Investigation
of Rabies Virus Strains in
Mexico with a Panel of Monoclonal
Antibodies
Used to Classify Lyssavivusl
ELIZABETH LOZA-RUBIO,~ RACL,VARGAS,~ ELISEO HERNANDEZ,~
DIODORO BATALLA,~ & ALVARO AGUILAR-SETIBN~
The purpose of this sfudy was to evaluate the abilify of a panel of eight anfinzicleocapsid rnonoclonal antibodies developed in Europe to assess rabies virus strains isolated from various animal species in geographically diverse areas of Mexico. Fifty-one rabies-positive brain fissue samples from animals and humans were examined. Material from these samples was used fo infect mice, whose brain tissue was subsequently tested by indirect immunofluorescence using the monoclonal antibodies described above. The study did nof turn up any strong evidence of Lyssavirus ofher than rabies virus, but did find four anfigenic variants differing from the classic rabies virus serofype. Samples of these laffer were sent to the Pasteur Institute in Paris for confirmation. Overall, the antibody panel was deemed useful for rapid typing
of rabies virus in Mexico. It also appears possible that autochfhonous anfigenic variations are now appearing in sfrains of the virus found in Mexico, which could explain some of the
failures observed with cerfain vaccines. These circumsfances appear to create a need for producing antinucleocapsid monoclonal antibodies with strains of rabies virus indigenous to the area.
A
pplication of the monoclonal anti- body (MA) technique has made it possible to better determine the epide- miologic characteristics of rabies by dem-‘Edited version of an article previously published in Spanish in the Bo/efin de fa Oficina Sanitaria Pnn- ameriomo (Vol. 119, No. 5, 1995, pp. 391-395) under the title “Evaluation de una serie de anticuerpos monoclonales para tipificar L~yssavirus en Mexico.” Reprint requests and other correspondence should be addressed to Dra. Elizabeth Loza-Rubio, Insti- tuto National de Investigaciones Forestales y Agro- pecuarias, Carretera Federal Mexico Toluca Km 15,5, Colonia Palo Alto, CP 05110, Mexico D.F., Mexico. 2National Institute of Forest, Agriculture, and
Livestock Research, Mexico City. Supported by CONACYT.
3National Autonomous University of Mexico, School of Veterinary Medicine and Zootechnics, Depart- ment of Preventive Medicine, Mexico City. “National Autonomous University of Mexico, Post-
graduate Unit, School of Advanced Studies- Cuautitlan, Mexico City.
5Mexican Social Security Institute, Medical Research Unit on Immunology, Mexico City.
onstrating the antigenic variability of the rabies virus throughout the world. Some authors have maintained that this varia- bility depends on the animal species har- boring the virus as well as the particular geographic area where the virus is indig- enous (1, 2, 3). However, other authors have refuted this assertion (4).
In the first MA tests conducted to iden- tify the rabies virus, researchers were able to fuse P3 x Ag8 myeloma cells with
splenocytes from rats that had been im- munized with the ERA strain. This hy- bridization process produced a large quantity of monoclonal antibodies able to recognize glycoprotein and nucleopro- tein in the envelope, although it was not possible to determine these antibodies’ specificity (5). Subsequently, the use of MA directed against the nucleocapsid (to distinguish between viruses belonging to the Lyssavirtls genus, including Lagos bat, Mokola, and Duvenhage) and against the glycoprotein (to distinguish between ra-
bies virus strains) was reported (6, 7). Later studies reported the use of various panels of antinucleocapsid and antinu- cleoprotein MA (I, 3, 4, 8, 9).
A WHO report (20) on the use of MA for diagnosing rabies showed the need to develop a reduced panel of these anti- bodies for identifying the principal anti- genie variants of the various Lyssavirtls strains related to classic rabies. The Pas- teur Institute in Paris developed four MA for identifying the serotypes of the Lys- savirus genus, plus an additional four permitting two Lyssavirus types found in European bats (EBL-1 and EBL-2) to be distinguished from other varieties (11).
In Mexico, as in all other countries of the Americas, no Lyssavirus other than the classic rabies virus has been detected. However, few studies have been con- ducted on the antigenic characteristics of the various strains of rabies virus found in Mexico (12). To facilitate such work, the study reported here assessed the abil- ity of a panel of eight MA developed in Europe to identify rabies viruses isolated in Mexico from a variety of animal spe- cies and geographic regions.
MATERIALS
AND METHODS
Initially, the investigators obtained 51 samples of brain tissue testing positive for the rabies virus, as determined by the results of a direct immunofluorescence test using a polyclonal conjugate. These samples, selected with an eye to the spe- cies and geographic diversity of the an- imal hosts, were obtained from the Centro National de Sanidad Animal (National Ani- mal Health Center), k&oratorio de Diagnds- tico Veterinario de1 Distrifo Federal (Federal District Veterinary Diagnostics Labora- tory), and Ranch0 del Molino (operated by the Veterinary Faculty of the University of Veracruz). The study was conducted be- tween May and November 1991.
The suspensions were subsequently thawed and centrifuged at 1 000 rpm for 10 minutes, and the supernatants were placed in prelabeled 2 mL vials. Subse- quently, 0.03 mL portions of each super- natant were injected intracerebrally into six albino mice (13). When the mice showed signs of distress and manifesta- tions characteristic of rabies, they were sacrificed and their brains extracted. This took place an average of 14 days follow- ing inoculation. The brains were then fro- zen and maintained at -70 “C.
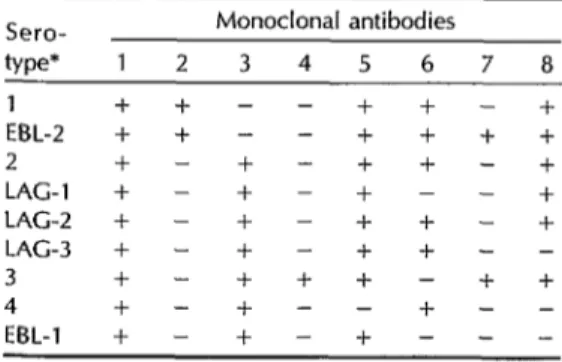
To detect the presence of rabies virus, the indirect immunofluorescence test (IIF) was employed (13). In addition, a panel of eight antinucleocapsid monoclonal antibodies (ANMA) was used to screen for variations in the Lyssavirus serotypes involved. The panel included the follow- ing monoclonal antibodies: MA 1, which is positive for all four Lyssavirus serotypes (serotype 1 type CVS, classic rabies; sero- type 2, Lagos; serotype 3, Mokola; and serotype 4, Duvenhage), as well as for the EBL-1 and EBL-2 viruses; MA 2, which is positive for serotype 1 and EBL-2 (a Lyssavirus isolated from Myotis dasy- cneme--14); MA 3, which is positive for serotypes 2, 3, and 4 and for EBL-1 (found in Eptesicus serotinus-15, 26); MA 4, which is positive only for serotype 3; MA 5, which is negative for serotype 4; MA 6, which is negative for Lagos type 1, sero- type 3, and EBL-1 but is positive for serotype 4; MA 7, which is positive for serotype 3 and EBL-2 but negative for serotype 1, and which was used to dis- tinguish EBL-2 from serotype 1; and MA 8, which is negative for Lagos type 3, serotype 4, and EBL-1. Table 1 reviews the response patterns exhibited by the eight antibodies to these Lyssavirus vari- eties (II).
The virus-positive brain tissue isolates Casts from normal mouse brains were were diluted 1:5 with phosphate-buffered used as negative controls, and mouse saline enriched with 0.75% of bovine al- bumin fraction V (BAPS) and were stored at -70 “C until used.
Table 1. Reactivity patterns obtained with a panel of monoclonal antibodies used to distinguish classic rabies virus from related
Lyssavirus serotypes.
Sero- Monoclonal antibodies
type* 1 2 3 4 5 6 7 8 1 + + - - + + - + EBL-2 + + - - + + + + 2 + - + - + + - + LAG-l + - + - + - - + LAG-2 + - + - + + - + LAG-3 + - + - + + - - 3 + - + + + - + + 4 + - + - - + - - EBL-1 + - + - + - - - *1 = serotype 1 Iclassic rabies virus); 2 = serotype 2 (La- gos); 3 = serotype 3 (Mokolaj; 4 = serotype 4 (Duvenhage); EEL-1 = European bat Lyssavrrus type 1 (isolated from Myotrs dasycneme and one human case); EBL-2 = European bat Lys- savrrus type 2 (isolated from Eptesicus serotinus and one hu- man case); LAG-1 = Lagos type 1; LAG-2 = Lagos type 2; and LAG-3 = Lagos type 3.
brains infected with fixed standard virus (CVS) were used as positive controls. Re- sults were expressed as - or + , with - indicating no observed fluorescence and
+ indicating fluorescence was observed. Certain samples in which antigenic variation was detected (see Table 3 foot- note) were sent to the Rabies Unit of the Pasteur Institute in Paris to be studied using a much larger panel of monoclonal antibodies (Wistar Institute, U.S.A.).
RESULTS
In order of decreasing frequency, the host creatures providing the 51 samples of rabies-positive brain tissue were as fol- lows: dogs (20 samples, 39%), cattle (17 samples, 33%), humans (5 samples, lo%), cats (4 samples, 8%), pigs (2 samples, 4%), horses (2 samples, 4%), and a goat (1 sample, 2%). Table 2 shows the geo- graphic distribution of the 51 samples. As may be seen, the samples came from 11 different states.
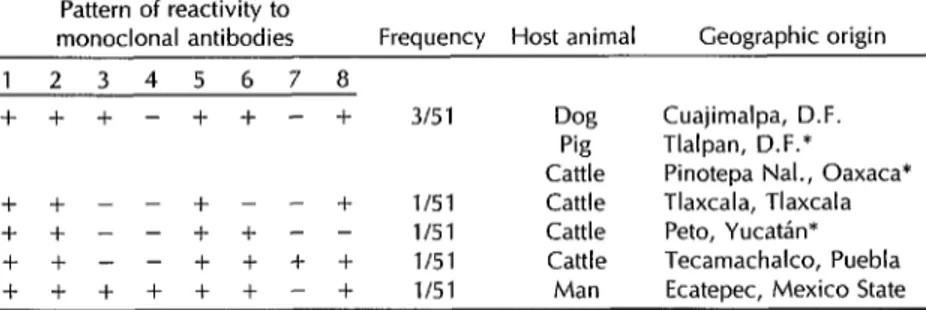
In all, 44 of these isolates yielded a reactivity pattern identical to that of sero- type 1, while the remaining seven ex- hibited small antigenic variations. Table 3 shows the antigenic responses obtained with the latter. One sample exhibiting such variation came from a dog in Cua- jimalpa, D.F., which tested positive for MA 3; however, the responsible virus could not be identified as serotype 2 La- gos type 2 because the sample tested pos- itive for MA 2.
Four variant strains were isolated from cattle. One of these, obtained from an animal in Pinotepa National, Oaxaca, ex- hibited the same antigenic variation as the dog isolate just described (positive for MA 3). A second, from Tlaxcala, tested negative for MA 6. A third, from Peto, Table 2. Geographic distribution of the rabies virus strains studied (n = 51).
Municipality or area No. of isolates State (No. of cases per region in parentheses) examined Chiapas Escuintla (2), Tuxtla Cutierrez (1) 3 Federal Drstrict Cuajimalpa (3), Nueva Atzacoalco (l), Rojo Cbmez (l), Tlalpan (11, 7
Culhuacan (1)
Durango Gomez Palacio (2) 2
Mexico State Cuautitlan (2), Coacalco (l), Ecatepec (3), Tejupilco (l), Texcoco (2), 10 Coatepec (1)
Guerrero Cutzamala (3) 3
Michoacan Coalcoman (2), Tmijaro (l), Tacambaro (l), Huetamo (1). Huacana (11, 10 Uruapan (l), Sta. Ana Maya (l), Apatzingan (l), Purudndiro (1)
Oaxaca Jamiltepec (l), Pinotepa National (2) 3 Puebla Puebla (2), Santiago Miahuatlan (1) Quecholac (11, Tlacotepec (11, 7
Tecamachalco (2)
Tlaxcala Tlaxcala (2) 2
Veracruz Medellin (l), Nautla (11, Santiago Tuxtla (1) 3
Yucatan Pet0 (1) 1
Table 3. Unusual antigenic reactivity patterns detected in the study strains, by geographic and animal origin of the samples. Mexico, 1991.
Pattern of reactivity to
monoclonal antibodies Frequency Host animal Geographic origin 1 2 3 4 5 6 7 8
+ + -I- - + + - + 315 1 Dog Cuajimalpa, D.F. Pig Tlalpan, D.F.* Cattle Pinotepa Nal., Oaxaca* + + - - + - - + l/51 Cattle Tlaxcala, Tlaxcala + + - - + -I- - - l/51 Cattle Peto, YucatAn* ++--++++ l/51 Cattle Tecamachalco, Puebla ++++++-+ l/51 Man Ecatepec, Mexico State
*The results obtained with these samples were confirmed by the Pasteur Institute of Paris
YucatBn, tested negative for MA 8. And the fourth, from Tecamachalco, Puebla, tested positive for MA 7, exhibiting the same antigenic pattern as EBL-2.
In addition, an isolate from a person in Ecatepec, Mexico State, differed from classic rabies by testing positive for MA 3 and MA 4. However, like most reactiv- ity patterns shown by the other variants, this pattern did not conform to that of any other known Lyssavirtls serotype.
DISCUSSION
AND
CONCLUSIONS
Testing of the 51 Mexican study sam- ples did not yield antigenic reactivity pat- terns suggesting the presence of a Lys- savirus other than the classic rabies virus. However, reactivity patterns other than those preestablished for the MA panel used and for known Lyssavirus serotypes were observed.
It was not possible to classify the strains exhibiting these variations with any cer- tainty. For example, the variant isolated from a Tlaxcala cow (see Table 3) could not even tentatively be identified as sero- type 2 Lagos type 2, since it tested pos- itive for MA 2. This uncertainty persists because the eight MA approach devised by Montafio-Hirose et al. (II) and em- ployed in our study had been used only to examine samples obtained from Eu- ropean animals, and there have been no
34 Bdetin of PAW0 30(l), 2996
reports in the Americas of any Lyssavirus not pertaining to the classic rabies virus group (serotype 1).
One variant strain, obtained from a cow in Peto, Yucat&, tested negative for MA 8. This did not suggest it was serotype 2 Lagos type 3 (see Table 3), because it also tested positive for MA 2 and negative for MA 3. The finding is somewhat unusual, however, because when this same MA panel was used previously to classify 25 Lyssavirus strains, only one, from an Ep- fesicus serofinus bat, tested negative for MA 8 (11).
Another variant, isolated from a cow in Tecamachalco, Puebla, yielded an an- tigenic pattern identical to that of Lyssa- virus from a European bat (EBL-2). How- ever, previous testing elsewhere has revealed this EBL-2 pattern on only two occasions, one of these being the testing of an isolate obtained from an infected person in Finland-a case in which re- searchers classified the responsible virus as Duvenhage type 8 (3, 24), now known as EBL-2.
ported here raise the possibility that au- tochthonous antigenic variations are be- ginning to appear among rabies strains found in Mexico (29). These circumstan- ces suggest a need to produce antinucleo- capsid monoclonal antibodies with strains of rabies virus indigenous to the area.
Acknowledgments. The authors wish to express their thanks for assistance pro- vided by Drs. Miguel Camara, Marcela Mercado, Carlos Gonzalez, Hector Cam- pos, Hector Campomanes, Nicolas de Miguel, and, especially, by Dr. Monique Lafon and Juan Antonio Montafio-Hirose for donating the monoclonal antibodies employed.
REFERENCES
1.
2.
3.
4.
5.
6.
7.
Schneider LG. Antigenic variants of ra- bies virus. Comp lrnmunol Microbial Infect Dis 1982;5:101-107.
Sureau P, Rollin PE, Chad1 S, Zeller H.
Etude 5 l’aide d’anticorps monoclonaux des caracteristiques de souches de virus
rabiques de Tunisie. Arch Insf Pasteur Tunis 1982;59:87-89.
Dietzschold B, Rupprecht CE, Tollis M, Lafon M, Mattei J, Wiktor TJ. Antigenic diversity of the glycoprotein and nucleo- capsid proteins of rabies and rabies re- lated viruses: implications for epidemiol- ogy and rabies control. Rev Infect Dis 1988;10:5285-S798.
German0 PLM, Silva EV, Miguel 0, Su- reau P. Variantes antigenicas de1 virus de la rabia aisladas en el nordeste y sudeste de Brasil: estudio preliminar. Bol Oficina Sanif Paanam 1990;108:39-44.
Wiktor TJ, Koprowski H. Monoclonal antibodies against rabies virus produced by somatic cell hybridization: detection of antigenic variants. PYOC Nat1 Acad Sci 1978; 75:3938-3942.
Flamand A, Wiktor TJ, Koprowski H. Use of hybridoma monoclonal antibodies in the detection of antigenic differences be- tween rabies and rabies related virus pro- teins: I, the nucleocapsid protein. ] Gen Virol 1980;48:97-104.
Flamand A, Wiktor TJ, Koprowski H. Use of hybridoma monoclonal antibodies in the detection of antigenic differences be- tween rabies and rabies related virus pro-
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
teins: II, the glycoprotein. J Gen Viral 1980;48:105-109.
Sureau P, Rollin I’, Wiktor TJ. Epidemi- ologic analysis of antigenic variations of street rabies virus: detection by mono- clonal antibodies. Am J Epidemiol 1983; 117:605-609.
Wiktor TJ, Koprowski H. Antigenic var- iants of rabies virus. J Exp Med 1980;152:99-
112.
World Health Organization. Repurf of the Fiffh WHO Consulfafion on Morwclonal Antibodies in Rabies Diagnosis. Geneva: WHO; 1989. Montario-Hirose JA, Bourhy H, Lafon M. A reduced panel of antinucleocapsid monoclonal antibodies for bat rabies virus identification in Europe. Res Viral 1990; 141:571-581.
Loza-Rubio E. Detection de variantes an- tigenicas de1 virus de la rabia proven- ientes de diversas regiones de Mexico, identificadas por anticuerpos monoclon- ales. [Master of Science thesis]. Mexico City: Universidad National Autonoma de Mexico; 1992.
Kaplan MM, Koprowski H. La rabia: t&- nicas de laboratorio. 3rd ed. Geneva: Or-
ganizacion Mundial de la Salud; 1976:389. King A, Crick J. Rabies related viruses. In: Campbell JB, Charlton KM, eds. Rabies USA. Boston: Kluwer Academic; 1988:165-
174.
Lumio J, Hillborn M, Roine R, et al. Hu- man rabies of bat origin in Europe. Lancet 1986;1:378.
King A, Davies I’, Laurie A. The rabies
viruses of bats. Vet Microbial 1990;23:165- 174.
Smith JS. Rabies virus epitopic variation: use in ecology studies. Adv Virus Res 1989;36:215-217.
Webster WA, Casey GA, Charlton KM, Wiktor TJ. Antigenic variants of rabies vi- rus in isolates from Eastern, Central and Northern Canada. Can J Camp Med 1985; 49:186-188.
Aguilar-Setien A, Loza-Rubio E, Ramos RL, Leon CY, Espinoza LE. Identification of an atypical rabies strain in Mexico. Arch Med Res 1994;1:25-54.
Manuscript submitted on 30 September 1994. Ac- cepted for publication in the Bolctiir dc In Ofirrrrn .Sn,fitnrin Pmmm+nira (following revision) on 1 June
1995. Accepted for publication in the Bzrlkfrrl of t/w Pm Afrrericnn Hdfh Orprh7fiorz on 28 June 1995.