ContentslistsavailableatScienceDirect
Behavioural
Brain
Research
j o ur na l h o me p a g e :w w w . e l s e v i e r . c o m / l o c a t e / b b r
Research
report
Quetiapine
treatment
reverses
depressive-like
behavior
and
reduces
DNA
methyltransferase
activity
induced
by
maternal
deprivation
Zuleide
M.
Ignácio
a,f,
Gislaine
Z.
Réus
a,∗,
Helena
M.
Abelaira
a,
Amanda
L.
Maciel
a,
Airam
B.
de
Moura
a,
Danyela
Matos
a,
Júlia
P.
Demo
a,
Júlia
B.I.
da
Silva
a,
Fernanda
F.
Gava
a,b,
Samira
S.
Valvassori
a,b,
André
F.
Carvalho
g,
João
Quevedo
a,c,d,eaLaboratoryofNeurosciences,GraduatePrograminHealthSciences,HealthSciencesUnit,UniversityofSouthernSantaCatarina,Criciúma,SC,Brazil bLaboratoryofNeuralSignalingandPsychopharmacology,GraduatePrograminHealthSciences,HealthSciencesUnit,UniversityofSouthernSanta
Catarina(UNESC),Criciúma,SC,Brazil
cCenterforTranslationalPsychiatry,DepartmentofPsychiatryandBehavioralSciences,MedicalSchool,TheUniversityofTexasHealthScienceCenterat
Houston,Houston,TX,USA
dCenterofExcellenceonMoodDisorders,DepartmentofPsychiatryandBehavioralSciences,MedicalSchool,TheUniversityofTexasHealthScienceCenter
atHouston,Houston,TX,USA
eNeuroscienceGraduateProgram,GraduateSchoolofBiomedicalSciences,TheUniversityofTexasHealthScienceCenteratHouston,Houston,TX,USA fLaboratoryofPhysiology,PharmacologyandPsychopathology,CampusChapecó,FederalUniversityofSouthFrontier,Chapecó,SantaCatarina,Brazil gDepartmentofClinicalMedicineandTranslationalPsychiatryResearchGroup,FacultyofMedicine,FederalUniversityofCeará,Fortaleza,CE,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received5November2016 Receivedinrevisedform 22November2016 Accepted25November2016 Availableonline29November2016
Keywords:
Quetiapine Epigenetic Antidepressant Maternaldeprivation Majordepressivedisorder
a
b
s
t
r
a
c
t
Stressinearlylifehasbeenappointedasanimportantphenomenonintheonsetofdepressionandpoor responsetotreatmentwithclassicalantidepressants.Furthermore,childhoodtraumatriggersepigenetic changes,whichareassociatedwiththepathophysiologyofmajordepressivedisorder(MDD). Treat-mentwithatypicalantipsychoticssuchasquetiapine,exertstherapeuticeffectforMDDpatientsand inducesepigeneticchanges.Thisstudyaimedtoanalyzetheeffectofchronictreatmentwithquetiapine (20mg/kg)ondepressive-likebehaviorofratssubmittedtomaternaldeprivation(MD),aswellasthe activityofhistoneacetylationbytheenzymeshistoneacetyltransferases(HAT)anddeacetylases(HDAC) andDNAmethylation,throughDNAmethyltransferaseenzyme(DNMT)intheprefrontalcortex(PFC), nucleusaccumbens(NAc)andhippocampus.Maternallydeprivedratshadadepressive-likebehaviorin theforcedswimmingtestandanincreaseintheHDACandDNMTactivitiesinthehippocampusand NAc.Treatmentwithquetiapinereverseddepressive-likebehaviorandreducedtheDNMTactivityin thehippocampus.Thisisthefirststudytoshowtheantidepressant-likeeffectofquetiapineinanimals subjectedtoMDandaprotectiveeffectbyquetiapineinreducingepigeneticchangesinducedbystress inearlylife.TheseresultsreinforceanimportantroleofquetiapineastherapyforMDD.
©2016ElsevierB.V.Allrightsreserved.
1. Introduction
Awidevarietyofresearchshowsthatthepathophysiologyof MDDinvolvesseveralbiologicalmechanismsboth,inthecentral nervoussystemandotherphysiologicalsystems[64].Thelow ther-apeuticresponsesofclassicalantidepressantssuggestthatMDDis anetiologicallyheterogeneousdisorder[21].Amongthevarious biologicalandpsychosocialfactors,stressduringchildhoodhistory, suchassexualabuse,physicalneglectandfamilyviolencewere
cor-∗ Correspondingauthorat:LaboratoryofNeurosciences,UniversityofSouthern SantaCatarinaCriciuma,SC,88806-000,Brazil.
E-mailaddress:gislainezilli@hotmail.com(G.Z.Réus).
relatedwithahighervulnerabilitytoMDDandanxietyinadulthood
[14,65].In addition,childhood traumahasalsobeenassociated
withpoorresponsetotreatment,similarlytotreatment-resistant depression(TRD)[41].Stressinchildhoodinduceschangesinthe hypothalamicpituitaryadrenal(HPA)axisactivity[6,22],aclassic physiologicalphenomenonassociatedtostress[63].Studieshave shownthatchangesinHPAaxisfunctionarefoundinMDD indi-vidualswhohaveexperiencedearlylifestress[1,54].
Despite the changes in theHPA axis functions,which seem inherentorinterrelatedtootherphysiologicalchanges,researches haveaddedanintensefocusonepigeneticphenomenaintricate in the processes that occuralong or much later in individuals exposed tostressat thebeginning oflife.Amongother mecha-nisms,epigeneticincludesprocesses,whicharebetterknownand
sistsof acetylation oflysine residues. Thisprocess reducesthe affinitybetweenproteinsandDNA,promotingarelaxationofthe chromatinstructureandalsoincreasingtherecruitment, activa-tionandstabilizationofthetranscriptionalmachinery[37].The acetylationlevelsalong ofthechromatinaredeterminedbythe balancebetweenHAT,whichaddsacetylgroups,andHDAC,which removesacetylgroupsfromlysineresiduesinhistone[30].The his-toneacetylationlevelisdeterminedbythebalancebetweenHAT andHDACenzymeactivity,andthusdeterminesthelevelof tran-scriptionofassociatedgene[29].Theresilience,aphenomenonof resistanceandabilitytocopewithadversitythroughoutlifecan beseriouslyimpairedwhenindividualsaresubjectedtotraumatic eventsduringcriticalperiodsforbraindevelopmental program-ming and can result in long-lasting detrimental consequences throughoutlifeandadulthood[34].Infact,recentliteraturehas pointedoutthatepigeneticchangesresultingfromstressin child-hoodareinherentinarepertoireofbiologicalprocessesinvolved indepression[12,34,58].Inhippocampusofsuicidevictims,who sufferedvariousformsof abuseandneglectinchildhood, stud-iesshowed increasedmethylation of gene promoter region for glucocorticoidreceptor(GR)in parallel witha reduction inthe GRmRNAlevelsandGRexpression[31,38].Thesefindings trans-lateresultsobtainedfromthe hippocampusofrat subjectedto maternaldeprivation,inwhichtheauthorsalsoobservedincreased depressive-likebehavior[67].OtherresearchersusingMD proto-cols,alsoobservedincreaseddepressive-likebehaviorsinparallel withincreaseofHDACactivityintheratNAc[48].Itisimportant tonotethattheincreaseinDNAmethylationanddepressive-like behaviorswerereversed withHDACinhibitors[67].Indeed,the inactivechromatinisassociatedwithmethylatedDNA[59],which supportsthefindingsaboutcorrelationbetweeninhibitionofHDAC andreversingofDNAmethylationanddepressivebehavior.Some studieshaveshownthatclassicalantidepressants,aswellas fast-actingdrugsseemtoreversetheepigeneticalterationsinvolvedin previousstressinchildhood,inanimalssubjectedtoMD[48].
Atypicalantipsychotic, also has epigenetic effects by reduc-ing the methylation of genes in the central nervous system
[19,20], and peripherally [23]. The Food and Drug
Administra-tion (FDA) approved quetiapine in 1997 for the treatment of schizophrenia [9]. Several studies have demonstrated the effi-cacy, safety, and tolerability of quetiapine in the treatment of MDD and bipolardisorders [3,56]. Noteworthy is thefact that TRDpatientsshowedantidepressantresponseswhentreatedwith quetiapine[13].Moreover,inanimalssubjectedtochronicstress, quetiapine,unlikefluoxetineaclassicalantidepressant,reversed depressive-likebehaviorandimprovedhippocampal neurogene-sis[66].Quetiapinehasserotonergicpropertiesasa5-HT1Apartial
agonist,potent ␣1 and ␣2-adrenoceptorsantagonist, as also is
an inhibitor of the norepinephrine transporter, through its N-desalkylquetiapinemetabolite[50,60].Theseaminergicactionsare believedtoplaya roleinitsantidepressantproperties[27] and increasethe therapeuticeffectof classical antidepressants[10]. Therefore,thestudyof drugstargeting neuroepigenetic mecha-nismscouldnotonlyprovideinsightsintotheunderstandingof themechanismsunderlyingneurobiologicalcausesoftheMDD, butshouldalsoacceleratethedevelopmentofnovel pharmacolog-icalagentswhichcanbemosteffectiveinthetreatmentofMDD.
Pregnant female Wistar rats (age of 3 months, weight of 250–280g)wereobtainedfromthebreedingcolonyof Universi-dadedoExtremoSulCatarinense(UNESC,Criciúma,SC,Brazil)and werehousedforoneweekinthepresenceofmalesforsexual expe-rience.Attheendof 7days,pregnantrats(n=10)werehoused individuallywithadlibitumaccesstofoodandwater.All moth-ersandpupswerekeptona12hlight/darkcycle(06:00a.m.to 06:00p.m.)atatemperatureof23±1◦C,includingthepups
sub-jectedtoMD.Maleandfemalepupswereusedduringthematernal deprivationperiod,butonlyhalfofmaleweresubjectedto depri-vation,accordingtotheprotocolbelow andschematic drawing (Fig.1).We takecaretoselectfemales withapproximatelythe samenumberofanimals,sothatthenumberofanimalsinthe sep-arationofthelitterwasnotmuch differentbetweenthem.The wholelitter,andthemotherratwerehousedinacleanbox,only afterthelastdayoftheMDprotocol.Afterthis,theanimalswere weanedinthe21thpostnataldayandhousedwithfiveanimalsper cage.FemalesweredonatedtotheUNESCvivariumforother stud-ies.Onlymaleratswereusedinthisresearchandwereprimarily dividedintotwoexperimentalgroups:(1)control,non-deprived (n=23),which receivednotreatmentwhatsoever; (2)deprived (n=21),whichweresubmittedtoMDasdescribed.All experimen-talproceduresinvolvinganimalswereperformedinaccordance withtheNIHGuidefortheCareandUseofLaboratoryAnimalsand theBrazilianSocietyforNeuroscienceandBehavior(SBNeC) rec-ommendationsforanimalcareandwithapprovalbylocalEthics Committeeunderprotocolnumber82/2012.
2.2. Maternaldeprivation(MD)protocol
Thepupsweredeprivedofthemotherfor3hperdayduringthe first10postnataldays(seeFig.1).Inthefirstpostnatalday,theMD protocolwasappliedto50%ofthemalepupsduring1–10postnatal days;othermaleswereusedascontrol.Themalepupswere ran-domlyselectedandafterthefirstwithdrawalprotocol,deprived pupsweremarkedforthedeprivationprotocolsinthefollowing days.Thematernaldeprivationprotocolconsistedofremovingthe pupsfromthenextbox.Non-deprivedanimalsremainundisturbed inahomecagewiththeirmother.
2.3. Drugsandexperimentaldesign
Fig.1.SchematicdrawingoftheMDprotocolandchronictreatmentwithquetiapine(14days;20mg/kg).MDprocedureswereperformedfor10daysafterthefirstpostnatal day.Thebehavioraltests(open-fieldandforcedswimming)wereperformedat62ndand63rdpostnataldays.Afterthebehavioraltestbrainsampleswereassessedtothe biochemicalanalysis.
reached 50th postnatal day, the drug treatment was initiated (seeFig.1).Theanimalsweredividedintofourgroups:(1) non-deprived+saline(n=11); (2) non-deprived+quetiapine (n=12); (3)deprived+saline(n=10);and(4)deprived+quetiapine(n=11).
2.4. Behavioraltests
All behavioral tests were conducted in the morning (8:00 a.m.–12:00p.m.),60minafterdrugtreatment,accordingtothe pro-tocolsdescribedinthemethodssection.Allbehavioraltestswere performedbyanobserverblindtotheexperimentalgroups.
2.4.1. Openfieldtest
Onthe62ndpostnatalday(Fig.1),and60minafter adminis-trationofquetiapineorsaline,theanimalsweresubjectedtothe open-fieldapparatus,in ordertoassesspossibleeffects ofdrug treatmentonspontaneouslocomotoractivity.Analysisoftherat’s spontaneousactivitywascarriedoutinanopenfieldapparatus, whichisanarena45×60cmsurroundedby50cmhighwallsmade ofbrownplywoodwithafront-facingglasswall.Thefloorofthe openfieldwasdividedinto9rectangles(15×20cmeach)byblack lines.Animalsweregentlyplacedontheleftrearrectangleandleft toexplorethearenafor5min.Thenumbersofhorizontal (cross-ing)andvertical(rearing)activitiesperformedbyeachratduring the 5min observationperiod were counted by an experienced observer.
2.4.2. Forcedswimmingtest
Theforcedswimmingtestwasconductedaccordingtothe previ-ousreports[42,45].Thetestinvolvestwoindividualexposurestoa cylindricaltankfilledwithwaterinwhichtheratscannottouchthe bottomofthetankorescape.Thetankismadeoftransparent Plexi-glas,80cmtall,30cmindiameter,andfilledwithwater(22–23◦C)
toa depthof 40cm. On the62thpostnatalday(Fig.1), 60min afterthequetiapineorsalinetreatment,andafterthelocomotor activitytestintheopenfield,theratswereindividuallyplacedin thecylindercontainingwaterfor15min(pre-testsession).Onthe 63rdpostnatalday(Fig.1),theratsreceivedtheirtreatment,and after60minwereagainsubjectedtotheforcedswimmingtestfor asessionof5min(testsession).Theimmobilitytimeoftherats wasrecordedinsecondsbyanexpertobserver(theresearcherwas blindtotheexperimentalgroups).Afterthebehavioraltests,all ratswerekilledbydecapitationandthebrainswereimmediately removed.ThePFC,hippocampusandNAcwerequicklyand man-uallyisolatedbyaskilledresearcher,usingamagnifyingglass,a spatulaandathinbrush.Thedissectionwasbasedonthe histo-logicaldistinctionsdescribedbyPaxinosandWatson[43].After theremovalofthestructures,theywereplacedinEppendorftube andstoredinafreezerat−80◦Cforposteriorbiochemical
analy-sis.Thebraintissueswereusedforanalysisofhistoneacetylation
andDNAmethylationlevelsbyobservingtheenzymaticactivityof HAT,HDACDNMT.
2.5. Histoneacetylatase,histonedesacetylaseandDNA methyltransferaseactivities
2.5.1. Nuclearextractionandcytosolicfractionseparation
ThePFC,hippocampusandNAcwererapidlyfrozenandstored at−80◦Cuntilnuclearproteinswereextracted.Thetissue sam-ples,PFC,hippocampusandNAc,frozenat−80◦C,weresubjected
to a nuclear extraction protocol from a Nuclear Extraction kit (Sigma,StLouis,USA).Briefly,sampleswerehomogenizedin cyto-plasmiclysisbuffercontainingdithiothreitol(DTT)andprotease inhibitors.Thesuspensionwaskeptonicefor15minandthen cen-trifugedat250xgfor5minat4◦C.Thesupernatantcontainingthe
cytosolicfractionwasdiscarded,andthepelletwasresuspendedin twovolumesofcoldcytoplasmiclysisbuffer.Thesuspensionwas homogenizedusingasyringewithasmallgaugeneedleand cen-trifugedat8000xgfor20minat4◦C.Thepelletwasresuspended
inanextractionbuffercontainingDTTandproteaseinhibitors.The resultingsamplewaskeptinslowagitationfor30–60mininan orbitalshakerat4◦C.After,thenuclearsuspensionwascentrifuged
at16,000xgfor5minat4◦Candthesupernatantcontainingthe
nuclearextractwastransferredtoanothertubeandstoredat−80◦C
untilfurtheranalysis.
NuclearextractsfromthePFC,hippocampusandNAcwere sub-jectedtotheHDAC,HATandDNMTenzymaticactivityassay(n=5 foreachbrainregionandenzyme)withtheuseofHDAC,HATand DNMTAssaykits(ColorimetricDetection)accordingtothe manu-facturer’sinstructions.Briefly,nuclearextractsamplesweremixed withHDAC(EPIGENTEK;Basecatalog#P-4034),HAT(EPIGENTEK; Base catalog #P-4003)or DNMT (EPIGENTEK;Base catalog #P-3009)assaybuffer,moreassaysubstrateofthesameenzymes,in a96wellplateandincubatedat30◦Cfor45min.Concomitantly,a
standardcurvewasperformedwithserialdilutionsofHDAC,HAT orDNMTsubstrates,asalsopositiveandnegativecontrolswere addedtotheplate.Afterwards,thedevelopersolutionwasadded tothewells,andtheplatewasincubatedatroomtemperaturefor 15min.Acolorimetricreadingwasperformedonafluorescence platereaderwith450nmforHDAC,HATandDNMTactivities.
2.6. Statisticalanalysis
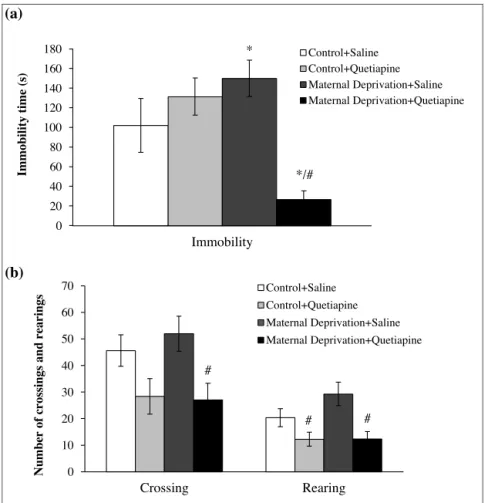
quetiapine,asmeasuredbyimmobilitytime(Fig.2a;F(1–41)=9.047,
p<0.001).Fig.2bshowstheeffectsofMDandquetiapineonthe locomotor activity evaluated in the open-field test. Locomotor activitywasnotdifferentbetweenMDandcontrolanimals (non-MD),asmeasuredbynumberofcrossingsandrearings.However, quetiapinereducedlocomotoractivityinMDandcontrolanimals (non-MD),butonlywhencomparedtosaline-treatedMDanimals, asmeasuredbycrossings(Fig.2b;F(3–40)=3.72;p<0.05)and
rear-ings(Fig.2b;F(3–40)=5.89;p<0.01).
3.2. Measurementofepigeneticparameters
3.2.1. Histonedeacetylases(HDAC)activity
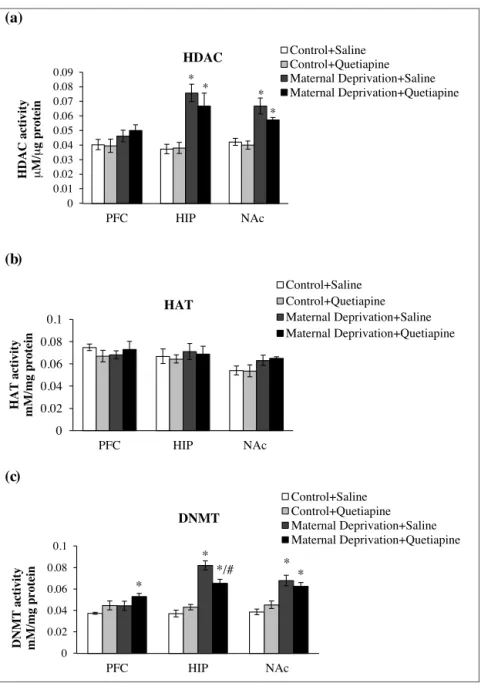
Fig.3aillustratestheinfluenceofMDandchronicquetiapine treatmentontheHDACactivity.Thestatisticalanalysisrevealed thatadulthoodMDratsdisplayedanincreaseintheHDAC activ-ityinthehippocampus(Fig.3a;F(3–16)=11.11;p<0.001)andNAc
(Fig. 3a;F(3–16)=13.81;p<0.001), but it didnot changein PFC
(Fig.3a;F(3–16)=1.61;p=0.227).However,chronictreatmentwith
quetiapinedidnotreducesignificantlytheincreaseofHDAC activ-ityinMDanimals.
3.2.2. Histoneacetyltransferases(HAT)activity
AsdepictedinFig.3b,thestresscausedbyMDdidnotinduce alterationofHATactivityinadultanimals.Similarly,chronic treat-mentwithquetiapinedidnotcauseanychangeintheHATactivity, whetherin relationtoMD animalstreated withsaline or con-trolanimals(non-MD).Statisticalanalysisrevealednosignificant changesinanyofbrainareas:Hippocampus(Fig.3b;F(3–16)=0.22;
p=0.88),NAc (Fig.3b;F(3–16)=2.05;p=0.15), and PFC(Fig.3b;
F(3–16)=0.58;p=0.63).
3.2.3. DNAmethyltransferases(DNMT)activity
Fig.3cshowstheinfluenceofMDandchronicquetiapine treat-mentontheDNMTactivity.Thestatisticalanalysisrevealedthat adulthoodMDratsdisplayedanincreaseofDNMTactivityinthe hippocampus.MDanimalstreatedwithquetiapinealsoshowedan increaseinthehippocampalDNMTactivity,whencomparedto ani-malswithoutstress(non-MD).Thequetiapinesignificantlyreduced DNMTactivitywhencomparedtoMDanimalstreatedwithsaline, indicatingthatquetiapinehasapharmacologicalprofileinorder toreversethehippocampalDNAmethylationinstressedanimals (Fig.3c;F(3–16)=36.56;p<0.001).IntheNAc,theDNMTactivity
increasedsignificantlyinMDanimals,bothtreatedwithsalineand quetiapine(Fig.3c;F(3–16)=13.92;p<0.001).Thechronictreatment
withquetiapinedidnotreducesignificantlytheincreaseofDNMT activityintheNAcofMDanimals.InthePFC,onlyMDanimals treatedwithquetiapinehadanincreaseintheDNMTactivity com-paredtoanimalsnotstressed(non-MD)andtreatedwithsaline (Fig.3c;F(3–16)=3.62;p<0.05).Theothergroupsshowedno
signif-icantchangesinenzymeactivityinthePFC.
changesinducedbystressinearlylife.Similartootherstudies,the MDincreasedimmobilitytimeintheforcedswimmingtest, indi-catingadepressive-likebehaviorinanimals,andconfirmingthat thetraumaticexperiencesinearlylifecanculminateindepression inadulthood[49].Chronictreatmentwithquetiapinesignificantly reduceddepressive-likebehavior.Theantidepressant-likeeffects ofquetiapinewerealsodemonstratedinotherstudiesinwhich adultanimalsweresubjectedtochronicstress[66,17].Additionally, quetiapinereversedthereductionofGABAlevelsinthe hippocam-pusaswellasitreducedtheconcentrationsofcorticosteronein theserumofanimalssubjectedtostress[17].In thestudiesby Wangetal.[66],theauthorsobservedthatquetiapineexercised antidepressant-likeactioninrodentresistanttotreatmentwith flu-oxetine.Inthepresentstudy,quetiapineinducedasignificanteffect inanimalssubjectedtostressinearlylife,aconditionthathasbeen highlightedasapivotofthepoorresponsetoclassical antidepres-santtreatmentsinMDDpatients[41,68],andinanimalsdisplaying depressive-likebehaviors[70].Itwasobservedthatanimals sub-jectedtoMDexhibiteddepressive-likebehaviorinadulthood,and escitalopram,aselectiveserotoninreuptakeinhibitor(SSRI),was lesseffectiveinreversingthedepressive-likebehavior[70].These andotherstudy[17]revealedthatquetiapine,asmonotherapyand chronicallyadministered exerted significantantidepressant-like effect.InMDDpatients,someauthorsalsoshowedthatquetiapine, aloneandchronicallyadministered,exercisedsignificant antide-pressanteffects[35,5],aswellasitreducedanxietyinMDDpatients withcomorbidanxiety[18].Thus,quetiapine,inadditionto func-tionasadjunctivetherapywithotherantidepressantdrugs,also exertsantidepressanteffectwhenadministeredasmonotherapy.
In locomotor activitytest, quetiapine reduced crossings and rearing activities, when compared with deprived animals and saline-treated,a resultthat suggest a sedativefunctionof dose administered60minbeforetesting.Indeed,quetiapinehasquick sedativeeffectafteritsadministrationinMDDpatients[61],being oneofitslimitationsastherapyforMDD[8].However,theeffecton locomotoractivityshouldbeinterpretedwithsomecaution,given thatinourpilotstudy,onlytheconcentrationof80mg/kgexerted sedativeeffect,oneofthereasonsthatpromptedustochoosethe lowestconcentrationforthisstudy.Somestudieshaveobserved thatmaternallydeprivedrats,inthe9thand10thpostnataldays, presentedanincreaseinthelocomotoractivityinadolescence[36]
(a)
(b)
*
*/#
0 20 40 60 80 100 120 140 160 180
Immobility
Immobility time (s)
Control+Saline Control+Quetiapine Maternal Deprivation+Saline Maternal Deprivation+Quetiapine
#
#
#
0 10 20 30 40 50 60 70
Crossing
Rearing
Number of crossings and rearings
Control+Saline Control+Quetiapine Maternal Deprivation+Saline Maternal Deprivation+Quetiapine
Fig.2.MDandquetiapine(20mg/kg,during14days)effectsontheimmobilitytimeintheforcedswimmingtest(2a)andlocomotoractivity(crossingsandrearingsnumbers) intheopenfield(2b).Valuesexpressedasthemeanandstandarderrorofthemean(M±SEM).*Differentfromcontrol+saline,p<0.05.#DifferentfromMD+saline,p<0,05.
byquetiapinetowardtheantidepressanttherapeutic,whenadded toclassicalantidepressantsorasmonotherapy[61].Other stud-ieshavefoundthatimipramine,aclassicantidepressant,reduced immobilityintheforcedswimmingtestindiabeticrats,whilealso reducedlocomotoractivityintheopenfield[7,40].Still, regard-ingtheeffects of locomotor activity,somestudiessuggest that reduction ofmotor activityin theopenfield is correlated with increasedimmobility,whereasotherauthorshaveshownthat loco-motor activityisnot underlyingtheperformance of animalsin theforced swimtest[11].Someauthorssuggestthatother fac-tors may be involved in motor behavior assessed in the open field.For example,in mice,themotoractivitywassignificantly decreasedbyimipraminechronicallyadministered,afterchronic stressfor27days,whiletheimmobilitytimealsowasreducedin theforcedswimtest.Ontheotherhand,after21daysofchronic stress,imipramine didnotchangelocomotoractivity,compared withthecontrolsorstressedanimals[71].
Althoughquetiapineactsthroughwidevarietyofmechanisms, anditisdifficulttoidentifythemechanismsmorecloselyrelated totheantidepressanteffect[51],itisimportanttonotethatthe drug increases the release of dopamine in brain regions such asPFC[46].Thisquetiapine effectissuggested asimportantto improveaffectiveandcognitiveeffectsunderlyingdepression[46]. Besidesdopamine,quetiapinemayalsohasatherapeuticbenefitto increaseextracellularlevelsofnorepinephrinethroughblockadeof 5-HT2Creceptors[2],whichexertatonic,inhibitoryinfluenceupon
dopaminergicandadrenergicpathwaysinthePFC[39].Blierand Blondeau[2]alsopostulatedthatMDD patientsnon-responsive toselectiveserotoninreuptakeinhibitors(SSRIs) mightbewith reduced noradrenergic activity, given that SSRIs reduces firing
of noradrenergic neurons in the locus coeruleus, by activating receptors 5-HT2A oninhibitory GABAergic neurons that
modu-latenoradrenergicneurons.Regardingthenoradrenergicactivity isimportanttonotethatquetiapinealsohasstrongblockingaction onthenorepinephrinetransporterandisapotent␣2-adrenoceptor
antagonist[50,10],whosemechanismsincreasethesynaptic avail-abilityofnoradrenaline.Inadditiontothenoradrenergicactivity,it isalsoimportanttoconsiderthatincreasingdopaminergicactivity maybeamechanismrelatedtotherapeuticeffectonindividuals whohavepoorresponsetoothertreatments,assuggestedby[24].
4.2. EffectofMDandtreatmentwithquetiapineontheenzymatic activityofHDAC,HATandDNMT
Inthiswork,adultanimals,whichwerematernallydeprived, showedanincreaseinHDACandDNMTactivityinthe hippocam-pusandNAc,confirmingotherfindingsdescribed[67,48].Increased activityoftheseenzymesisassociatedwithlowergene
transcrip-tion [28,29]. Noteworthy is thefact that increasingfunction of
HDACsisassociatedwithdepressioninanimalmodelsandreduced therapeuticresponsetoantidepressants[15,62].Also,itis impor-tanttonotethatchronictreatmentwithantidepressantscanalter theepigeneticchanges[48,57],andtomitigatethelossesof hip-pocampalneurogenesispossiblyunderlyingtoepigeneticchanges resultingfromstressinearlylife[57].
(b)
(c)
0 0.01 0.02 0.03
PFC HIP NAc
HDAC activity μM/
0 0.02 0.04 0.06 0.08 0.1
PFC HIP NAc
HAT activity
mM/mg protein
HAT
Control+Saline Control+Quetiapine Maternal Deprivation+Saline Maternal Deprivation+Quetiapine
*
*
*
*/#
*
0 0.02 0.04 0.06 0.08 0.1
PFC HIP NAc
DNMT activity mM/mg protein
DNMT
Control+Saline Control+Quetiapine Maternal Deprivation+Saline Maternal Deprivation+Quetiapine
Fig.3. MDandquetiapine(20mg/kg,during14days)effectsontheenzymeactivities:histonedeacetylase(HDAC)(3a),histoneacetyltransferase(HAT)(3b)andDNA methyltransferase(DNMT)(3c).Valuesexpressedasthemeanandstandarderrorofthemean(M±SEM).*Differentfromcontrol+saline,p<0.05.#DifferentfromMD+saline, p<0,05.
inthehippocampusandpromotedaslightreductioninenzymatic activityintheNAc.Somestudieshaveshownthatquetiapine,as wellasotherdibenzepinederivativessuchasclozapineand olan-zapinealsohasepigeneticeffect,reducingthemethylationofgenes inthecentralnervoussystem,unlikebutyrophenonederivative haloperidolandthepiperidyl-benzisoxazolederivativerisperidone
[19,20].Anotherimportantstudyfromhumanneuroblastomacell
cultureshowedthatquetiapinepromoteshypomethylationinwide varietyof genes,withthe mosthypomethylatedgenes belongs to promoter regions located within CpG island [55]. Another importantfindingof theselaststudieswasone decreased DNA methylationin thespecificCpG site onthepromoter regionof theserotonintransportergene(5-HTT).Geneticpolymorphismsin theseregions areassociatedwithincreasedmethylationof pro-moterregionsofthe5-HTTgeneinlymphoblasticcelllinesand withreduced transcriptionalactivityforthemRNAofserotonin transporter[44].Moreover,literaturereportsthatthepolymorphic variantassociatedwithlowertranscriptionalactivity,alsopresents greaterinteractionbetweengenexstressinearlylife,resulting
inlifelongdepression[25].Importantlyisthefactthatgenotype thatpresentreducedtranscriptionalactivityalsoisassociatedwith poorresponseofpatientstotreatmentwithclassical antidepres-sants[53].Anotherimportantevidenceis thatthepolymorphic variant,whichshowedahigherlevelofmethylationandlower tran-scriptionalactivity,wasalsoassociatedwithsmallerhippocampal volumeinMDDpatients[16].
leastinpart,berelatedtoitsabilitytoreducetheactivityofDNMT enzymes.
TheDNMTactivityincreasedinthePFCofMDanimalstreated withquetiapine.Thisresultseemsparadoxicalwhencomparedto theresultsobservedinthehippocampus,inthisstudyorresults fromstudies, which observed other brainregions. However, in studieswhich observed a reduction inDNA methylation inthe PFC, theresearch was directed tospecific promoter regions of genesassociatedwithotherdisorders,wherethemethylationand demethylationprocessesappeartorequireintermediationofother molecularmarkers[20].Asdiscussedbytheauthors,inneuronsthe promotermethylationisadynamicprocess,involvingmany fac-tors,amongwhichmaybethepsychopathologyandbrainregions involvedwithfunctionsrelevanttorespectivedisorder.In addi-tiontolackingdataaboutthefunctionofquetiapineonepigenetic parameters,thescientificliteraturealsolacksdatainvolvingthe profileof quetiapine and otheratypical antipsychotics onDNA methylationmechanismsinthePFC.Onestudyshowedthat cloza-pineandrisperidoneincreasedmethylationofsomegenes,while inothergenes,itappearstoshowreducedmethylationinthePFC, possiblyasapositiveantipsychoticeffectonbehaviorsrelatedto schizophrenia,orotherpsychiatricdisorders[52].Therefore, fur-therstudiesareneededinordertouncoverapossibleprotective orharmfulroleofquetiapineandotheratypicalantipsychoticsin epigeneticparametersinthePFC.
Inconclusion, theresultsofthis studyshowedthat quetiap-ineexertedantidepressant-likeeffectsinanimalssubjectedtoMD stress,aneventunderlyingtoMDD.Additionally,ratifyingresults ofthescientificliterature,thisworkdataalsoindicatethat previ-ouslifestressinducesalterationsinepigeneticpatterns,whichcan culminateinareductioningenetranscriptionprocess.Amost sig-nificantfindingwasthefactthatquetiapineinducedasignificant decreaseinDNMTactivityinthehippocampus,DNAmethylation mechanisms,possiblyreversinga hypermethylationinducedby stressinchildhood.
Acknowledgements
TheTranslationalPsychiatryProgram(USA)isfundedbythe DepartmentofPsychiatryandBehavioralSciences,McGovern Med-ical School, The University of Texas Health Science Center at Houston(UTHealth).LaboratoryofNeurosciences(Brazil)isone of thecenters of theNational Institutefor MolecularMedicine (INCT-MM)andoneofthemembersoftheCenterofExcellence inAppliedNeurosciencesofSantaCatarina(NENASC).Itsresearch issupportedbygrantsfromCNPq(JQ)FAPESC(JQ);Instituto Cére-broeMente(JQ)andUNESC(JQandGZR).JQandAFCare1ACNPq ResearchFellow.
References
[1]C.V.Baes,C.M.Martins,S.M.Tofoli,M.F.Juruena,Earlylifestressindepressive patients:HPAaxisresponsetoGRandMRagonist,Front.Psychiatry5(2014) 2.
[2]P.Blier,C.Blondeau,Neurobiologicalbasesandclinicalaspectsoftheuseof aripiprazoleintreatment-resistantmajordepressivedisorder,J.Affect. Disord.128(Suppl.1)(2011)S3–S10.
[3]B.T.Baune,Newdevelopmentsinthemanagementofmajordepressive disorderandgeneralizedanxietydisorder:roleofquetiapine,Neuropsychiatr. Dis.Treat.4(6)(2008)1181–1191.
[4]S.Boku,H.Toda,S.Nakagawa,A.Kato,T.Inoue,T.Koyama,N.Hiroi,I.Kusumi, Neonatalmaternalseparationaltersthecapacityofadultneuralprecursor cellstodifferentiateintoneuronsviamethylationofretinoicacidreceptor genepromoter,Biol.Psychiatry77(4)(2015)335–344.
[5]B.Bortnick,N.El-Khalili,M.Banov,D.Adson,C.Datto,S.Raines,W.Earley,H. Eriksson,Efficacyandtolerabilityofextendedreleasequetiapinefumarate (quetiapineXR)monotherapyinmajordepressivedisorder:a
placebo-controlled,randomizedstudy,J.Affect.Disord.128(1–2)(2011) 83–94.
[6]L.L.Carpenter,J.P.Carvalho,A.R.Tyrka,L.M.Wier,A.F.Mello,M.F.Mello,G.M. Anderson,C.W.Wilkinson,L.H.Price,Decreasedadrenocorticotropichormone andcortisolresponsestostressinhealthyadultsreportingsignificant childhoodmaltreatment,Biol.Psychiatry62(10)(2007)1080–1087. [7]L.B.Ceretta,G.Z.Réus,R.B.Stringari,K.F.Ribeiro,G.Zappellini,B.W.Aguiar,B.
Pfaffenseller,C.Lersh,F.Kapczinski,J.Quevedo,Imipraminetreatment reversesdepressive-likebehaviorinalloxan-diabeticrats,DiabetesMetab. Res.Rev.28(2)(2012)139–144.
[8]D.S.Cha,R.S.McIntyre,Treatment-emergentadverseeventsassociatedwith atypicalantipsychotics,Expert.Opin.Pharmacother.13(11)(2010) 1587–1598.
[9]S.M.Cheer,A.J.Wagstaff,Quetiapine−Areviewofitsuseinthemanagement ofschizophrenia,CNSDrugs18(3)(2004)173–199.
[10]O.Chernoloz,M.ElMansari,P.Blier,Effectsofsustainedadministrationof quetiapinealoneandincombinationwithaserotoninreuptakeinhibitoron norepinephrineandserotonintransmission,Neuropsychopharmacology37 (7)(2012)1717–1728.
[11]J.N.Crawley,J.K.Belknap,A.Collins,J.C.Crabbe,W.Frankel,N.Henderson,R.J. Hitzemann,S.C.Maxson,L.L.Miner,A.J.Silva,J.M.Wehner,A.Wynshaw-Boris, R.Paylor,Behavioralphenotypesofinbredmousestrains:implicationsand recommendationsformolecularstudies,Psychopharmacology(Berl.)132(2) (1997)107–124.
[12]V.S.Dalton,E.Kolshus,D.M.Mcloughlin,Epigeneticsanddepression:return oftherepressed,J.Affect.Disord.155(2014)1–12.
[13]E.J.Daly,M.H.Trivedi,Areviewofquetiapineincombinationwith antidepressanttherapyinpatientswithdepression,Neuropsychiatr.Dis. Treat.3(6)(2007)855–867.
[14]N.P.Daskalakis,R.C.Bagot,K.J.Parker,C.H.Vinkers,E.R.deKloet,Thethree-hit conceptofvulnerabilityandresilience:towardunderstandingadaptationto early-lifeadversityoutcome,Psychoneuroendocrinology38(9)(2013) 1858–1873.
[15]M.Dyrvig,H.H.Hansen,S.H.Christiansen,D.P.Woldbye,J.D.Mikkelsen,J. Lichota,EpigeneticregulationofArcandc-Fosinthehippocampusafteracute electroconvulsivestimulationintherat,BrainRes.Bull.88(5)(2012) 507–513.
[16]M.C.Eker,O.Kitis,H.Okur,O.D.Eker,E.Ozan,S.Isikli,N.Akarsu,A.S.Gonul, Smallerhippocampusvolumeisassociatedwithshortvariantof5-HTTLPR polymorphisminmedication-freemajordepressivedisorderpatients, Neuropsychobiology63(1)(2011)22–28.
[17]S.M.K.S.ElDine,AnincreaseinGABAcontentinHippocampusofAlbinorats exposedtochronicrestraintmodelandtreatedbyQuetiapinefor3weeks,J. Pharm.Innov.3(12)(2015)89–93.
[18]I.Galynker,A.Khan,Y.Grebchenko,A.Ten,L.Malaya,P.Yanowitch,L.J.Cohen, Low-doserisperidoneandquetiapineasmonotherapyforcomorbidanxiety anddepression,J.Clin.Psychiatry66(4)(2005)544.
[19]A.Guidotti,J.Auta,Y.Chen,J.M.Davis,E.Dong,D.P.Gavin,D.R.Grayson,F. Matrisciano,G.Pinna,R.Satta,R.P.Sharma,L.Tremolizzo,P.Tueting, EpigeneticGABAergictargetsinschizophreniaandbipolardisorder, Neuropharmacology60(7–8)(2011)1007–1016.
[20]A.Guidotti,D.R.Grayson,DNAmethylationanddemethylationastargetsfor antipsychotictherapy,DialoguesClin.Neurosci.16(3)(2014)419–429. [21]G.Hasler,Pathophysiologyofdepression:dowehaveanysolidevidenceof
interesttoclinicians?WorldPsychiatry9(3)(2010)155–161.
[22]C.Heim,D.J.Newport,S.Heit,Y.P.Graham,M.Wilcox,R.Bonsall,A.H.Miller, C.B.Nemeroff,Pituitary-adrenalandautonomicresponsestostressinwomen aftersexualandphysicalabuseinchildhood,JAMA284(5)(2000)592–597. [23]L.C.Houtepen,A.H.vanBergen,C.H.Vinkers,M.P.Boks,DNAmethylation
signaturesofmoodstabilizersandantipsychoticsinbipolardisorder, Epigenomics8(2)(2016)197–208.
[24]M.C.Hsiao,K.J.Lin,C.Y.Liu,D.B.Schatz,Theinteractionbetweendopamine transporterfunction,genderdifferences,andpossiblelateralityindepression, PsychiatryRes.211(1)(2013)72–77.
[25]Z.M.Ignácio,G.Z.Réus,H.M.Abelaira,J.Quevedo,Epigeneticandepistatic interactionsbetweenserotonintransporterandbrain-derivedneurotrophic factorgeneticpolymorphism:insightsindepression,Neuroscience275 (2014)455–468.
[26]Z.M.Ignácio,G.Z.Réus,H.M.Abelaira,S.E.Titus,A.S.Carlessi,J.R.daLuz,B.I. Matias,L.Bruchchen,M.Carvalho-Silva,L.M.Gomes,J.Rebelo,E.L.Streck,J. Quevedo,Acuteandchronictreatmentswithquetiapineincrease mitochondrialrespiratorychaincomplexactivityintheratbrain,Curr. Neurovasc.Res.12(3)(2015)283–292.
[27]N.H.Jensen,R.M.Rodriguiz,M.G.Caron,W.C.Wetsel,R.B.Rothman,B.L.Roth, N-desalkylquetiapine,apotentnorepinephrinereuptakeinhibitorandpartial 5-HT1Aagonist,asaputativemediatorofquetiapine’santidepressant activity,Neuropsychopharmacology33(10)(2008)2303–2312. [28]P.Jones,D.Takai,TheroleofDNAmethylationinmammalianepigenetics,
Science293(2001)1068–1070.
[29]T.Kouzarides,Chromatinmodificationsandtheirfunction,Cell128(2007) 693–705.
[30]M.H.Kuo,C.D.Allis,Rolesofhistoneacetyltransferasesanddeacetylasesin generegulation,Bioessays20(8)(1998)615–626.
monotherapyinacutephaseformajordepressivedisorder:ameta-analysis ofrandomized,placebo-controlledtrials,BMCPsychiatry12(2012)160. [36]E.M.Marco,W.Adriani,R.Canese,F.Podo,M.P.Viveros,G.Laviola,
Enhancementofendocannabinoidsignallingduringadolescence:modulation ofimpulsivityandlong-termconsequencesonmetabolicbrainparametersin earlymaternallydeprivedrats,Pharmacol.Biochem.Behav.86(2007) 334–345.
[37]R.Marmorstein,R.C.Trievel,Histonemodifyingenzymes:structures, mechanisms,andspecificities,Biochim.Biophys.Acta(BBA)GeneRegul. Mech.1789(1)(2009)58–68.
[38]P.O.McGowan,A.Sasaki,A.C.D’Alessio,S.Dymov,B.Labonté,M.Szyf,G. Turecki,M.J.Meaney,Epigeneticregulationoftheglucocorticoidreceptorin humanbrainassociateswithchildhoodabuse,Nat.Neurosci.12(3)(2009) 342–348.
[39]M.J.Millan,A.Gobert,F.Lejeune,A.Dekeyne,A.Newman-Tancredi,V. Pasteau,J.M.Rivet,D.Cussac,Thenovelmelatoninagonistagomelatine (S20098)isanantagonistat5-hydroxytryptamine2Creceptors,blockadeof whichenhancestheactivityoffrontocorticaldopaminergicandadrenergic pathways,J.Pharmacol.Exp.Ther.306(2003)954–964.
[40]R.I.Nadeem,H.I.Ahmed,E.E.El-Denshary,Effectofimipramine,paroxetine, andlithiumcarbonateonneurobehavioralchangesofstreptozotocininrats: impactonglycogensynthasekinase-3andbloodglucoselevel,Neurochem. Res.40(9)(2015)1810–1818.
[41]V.Nanni,R.Uher,A.Danese,Childhoodmaltreatmentpredictsunfavorable courseofillnessandtreatmentoutcomeindepression:ametaanalysis,Am.J. Psychiatry169(2)(2012)141–151.
[42]C.F.Ortmann,G.Z.Réus,Z.M.Ignácio,H.M.Abelaira,S.E.Titus,P.deCarvalho, C.O.Arent,M.A.DosSantos,B.I.Matias,M.M.Martins,A.M.deCampos,F. Petronilho,L.J.Teixeira,M.O.Morais,E.L.Streck,J.Quevedo,F.H.Reginatto, EnrichedflavonoidfractionfromCecropiapachystachyaTréculleavesexerts antidepressant-likebehaviorandprotectsbrainagainstoxidativestressin ratssubjectedtochronicmildstress,Neurotox.Res.29(4)(2016)469–483. [43]G.Paxinos,C.Watson,TheRatBrain:StereotaxicCoordinates,2nded.,
AcademicSanDiego,1986.
[44]R.Philibert,A.Madan,A.Andersen,R.Cadoret,H.Packer,H.Sandhu,Serotonin transportermRNAlevelsareassociatedwiththemethylationofanupstream CpGisland,Am.J.Med.Genet.BNeuropsychiatr.Genet.144B(2007)101–105. [45]R.D.Porsolt,A.Bertin,M.Jalfre,Behaviouraldespairinmice:aprimary
screeningtestforantidepressants,Arch.Int.Pharmacodyn.Ther.229(1977) 327–336.
[46]E.Prieto,J.A.Micó,J.J.Meana,S.Majadas,Neurobiologicalbasesof
quetiapineantidepresanteffectinthebipolardisorder,ActasEsp.Psiquiatr.38 (1)(2010)22–32.
[47]G.Rentesi,K.Antoniou,M.Marselos,M.Syrrou,Z.Papadopoulou-Daifoti,M. Konstandi,Earlymaternaldeprivation-inducedmodificationsinthe neurobiological,neurochemicalandbehavioralprofileofadultrats,Behav. BrainRes.244(2013)29–37.
[48]G.Z.Réus,H.M.Abelaira,M.A.dosSantos,A.S.Carlessi,D.B.Tomaz,M.V. Neotti,J.L.Liranc¸o,C.Gubert,M.Barth,F.Kapczinski,J.Quevedo,Ketamine andimipramineinthenucleusaccumbensregulatehistonedeacetylation inducedbymaternaldeprivationandarecriticalforassociatedbehaviors, Behav.BrainRes.256(2013)451–456.
[49]G.Z.Réus,A.S.Carlessi,S.E.Titus,H.M.Abelaira,Z.M.Ignácio,J.R.daLuz,B.I. Matias,L.Bruchchen,D.Florentino,A.Vieira,F.Petronilho,J.Quevedo,A singledoseofS-ketamineinduceslong-termantidepressanteffectsand decreasesoxidativestressinadulthoodratsfollowingmaternaldeprivation, Dev.Neurobiol.75(11)(2015)1268–1281.
[50]E.Richelson,T.Souder,Bindingofantipsychoticdrugstohumanbrain receptorsfocusonnewergenerationcompounds,LifeSci.68(2000)29–39.
regulationandlatermentaldisorders,Neurosci.Biobehav.Rev.38(2014) 17–37.
[55]H.Sugawara,M.Bundo,T.Asai,F.Sunaga,J.Ueda,J.Ishigooka,K.Kasai,T. Kato,K.Iwamoto,EffectsofquetiapineonDNAmethylationinneuroblastoma cellsProg,Neuropsychopharmacol.Biol.Psychiatry56(2015)117–122. [56]T.Suppes,E.Vieta,S.Liu,M.Brecher,B.Paulsson,Trial127Investigators.,.
MaintenancetreatmentforpatientswithbipolarIdisorder:resultsfroma northamericanstudyofquetiapineincombinationwithlithiumor divalproex(trial127),Am.J.Psychiatry166(4)(2009)476–488.
[57]D.Suri,V.Veenit,A.Sarkar,D.Thiagarajan,A.Kumar,E.J.Nestler,S.Galande, V.A.Vaidya,Earlystressevokesage-dependentbiphasicchangesin hippocampalneurogenesis,BDNFexpression,andcognition,Biol.Psychiatry 73(7)(2013)658–666.
[58]M.Szyf,Theearly-lifesocialenvironmentandDNAmethylation,Clin.Genet. 81(2012)341–349.
[59]M.Tachibana,Y.Matsumura,M.Fukuda,H.Kimura,Y.Shinkai,G9a/GLP complexesindependentlymediateH3K9andDNAmethylationtosilence transcription,EMBOJ.27(20)(2008)2681–2690.
[60]Y.Tanibuchi,Y.Fujita,M.Kohno,T.Ishima,Y.Takatsu,M.Iyo,K.Hashimoto, Effectsofquetiapineonphencyclidine-inducedcognitivedeficitsinmice:a possibleroleofalpha1-adrenoceptors,Eur.Neuropsychopharmacol.19(12) (2009)861–867.
[61]D.Todder,S.Caliskan,B.T.Baune,Nightlocomotoractivityandqualityof sleepinquetiapine-treatedpatientswithdepression,J.Clin.
Psychopharmacol.26(6)(2006)638–642.
[62]N.M.Tsankova,O.Berton,W.Renthal,A.Kumar,R.L.Neve,E.J.Nestler, Sustainedhippocampalchromatinregulationinamousemodelofdepression andantidepressantaction,Nat.Neurosci.9(4)(2006)519–525.
[63]C.Tsigos,G.P.Chrousos,Hypothalamic-pituitary-adrenalaxis,
neuroendocrinefactorsandstress,J.Psychosom.Res.53(4)(2002)865–871. [64]J.Verduijn,Y.Milaneschi,R.A.Schoevers,A.M.vanHemert,A.T.Beekman,
B.W.Penninx,Pathophysiologyofmajordepressivedisorder:mechanisms involvedinetiologyarenotassociatedwithclinicalprogression,Transl. Psychiatry5(2015)e649.
[65]A.M.Vranceanu,S.E.Hobfoll,R.J.Johnson,Childmulti-typemaltreatmentand associateddepressionandPTSDsymptoms:theroleofsocialsupportand stress,Child.AbuseNegl.31(1)(2007)71–84.
[66]Y.Wang,T.Chang,Y.C.Chen,R.G.Zhang,H.N.Wang,W.J.Wu,Z.W.Peng,Q.R. Tan,Quetiapineadd-ontherapyimprovesthedepressivebehaviorsand hippocampalneurogenesisinfluoxetinetreatmentresistantdepressiverats, Behav.BrainRes.253(2013)206–211.
[67]I.C.Weaver,N.Cervoni,F.A.Champagne,A.C.D’Alessio,S.Sharma,J.R.Seckl,S. Dymov,M.Szyf,M.J.Meaney,Epigeneticprogrammingbymaternalbehavior, Nat.Neurosci.7(8)(2004)847–854.
[68]L.M.Williams,C.Debattista,A.M.Duchemin,A.F.Schatzberg,C.B.Nemeroff, Childhoodtraumapredictsantidepressantresponseinadultswithmajor depression:datafromtherandomizedinternationalstudytopredict optimizedtreatmentfordepression,Transl.Psychiatry.6(2016)e799. [69]H.Xu,Z.Chen,J.He,S.Haimanot,X.Li,L.Dyck,X.M.Li,Synergeticeffectsof
quetiapineandvenlafaxineinpreventingthechronicrestraintstress-induced decreaseincellproliferationandBDNFexpressioninrathippocampus, Hippocampus16(6)(2006)551–559.
[70]Y.Zhang,Y.Wang,L.Wang,M.Bai,X.Zhang,X.Zhu,DopaminereceptorD2 andassociatedmicroRNAsareinvolvedinstresssusceptibilityandresistance toescitalopramtreatment,Int.J.Neuropsychopharmacol.18(8)(2015)(pii, pyv025).