various mechanisms that control phagocytosis, such as inhibition of chemotaxis and immunosuppressive and cytotoxic factors that suppress both the
unspeciic and speciic immune responses, as well as preventing ibroblast proliferation. They also
present the additional capacity to invade epithelial and endothelial cells15. Baehni, et al.3 (1979)
observed by electron microscopy that the cytotoxic effects on PMN by strains of Aa were independent of phagocytosis. The authors suggested that soluble bacterial products may be released by bacteria. Carvalho, et al.4 (2009) found high frequency
of P. gingivalis, Tannerella forsythia and Aa in individuals with aggressive periodontitis. They observed that in these individuals the frequency and quantity of P. gingivalis and T. forsythia presented negative correlation with phagocytosis by PMN. The depression of phagocytosis observed in the present study in the periodontal disease group can
be justiied by the action of these pathogens usually
found in more severe forms of periodontal disease. In the analysis of phagocytosis by neutrophils using yeasts sensitized with fresh serum from the individual, although the PhI in individuals from the periodontitis group presented a trend toward reduction, the results did not differ statistically from those of the control group (Figure 2A). This suggests that there was no considerable change in complement an immunoglobulin receptors on
neutrophils, as well as considerable inluence of
other serological factors in this group.
Different from the present results, studies in individuals with more severe forms of periodontal disease demonstrate that regulatory factors in serum may modulate functions of PMNs. Depression of the chemotactic response in patients with localized aggressive periodontitis may not be an abnormality associated with the cell, but rather a consequence of the elevation of the serum concentration of cytokines produced during the host-parasite interaction. The TNF-alpha and IL-1 cytokines may cause a reduction in chemotactic receptors1. However, increased local production
of cytokines in response to pathogens in the periodontium may result in bone loss and tissue damage typically observed in more severe forms of periodontitis10,30. Although there are no studies
showing the relationship between serum levels of
inlammatory mediators and phagocytosis, there
may be an inverse correlation between them because depressed phagocytosis is always found in periodontal pockets9,24.
Although with small difference, the present data demonstrated that the serum level of C-reactive protein (CRP) was increased in individuals with periodontitis (Table 2). In more severe forms of periodontal disease, the association between the LPS of periodontopathogenic bacteria and
inlammatory mediators has been well established8,
which leads to the increase of CRP with consequent cardiovascular alterations18. Similarly, it is possible
that systemic immunosuppression of phagocytic cells, as observed in this study, may reduce the defense against bacteria and fungi. It is also a possibility the fact that the absence of systemic manifestations, observed in the present study, occur due to the redundancy of immune system functions. The long-term consequences of the reduction of phagocytosis in the group with periodontitis should be further evaluated.
CONCLUSIONS
This study showed for the irst time that the
monocytes of peripheral blood from individuals with periodontitis present decreased phagocytosis of opsonized and non-opsonized S. cerevisiae in comparison with control individuals. Concerning the neutrophils, decreased phagocytosis was observed only for non-opsonized yeasts. Although the individuals did not show clinical parameters of immunodeficiency, a laboratorial decreased function of phagocytes was characterized in this work, which may be a consequence, not the cause, of periodontitis. More studies should be conducted
to investigate the immune inlammatory events
implicated in phagocytosis by neutrophils and monocytes in periodontitis.
ACKNOwLEDgMENTS
The authors gratefully acknowledge Mrs. Shirley Claudino Couto for the excellent technical assistance and Dr. Raquel de Menezes Barbosa and Dr. Mariah Bastos Braun for helping with the phagocytosis
test evaluation. The authors report no conlicts of
REFERENCES
1- Agarwal S, Suzuki JB, Riccelli Ae. Role of cytokines in the modulation of neutrophil chemotaxis in localized juvenile periodontitis. J Periodontal Res. 1994;29:127-37.
2- Asif K, Kothiwale SV. Phagocytic activity of peripheral blood and crevicular phagocytes in health and periodontal disease. J Indian Soc Periodontol. 2010;14:8-11.
3- Baehni P, Tsai CC, McArthur WP, Hammond BF, Taichman NS. Interaction of inlammatory cells and oral microorganisms. VIII. Detection of leukotoxic activity of a plaque-derived gram-negative microorganism. Infect Immun. 1979;24:233-43.
4- Carvalho RP, Mesquita JS, Bonomo A, elsas PX, Colombo AP. Relationship of neutrophil phagocytosis and oxidative burst with the subgingival microbiota of generalized agressive periodontitis. Oral Microbiol Immunol. 2009;24:124-32.
5- Cortelli JR, Roman-Torres CV, Aquino DR, Franco GC, Costa FO, Cortelli SC. Occurrence of Aggregatibacter actinomycetemcomitans
in Brazilians with chronic periodontitis. Braz Oral Res. 2010;24:217-23.
6- Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, Coats SR, et al. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect Immun. 2004;72:5041-51.
7- Deas De, Mackey SA, McDonnell HT. Systemic disease and periodontitis: manifestations of neutrophil dysfunction. Periodontol 2000. 2003;23:82-104.
8- Duarte PM, Rocha M, Sampaio e, Mestnik MJ, Feres M, Figueiredo LC, et al. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: a pilot study. J Periodontol. 2010;81:1056-63.
9- Eick S, Pister W, Sigusch B, Straube E. Phagocytosis of periodontopathogenic bacteria by crevicular granulocytes is depressed in progressive periodontitis. Infection. 2000;28:301-4. 10- Figueredo CM, Ribeiro MS, Fischer RG, Gustafsson A. Increased interleukin-1beta concentration in gingival crevicular luid as a characteristic of periodontitis. J Periodontol. 1999;70:1457-63. 11- Gelani V, Fernandes AP, Gasparoto TH, Garlet TP, Cestari TM, Lima HR, et al. The role of toll-like receptor 2 in the recognition of Aggregatibacter actinomycetemcomitans. J Periodontol. 2009;80:2010-9.
12- Gomez RS, Costa Je, Lorentz TM, Garrocho AA, Nogueira-Machado JA. Chemiluminescence generation and MTT dye reduction by polymorphonuclear leukocytes from periodontal disease patients. J Periodontal Res. 1994;29:109-12.
13- Guentsch A, Puklo M, Preshaw PM, Glockmann E, Pister W, Potempa J, et al. Neutrophils in chronic and aggressive periodontitis in interaction with Porphyromonas gingivalis and
Aggregatibacter actinomycetemcomitans. J Periodontal Res. 2009;44:368-77.
14- Kantarci A, Oyaizu K, Van Dyke Te. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: indings from localized aggressive periodontitis. J Periodontol. 2003;74:66-75.
15- Kurita-Ochiai T, Ochiai K, Ikeda T. Immunosuppressive effect induced by Actinobacillus actinomycetemcomitans: effect on immunoglobulin production and lymphokine synthesis. Oral Microbiol Immunol. 1992;7:338-43.
16- Lins RD, Figueiredo CR, Queiroz LM, Silveira eJ, Freitas RA. Immunohistochemical evaluation of the inlammatory response in periodontal disease. Braz Dent J. 2008;19:9-14.
17- Loss BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000;71:1528-34.
18- Marcaccini AM, Meschiari CA, Sorgi CA, Saraiva MC, Souza AM, Faccioli LH, et al. Circulating interleukin-6 and high-sensitivity C-reactive protein decrease after periodontal therapy in otherwise healthy subjects. J Periodontol. 2009;80:594-602.
19- Muniz-Junqueira MI, Peçanha LM, Silva-Filho VL, Cardoso MCA, Tosta Ce. Novel microtechnique for assessment of postnatal maturation of the phagocytic function of neutrophils and monocytes. Clin Diagn Lab Immunol. 2003;10:1096-102. 20- Muniz-Junqueira MI, Prata A, Tosta Ce. Phagocytic and bactericidal function of mouse macrophages to Salmonella typhimurium in schistosomiasis mansoni. Am J Trop Med Hyg. 1992;46:132-6.
21- Nibali L, O’Dea M, Bouma G, Parkar M, Thrasher AJ, Burns S, et al. Genetic variants associated with neutrophil function in aggressive periodontitis and healthy controls. J Periodontol. 2010;81:527-34.
22- Page RC, Kornman KS. The pathogenesis of human periodontitis: an introduction. Periodontol 2000. 1997;14:9-11. 23- Papapanou PN, Sedaghatfar MH, Demmer RT, Wolf DL, Yang J, Roth GA, et al. Periodontal therapy alters gene expression of peripheral blood monocytes. J Clin Periodontol. 2007;34:736-47. 24- Sigusch B, Klinger G, Holtz H, Süss J. In vitro phagocytosis by crevicular phagocytes in various forms of periodontitis. J Periodontol. 1992;63:496-501.
25- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134-44.
26- Tsai CC, McArthur WP, Baehni PC, Hammond BF, Taichman NS. extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect Immun. 1979;25:427-39.
27- Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825-52.
28- Van Dyke Te. The etiology and pathogenesis of periodontitis revisited [editorial]. J Appl Oral Sci. 2009;17:pii.
29- Van Dyke TE, Zinney W, Winkel K, Tauiq A, Offenbacher S, Arnold RR. Neutrophil function in localized juvenile periodontitis. Phagocytosis, superoxide production and speciic granule release. J Periodontol. 1986;57:703-8.