REVISTA
BRASILEIRA
DE
REUMATOLOGIA
www . r e u m a t o l o g i a . c o m . b r
Review
article
The
analgesic
effect
of
intravenous
lidocaine
in
the
treatment
of
chronic
pain:
a
literature
review
Maiara
Ferreira
de
Souza
∗,
Durval
Campos
Kraychete
MedicineSchool,UniversidadeFederaldaBahia,Salvador,BA,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received16October2013 Accepted28January2014 Availableonline21August2014
Keywords:
Lidocaine
Intravenouslidocaine Chronicpain
a
b
s
t
r
a
c
t
Background:Painisapublichealthproblem,greatlyimpairingqualityoflife.Almost80%of patientswithchronicpainreportedthattheirpaininterfereswithactivitiesofdailyliving, andtwothirdsreportedthatthepaincausesnegativeimpactontheirpersonalrelationships. Thephysicalandfunctionaldisability,whethertemporaryorpermanent,compromisesthe professionalactivityandcausesworkabsenteeism,increasingcostsofhealthsystems.
Objectives:Theaimofthisreviewistoanalyze,basedontheliterature,theanalgesiceffect oflidocaineadministeredintravenouslyforthetreatmentofchronicpainandtoevaluate thereductionofpainintensityinpatientswithchronicpain,focusingonmusculoskeletal andneuropathicetiology.
Methodology:Themethodusedwasareviewoftheliterature,consistinginsearchingthe scientificliteratureontheefficacyofintravenouslidocaineinfusioninthetreatmentof patientswithchronicpain.
Content:Ofthe19studiesreviewed,12hadresultsthatconfirmtheanalgesiceffectof intra-venouslidocaineinpatientswithchronicpain.Mostauthorsuseddosesof5mg/kginfused for30minutesormore,producingsignificantanalgesiawithvariableduration(minutesto weeks).
Conclusions:Basedontheliteraturereview,itisnotpossibletouniformlyspecifythemost effectiveandsafedoseoflidocaineadministeredintravenouslyforthetreatmentof neuro-pathicormusculoskeletalpain.Asforeffectiveness,theintravenousinfusionoflidocaineas analternativeforthetreatmentofchronicpainofvariousetiologiesseemsverypromising, butfurtherstudiesneedtobeconducted.
©2014ElsevierEditoraLtda.Allrightsreserved.
DOIoforiginalarticle:http://dx.doi.org/10.1016/j.rbr.2014.01.010.
∗ Correspondingauthor.
E-mail:maifsmr@hotmail.com(M.F.deSouza).
http://dx.doi.org/10.1016/j.rbre.2014.01.002
A
ac¸ão
analgésica
da
lidocaína
intravenosa
no
tratamento
da
dor
crônica:
uma
revisão
de
literatura
Palavras-chave:
Lidocaína
Lidocaínaintravenosa Dorcrônica
r
e
s
u
m
o
Justificativa: Adoréumproblemadesaúdepública,comprometendosobremaneiraa qual-idadedevida.Quase80%dospacientescomdorcrônicarelataramqueadorinterfereem suasatividadesdavidadiária,edoisterc¸osafirmaramqueadorprovocaimpactonegativo nasrelac¸õespessoais.Aincapacidadefísicaefuncional,sejatemporáriaoupermanente, comprometeaatividadeprofissionalecausaabsenteísmoaotrabalho,elevandooscustos dossistemasdesaúde.
Objetivos: Oobjetivodestarevisãoéanalisar,combasenaliteratura,oefeitoanalgésicoda lidocaínaadministradaporviaintravenosanotratamentodadorcrônicaeavaliarareduc¸ão daintensidadedadorempacientescomdorcrônica,focandoaetiologiamusculoesquelética eneuropática.
Metodologia:Ométodoadotadofoioderevisãodaliteratura,consistindonabuscadeartigos científicossobreaeficáciadainfusãointravenosadelidocaínanotratamentodepacientes comdorcrônica.
Conteúdo: Dos19estudosrevisados,12apresentaramresultadosqueconfirmama ac¸ão analgésicadalidocaínaporviaintravenosaempacientescomdorcrônica.Amaioriados autoresutilizoudosesde5mg/kginfundidaspor30minutosoumais,produzindoanalgesia significativacomdurac¸ãovariável(deminutosasemanas).
Conclusões: Combasenarevisãodaliteratura,nãoépossíveluniformementeespecificara dosemaiseficazeseguradelidocaína administradaporviaintravenosanotratamento da dorneuropáticaoumusculoesquelética.Quantoà eficácia,a infusãointravenosada lidocaínacomoalternativaparaotratamentodadorcrônicadeetiologiasdiversasparece bastantepromissora,emboraestudosadicionaisnecessitemserrealizados.
©2014ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
Chronicpainaffectsapproximately 7%to40% ofthe world population.1 InBrazil,astudy conductedbyWHO, in1998,
showedaprevalenceof31%(datafromRiodeJaneiro);2onthe
otherhand,inSalvador,Bahia,itisestimatedthat41.4%ofthe populationsuffersfromchronicpain.1
Painisapublichealthproblem,3–5 greatly impairingthe
qualityoflife.Severalfactors,suchasdepression,sleep dis-turbances,difficultyconcentrating,hopelessness,feelingsof deathandothers,areassociatedwiththissymptom.Theloss ofqualityoflifeisafact,asthepainbeginstoguideandlimit thebehaviorand activitiesofthesubject, generatingsocial withdrawal,changesinsexuality,changesinfamily dynam-icsand economic imbalance.3 Nearly80% ofpatients with
chronicpainreportedthattheirpaininterferesinactivitiesof dailyliving,andtwothirdssaidthatthepaincausesnegative impact onpersonal relationships.6 Physical and functional
disability,whethertemporaryorpermanent,jeopardizesthe professionalactivity7andcausesworkabsenteeism,
increas-ing the costs ofhealth systems.5 Inthe United States, for
example,itisestimatedthatover50millionworkingdaysare losteachyear.8Thus,chronicpainisanimportantmedical
andsocialproblem,andopioidabuseisofgreatconcern,due fortheproblemsstemmingfromtheirmultiplesideeffects, includingaddiction.
Often the complexity ofthe pathophysiological mecha-nismsthat explaintheinitiation and maintenanceofpain
makesdifficultthe assessment,diagnosisandtreatmentof painsyndromesthatmaypresentinflammatory,neuropathic or mixed components. Thus, there are several classes of drugs used in the treatment of chronic pain patients, in an attempt to reduce the intensity of pain and improve their qualityoflife.Amongthelocalanesthetics,lidocaine [2-(diethylamino)-N-(2,6dimethylphenyl)acetamide],aweak basewithantiarrhythmicproperties,9hasbeenusedby
vari-ousroutes,includingintravenous.
Lidocaine alters the transmembrane conductance of cations,especiallysodium,potassiumandcalcium,bothin neurons and myocytes.10 Voltage-dependent sodium
chan-nels constitute its classical targets, and the affinity of the drugforthechannelisgreaterwhenitisopened(activated or inactive).9 Thus, the degreeofblockingvaries according
to the neuronal stimulation frequency.6,11 However, other
mechanismsare alsoinvolvedintheanalgesia providedby lidocaine9,12as,forinstance,theinteraction,whetherdirector
indirect,withdifferentreceptorsandpathwaysofnociceptive transmission,likethemuscarinicagonists,glicineinhibitors, releaseofendogenousopioidsandofadenosinetriphosphate, andthereducedproductionofexcitatoryaminoacids, neu-rokininsandthromboxaneA2.12
Althoughlidocaineistypicallyadministeredthroughlocal injections,itisalsousedintravenouslyforvariouspurposes, suchas regionalanesthesia,asananti-dysrhythmic agent, inthereliefofperipheralandcentralneuropathicpain,13,14
fibromyalgiatreatment15andasanadjuvantinpostoperative
For thesereasons,lidocaineisused inthe treatmentof patients with fibromyalgia, arthrosis, cancer, postherpetic neuralgia,neuropathicpain,andofpatientswithseveralother disorderscausingchronicpain.Althoughcontrolofchronic painisdifficult,manyeffortshavebeendirectedtowardsthe developmentofincreasinglyeffectivetreatments,especially pharmacologicalones, inreducing the intensity ofpain in thesepatientsandinprovidinglongerperiodsofanalgesia.
Theaimofthisreviewistoanalyze,basedontheliterature, theanalgesiceffectoflidocaineadministeredintravenously forthetreatmentofchronicpain.Themethodadoptedwas to review the literature, consisting in the search for sci-entificarticles, inthis case, on the efficacy of intravenous lidocaineinfusioninthetreatmentofpatientswithchronic pain.Tothisend,bibliographicdatabases,suchasCENTRAL, MEDLINE/PubMed,LILACSandSciELOweresearched.Inthe searchingstrategy,weusedthefollowingkeywords
“lidocaine”,“intravenousandchronicpain”,oralso “lido-caine,infusion and chronic pain”. Another strategy was a manual search in reference lists of those identified and selected articles by electronic search. We used as criteria forselecting thestudies:publicationsuntilDecember 2012, withdesignsoftherandomizedclinicaltrialonhumanstype, whichhavebeenpublishedinPortugueseorEnglish, exclud-ingotherlanguages.Articlesofrelevancetolidocaine,aswell as on disorders that present with chronic pain, were also included. Studies whichevaluated the efficacy oflidocaine inrelievingonlyevokedpain inanimals,or thatevaluated theefficacy oflidocainewithanother drug,were discarded (exclusioncriteria).
Intravenous
lidocaine
in
the
treatment
of
chronic
pain
conditions
Fibromyalgia
Severalstudiessuggestthatintravenouslidocainecanreduce the pain associated with fibromyalgia, although this is a condition refractoryto other analgesic drugs. In a double-blind placebo-controlled trial conducted in the 90s, there wasadecrease inpain scoresduringand afterinfusionof lidocaine.15 This finding is confirmed by subsequent
stud-ies,inthatthedurationofreliefexceededboththeinfusion time as the half-life of the drug.15 In an uncontrolled
trial, five consecutive infusions of intravenous lidocaine with increasing doses of 2mg/kg to 5mg/kg resulted in a reduction in pain scores that was significant after the fifth day and persisted after 30 days.15 In another study,
the reductionin pain scoreswas also maintained even30 days after the last infusion of lidocaine.15 In a
double-blindcrossovertrialinvolving75patientswithfibromyalgia, a lasting analgesic effect of the drug was confirmed.15
On the other hand, other studies did not achieve posi-tive results after the intravenous lidocaine infusion: after fourinfusionsofthedrugatweeklyintervals,theobserved reductionin pain scores was not statisticallysignificant.16
In another study, which combined 3mg/kg of intravenous lidocaineadministered weekly with 25mg ofamitriptyline for 4 weeks, there was no change in pain intensity in
patientswithfibromyalgia,whencomparedwith amitripty-linemonotherapy.17
Myofascialpainsyndromes
Most studies on intravenous lidocaine were performed in patientswithneuropathicpain,whilemyofascialpain carri-ersaregenerallytestedwithanintramuscularinfusionofthe drug.Inastudycarriedoutin2005,whichinvolvedinfusions oflidocaine,ketamineandmorphine,among30patientswith chronicpainassociatedwithwhiplashlesion(cervical deceler-ationinjury),11of18respondersexperiencedpainreduction afterlidocaineinfusionatadoseof5mg/kg.18
Neuropathicpain
Peripheralneuropathies
Wallaceetal.achievedsignificantanalgesiainplasma concen-trationsoflidocainebetween1.5-2.5mg/mL.Inanotherstudy, areductioninVASscoreforcontinuouspainoccurredwhen lidocaine was infused at high (5mg/kg) and low (1mg/kg) dosesforperiodsoftwohoursormore.However,therewere nodifferencesincomparisonwithplacebo.19
InthetrialbyFerranteetal.,theinfusionofintravenous lidocainedemandedaboutfiveminutestoachievemaximal analgesia,andthe analgesiaincreasedabruptly froma cer-tainplasmaconcentration(0.62g/mL).20Inotherstudies,the
effectbegan30minutesafterstartingtheinfusion,reaching thepeakeffectwithin60to120minutes.21,22Inaddition,there
wassignificantanalgesiacomparedtoplaceboformorethan 6hoursafterinfusion,andasubsetofpatientsreportedrelief forastilllongerperiod,greaterthan7days.21
Reductioninpainintensitywasalsoreportedinpatients with postherpeticneuralgia.The druginfusion atdosesof 5mg/kgoreven1mg/kgforvaryingperiodscauseda signifi-cantreductioninpainscores,6without,however,establishing
acorrelationbetweenreliefandplasmaconcentrationsofthe drug.
Inpatientswithpainfuldiabeticneuropathy,theduration oftheindividualeffectvariedbetween3and28daysatdoses of5or7.5mg/kginfusedoveraperiodupto4hours,23,24with
atrendtoagreaterresponsetolidocaine7.5mg/kgcompared to5mg/kg,butthisdifferencedidnotreachsignificance.The qualitativenatureofpainwassignificantlymodifiedbythe drug,comparedwithplacebo.24
Althoughseveralstudiesconfirmtheefficacyoflidocaine oncontinuousspontaneouspaincausedbyperipheralnerve injury,otherexperimentshavefailedtoaccordinglyreaffirm the beneficial effectofthedrug. Atrialconductedin2006, for example, demonstrated a significant reduction in pain evokedbyrepetitivestimuli,butnotinspontaneouspain,after theinfusionof5.0mg/kgfor30minutes.25Thesamenegative
resultwasobservedbyGormsenetal.26Inastudycomparing
increasingdosesofthedrug(1,3and5mg/kg)alsofailedto showanalgesiceffectoflidocaineatlow doses,provingits effectivenessonlyatthehighestconcentration.28
Lidocainedosesbetween1.5and5.0mg/kgprovedeffective tosuppress ectopic discharge without blocking nerve con-duction,correspondingtoplasmalevelsof0.62to5.0mg/mL. Furthermore,theeffectofsystemiclidocaineonneuropathic painmaybedifferent,dependingonthesourcecausingthe pain.Thus,itseffectivenessmaybegreaterinpatientswith peripheralnerveinjurythaninthosewithpainduetodamage tothecentralnervoussystemorofunknownetiology.29
Incasesofcomplexregionalpainsyndrome(CRPS),theuse ofintravenouslidocainewasreportedtobebeneficialinsome patientswhenstudiedretrospectively,bothinadultsandin children.30,31 Controlled studies,however, failedto confirm
thesedata.Inanexperimentthatusedacomputer-controlled infusionpump,therewasasignificantdecreaseinthescores forspontaneouspain onlywhenplasma lidocainereached thehighestlevel(3mg/mL);plasmalevelsof1and2mg/mL didnotcauseasignificanteffectonspontaneouspain,which canbeexplainedbythefactthatthisstudyinvolvedintense neurosensorytesting,thatmayhavemaskedtheeffectofthe drug.32
Centralpain
Systemically, lidocainecaninduce significant and selective reductionofvariouscomponentsofpaincausedbylesionsof thecentralnervoussystem,includingspontaneouspain,and twoof16patientshadreliefforover45minutesafteran infu-sionof5mg/kgoftheactivedrug,comparedwithplacebo.33
Inanotherrandomized,placebo-controlledtrial,thedrug alle-viatedneuropathic pain eitherbelowor atthe levelofthe spinallesion.34Theseresultsareoppositetothosefoundina
studycarriedoutin2004inwhichonlyketamine,andnot lido-caine,wasshowntoreducespontaneouscontinuouspainin patientswithpainsecondarytoinjurytothecentralnervous system.6
The three aforementioned placebo-controlled studies evaluatedtheefficacyofintravenouslidocaineincentral neu-ropathic pain. Two of them,using high doses of the drug (5mg/kgIV)andincludingatotalof32patientswithtraumatic spinalinjuryorsyringomyelia,testedpositiveforspontaneous pain(bothatthelevelofthespinalinjuryandbelowit),while thestudyusingasmallerdose(1.5mg/kgIV)hadanegative outcome.
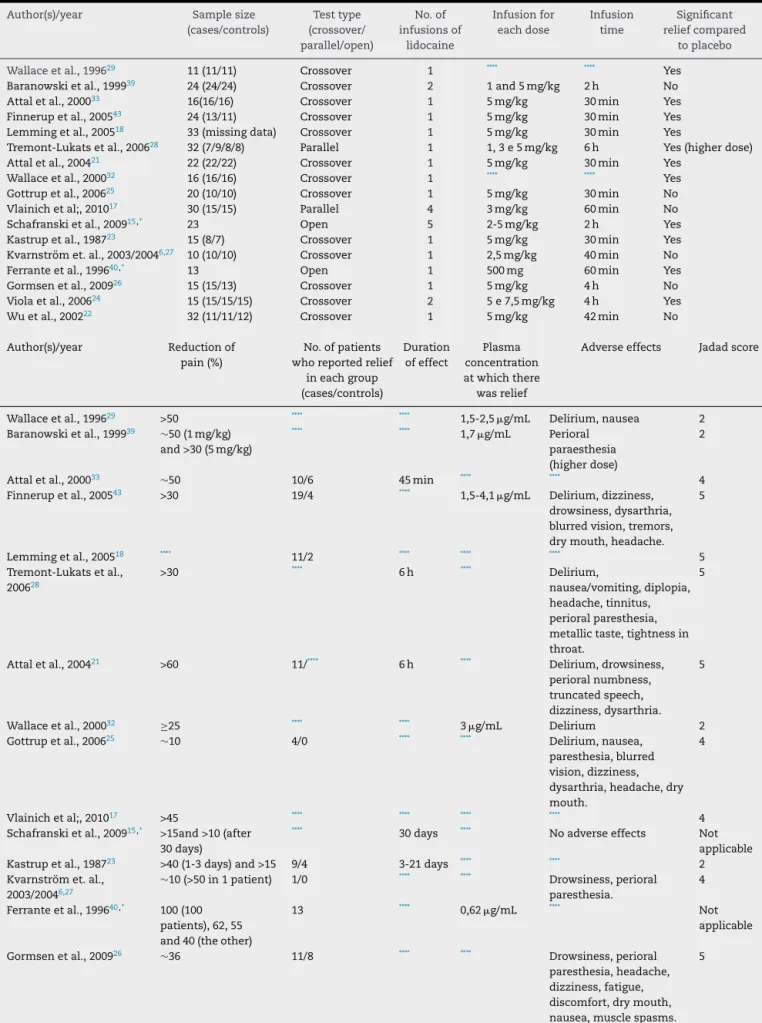
TheanalysisofTable1demonstratesthe lackof unifor-mityofthestudiesinrelationtothedatapresented:ofthe 18articlesanalyzed,notallreportedthedoseoflidocaineor theinfusiontime; justsomeofthemmadesome measure-mentsofplasmaconcentrationofthedrugduringinfusion, andofthosewhichdidit,fewreportedtheminimalplasma levelinwhichtherewaseffect.Althoughtheoccurrenceof adverseeffectshavebeenreportedinmoststudies,someof themdidnotspecifywhichreactionswereobserved.Thevast majorityofstudieswaslimitedtosearchtheanalgesiceffect oflidocaineshortlyaftertheinfusionor,atmost,afewhours later,refrainingfrom investigating whether therewas pro-longedrelief, acriticalfactorinpatientswithchronicpain. Smallsamplesizewasacommonlimitationofmanyofthe studiesreviewed,and,atleastinoneofthem,adifference
betweengroups wasgenerated(in termsofcharacteristics: mean ageand proportionofmenand women),despitethe randomallocation.28
Twoofthestudiesincludedinthereviewdidnotinvolvea controlgroup;however,themaintenanceofapositiveeffect forover30daysafterthelasttherapeuticintervention,asit occurred inanexperimentconductedbySchafranskiet al., makes unlikely aplacebo effect.15 Conversely, acontrolled
studyfailedtodemonstratetheanalgesicefficacyoflidocaine infibromyalgiapatients,becausethereductioninpainscores didnotreachstatisticalsignificance,althoughmore impor-tantreliefshavebeenobtainedinthegroupreceivingthedrug, comparedtoplacebo.16
According to most studies reviewed, the lasting effect would be obtained after repeated infusions of the drug, althoughthepossibilityofreliefafterasingleinfusioncould notberuledout.Ofthesixexperimentsrelatedto fibromyal-gia, only two have not confirmed the analgesic power of intravenouslidocaine,havingstrongevidenceofitsefficacy forpaincontrolinthesepatients.
Withregardtomusculoskeletalpain,thepositiveeffects ofdrugstendtobepoorandgenerallydonotimprove sig-nificantly thepatients’ disabilitynorquality oflife,despite thepainreliefproduced.20Thus,agreaternumberofstudies
toevaluatetheeffectofsystemiclidocaineinthesepatients todetermineits actualusefulness andeffectivenessisstill needed.
Boas et al. reported areductionofpain by deafferenta-tionandofcentralpainwiththeuseofintravenouslidocaine, indicatingapossibletherapeuticvalueofthisroutefor lido-caine in the management of intractable neuropathic pain syndromes.35Sincethen,severalstudieshavedemonstrated
thatsystemiclidocainemaybeeffectiveintreatingvarious disordersthatpresentwithneuropathicpain,atdosesthatdo notproducefrankanesthesiaandinplasmaconcentrations belowthoserequiredtoblockaxonalconduction.23,35–38The
analgesiceffectsofthedrugmaybeobservedinpatientswith diabetic neuropathy, postherpetic neuralgia and in several neuropathic disorders,suchascomplex regionalsyndrome typeIandIIandpost-strokepain.28
Incasesofperipheralneuropathicpain,plasma concen-trationsoflidocainebetween1.5-2.5mg/mLappearsufficient topromoteanalgesia.29 However,thereisevidencethatthe
duration ofexposurecanbemoreimportantthan thedose itself,leadingtoareductioninVASscoreforcontinuouspain afterinfusionofhigh(5mg/kg)andlow(1mg/kg)dosesof lido-caineforperiodsoftwohoursormore,althoughtherewasno differencefromplacebo.39Thefactthatlidocainehasreached
maximumanalgesiawithinfiveminutesafterthestartof infu-sion, withanabruptincreaseinits analgesiceffectaftera certainplasmaconcentration(0.62g/mL),suggeststhat its effect byvenousroute doesnotaccompanies adose-effect curve,suddenlyblockingthepainfulstimulus.40
Althoughthehalf-lifeofthedrugisonly120minutes,the analgesiaprovidedbysystemiclidocaineisprolonged,maybe extendingoverdaysorevenweeks.21Thismaybeduetothe
Table1–Revisedarticles’data.
Author(s)/year Samplesize (cases/controls)
Testtype (crossover/ parallel/open)
No.of infusionsof
lidocaine
Infusionfor eachdose
Infusion time
Significant reliefcompared
toplacebo
Wallaceetal.,199629 11(11/11) Crossover 1 **** **** Yes
Baranowskietal.,199939 24(24/24) Crossover 2 1and5mg/kg 2h No
Attaletal.,200033 16(16/16) Crossover 1 5mg/kg 30min Yes
Finnerupetal.,200543 24(13/11) Crossover 1 5mg/kg 30min Yes
Lemmingetal.,200518 33(missingdata) Crossover 1 5mg/kg 30min Yes
Tremont-Lukatsetal.,200628 32(7/9/8/8) Parallel 1 1,3e5mg/kg 6h Yes(higherdose)
Attaletal.,200421 22(22/22) Crossover 1 5mg/kg 30min Yes
Wallaceetal.,200032 16(16/16) Crossover 1 **** **** Yes
Gottrupetal.,200625 20(10/10) Crossover 1 5mg/kg 30min No
Vlainichetal;,201017 30(15/15) Parallel 4 3mg/kg 60min No
Schafranskietal.,200915,* 23 Open 5 2-5mg/kg 2h Yes
Kastrupetal.,198723 15(8/7) Crossover 1 5mg/kg 30min Yes
Kvarnströmet.al.,2003/20046,27 10(10/10) Crossover 1 2,5mg/kg 40min No
Ferranteetal.,199640,* 13 Open 1 500mg 60min Yes
Gormsenetal.,200926 15(15/13) Crossover 1 5mg/kg 4h No
Violaetal.,200624 15(15/15/15) Crossover 2 5e7,5mg/kg 4h Yes
Wuetal.,200222 32(11/11/12) Crossover 1 5mg/kg 42min No
Author(s)/year Reductionof pain(%)
No.ofpatients whoreportedrelief
ineachgroup (cases/controls)
Duration ofeffect
Plasma concentration atwhichthere wasrelief
Adverseeffects Jadadscore
Wallaceetal.,199629 >50 **** **** 1,5-2,5
g/mL Delirium,nausea 2
Baranowskietal.,199939 ∼50(1mg/kg)
and>30(5mg/kg)
**** **** 1,7
g/mL Perioral
paraesthesia (higherdose)
2
Attaletal.,200033 ∼50 10/6 45min **** **** 4
Finnerupetal.,200543 >30 19/4 **** 1,5-4,1g/mL Delirium,dizziness,
drowsiness,dysarthria, blurredvision,tremors, drymouth,headache.
5
Lemmingetal.,200518 **** 11/2 **** **** **** 5
Tremont-Lukatsetal., 200628
>30 **** 6h **** Delirium,
nausea/vomiting,diplopia, headache,tinnitus, perioralparesthesia, metallictaste,tightnessin throat.
5
Attaletal.,200421 >60 11/**** 6h **** Delirium,drowsiness,
perioralnumbness, truncatedspeech, dizziness,dysarthria.
5
Wallaceetal.,200032 ≥25 **** **** 3g/mL Delirium 2
Gottrupetal.,200625 ∼10 4/0 **** **** Delirium,nausea,
paresthesia,blurred vision,dizziness, dysarthria,headache,dry mouth.
4
Vlainichetal;,201017 >45 **** **** **** **** 4
Schafranskietal.,200915,* >15and>10(after
30days)
**** 30days **** Noadverseeffects Not
applicable
Kastrupetal.,198723 >40(1-3days)and>15 9/4 3-21days **** **** 2
Kvarnströmet.al., 2003/20046,27
∼10(>50in1patient) 1/0 **** **** Drowsiness,perioral paresthesia.
4
Ferranteetal.,199640,* 100(100
patients),62,55 and40(theother)
13 **** 0,62
g/mL **** Not
applicable
Gormsenetal.,200926 ∼36 11/8 **** **** Drowsiness,perioral
paresthesia,headache, dizziness,fatigue, discomfort,drymouth, nausea,musclespasms.
Table1(Continued).
Author(s)/year Reductionof pain(%)
No.ofpatientswho reportedreliefin
eachgroup (cases/controls)
Duration ofeffect
Plasma concentrationat whichtherewas
relief
Adverseeffects Jadadscore
Violaetal.,200624 **** **** 28days **** Delirium(higherdose) 3
Wuetal.,200222 ∼30 **** **** **** **** 4
∗ Uncontrolledstudies.
∗∗∗∗Missingdata.
totheseverepainproducedbysuchlesions.14,41Thecentral
hyperalgesia,ontheotherhand,isrelatedtosodium chan-nelslocatedattheendsofmechanoreceptors,inthespinal cord,andindorsalrootganglia.12Theblockingofsuchsodium
channelscauseinhibitionofthespontaneousandevoked neu-ronalactivity and reducestheneuronal hyperactivity, with greater pain relief and for a longer time, compared with thatrelatedtothedrug’spharmacokineticpatterns.Lidocaine alsopromotesthereductionofallodyniaandhyperalgesia.A decreaseinspontaneouspain,dysesthesia,mechanical hyper-algesiaandmechanicalallodyniaoccurs.9
Inadditiontoits wellestablishedanesthetic and antiar-rhythmicactions,intravenouslidocainealsohassignificant anti-inflammatory properties, by inhibiting the release of cytokines and interfering with the action of inflammatory cells,suchasmacrophages,monocytesand polymorphonu-clearcells.9,12,42 Thislatterformofactionisbeingcurrently
testedinseveralstudiesandseemsverypromising.
Inpatientswithpainfuldiabeticneuropathy,lidocainecan reduce spontaneousactivity in small myelinated fibers by stabilizingthedamagednervemembrane;thishasbeen pro-posedasthecauseofneuropathicpain.23 Furthermore,the
effectofsystemiclidocaineonneuropathicpainmaybe dif-ferent,dependingonthesourceofpaingeneration.Thus,its effectivenessmaybegreaterinpatientswithperipheralnerve injurythaninthosewithpainduetodamagetotheCNSorof unknownetiology.29
InthestudybyViolaetal.(2006),therewasatrendfora greaterresponsetolidocaineindosesof7.5mg/kgcompared with5mg/kg,butwithoutstatisticalsignificance.24Thisresult
mayindicatethatthedosesexaminedwerenearthetopofthe dose-responsecurveforthistherapy.Althoughseveralstudies confirmtheefficacyoflidocaineincasesofcontinuous spon-taneouspaincausedbyperipheralnerveinjury,othertrials failedtoaccordinglyreaffirmthebeneficialeffectofthedrug. Theliteraturesuggeststhat,infact,intravenouslidocaine iseffectiveinthemanagement ofchronicpain.Tenofthe 14studiesinpatientswithperipheralneuropathiesobtained favorableoutcomeswiththesystemicuseofthedruginthe treatmentofdiseaseswithneuropathicpain,including pos-therpeticneuralgiaandpainfuldiabeticneuropathy.Thefour remainingstudiesfailedtodemonstratetheanalgesiceffectof lidocaine,mostlikelyduetomethodologicalissues,for exam-ple,insufficientnumbersofpatients,26oreventheproduction
ofevoked pain shortlyafter the startofinfusion, masking possiblepositiveresults.25
InpatientswithCNSinjury,lidocainerelievedneuropathic paineitherbeloworatthelevelofthespinallesion,suggesting
an effect on the generating mechanisms of central pain, althoughit isnotpossibletodeterminewhether theeffect occursatthespinalorbrainlevel.43
The lidocainedose used byseveral investigators varies, most often from 1 to 5mg/kg, administered over a period of 30 to 60minutes. In several randomized clinical trials, researchers measured the plasma levels of the drug in an attempt to find a relationship between concentration and response.32,40 Although some authors state that the
minimumplasmaconcentrationabletoproducesignificant analgesia is1.5mL/L(achievedwith2-5mg/kginfused over 30-60minutes),22 noinformationonthespecifictherapeutic
concentrationisavailable.
Conclusion
Theintravenousinfusionoflidocaineasanalternativeforthe treatmentofchronicpainofvariousetiologiesseemstobe verypromising,butfurtherstudiesneedtobeperformed.
Regarding theneuropathic ormusculoskeletalpain, itis notpossibletouniformlyspecifythemosteffectiveandsafe doseofintravenouslidocainetobeusedinitstreatment.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.SáK,BaptistaAF,MatosMA,LessaI.Prevalênciadedor crônicaefatoresassociadosnapopulac¸ãodeSalvador,Bahia. RevSaúdePública.2009;43:622–30.
2.GurejeO,VonKorff,SimonG,GalerR.Persistentpainand well-being:aWorldHealthOrganizationstudyinprimary care.JAMA.1998;280:147–51.
3.DellarozaMSG,PimentaCAM,MatsuoT.Prevalênciae caracterizac¸ãodadorcrônicaemidososnão
institucionalizados.CadSaúdePública[serialnainternet]. 2007;23:1151–60,citado30junho2011.
4.KrelingMCGD,CruzDALM,PimentaCAM.Prevalenciade dolorcrônicoenadultos.RevBrasEnferm.2006;59:509–13.
5.PicavetHS,SchoutenJS.Musculoskeletalpaininthe Netherlands:prevalences,consequencesandriskgroups,the DMC3-study.Pain.2003;102:167–78.
6.KvarnströmA,KarlstenR,QuidingH,GordhT.Theanalgesic effectofintravenousketamineandlidocaineonpainafter spinalcordinjury.ActaAnaesthesiolScand.2004;48:498–506.
PimentaCAM(orgs.).Dor:conceitosgerais.SãoPaulo:Limay, 1995.
8. StewartWF,RicciJA,CheeE,MorgansteinD,LiptonR.Lost productivetimeandcostduetocommonpainconditionsin theUSworkforce.JAMA.2003;290:2443.
9. OliveiraCMB,IssyAM,SakataRK.Lidocaínaporviavenosa intraoperatória.RevBrasAnestesiol.2010;60:325–32.
10.DiasRR,DalvaM,SantosB,KwasnickaKL,SarraffAP,DiasAR. Influênciadalidocaínanaprotec¸ãomiocárdicacomsoluc¸ão cardioplégicasanguínea.RevBrasCirCardiovasc,SãoJosédo RioPreto.2002;17(3).
11.HeavnerJE.Localanesthetics.CurrOpinAnaesthesiol. 2007;20:336–42.
12.LaurettiGR.Mecanismosenvolvidosnaanalgesiada lidocaínaporviavenosa.RevBrasAnestesiol.2008;58:280–6.
13.CahanaA,CarotaA,MontadonML,AnnoniJM.Thelong-term effectofrepeatedintravenouslidocaineoncentralpainand possiblecorrelationinpositronemissiontomography measurements.AnesthAnalg.2004;98:1581–4.
14.NikolajsenL,BlackJA,KronerK,JensenTS,WaxmanSG. Neuromaremovalforneuropathicpain:efficacyand predictivevalueoflidocaineinfusion.ClinJPain. 2010;26:788–93.
15.SchafranskiMD,MalucelliT,MachadoF,TakeshiH,KaiberF, SchmidtC,etal.Intravenouslidocaineforfibromyalgia syndrome:anopentrial.ClinRheumatol.2009;28:853–5.Epub 5mar2009.
16.PosnerIA.Treatmentoffibromyalgiasyndromewith intravenouslidocaine:aprospective,randomizedpilotstudy. 1994.
17.VlainichR,IssyAM,GerolaLR,SakataRK.Effectof
intravenouslidocaineonmanifestationsoffibromyalgia.Pain Pract.2010;10:301–5.
18.LemmingD,SörensenJ,Graven-NielsenT,Arendt-NielsenL, GerdleB.Theresponsestopharmacologicalchallengesand experimentalpaininpatientswithchronic
whiplash-associatedpain.ClinJPain.2005;21:412–21.
19.BackonjaM.Neuromodulatingdrugsforthesymptomatic treatmentofneuropathicpain.CurrPainHeadacheRep. 2004;8:212–6.
20.CuratoloM,BogdukN.Pharmacologicpaintreatmentof musculoskeletaldisorders:currentperspectivesandfuture prospects.ClinJPain.2001;17:25–32.
21.AttalN,RouaudJ,BrasseurL,ChauvinM,BouhassiraD. Systemiclidocaineinpainduetoperipheralnerveinjuryand predictorsofresponse.Neurology.2004;62:218–25.
22.WuCL,TellaP,StaatsPS,VaslavR,KazimDA,WesselmannU, etal.Analgesiceffectsofintravenouslidocaineandmorphine onpostamputationpain:arandomizeddoubleblind,active placebo-controlled,crossovertrial.Anesthesiology. 2002;96:841–8.
23.KastrupJ,PetersenP,DejgårdA,AngeloHR,HilstedJ. Intravenouslidocaineinfusion:anewtreatmentofchronic painfuldiabeticneuropathy?1987.
24.ViolaV,NewnhamHH,SimpsonRW.Treatmentofintractable painfuldiabeticneuropathywithintravenouslignocaine. 2006.
25.GottrupH,BachFW,JuhlG,JensenTS.Differentialeffectof ketamineandlidocaineonspontaneousandmechanical evokedpaininpatientswithnerveinjurypain.
Anesthesiology.2006;104:527–36.
26.GormsenL,FinnerupNB,AlmqvistPM,JensenTS.The efficacyoftheAMPAreceptorantagonistNS1209and lidocaineinnerveinjurypain:arandomized,double-blind, placebo-controlled,three-waycrossoverstudy.AnesthAnalg. 2009;108:1311–9.
27.KvarnströmA,KarlstenR,QuidingH,EmanuelssonBM, GordhT.Theeffectivenessofintravenousketamineand lidocaineonperipheralneuropathicpain.ActaAnaesthesiol Scand.2003;47:868–77.
28.Tremont-LukatsIW,HutsonPR,BackonjaMM.Arandomized, double-masked,placebo-controlledpilottrialofextendedIV lidocaineinfusionforreliefofongoingneuropathicpain.Clin JPain.2006;22:266–71.
29.WallaceMS,DyckJB,RossiSS,YakshTL.Computer-controlled lidocaineinfusionfortheevaluationofneuropathicpain afterperipheralnerveinjury.Pain.1996;66:69–77.
30.SchwartzmanRJ,PatelM,GrothusenJR,AlexanderGM. Efficacyof5-daycontinuouslidocaineinfusionforthe treatmentofrefractorycomplexregionalpainsyndrome.Pain Med.2009;10:401–12.
31.SureshS,WheelerM,PatelA.Caseseries:IVregional anesthesiawithketorolacandlidocaine:isiteffectiveforthe managementofcomplexregionalpainsyndrome1in childrenandadolescents?AnesthAnalg.2003;96:694–5.
32.WallaceMS,RidgewayBM,LeungAY,GerayliA,YakshTL. Concentration-effectrelationshipofintravenouslidocaineon theallodyniaofcomplexregionalpainsyndrometypesIand II.Anesthesiology.2000;92:75–83.
33.AttalN,GaudéV,BrasseurL,DupuyM,GuirimandF,ParkerF, etal.Intravenouslidocaineincentralpain:adouble-blind, placebo-controlled,psychophysicalstudy.Neurology. 2000;54:564–74.
34.FinePG.Theuseofopioidsinpainmanagement.CitaP,Marx S,PenlesL.Health-relatedqualityoflife(HRQoL)among patientsexperiencingacuteandchronic
moderate-to-moderately-severepain:resultsfromasurveyof 606painpatientsintheUnitedStates.Paperpresentedat: AmericanPainSocietyAnnualMeeting;May8-10,2008; Tampa,Florida.Availablefrom:
http://www.accesscme.org/PDFs/PN808.pdf
35.BoasRA,CovinoBG,ShahnarianA.AnalgesicresponsestoI.V. lidocaine.BrJAnesth.1982;54:501–5.
36.BachFW,JensenTS,KastrupJ,StigsbyB,DejgårdA.Theeffect ofintravenouslidocaineonnociceptiveprocessingindiabetic neuropathy.Pain.1990;40:29–34.
37.LeeE,DonovanK.Reactivationofphantomlimbpainafter combinedinterscalenebrachialplexusblockandgeneral anesthesia:successfultreatmentwithintravenouslidocaine. Anesthesiology.1995;82:295–8.
38.TanelianDL,BroseWG.Neuropathicpaincanberelievedby drugsthatareuse-dependentsodiumchannelblockers: lidocaine,carbamazepine,andmexiletine.Anesthesiology. 1991;74:949–51.
39.BaranowskiAP,DeCourceyJ,BonelloE.Atrialofintravenous lidocaineonthepainandallodyniaofpostherpeticneuralgia. JPainSymptomManage.1999;17:429–33.
40.FerranteFM,PaggioliJ,CherukuriS,ArthurGR.Theanalgesic responsetointravenouslidocaineinthetreatmentof neuropathicpain.AnesthAnalg.1996;82:91–7.
41.AmirR,ArgoffCE,BennettGJ,CumminsTR,DurieuxME, GernerP,etal.Theroleofsodiumchannelsinchronic inflammatoryandneuropathicpain.JPain.2006;7: S1–29.
42.KraycheteDC,GuimarãesAC,CarvalhoMG,CarvalhoEM. Papeldalidocaínaporviavenosanotratamentodadorna esclerodermia:relatodecaso.RevBrasAnestesiol. 2003.