Toward a Vaccine against Argentine
Hemorrhagic Fever1
JULIO
G. BARRERA0~02 & KELLY T. MCKEE,
JR.~A vaccine against Argentine hemorrhagic feven the “ma1 de 10s rastrojos” of the pampas, has been a dream of physicians and scientists involved with the disease since its recognition in the 1950s. Several killed and live immunogens have been produced and tested in pursuit of this goal, none of which has proved suitable for
widespread human use. Recently, a new live-attenuated Junin virus vaccine, Candid #I, was developed through a cooperative international effort. Testing conducted to date indicates that this vaccine holds considerable promise.
A
rgentine hemorrhagic fever (AHF) isa clinically severe, rodent-borne zoonosis restricted in nature to the fertile
pampas of north-central Argentina. The
causative agent, Junin virus, is an arena-
virus that induces chronic infections
among reservoir rodents of the family
Cricetidae and is transmitted to man via fomites and aerosols of contaminated ex- creta. The region’s rural inhabitants and agricultural workers constitute the popu- lation at greatest risk (I). Cases of AHF, typically fatal among 5% to 30% of those
afflicted and generally more severe in
older persons, are recognized through- out the year; but the vast majority of the illnesses occur during a well-defined epi-
‘This article will also be published in Spanish in the Boletin de la Oficina Sanitaria Panamericana, vol. 110. Correspondence should be addressed to Dr. McKee, Division of Medicine, USAMRIID, Ft. De- trick, Frederick, Maryland, 21701-5011, USA. The views of the authors do not purport to reflect the views of the U.S. Department of the Army or De- partment of Defense.
2The Salk Institute Government Services Division, Swiftwater, Pennsvlvania, U.S.A.
aDivision of Medicine, United States Army Medical Research Institute of Infectious Diseases, Fort De- trick, Frederick, Maryland, U.S.A.
demic season that lasts from February through July and coincides with the an- nual grain harvests.
AHF epidemiology indicates an ex-
panding disease with changing inci-
dence. When the disease first was recog- nized in the 195Os, its endemic area was limited to a region 16,000 km2 within the province of Buenos Aires (I). Since then it has spread to involve three adjacent
provinces; it currently covers an area
nearly eight times that originally de-
scribed (2), and appears destined to con-
tinue extending outward in a north-
easterly direction (3).
During the 1960s and 197Os, epidemic outbreaks exceeding 1,000 cases per year were frequent. Since 1979, however, the annual incidence has rarely exceeded 400 cases (4; J. I. Maiztegui, unpublished data). One reason is that the continually advancing disease front has created “his- toric” areas where AHF has been seen for 10 years or more. While cases con- tinue to occur in such regions, the annual disease incidence is much lower than that seen in more recently affected regions
(2). Curiously, this phenomenon occurs
despite a continued population suscep-
tibility that exceeds 95% (5).
HISTORY OF VACCINE
DEVELOPMENT
Inactivated Vaccines
The need for a widely available vaccine to protect against Al% has long been rec- ognized. Initial efforts focused on for-
malin-inactivated mouse brain prepara-
tions (6, 7) that induced neutralizing
antibodies and protected mice and
guinea pigs against virulent Junin chal- lenge (Table 1). One such product was inoculated into more than 15,000 persons between 1959 and 1962, although its effi- cacy was never assessed (8). However, the need to administer 3-4 weekly doses
with or without a “booster,” together
with concerns about the virus substrate (i.e., mouse brain), severely limited these vaccines’ potential for general use. Re- cently, some of these deficiencies were
addressed through production of a cell
culture-derived, formalin-inactivated im-
munogen. Neutralizing antibodies were
induced successfully with this product; however, vaccinated animals failed to re- sist challenge with a virulent Junin virus strain (9).
Additional inactivation strategies have
included exposure of Junin virus to
photoactive dyes (10, Zl), ultraviolet irra- diation (E’), heating, and acetone (23). Though at least one resulting product has
been administered to humans in limited studies (Table l), all such products have proved of limited utility. A subunit im-
munogen composed of purified G38
envelope glycoprotein emulsified in
Freund’s complete adjuvant has induced neutralizing antibodies in rabbits and has protected guinea pigs against virulent Ju- nin virus challenge (14). While this ap- proach has shown some limited promise, additional studies are still needed to as- sess its potential for human vaccination.
Live Vaccines
The alternative to virus inactivation
and classical vaccine development in-
volves production of a live-attenuated immunogen. As Table 2 indicates, a num-
ber of such immunogens, derived from
both heterologous and homologous vi-
ruses, have been produced and tested.
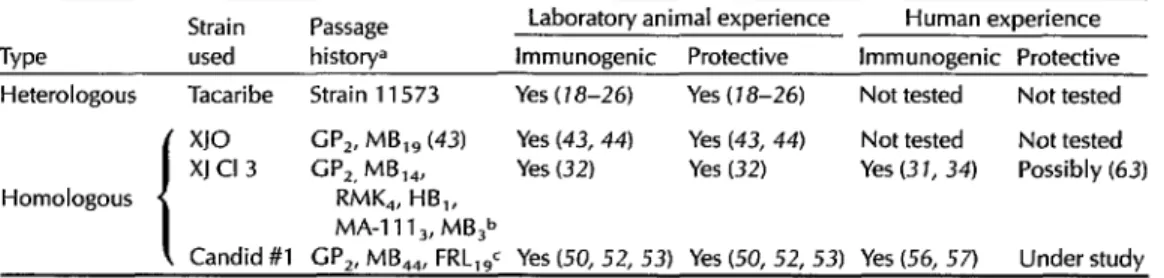
Heterologous live vaccines. Viruses
comprising the family Arenaviridae fall
serologically into two groups: the so-
called “Old World” (lymphocytic cho-
riomeningitis, Lassa, Mopeia, Mobala,
and Ippy) and “New World” (Machupo,
Junin, Tacaribe, Amapari, Parana, Lat-
ino, Tamiami, Pichinde, and Flexal)
arenaviruses.
The type-member of the “New World” complex, Tacaribe virus (1.5), is closely
Table 1. Candidate Argentine hemorrhagic fever vaccines (inactivated).
Laboratory animal experience Human experience Type Strain used immunogenic Protective Immunogenic Protective
Formalin-
l
Multiple Not tested Yes (6) Not tested Not determined (8) killed MC2 Yes (7) Yes (7) Not tested
Not tested XJ Clone 3 Yes (9) No (9) Not tested Not tested
Photo-
i
Xl Not tested Partial (81 Not tested Not tested inactivated RC Not tested Yes (IS) No (18, 6W No (61)
Subunit MC, Yes (14) Yes (14) Not tested Not tested dBoosted seropositives.
Table 2. Candidate Argentine hemorrhagic fever vaccines (live).
Strain Passage Laboratory animal experience Human experience Type used historya Immunogenic Protective Immunogenic Protective Heterologous Tacaribe Strain 11573 Yes ( 18-26) Yes (18-26) Not tested Not tested
x10 GP,. MB,, (43) Yes (43, 44) Yes (43, 44) Not tested Not tested XJ Cl 3 G”,, MB,,, Yes (32) Yes (32) Yes (3 1, 34) Possibly (63) Homologous RMK,, HB,,
MA-l 11 3, MB,b
Candid #l GP,, MB,,, FRL,$ Yes (50, 52, 53) Yes (50, 52, 53) Yes (56, 57) Under study GP = guinea pig; MB = mouse brain; RMK = rhesus kidney cell culture; HB = hamster brain; MA-1 11 = rabbit kidney cell line; FRL = fetal rhesus lung cell culture. Subscripts indicate passage numbers.
bPortions of passage history uncertain; lineage reconstructed from written documents provided by Dr. N. Wiebegna and (31)
(see text).
cJ.C. Barrera Oro, manuscript in preparation.
related both serologically and bio-
chemically to Junin virus (26, 17). Cross- protection against Junin virus has been
demonstrated repeatedly in guinea pig
(28-22) and nonhuman primate (23-26)
model systems. Adverse reactions have not been reported among animals receiv- ing Tacaribe virus in doses suitable for
vaccination. A single human infection
with Tacaribe virus has been recognized, this being characterized by fever, head-
ache, and constitutional symptoms that
resulted in hospitalization (noted in 27). However, this infection resulted from ex- posure to laboratory virus preparations of possibly high titer, making generaliza-
tions regarding human pathogenicity of
Tacaribe difficult. There is no evidence that the virus induces chronic or persis- tent infections in mammalian models (25, reviewed in 17). Heterologous immunity induced by Tacaribe appears to be long lasting (25, 28). On the basis of these ob- servations, Tacaribe virus has been pro-
posed as a candidate heterologous AHF
vaccine (27, 25). No human studies have yet been conducted.
Homologous live vaccines. A fortuitous
observation at the Rockefeller Founda-
tion Virus Laboratories provided the key to developing a live-attenuated homolo-
gous vaccine. Investigators there noted
that the prototype XJ strain of Junin virus
120 Bulletin of PAHO 25(2), 1992
seemed to lose its pathogenicity for
guinea pigs after about 40 passages
through newborn mouse brain (29)-al-
though this was thought initially to be due to host resistance rather than viral attenuation (30). Subsequently, a virus pool was created by Wiebenga at the U.S. National Institutes of Health by passing a plaque-purified isolate (purified in pri- mary rhesus monkey kidney cells) from mouse brain passage 14 of XJ into suck- ling hamster brain. This strain, desig- nated XJ Clone 3 (N. Wiebenga, written
communication), was passed further in
continuous MA-104 and MA-111 cells,
one passage (from MA-111 cells) being further passed in suckling mouse brain
and evaluated as a prospective vaccine
(39
XJ Clone 3 proved attenuated and im- munogenic after intraperitoneal inocula- tion of guinea pigs, and was found to
protect animals against virulent virus
challenge (32). Its safety for man then
was evaluated in seven individuals in-
volved in Junin virus research. Mild,
transitory clinical reactions occurred
among the volunteer group (33), and
neutralizing antibodies were elicited in all (34).
On the basis of these encouraging re- sults, XJ Clone 3 was administered to an
additional 629 Argentine volunteers in
were recorded in 75.6% of 213 intensively
studied individuals: 44% developed fe-
ver, often accompanied by asthenia,
headache, retro-ocular pain, and myalgia which appeared 3-10 days after immu-
nization and resolved spontaneously
within 3 days; 29.1% developed asthenia, headache with retro-ocular pain, and my- algia unaccompanied by fever that lasted 1 to 4 days; and 2.4% developed a local reaction at the site of inoculation that dis- appeared within 48 hours. The remaining
volunteers (24.4%) remained asympto-
matic (35). Out of 57 individuals given
hematologic analyses, 29 (50.9%) dis-
played mild leukopenia, 5 (8.8%) mildly decreased platelet counts, and 11(19.2%) both; no alterations were found in the re- maining 12 (21.1%) (31, 35). Neutralizing antibodies were found in 97% of 165 indi- viduals examined 1-3 months after vac-
cination (31). Follow-up studies con-
ducted 7-9 years later on 267 of these volunteers revealed no long-term side ef- fects; 153 of 165 (90%) retained measur- able neutralizing antibodies against Junin virus (31).
Because of uncertainties in the passage history of XJ Clone 3 (its previous pas-
sage through heteroploid cells and
mouse brain substrates), administration of the vaccine was discontinued in 1971
(17). Further characterization of this
product continued, however, and addi-
tional passages of the prototype XJ strain were evaluated for attenuation.
It was clear that XJ Clone 3 was signifi-
cantly attenuated for guinea pigs when
compared with naturally occurring Junin virus strains (36). Nevertheless, residual
peripheral virulence, neurotropism, and
neurovirulence could be demonstrated in
these animals and in primates (37-39).
Moreover, by using sensitive tech-
niques-such as co-cultivation and im-
munoperoxidase staining for antigens-
persistence of the virus in guinea pig tis-
sues could be demonstrated for up to
three months (39-41), and antigen could be found in primate organs over one year after infection (42).
Another attenuated Junin virus strain,
derived from prototype XJ and desig-
nated XJO, was maintained in newborn
mouse brain and not passaged in cell cul- ture (43). Although XJO reportedly con- ferred complete protection against viru-
lent Junin virus challenge for guinea
pigs, it also exhibited persistence, neuro-
tropism, and neurovirulence similar to
those exhibited by XJ Clone 3 (41,43-47).
CANDID #1
Encouraging discussions held in 1976
during the First International Seminar on
Hemorrhagic Fevers Produced by Arena-
viruses (48) led to a joint effort directed at developing an acceptable live-attenuated Junin virus vaccine against AHF. This was begun as a joint effort in 1979 by the U.S. Army Medical Research Institute of
Infectious Diseases and the Argentine
Ministry of Health and Social Action, un- der auspices of the United Nations De-
velopment Program and PAHO. The
goals were straightforward: identify a vi- rus at least as attenuated as XJ Clone 3 for humans with an acceptable passage his- tory; adapt the virus to replication in cer- tifiable cell substrates; select attenuated variants with minimal residual virulence that are immunogenic and confer protec-
tion upon laboratory animals; and de-
velop and test this candidate vaccine in human volunteers under the supervision
of U.S., Argentine, and PAHO regula-
tory agencies.
Two approaches to vaccine develop-
ment were undertaken concurrently. The
first, identification of an already attenu- ated Junin virus strain that could be used as a parent virus for further modification, provided a direct route to a highly atten- uated virus that could be evaluated for
acceptability as a vaccine candidate by
appropriate methods. The second en-
tailed isolation and passage in certified cell substrates of a novel, naturally occur- ring Junin virus strain in order to enrich for attenuated variants. The latter effort yielded viruses with reduced but still sig- nificant virulence, and this approach was abandoned.
The development initiative culminated in the production of Candid #1 vaccine.
The virus strain involved was derived
from a documented (mouse brain and
guinea pig passaged) descendant of pro- totype XJ Junin virus that had been pas- saged and cloned in certified diploid mammalian cells (Figure 1) (49; J.G. Bar- rera Or0 et al, manuscript in prepara- tion) .
Candid #l is more attenuated for new- born mice and guinea pigs than XJ Clone 3 (50; J.G. Barrera Oro et al, manuscript
in preparation). Neuroinvasiveness and
neurovirulence in nonhuman primates
are minimal, being less than what is ex- hibited by XJ Clone 3 (51-53). Candid #l
has demonstrated immunogenicity and
protective efficacy in both guinea pigs and nonhuman primates (26, 50, 52, 53).
Parodl
Strain XJ
-1
Passaqes
Casals
XJll -L
GPp2MBpll
USAMRIID
XJ43 -1
GPp2MBp43
Barrera Or0
XJ44 -1
GPp2MBp44
Candld #l GPp2MBp44FRLplS
Figure 1. Passage history of Candid #l Junin virus vaccine. GPp = guinea pig passage, MBp
= newborn mouse brain passage, FRLp = fetal rhesus lung cell culture passage.
It has also been found to protect ma-
caques against heterologous challenge
with virulent Machupo virus, the closely related etiologic agent of Bolivian hemor- rhagic fever (54, 55).
Candid #1 has been evaluated for
safety and immunogenicity in more than
200 human volunteers from both the U.S. and Argentina (56, 57). To date, no signif- icant adverse reactions related to vaccina- tion have been observed, and more than 95% of the recipients have developed an-
tibodies after immunization. The vac-
cine’s efficacy is currently being studied by means of a large, double-blind, pla- cebo-controlled field trial in Argentina’s Santa Fe Province (J.I. Maiztegui, K.T. McKee, Jr., personal communication).
CONCLUSIONS
The history of vaccination against AHF spans a period of more than 30 years. During that time, a variety of classical ap-
proaches to vaccine development have
been employed in an attempt to produce a safe and effective human immunogen. Development of the current most promis- ing candidate, Candid #l, has depended upon experience gleaned from years of
experimentation in laboratories and
clinics on two continents. Specifically, ex- tensive testing of XJ Clone 3 in animal models and humans provided a wealth of scientific information permitting contin- ued modification of the prototype XJ Ju- nin virus strain to ultimately produce Candid #l.
If successful, Candid #l will become the first vaccine widely employed against an arenavirus. It thus appears that expe- rience gained in the e forts to develop it P
should have broader applicability to
other arenaviruses pathogenic for man,
most notably Lassa virus. Recently, vac- cine research on Lassa fever prophylaxis has focused upon molecular cloning and vector insertion (58-61). While this excit-
ing new approach holds great promise for vaccine development, it has not yet
produced a product suitable for human
use. Should the potential of this new 10.
technology fail to be realized, the experi- ence gained through Junin virus vaccine
development will be of great utility in 11.
planning and implementing a classical
Lassa vaccine program.
12.
REFERENCES
1.
2.
3.
4.
5.
6.
7.
8.
9.
Maiztegui JI. Clinical and epidemiological patterns of Argentine haemorrhagic fever. Bull WHO. 1975;52:567-75.
Maiztegui J, Feuillade M, Briggiler A. Progressive extension of the endemic area and changing incidence of Argentine hemorrhagic fever. Med Microbial Immu- nol. 1986;175:149-52.
Mills J, Ellis B, Maiztegui J, Ksiazek T, McKee K, Childs J. Prevalence of Junin virus antigen and antibody among ro- dents in endemic and non-endemic areas of Argentine hemorrhagic fever. Hon- olulu, Hawaii: 38th Annual Meeting of the American Society of Tropical Medi- cine and Hygiene; 1989. (Abstract). Carballal G, Videla CM, Merani MS. Epi- demiology of Argentine hemorrhagic fe- ver. Eur J Epidemiol. 1988;4:259-74.
Weissenbacher MC, Sabattini MS, Avila MM, et al. Junin virus activity in two rural populations of the Argentine hemor- rhagic fever (AHF) endemic area. ] Med Viral. 1983;12:273-280.
Pirosky I, Martini P, Zuccarini J, et al. Vir- osis hemorrrigica de1 noroeste bonaerense (endemo-epidemica, febril, exantemitica y leucop&ica): V, la vacuna especffica y ~43apaci6n. Orientac Med. 1959; 8:
Barrera Oro JG, Girola RA, Frugone G. Estudios immunoldgicos con virus Junfn: II, inmunidad adquirida por cobayos inoc- ulados con virus inactivado por formol. Medicina (Buenos Aires). 1967;27:279-82. Mettler N. Argentine hemorrhagic f#er: cur-
rent knowledge. Washington, DC: Pan American Health Organization; 1969. (PAHO scientific publication no. 183). Videla C, Carballal G, Remorini P, La
13.
14.
15.
16.
17.
18.
19.
20.
21.
Torre J. Formalin inactivated Junin virus: immunogenicity and protection assays. I Med Viral. 1989;29:215-20.
Parodi AS, de Guerrero LB, Weissen- bather M. Fiebre hemorr&ica Argentina: vacunaci6n con virus Junfn inactivado. Cienc Invest. 1965;21:132-33.
De Martinez Segovia ZM, Arguelles G, Tokman A. Capacidad antigknica de1 vi- rus Junfn inactivado mediante oxidaci6n fotodin&mica. Medicina (Buenos Aires). 1980;40:156-60.
D’Aiutolo AC, Lampuri JS, Coto CE. Re- activaci6n de1 virus Junfn inactivado por la luz ultravioleta. Medicina (Buenos Aires). 1979;39:801.
Carballal G, Videla C, Oubiiia JR, Frigerio MJ. Antigenos inactivados de virus Junin. Medicina (Buenos Aires). 1985;45:153-58. Cresta 8, Padula P, de Martinez Segovia ZM. Biological properties of Junin virus proteins: I, identification of the immu- nogenic glycoprotein. Intervirology. 1980; 13:284-88.
Downs WC, Anderson CR, Aitken THG, Spence L, Greenhall AH. Tacaribe virus, a new agent isolated from Artibeus bats and mosquitos in Trinidad. Am ] Trop Med Hyg. 1963;12:639-46.
Damonte EB, Mersich SE, Candurra NA, Coto CE. Cross reactivity between Junin and Tacaribe viruses as determined by neutralization test and immunoprecipita- tion. Med Microbial Immunol. 1986; 175:85-88.
Weissenbacher MC, Laguens RF’, Coto CE. Argentine hemorrhagic fever. Curr Top Microbial Immunol. 1987;134:79-116. Parodi AS, Coto CE. Inmunizacidn de co- bayos contra el virus Junin por inocula- cidn de1 virus Tacaribe. Medicina (Buenos Aires). 1964;24:151-53.
Tauraso N, Shelokov A. Protection against Junin virus by immunization with live Tacaribe virus. Proc Sot Exp Biol Med. 1965;119:608-11.
Weissenbacher MC, Coto CE, Calello MA. Cross-protection between Tacaribe com- plex viruses: presence of neutralizing an- tibodies against Junin virus (Argentine hemorrhagic fever) in guinea pigs infec- ted with Tacaribe virus. Interoirology. 1975-76;6:42-49.
Coto CE, Damonte EB, Calello MA, Weissenbacher MC. Protection of guinea
?igs inoculated with Tacaribe virus
igainst’lethal doses of Junin virus. J Infect
3is. 1980;141:389-93.
22. 3amonte EB, Calello MA, Coto CE, Weissenbacher MC. Inmunizacion de co- >ayos contra la fiebre hemorragica Argen- bina con virus Tacaribe replicado en cd-
ulas diploides humanas. Medicina (Buenos Aires). 1981;41:467-70.
23. Weissenbacher MC, Coto CE, Calello MA, Rondinone SN, Damonte EB, Frigerio MJ. Cross-protection in nonhuman primates against Argentine hemorrhagic fever. In- fect Immun. 1982;35:425-30.
24. Samoilovich SR, Pecci Saavedra J, Frig- erio MJ, Weissenbacher MC. Nasal and intrathalamic inoculations of primates with Tacaribe virus: protection against Ar- gentine hemorrhagic fever and absence of neurovirulence. Acfa Virol. 1984;28: 277-81.
25. Samoilovich SR, Calello MA, Laguens RR Weissenbacher MC. Long-term protection against Argentine hemorrhagic fever in Tacaribe virus infected marmosets: vir- ologic and histopathologic findings. J Med Virol. 1988;24:229-36.
26. Kenyon RH, McKee K, Barrera Oro J, Ragland D, Crabbs C, Higgins Y. Protec- tive efficacies in rhesus monkeys of Can- did 1 strain Junin virus (JV) and Tacaribe virus against aerosol challenge with viru- lent JV Honolulu, Hawaii: 38th Annual Meeting of the American Society of Tropi- cal Medicine and Hygiene; 1989. (Abstract #136).
27. Peters CJ, Jahrling PB, Liu CT, Kenyon RH, McKee KT Jr, Barrera Oro JG. Experi- mental studies of arenaviral hemorrhagic fevers. Curr Top Microbial lmmunol. 1987;134:5-68.
28. Weissenbacher MC, Damonte EB. Fiebre hemorragica Argentina. Adel Microbial Enferm Infect. 1983;2:119-71.
29. Mettler NE, Casals J, Shope RE. Study of the antigenic relationships between Junin virus, the etiological agent of Argentinean hemorrhagic fever, and other arthropod- borne viruses. Am j Trop Med Hyg. 1963;12:647-52.
30. Casals J. Serological studies on Junin and Tacaribe viruses. Am J Trop Med Hyg. 1965; 14:794-95.
31. Ruggiero HA, Magnoni C, Guerrero LB 32.
33.
34.
35.
36.
37.
38.
39.
40.
de, et al. Persistence of antibodies and clinical evaluation in volunteers 7 to 9 years following the vaccination against Argentine hemorrhagic fever. J Med Virol. 1981;7:227-32.
Guerrero LB de, Weissenbacher MC, Par- odi AS. Inmunizacion contra la fiebre hemorrdgica Argentina con una cepa at- enuada de1 virus Junin: I, estudio de una cepa modificada de1 virus Junfn, in- munizacion de cobayos. Medicina (Buenos Aires). 1969;29:1-5.
Ruggiero HR, Astarloa L, Gonzalez Cam- baceres C, Maglio F, Squassi G. In- munizacion contra la fiebre hemorrdgica Argentina con una cepa atenuada de virus Junfn: II, inmunizacion de voluntarios, anglisis clinic0 y de1 laboratorio. Medicina (Buenos Aires). 1969;29:81-87.
Weissenbacher M, Guerrero LB de, Help G, Parodi AS. Inmunizacion contra la fiebre hemorragica Argentina con una cepa atenuada de virus Junin: III, reac- ciones serologicas en voluntarios. Medic- ina (Buenos Aires). 1969;29:88-92.
Ruggiero HA, Magnoni C, Cintora FA, et al. Inmunizacion contra la fiebre hemor- rdgica Argentina con una cepa atenuada de virus Junfn: anAlisis de 636 vacunados. Prensa Med Argenf. 1974;61:231-40. Contigiani MS, Sabattini MS. Virulencia diferencial de cepas de virus Junfn por marcadores biologicos en ratones y co- bayos. Medicina (Buenos Aires). 1977; 37:244-51.
Avila MM, Samoilovich SR, Weissen- bather MC. Infection de1 cobayo con la cepa atenuada de1 virus Junfn XJCl,. Me- dicina (Buenos Aires). 1979;39:597-603. Carballal G, Cossio PM, de la Vega MI, et al. Neurovirulencia de la cepa XJCI, de virus Junfn en un primate sudamericano. Cordoba, Argentina: III Congreso Argen- tino de Microbiologfa; 1982. (Abstract #186).
Laguens RM, Avila MM, Samoilovich SR, Weissenbacher MC, Laguens RI?. Patho- genicity of an attenuated strain (XJCl,) of Junin virus: morphological and virological
studies in experimentally infected guinea pigs. Infervirology. 1983;20:195-201. Avila MM, Laguens RM, Laguens Rl’, Weissenbacher MC. Selectividad tisular e indicadores de virulencia de tres cepas de1
virus Junk Medicina (Buenos Aires). 1981;41:157-66.
41. Malumbres E, Boxaca MC, Guerrero LB de, Berria M, Lascano EF. Persistence of attenuated Junin virus strains in guinea pigs infected by IM or IC routes. 1 Infect Dis. 1984;149:1022.
42. Avila MM, Frigerio MJ, Weber EL, et al. Attenuated Junin virus infection in Cal- lifhrixjacchus. 1 Med Viral. 1985;15:93-100. 43. Guerrero LB de, Boxaca MC. Estudio pre-
liminar de una variante atenuada de1 vi- rus Junfn derivada de la cepa prototipo
XJ.
Medicina (Buenos Aires). 1980; 40:267-74.44. Boxaca MC, Guerrero LB de, Weber EL, Malumbres E. Protection inducida en co- bayo por la variante XJa de1 virus Junfn. Medicina (Buenos Aires). 1981;41:25-34. 45. Guerrero LB de, Boxaca MC, Rabinovich
RD, Malumbres E. Evolution de la infec- cion en cobayos infectados con la variante atenuada XJ, de1 virus Junfn. Rev Argenf Microbial. 1983;15:205-12.
46. Boxaca MC, Guerrero LB de, Malumbres E. Modification of Junin virus neurotrop- ism in the guinea pig model. Acfa Viral. 1984;28:198-203.
47. Guerrero LB de, Boxaca MC, Malumbres E, Mercedes Gomez M de las. Patho- genesis of attenuated Junin virus in the guinea pig model. 1 Med Virol. 1985; 15:197-202.
48. Eddy GA, Barrera Oro JG. Discusion gen- eral. Medicina (Buenos Aires). 1977; 37(Suppl3):257-59.
49. Barrera Oro JG, Eddy GA. Characteristics of candidate live attenuated Junin virus vaccine. Banff, Alberta, Canada: Interna- tional Conference on Comparative Virol- ogy; 1982. (Abstract #S4-10).
50. Lupton Hw, Cole FE, Moe J, et al. Safety and efficacy of a live, attenuated vaccine against Argentine hemorrhagic fever. Sendai, Tanan: Sixth International Con- gress of I Virology; 1984. (Abstract #P27-22).
51. McKee KT Jr, Green DE, Mahlandt BG, et al. Infection of Cebus monkeys with Junin virus. Medicina (Buenos Aires). 1985; 45~144-52.
52. McKee KT, Barrera Oro JG, Kuehne AI, Spisso J, Mahlandt BG. Immunogenicity and protective capacity of a live, attenu-
ated Argentine hemorrhagic fever (AHF) vaccine in primates. Calgary, Canada: XI International Congress for Tropical Medi- cine and Malaria; 16-22 September 1984. (Abstract).
53. Barrera Oro JG, McKee KT Jr, Kuehne AI, et al. Preclinical trials of a live, attenuated Junin virus vaccine in rhesus macaques. Paris, France: Institut Pasteur Sympo- sium on Vaccines and Vaccinations; 1985. (Abstract #l?28).
54. Lupton Hw, Jahrling PB, Barrera Oro JG, Peters CJ. Cross-protection against Ma- chupo virus with Candid #l live-attenu- ated Junin virus vaccine: II, postchallenge virological and immunological findings. Mar de1 Plata, Argentina: Second Interna- tional Conference on the Impact of Viral Diseases on the Development of Latin American Countries and the Caribbean Region; 1988. (Abstract #PE2).
55. Jahrling PB, Trotter RW, Barrera Oro JG, et al. Cross-protection against Machupo virus with Candid #1 Junin virus vaccine: III, postchallenge clinical findings. Mar de1 Mata, Argentina: Second Interna- tional Conference on the Impact of Viral Diseases on the Development of Latin American Countries and the Caribbean Region; 1988. (Abstract #PE3).
56. MacDonald C, McKee K, Peters C, Fein- sod F, Cosgriff T, Barrera Oro J. Initial clin- ical assessment of humans inoculated with a live-attenuated Junin virus vaccine. Edmonton, Alberta, Canada: VII Interna- tional Congress of Virology; 1987. (Ab- stract #R3.27).
57. Maiztegui JI, McKee KT Jr. Inoculation of human volunteers with a vaccine against Argentine hemorrhagic fever. Banff, Al- berta, Canada: Sixth International Con- ference on Comparative and Applied Vir- ology; 1989. (Abstract #S4).
58. Clegg JC, Lloyd G. Vaccinia recombinant expressing Lassa-virus internal nucleo- capsid protein protects guinea pigs against Lassa fever. Lancef. 1987; 2(8552):186-88.
59. Auperin DD, Esposito JJ, Lange JV, et al. Construction of a recombinant vaccinia vi- rus expressing the Lassa virus glycopro- tein gene and protection of guinea pigs from a lethal Lassa virus infection. Virus Res. 1988;9:233-48.
60. Morrison HG, Bauer SF’, Lange JV, Es- posito JJ, McCormick JB, Auperin DD. Protection of guinea pigs from Lassa fever by vaccinia virus recombinants expressing the nucleoprotein or the envelope gly- coproteins of Lassa virus. Virology.
1989;171:179-88.
a recombinant vaccinia virus containing the Lassa virus glycoprotein gene. Proc
Nat1 Acad Sci USA. 1989;86:317-21. 62. Guerrero LB de. Vacunas experimentales
contra la fiebre hemorrsigica Argentina.
Medicina (Buenos Aires) 1977;37(Suppl 3):252-259.
61. Fisher-Hoch SF’, McCormick JB, Auperin 63. Johnson KM. Status of arenavirus vac- D, et al. Protection of rhesus monkevs cines and their application. Bull WHO.
from fatal Lassa fever by vaccination w&h 1975;52:729-35. - -
Cholera Takes Hold in the Americas
The epidemic of cholera that is currently raging in South America has spread from its initial focus on the Pacific coast of Peru to areas throughout that country and to parts of Chile, Colombia, and Ecuador. From the start of the epidemic in January to 2 May 1991, almost 175,000 cases, of which 1,390 were fatal, had been notified by health authorities in those four countries, as follows: 169,255 cases and 1,244 deaths in Peru, 5,005 cases (1,107 confirmed) and 140 deaths in Ecuador, 189 cases and 5 deaths in Colombia, and 26 cases and 1 death in Chile. In addition, five cases have been reported from Amazonas State in Brazil, and five imported cases have occurred in the United States.
Cholera is an acute bacterial enteric disease, usually of sudden onset, that causes profuse watery stools, vomiting, rapid dehydration, acidosis, and circulatory collapse. Mild cases may be indistinguishable from other types of diarrhea except by fecal cultures. Without treatment, the case- fatality rate of serious cholera can reach 50%. With adequate treatment, mortality is around 1%. Management of the disease focuses on replacing fluids and electrolytes.
The prognosis for the course of the epidemic in the affected countries and in other countries in the Region is guarded, since it is not possible to prevent the spread of cholera from one country to another. The living con- ditions of the population are a crucial factor in determining the intensity with which cholera epidemics spread.
A comprehensive report on cholera in the Americas will appear in the next issue of the Bulletin ufPAH0.
Source: WHO, wkly Epidemiol Ret 66(18):132,1991; and PAHO, Epidemiol Bull 12(l): 1,2,14-15,199l.