UNIVERSIDADE FEDERAL DO CEARÁ CENTRO DE CIÊNCIAS
DEPARTAMENTO DE BIOQUÍMICA E BIOLOGIA MOLECULAR PROGRAMA DE PÓS-GRADUAÇÃO EM BIOQUÍMICA
FELIPE DOMINGOS DE SOUSA
CLONAGEM E EXPRESSÃO DA LECTINA FRUTAPINA EM ESCHERICHIA COLI: ANÁLISES ESTRUTURAIS
CLONAGEM E EXPRESSÃO DA LECTINA FRUTAPINA EM ESCHERICHIA COLI: ANÁLISES ESTRUTURAIS
Tese apresentada ao Programa de Pós-Graduação em Bioquímica da Universidade Federal do Ceará, como parte dos requisitos para obtenção do título de Doutor em Bioquímica. Área de concentração: Bioquímica Vegetal.
Orientador:
Prof. Dr. Renato de Azevedo Moreira. Co-orientadora:
Profa. Drª. Ana Cristina de Oliveira Monteiro Moreira.
Dados Internacionais de Catalogação na Publicação Universidade Federal do Ceará
Biblioteca Universitária
Gerada automaticamente pelo módulo Catalog, mediante os dados fornecidos pelo(a) autor(a)
S696c Sousa, Felipe Domingos de.
Clonagem e expressão da lectina frutapina em Escherichia coli : Análises estruturais / Felipe Domingos de Sousa. – 2018.
96 f. : il. color.
Tese (doutorado) – Universidade Federal do Ceará, Centro de Ciências, Programa de Pós-Graduação em Bioquímica , Fortaleza, 2018.
Orientação: Prof. Dr. Renato de Azevedo Moreira.
Coorientação: Prof. Dr. Ana Cristina de Oliveira Monteiro Moreira. 1. Lectinas. 2. Fruta-pão. 3. Cicatrização. 4. Receptor Toll-like 4. I. Título.
CLONAGEM E EXPRESSÃO DA LECTINA FRUTAPINA EM Escherichia coli:
ANÁLISES ESTRUTURAIS
Tese apresentada ao Programa de Pós-Graduação em Bioquímica da Universidade Federal do Ceará, como parte dos requisitos para obtenção do título de Doutor em Bioquímica. Área de concentração: Bioquímica Vegetal.
Aprovada em 04/05/2018.
BANCA EXAMINADORA
________________________________________________________ Prof. Dr. Renato de Azevedo Moreira (Orientador)
Universidade Federal do Ceará
________________________________________________________ Profª. Drª. Ana Cristina de Oliveira Monteiro Moreira (Co-orientadora)
Universidade de Fortaleza
_______________________________ Prof. Dr. Márcio Viana Ramos Universidade Federal do Ceará
______________________________ Prof. Dr. José Tadeu Abreu de Oliveira
Universidade Federal do Ceará
_______________________________ Profª. Drª. Ilka Maria Vasconcelos
Universidade Federal do Ceará
______________________________ Prof. Dr. Gilvan Furtado Pessoa
Federal do Ceará – UFC; Universidade Estadual do Ceará – UECE; Universidade de Fortaleza – UNIFOR e University College London (UCL). Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico – FUNCAP; Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq, pela concessão da bolsa para período sanduíche na UCL; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES, pela concessão da bolsa de estudos; Rede Nordeste de Biotecnologia -RENORBIO.
De todos os desafios que aceitei fazer nessa vida, até agora, esse é o de mais longa e complexa jornada. Trabalhar com biologia molecular envolve uma cascata de sentimentos, com a felicidade oscilando entre picos e vales dia-a-dia. Admiro hoje, muito mais, os profissionais desse campo e deixo aqui um conselho aos que um dia se aventurarão na mesma jornada: força, foco e fé. Esse trabalho não teria acontecido sem o acesso à brilhante equipe de profissionais que eu tive. A todos os envolvidos, estimo a minha mais profunda gratidão.
Agradeço aos meus orientadores Prof. Dr. Renato de Azevedo Moreira e Profa. Dra. Ana Cristina de Oliveira Monteiro Moreira pelo desafio, confiança no trabalho e por me fazerem crer que era possível chegar onde eu cheguei. Em especial ao Prof. Renato, que me desafiou a fazer dois doutorados ao mesmo tempo e acreditou que eu tinha potencial para isso. Desafio aceito e agora concluído com sucesso. Muito obrigado por todos esses anos de ensinamento e companheirismo, dos cafés às cervejas. À profa. Ana Cristina pela solicitude em atender minhas demandas e amizade nos momentos difíceis. É sempre um prazer trabalhar com vocês. O que aprendi nesse período será perpetuado em toda minha carreira acadêmica.
Agradeço a todos os membros da banca que contribuíram substancialmente para a melhoria desse trabalho, com suas sugestões e considerações finais na avaliação do manuscrito.
infraestrutura disponibilizada. Aos meus amigos Igor de Sá Carneiro e Gilvan Furtado Pessoa que se somaram e me fizeram ir além quando eu pensei que nada fazia mais sentido. Agradeço à Pedrinha Vasconselos e Tais Luz que me acompanharam também no início dessa jornada e hoje perseveram em caminhos além. A vocês eu agradeço as várias horas dedicadas ao desenvolvimento desse trabalho.
Durante esse trabalho fui contemplado com uma bolsa de estudos do programa Ciências sem Fronteiras. Por intermédio do Prof. James S. Owen, tive acesso aos pesquisadores David J. Abraham, Xu Shiwen e Korsa Khan do Centre
for Rheumatology and Connective Tissue Diseases, na University College London,
além dos pesquisadores Alun R. Coker, Jingxu Guo e Yiwei Guan, do Wolfson Institute for Biomedical Research. Sem vocês este trabalho não teria chegado tão
longe. Agradeço também às amigas que fiz durante esse período: Johanna Verneau, Athina Dritsoula, Bahja Abdi e Geerthana Jeyathas.
Ao Prof. Marcos Lourenzoni pelo suporte nos experimentos em bioinformática. Às minhas amigas, companheiras de pesquisa: Ayrles Brandão, Carol Viana e Mayara Matos. Aos meus grandes amigos Rogênio Mendes e Rosueti Diógenes filho, pela amizade que contribuiu na manutenção da minha sanidade mental.
"Permita-se sonhar um pouco. E sonhe alto sobre todas as coisas que você pode ser. Mas, sobretudo, permita-se."
RESUMO
As lectinas vegetais são proteínas que se ligam reversivelmente a carboidratos ou glicoconjugados, sem alterar a estrutura covalente dos mesmos. Devido a estas características inerentes, elas podem interagir com a superfície celular e exibir tanto propriedades inflamatórias e anti-inflamatórias, além de imunomoduladoras e imunoestimulantes. Artocarpus incisa é uma espécie comum em regiões
pantropicais, conhecida popularmente no Brasil como fruta-pão, onde é consumida cozida pela população. A frutapina (FTP) é a segunda lectina mais abundante em sementes de A. incisa, pertencente à subfamília de lectinas manose ligantes da
família das jacalin-like. Em estudos preliminares, FTP foi de difícil purificação, com
rendimentos muito baixos e contaminação com frutalina (a lectina mais abundante e de caráter multivalente nas sementes da mesma espécie). Neste trabalho, foi desenvolvido um sistema de expressão heteróloga da rFTP em Escherichia coli
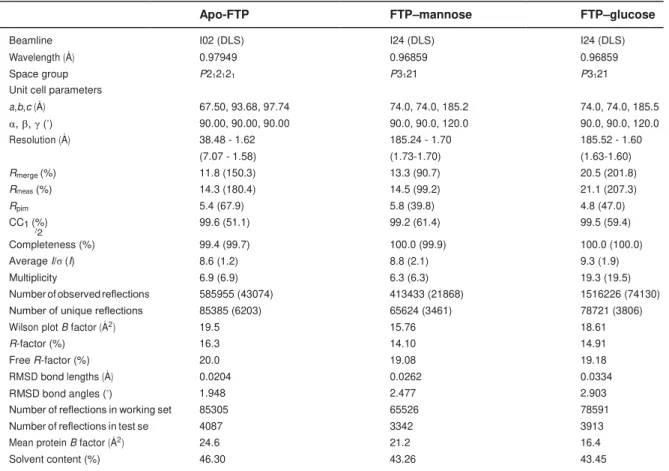
(BL21DE) usando a estratégia SUMO-tag, com rendimento médio superior a 40 mg de proteína solúvel por litro de cultura. Cristais da Apo-FTP, manose e FTP-glucose foram obtidos e submetidos à difração de raio-x , apresentando resolução de 1,58 (P212121), 1,70 (P3121) e 1,60 (P3121) Å, respectivamente. A melhor solução mostrou quatro monômeros por unidade assimétrica. Simulações com dinâmica molecular demonstraram que FTP exibe maior afinidade por manose do que glucose. A capacidade da rFTP em interagir com o receptor TLR4 foi avaliada (HEK-Blue™-hTLR4). Além disso, o efeito da FTP na fisiologia de fibroblastos, de pele humana, foi avaliado por meio de ensaios de migração celular, produção de IL-6 e
Western blot. A rFTP não mostrou citotoxicidade para os fibroblastos (<1 000 µg /
mL). A partir de 8 h de tratamento, amostras tratadas com FTP tiveram um número significativo de células migradas quando comparadas ao controle. A rFTP estimulou o TLR4 de maneira similar àquela observada com o LPS. Concentrações elevadas de IL6 foram observadas em fibroblastos tratados. Os níveis de pERK 1/2 e MyD88 foram significativamente maiores quando comparados com os dos controle, durante 24 h de tratamento (p< 0,05). Assim, este estudo mostrou que rFTP não foi citotóxica para fibroblastos humanos. Sabendo que fibroblastos desempenham um importante papel no reparo de tecidos, rFTP representa uma biomolécula com potencial terapêutico para a cicatrização de feridas e outras doenças de pele.
reversible binding to selective sugars in carbohydrates or glycoconjugates without altering their covalent structure. Due to these inherent features, lectins can interact with cell surface moieties and display both inflammatory and anti-inflammatory, as well as immunomodulatory and immunostimulatory, properties. Artocarpus incisa is a
widespread plant, common in pan-tropical regions, and popularly known as “ fruta-pão” (breadfruit) in Brazil, where it is consumed cooked by local populations. Frutapin
(FTP) is the second most abundant lectin in A. incisa seeds, belonging to the
mannose-binding subfamily of jacalin-related lectins (JRL). FTP proved difficult to purify with very low yields and contamination with Frutalin (a multiple-binding lectin and most abundant in the same species), which have frustrated its characterization so far. In this work, we developed a high-level expression system of biologically active recombinant FTP in Escherichia coli BL21 using a SUMO-tag strategy,
optimizing conditions with the best set yielding >40 mg/l culture of soluble active rFTP. Apo-FTP, FTP–mannose and FTP–glucose crystals were obtained, and they diffracted X-rays to a resolution of 1.58 (P212121), 1.70 (P3121) and 1.60 (P3121) Å, respectively. The best solution showed four monomers per asymmetric unit. Molecular dynamics (MD) simulation suggested that FTP displays higher affinity for mannose than glucose. The ability of rFTP to interact with TLR4 was evaluated using HEK-Blue™-hTLR4 cells. Also, the effect of FTP on fibroblast biology was assessed through cell migration assay, IL-6 production and western blot analysis. rFTP did not show cytotoxicity to human skin fibroblasts (<1,000 µg/mL). After 8 h, samples treated with FTP had significant numbers of migrated cells when compared to controls, migrating across the denuded area and effecting efficient wound closure. rFTP was able to potently stimulate the TLR4 pathway in a manner similar to that observed with LPS. High levels of IL6 production were observed when compared to untreated cells (p<0.0001). The levels of pERK 1/2 and MyD88 were significantly higher when compared to untreated cells after 24 h (p<0.05). In conclusion, this study showed that rFTP was non-cytotoxic to human fibroblasts. Given that fibroblasts play an important role in tissue repair, rFTP may represent a potential therapeutic biomolecule for wound healing and other related skin diseases.
SUMÁRIO
1 INTRODUÇÃO ... 14
2 AN OVERVIEW ON ARTOCARPUS LECTINS: PURIFICATION AND
BIOLOGICAL PROPERTIES ... 16
3 FRUTAPIN, A LECTIN FROM ARTOCARPUS INCISA (BREADFRUIT):
CLONING, EXPRESSION AND MOLECULAR INSIGHTS ... 51
4 FRUTAPIN, A BREADFRUIT (ARTOCARPUS INCISA) SEED-DERIVED
LECTIN, INTERACTS WITH TLR4 AND INCREASES IL-6 PRODUCTION BY SKIN FIBROBLASTS ... 69
5 CONSIDERAÇÕES FINAIS... 85
1 INTRODUÇÃO
Bastante difundidas na comunidade científica, as lectinas vegetais possuem uma grande versatilidade em aplicações biotecnológicas envolvendo atividade antimicrobiana, antiviral e inseticida, além de serem imunoestimulantes e imunomoduladoras, apresentando propriedades tanto pró-inflamatória como anti-inflamatória. Essa versatilidade é devida à definição intrínseca das próprias lectinas, a qual engloba proteínas ou glicoproteínas capazes de se ligar reversivelmente a glicanos sem alterar a estrutura covalente dos mesmos.
Curiosamente, nas sementes de Artocarpus incisa, conhecida popularmente
como fruta-pão, é possível encontrar três lectinas com reconhecimento distinto a carboidratos, citadas aqui em ordem de abundância nas sementes: frutalina, frutapina e frutaquina. Esse padrão também foi observado nas sementes de jaca, a
Artocarpus integrifolia, com as lectinas jacalina, artocarpina (KM+ ou ArtinM) e
jacaquina. Frutalina e Jacalina são as lectinas mais abundantes nessas espécies, com alta homologia estrutural, porém com diversos estudos demonstrando diferentes aplicações biológicas. Da mesma forma, se supunha frutapina e ArtinM, as lectinas glucose/manose ligantes, como homólogas estruturalmente. ArtinM já havia sido reportada como potente estimuladora do sistema imune, além de apresentar potencial para aplicações em cicatrização de feridas.
Por outro lado, a frutapina, apesar de ser a segunda lectina mais abundante em extratos de sementes de fruta-pão, foi pouco estudada devido ao seu baixo rendimento e laborioso processo de isolamento resultante de sucessivas cromatografias até eliminação total da frutalina em preparações. Isso se deve ao fato da frutalina, apesar de grande afinidade por glicanos contendo α-D-galactose, ser também capaz de reconhecer glucose e manose, o que impactou no isolamento e caracterização da frutapina até então.
Com o advento da tecnologia do DNA recombinante, associada à facilidade de se manipular microrganismos, tais como E. coli para clonagem e expressão
O que permite reunir material que fomenta o estudo da estrutura e função das proteínas obtidas, para aplicações clínicas ou industriais. Dessa forma, essa abordagem impulsionou os objetivos deste trabalho, divididos aqui em três capítulos, a fim de se promover um melhor entendimento do assunto a ser abordado.
No capítulo I, uma revisão com um histórico sobre as lectinas encontradas no gênero Artocarpus é apresentada, a fim de situar o leitor no escopo do trabalho.
O capítulo II traz como objetivo a clonagem e expressão da frutapina recombinante. A metodologia foi otimizada para a produção heteróloga da lectina em Escherichia coli, com alto rendimento (>40 mg de frutapina/L de cultura) e preservação da
funcionalidade da mesma. E por fim, resultados prévios in vitro demonstraram o
2 AN OVERVIEW ON ARTOCARPUS LECTINS: PURIFICATION AND
BIOLOGICAL PROPERTIES
Felipe Domingos de Sousa1,2, Ana Cristina de Oliveira Monteiro-Moreira1 and
Renato de Azevedo Moreira1,2
1Northeast Biotechnology Network (RENORBIO), Center of Experimental Biology (Nubex), University
of Fortaleza (UNIFOR), Av. Washington Soares, 1321, Edson Queiroz, CEP 60811-905, Fortaleza-Ceará, Brazil; 2Department of Biochemistry and Molecular Biology, Federal University of Ceará (UFC),
Campus do Pici, S/N, Bloco 907, CEP 60451-970, Fortaleza-Ceará, Brazil.
Resumo
O gênero Artocarpus (Moraceae) compreende cerca de 60 espécies de árvores
perenes e caducifólias, incluindo aquelas de notável valor como fonte de frutos comestíveis e usos na medicina popular. Entre elas, a jaca é a mais estudada como fonte da lectina jacalina, que após sua descoberta, deu nome à família de lectinas relacionada à Jacalina (JRL). Aqui, nós revisamos este gênero e família com ênfase especial no padrão incomum de espécies de Artocarpus, discutindo diferentes
lectinas com três propriedades distintas de reconhecimento de carboidratos e focando em lectinas de jaca, fruta-pão e chempedak. Apesar de sua alta homologia estrutural, as lectinas de Artocarpus apresentam uma ampla gama de atributos bioquímicos e biológicos com diferentes aplicações.
Abstract
The genus Artocarpus (Moraceae) comprises about 60 species of evergreen and
deciduous trees, including those of notable value as sources of edible fruit and uses in folk medicine. Among these, jackfruit is the most studied as the source of the lectin jacalin, which after its discovery gave name to the Jacalin-related lectin (JRL) family. Here, we review this genus and family with special emphasis on the uncommon pattern of Artocarpus species, discussing different lectins with three distinct
carbohydrate recognition properties and focusing on jackfruit, breadfruit and chempedak lectins. Despite their high structural homology Artocarpus lectins display
Introduction
Artocarpus is a genus comprising about 60 trees and shrubs of Southeast Asian and
Pacific origin, belonging to the Moraceae family; all species are lactiferous with
leaves, twigs and stems producing milky sap. The name is derived from the Greek
words artos (“bread”) and karpos (“fruit”). Although this flora type produces unisexual
flowers both sexes can be found in the same plant. After pollination, the female
flowers can grow into a syncarpous fruit, and these can reach very large sizes.
Leaves vary from relatively small and entire such as Artocarpus integer to large
divided ones such as Artocarpus altilis in which each division ends in long and sharp
tips. Indeed, in the most recent revision of the genus, the highly variable species A.
communis is a complex of three species of breadfruit: A. altilis, A. mariannesis, and
A. camansi [1]. All these species are similar in terms of the general morphology of the
fruit, but upon ripening they differ both in taste and in composition.
Although most species of Artocarpus are restricted to Southeast Asia, such as A.
hypargyreus (kwai muk), A. lakoocha (lakoocha), A. kemando (pudau), A. hirsutus
(anjily), A. chama (chaplaish), and A. odoratissimus (marang), several species are
widely distributed and cultivated throughout the tropics due to their edible fruits and
timber. These include A. heterophyllus (jackfruit), A. altilis, and A. integer (cempedak,
also known as chempedak) [1–3], well-known species conspicuous as substantial
sources of plant lectins which are readily recovered from their seed flour.
Lectins (from Latin “legere”, to pick out or choose) are proteins or glycoproteins
found in nonimmune nature which can specifically recognize and reversibly bind
Indeed, this attractive characteristic distinguishes lectins from other carbohydrate
binding proteins and enzymes. They are also widely distributed in the plant kingdom,
usually from leguminous seed origin and play crucial roles and functions in biological
processes such as molecular recognition, storage proteins and plant defence
mechanisms. Their interaction with glycosyl ligands occurs mainly through hydrogen
bonds, van der Waals’ forces, hydrophobic interactions and, less frequently,
electrostatic interactions [4]. Here, we focus on the structural features of lectins from
jackfruit, breadfruit and chempedak (Figure 1) and their biological applications.
Artocarpus heterophyllus
Also known as A. intrifolia or A. integrifolius, the jackfruit is one of the most studied
species within the Artocarpus genus. The exemplars of this species are monoecious,
presenting both male and female flowers on the same tree, and growing in several
tropical countries. Jackfruit trees can reach about 25 m high bearing one of the
biggest fruits in the world with a length up to 90 cm and 50 cm diameter, weighing up
to 40 kg. About 100-500 seeds are enclosed in each fruit to comprise about 15% of
the weight [5,6]. These seeds contain not only carbohydrates (~50%) and proteins
(~20%), but also antinutritional factors such as trypsin inhibitors, saponin, tannin and
oxalate [7,8]. As regards the ripening process, the fruits are found in two forms: some
trees produce hard fruits in which the perianth remains firm even at full ripeness,
while others produce fruits whose perianth become soft and pulpy on ripening. The
textural differences between the two forms appears due to different capacities to
produce pectic enzymes and control cell wall degradation [9].
asthma, dermatitis, anemia, diarrhea and fever; antisyphilitic and anthelmintic
properties; sedative effects in convulsions; and also wound healing properties [10].
Indeed, jackfruit are in high demand in areas such as cosmeceutical, pharmaceutical
and natural food handling for supplement markets, due to numerous studies on
phytochemical and pharmacological properties of all parts of the plant (pulp, leaf, root
and bark) [11–13].
Extracts and metabolites from jackfruit possess several useful bioactive
compounds and may confer multiple health-promoting effects for heart and skin
disorders, and to treat ulcers and cancer [14]. Moreover, new studies of jackfruit
properties provide additional biological findings consistent with antibacterial,
antitubercular, antiviral, antifungal, antiplatelet and antiarthritic actions, thereby
indicating therapeutic applications [10]. It is noteworthy that crude extracts of jackfruit
are rich in jacalin, a lectin with wide-ranging bioactivities as discussed in later
sections of this review.
Artocarpus altilis (Parkinson) Fosberg
The Pacific Islands are the centre of origin and diversity of breadfruit (A. altilis),
sometimes referred to as A. communis or A. incisa. The species was derived from a
seeded, diploid ancestor, A. camansi, giving two varieties, one seeded (var.
seminifera) whose rind spines are as prominent as those on jackfruit, and one
seedless (var. apyrena), which presents a spineless outer layer. The latter is
well-appreciated by local Brazilians when cooked, because of its large starchy content of
compound fruits with high levels of minerals and provitamin A carotenoids [1,3,15–17].
lost the ability to produce viable seed [18].
Also found is a monoecious species, in which breadfruit flowers fuse together to
give the edible portion of the fruits. These are usually oval with weight ranging from
0.25 to 5.0 Kg. Exemplars of the A. altilis var. seminifera do not present an edible
pulp, and the seeds are consumed toasted in Northeast Brazil [3,15,19]. Fruits are
usually cooked at the starchy stage, which is reached when the fruit is totally mature,
becoming soft and sweet. The species begins producing fruits after 3-5 years and
can keep producing nutritious fruit for decades. Breadfruit is an excellent dietary
staple, often compared with other starchy crops such as sweet potato, corn and rice.
Indeed, it is an energy-rich food, presenting high levels of complex carbohydrates,
fibre and minerals [3]. Due to these features, its flour was approved in 2016 by the US
Food and Drug Administration (FDA) as a food Generally Recognized as Safe
(GRAS).
Several studies have identified bioactive compounds within A. altilis extracts and
explored their pharmacological or biological effects. Thus, although the species is
extensively used in traditional medical practices throughout the tropics, many have
been successfully proven scientifically. For example, based on acute toxic studies in
rats [20], A. altilis leaf and bark extracts are considered safe for therapeutic use, while
the traditional use of breadfruit leaves to treat hypertension and diabetes is supported
by experimental data [21,22].
Artocarpus integer
Although widely known in the tropics as chempedak or campedak, Artocarpus integer
species is a monoecious tree reaching heights of 10-30 m, bearing fruits containing
from 100 to 500 seeds. The fruit is syncarp and cylindrical growing up to 30 cm long,
remaining attached to the trunk or main branch by a peduncle of 7-12 cm long. The
pulp covering the seeds within the fruit is popular for its fragrant taste. However, the
hard seeds can also be cooked and eaten [2], having a pleasant, nutty flavor and
comprised mainly of carbohydrate, protein, and dietary fibre [23].
The A. integer species is rich in phenolic compounds such as flavones possessing
cytotoxic and antimalarial activities. Artoindonesianin A-2, artoindoneisianin A-3,
heterophyllin, cudradaflavone C, artoindonesianin T have all been isolated,
presenting a strong cytotoxic activity against murine leukemia P-388 [24]. Among
these, the potent inhibitory activity of heteroflavanone C against growth of the
Plasmodium falciparum 3D7 clone supports folk medicine use of chempedak dried
stem bark as an antimalarial [25]. Likewise, a chempedak paste of the inner bark
prevents infection and helps healing when applied on wounds, as well as heated leaf
extract. The fruits and products of A. interger are not only conspicuous as sources of
pharmacoactive molecules but also as providers of natural and readily available
instant energy [26,27].
Jacalin-related lectin family
Lectins were first discovered in plants and, although ubiquitously distributed in nature
(animals, insects, viruses, fungi and bacteria), the majority have been characterized
from plant protein extracts, reflecting ease of extraction and relatively high yields
usually via a simple one-step affinity chromatographic method [28]. Due to this
carbohydrate specificity, evolutionary relationships and sequence or tri-dimensional
structural similarities. As regards amino acid sequences, the carbohydrate binding
property of most lectins consists mainly of hydrophobic interactions involving five to
six amino acids residues that bind sugar hydroxyl groups. This
carbohydrate-recognition domain within a polypeptide sequence is termed the Carbohydrate
Binding Site (CBS). Generally, lectins are classified into five groups based on their
affinity for (i) Glucose/Mannose; (ii) Galactose and acetyl-D-galactosamine; (iii)
N-acetylglucosamine; (iv) L-fucose, and (v) Sialic acid [29].
After the discovery of jacalin, novel lectins sharing high homology to it were placed
into a family of jacalin-related lectins (JRL), now divided into two different subgroups.
The first comprises galactose-specific lectins (gJRL) and a few other Moraceae
lectins, which exhibit specificity towards galactose and are built up of subunits
consisting of a short β chain and a long α chain. The second is the mannose-binding
subgroup (mJRL), which occurs in different plant families with the lectins exhibiting
exclusive specificity towards glucose/mannose residues with the binding subunits
contained within a single polypeptide chain [30,31].
Jacalin itself is synthesized as a preproprotein on the rough endoplasmic reticulum
and follows a secretory pathway comprising a complex series of co- and
post-translational modifications: signal peptide removal, a glycosylation, removal of an
N-terminal propeptide, and excision of a linker tetrapeptide. The immunocytological
analysis of jackfruit cotyledons revealed that jacalin accumulates in small punctuate
structures distributed throughout the cytoplasm. On the other hand, mannose-specific
mature lectin polypeptides comprise the entire open reading frame of the
corresponding genes [32,33]. In contrast, Helja, a JLR from Helianthus annuus
extracellular fluid is found as an unconventionally secreted lectin, being detected in
apoplastic vesicles. Evidence suggests that Helja is likely associated with
multivesicular bodies released after fusion of these vesicles with the plasma
membrane [34].
From another perspective, glucose and galactose are epimers of each other,
differing only in the hydroxyl group position at C4, which is important for sugar
recognition by galactose-binding lectins. Glucose and mannose differ in the position
of the hydroxyl group at C2, placing the glucose/mannose-binding lectins in a
different JRL subgroup family [30]. Additionally, comparative biochemical studies
highlight lectins as a very heterogeneous group of proteins based on their
carbohydrate recognition pattern, which reflects their biochemical/ physicochemical
properties, molecular structure, and biological activities [35].
Galactose-binding lectins
Jacalin
The occurrence of jacalin, the D-galactose-binding lectin from A. integrifolia seeds
was first reported in 1979 [36] and found to exceed 50% of the protein in jackfruit
crude seed extracts. This is also the case for galactose-binding lectins in the
Artocarpus genus such as frutalin and CGB (champedak galactose-binding). Jacalin
is a tetrameric two-chain lectin of 65 kDa combining a heavy α-chain of 133 amino
acids and light β-chain of 20–21 amino acid to form a 3D structure as a single
which correspond to glycosylated and slightly or non-glycosylated forms, respectively
[37].
Jacalin presents a characteristic subunit fold, known as type-1 β-prism fold. This
type of subunit fold has three four-stranded β-sheets parallel to each other arranged
in a prism. The subunit structure shows a tetramer with 222 symmetry and the
asymmetric unit of the crystal consists of four subunits, two each from two
independent tetramers that occupy crystallographic two-fold special positions. This
prismatic organization of the subunit is stabilized by hydrophobic interactions in the
core of the subunit and a small number of hydrogen bonds involving main-chain, as
well as side-chains, atoms [38].
The CBS of jacalin mainly involves residues Gly1, Tyr78, Val80, Gly121, Tyr122,
Trp123 and Asp125. In D-galactose-jacalin complexes, the O4 hydroxyl group of the
galactose axial position forms hydrogen bonds with the side chain of Asp125 and the
terminal amino group of Gly1. If O4 is at the equatorial position, as in glucose and
mannose, Asp125 can still interact with O4, but not the amino group. This explains
the high specificity of jacalin for galactose compared to glucose and mannose at the
primary binding site. Additionally, the post-translational cleavage and removal of the
“T-S-S-N” peptide linker assure a stronger hydrogen bond connecting the α- and β-
chains, as non-cleavage leaves a neutral peptide NH group [38,39].
The whole CBS of jacalin is assumed to have a secondary binding site A, the
primary site and secondary binding site B which give an extended anchoring domain
for the sugars. Hence, the primary binding site is made up of the side chains of
Trp123 and the free amino group of Gly1 of the α-chain. Among these, the side chain
of Tyr78 stacks against the B-face of the galactose ring. The aromatic side chains of
Tyr78, Tyr122, and Trp123 form the secondary binding site A, which is important to
binding of methyl α-mannose (Me-α-Man), facilitating the weak lectin-carbohydrate
interaction of the methyl group with this wholly hydrophobic region. Lastly, at the
secondary site B, the ligand is involved only in water mediating interactions with the
main chain nitrogen and oxygen atoms of Val79, Ser119 OG, and the carboxyl
terminal region of the β-chain [40,41]. This acquired plasticity of the CBS has been fully
documented using molecular dynamics and X-rays studies [42].
Frutalin
As of 1983, our group started to survey lectins in A. incisa seeds and found lectins
with similar behaviour to those in jackfruit seeds [43]. Breadfruit seeds are composed
of a high water content (up to 60%) and moderate protein content (12.25% of dry
weight). Most of this protein is recovered as frutalin in chromatographic methods
using crude extracts of seed flour. Therefore, frutalin is the most abundant lectin of
this species, presenting multiple-binding properties in which the same CBS
recognizes a range of different ligands, although it has higher affinities for α
-D-galactose monosaccharides and complex carbohydrates that contain Galα1–3
glycans [17].
Frutalin has a broad band pattern by isoelectric focusing with pI between 8.8 and
9.0, and a deconvoluted mass spectrum with different masses within 16.5 kDa,
consistent with the presence of glyco-isoforms of identical monomers. These
carbohydrate content can reach 2.1% of the total protein mass. The SDS-PAGE
pattern of frutalin presents a jacalin-like pattern of two bands between 20 and 14 KDa
that correspond to glycosylated and non-glycosylated fractions, respectively. Indeed,
three sites of N-glycosylation are predicted for jacalin based on necessary
Asn-Xaa-Ser/Thr consensus sequences and also on other gJRL lectins. In solution, frutalin
may form homotetramers in which each monomer contains an α-chain (16 kDa) and
a β-chain (2 kDa) [17,44]. This tetrameric form can be disturbed by thermal and
chemical denaturation as was described by spectroscopy and chromatographic
studies [45]. It was shown that the sugar-binding site serves as a nucleus at the
monomer level to increase the stability of D-galactose-frutalin complexes during the
unfolding process [45].
The CBS of frutalin in the binding of galactose is dominated by hydrogen bonding
through O1, O3, O5 and O6, and backbone/side chain hydroxyl groups. Similar to the
Moraceae lectins, the CBS of frutalin is located in a domain close to the N-terminus
of the α chain, consisting of four key residues Gly25, Tyr146, Trp147 and Asp149.
About ten interactions occur, involving the C1 hydroxyl to residue Tyr146, hydroxyl
C3 to residue Gly25, hydroxyl C4 to residues Gly25 and Asp149, and hydroxyl C6 to
residues Tyr146, Trp147 and Asp149 [46]. Moreover, there is evidence to suggest that
frutalin possesses stereospecificity, capable of specifically binding α-D-galactose,
since it was previously isolated on a cross-linked galactomannan column, but not on
β-galactosyl-immobilized matrices [17,47].
Champedak galactose-binding lectin (CGB)
high levels in extracts from its seed flour. The lectin’s bioactivity was first noted when
extracts were tested for selective stimulation of peripheral blood mononuclear cells;
at 20 µg/mL CGB stimulated proliferation of T-lymphocyte populations [48,49]. Like
frutalin and jacalin, CGB is transcribed as a propeptide and then post-translationally
processed into a typical gJRL lectin, having a 13-kDa α-chain (133 amino acids) and
a 2.1-kDa β-chain (21 amino acids). CGB has 6 different amino-acids compared to
jacalin, giving 97% identity for their amino-acid sequences [50].
As part of structural analyses, CGB lectin complexes with galactose (Gal) and
galactose-β1–3-galactose-NAc (GalNac) were obtained by crystal soaking
techniques, though attempts to obtain a CGB-mannose complex was unsuccessful
[51]. As with jacalin complexes, the interactions are well conserved, presenting the
same CBS. In Gal-CGB complexes, the O atoms on the sugar ring are bound with
the side-chain and main-chain N and O atoms on the α chain (O3 and Gly1 N; O4
and Gly1 N and Asp125 OD1; O6 and Trp123 O, Trp123 N and Tyr122 N; and O5
and Tyr122 N). Similarly, GalNac-CGB complexes present bound disaccharide in the
same region though hydrogen bonds (O3 and Gly1 N; O4 and Gly1 N and Asp125
OD1; and O6 and Asp125 OD1). Also, there are a number of hydrophobic
interactions contributed by Tyr78, Gly121 and Tyr122. Although closely related to
jacalin’s structure, CGB was unable to bind mannose as judged by isothermal
calorimetry and co-crystallization studies. It is believed that this change in CGB
specificity is caused by subtle differences in the environment near the sugar-binding
Mannose-binding lectins
Artocarpus seeds are also the source of several mannose-binding jacalin-related
lectins (mJRLs). These proteins have potential utility based on their carbohydrate
recognition, which display specificity toward Man and oligomannosides. However,
biological application of these molecules is often impaired due to their low
concentrations in extracts, in addition to contamination with gJRLs lectins from the
same source, some being multiple-binding lectins.
ArtinM
Further studies post-discovery of jacalin revealed that jackfruit seed extracts also
possess small amounts of a D-mannose-binding lectin [52]. Previously named
artocarpin, the term was provisionally replaced by the name KM+; the lectin had
become confused with distinct substances from Artocarpus spp. which were similarly
designated. Additionally, artocarpin was used to name the galactose-binding lectin in
Artocarpus lakoocha seeds [53].
The origin of the name KM+ comes from the successive affinity chromatography
steps in immobilized D-galactose matrices to remove jacalin (retained fraction J). The
unretained fraction was called K, while M+ refers to the retained fraction on
immobilized mannose matrices. Nevertheless, there remained confusion over this
adopted nomenclature, which led to the proposal for a rational name change to
ArtinM based on the universal code for plant proteins. This takes into account both a
lectin’s origin and specificity of sugar recognition [54]. Hereafter, we will apply ArtinM
ArtinM is a single polypeptide of 150 amino acids devoid of covalently attached
carbohydrates with four isolectins and a pI range of 5–6.5, sharing 52% sequence
identity with jacalin [55]. In solution, the protein is built up in a homotetrameric complex
with one CBS per protomer. Isothermal titration calorimetry was employed to
determine the thermodynamics of the carbohydrate-protein binding reaction,
revealing that mannose (1640 ± 36 M-1, K
b) is preferred over glucose (150 ± 20 M-1, Kb). However, ArtinM presents low affinity for simple sugars, interacting potentially with mannotriose (20100 ± 900 M-1, K
b) and a mannopentaose (21200 ± 600 M-1, Kb)
[56,57].
Unlike jacalin, there are no aromatic residues on the CBS of ArtinM, comprising
Gly15, Asp138, Leu139, and Asp141. Indeed, it is believed that the galactose
specificity of jacalin was achieved by a two-step process with ArtinM as its putative
precursor: mutation of key aliphatic residues close to the sugar binding pocket to
aromatic ones; and then cleavage of a short loop, a key step as it creates a positively
charged N-terminal which interacts specifically with O4 at the axial position. The
structures of ArtinM complexed with mannotriose and mannopentose display an
involvement of three loops in the sugar recognition. Two of them compose the
primary binding site through hydrogen bonds, while the third one is involved in van
der Waals interactions. Mannotriose in its complex with the lectin interacts through all
the three mannopyranosyl residues, while mannopentose interacts with the lectin
using at least three of its five mannose residues. Compared to Heltuba lectin, this
Frutapin
Frutapin (FTP) is the next most abundant lectin in breadfruit seed extracts.
Preliminary FTP investigations began in 1998, describing three different lectins with
distinct carbohydrate recognition within the same species [60]. However, further
studies proved difficult since native FTP was hampered by very low yields and
contamination with Frutalin, a notable factor as frutalin binds diverse sugar moieties
and has high abundance in plant extracts. Recombinant FTP production is now a
feasible approach to circumvent this problem, allowing large-scale heterologous
expression to give continuous supplies for further characterization and to improve
potency, particularly for biomedical applications [61].
Recognizing this possibility, we optimized a high-yield production protocol to
isolate biologically active recombinant FTP in Escherichia coli BL21 (>40 mg/L
culture) [62]. Moreover, molecular insights of carbohydrate recognition by FTP were
obtained through crystal structures and molecular dynamics (MD) using mannose
and glucose-FTP complexes. FTP shares 91% of sequence identity with ArtinM,
shows a β-prism I fold similar to that in jacalin, and has high structural homology
except for a few loop areas around residues Leu90, Glu36 and Lys106.
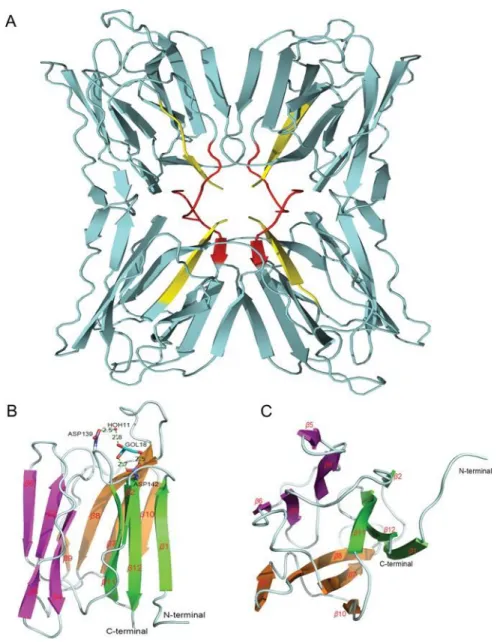
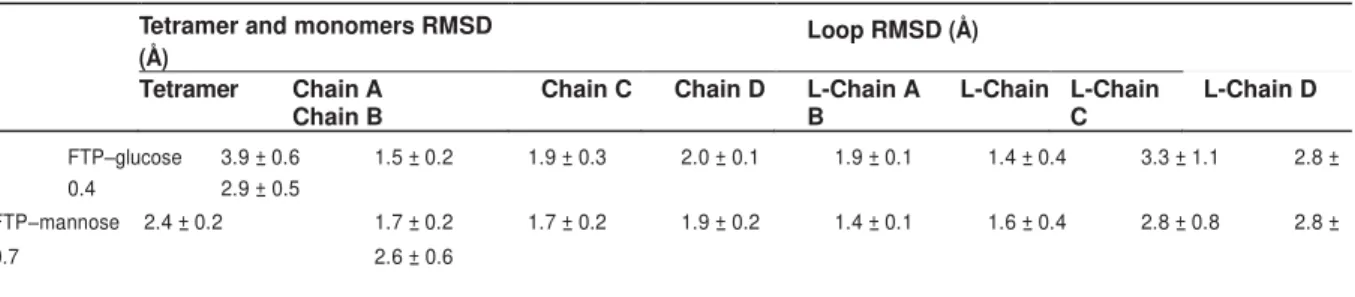
FTP is a hololectin defined as a homotetramer with an identical CBS per protomer,
able to bind either the same or structurally similar sugars. The CBS is formed of four
residues, namely Gly16, Asp139, Leu140 and Asp142, distributed in a few loops
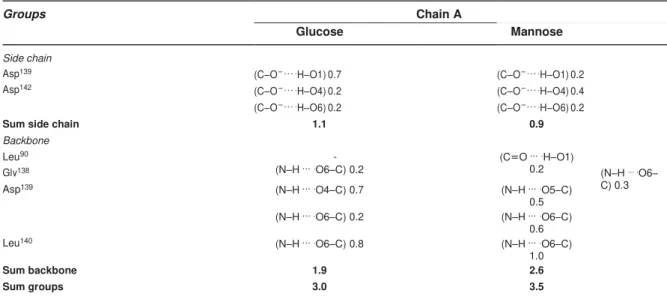
connecting the strands β5 and β6, β7 and β8, and β11 and β12. Several hydrogen
bonds (HB) occur in FTP–glucose and FTP–mannose complexes involving Gly16,
In MD simulations, Lys60 plays an important role forming salt bridges with Asp139 in
FTP-glucose complexes, reducing the interaction between this former residue and
mannose and minimizing the repulsion of the mannose hydroxyl groups with oxygen.
In this case, mannose was more completely surrounded in the carbohydrate-binding
site and also stabilized by indirect interaction with Asp139 through water molecules.
This local structuring is more stable in the case of mannose than glucose, indicating
a higher affinity of FTP for mannose residues than glucose [62].
Champedak mannose-binding (CMB)
Extracts of Artocarpus integer seeds are a source of CMB, previously termed
champedak lectin-M [63]. The lectin is ~20-fold more abundant than ArtinM in crude
extracts of A. heterophyllus seeds. Structural studies reveal CMB to be a 64-kDa
tetramer with some of the polypeptides being disulphide-linked to give dimers. The
functional activity of CMB was assessed by studying interactions with different
isotypes of human immunoglobulins: strong binding to IgE and IgM was noted, unlike
CGB and jacalin which strongly interact with IgA1. The lectin also shows a similar
pattern of carbohydrate binding specificity to ArtinM with Man-α-1–3Man the most
potent inhibitor, followed by methyl-α-D-mannopyranose and D-mannose [63].
Further studies allowed full characterization of the CMB sequence and structure.
At least 8 amino acids were different in CMB compared to ArtinM, though 97%
identity was reached, while their crystals belonged to space group P212121. As with
CGB, the CMB monomer is a β-prism made up of a single polypeptide chain, with
four chains forming the asymmetric unit. The sugar-binding properties of CMB differ
only a weak interaction with mannose (0.18 x 10-3 M,K
b), and no sign of binding to galactose. Indeed, a crystal structure of CMB with mannose is not yet available.
Based on the high sequence similarity between CMB and ArtinM, with all
sugar-binding residues (Gly15, Asp138, Leu139 and Asp141) conserved, it is hypothesized
that the two have similar binding characteristics; for example, that the binding site is
formed by the loops between β5 and β6, β7 and β8, and β11 and β12, as in the CGB
lectin. In this CBS, modelled mannose implies hydrogen bonds between O6 and
Asp139, Leu140 and Asp142, O4 and Asp142, and O3 and Gly16. There are also
hydrophobic interactions between mannose and residues Gly15 and Gly138 [50,64].
Chitin-binding lectins
Jackin and Frutackin
Chitin is a key component on the cell wall of fungi. As a result, chitin-binding lectins
have been isolated from diverse sources and screened for antifungal activity against
phytopathogenic species. Such lectins are particularly attractive, since they affect
plant-pathogen interactions at the first point of contact. Although generally accepted
that they are unable to penetrate into the fungal cytoplasm, owing to the cell wall
barrier, they are expected to affect fungal growth and development, disturbing the
synthesis and/or deposition of chitin in the cell wall [28,65].
Besides jacalin and ArtinM, jackfruit seeds are the source of a third lectin named
jackin due to its affinity for chitin. Further studies of breadfruit seeds also revealed a
similar lectin, named frutackin. Both lectins have a 14 kDa polypeptide chain, built up
lectins showing their homology to each other in terms of molecular mass, secondary
structure, and primary sequence, but not to other plant chitin-binding proteins. Both
jackin and frutackin inhibit the growth of F. moniliforme and S. cerevisiae [66].
Nevertheless, their low yield from natural sources still hampers further
characterization, though this might soon be overcome with high-yield heterologous
production in microbial systems.
Summary: despite high structural homology, Artocarpus lectins differ in
biological properties
It appears that the Artocarpus genus employs a plurality of lectins, though to date few
of these lectins have been purified and functionally characterized. Nevertheless, a
consistent pattern is that Artocarpus seeds contain more than one lectin with distinct
carbohydrate recognition features [5,60,66,67]. Altogether, the JRL family is complex with
a vast diversity in biochemical properties and activities, which have drawn much
attention because of their pivotal biomedical applications. Owing to
carbohydrate-binding interactions of plant lectins with cell wall glycoproteins, they are promising
targets to selectively modulate immune responses in plants. Hence, it is important to
delineate the molecular details of lectin binding to CBS domains and how
downstream activation of cellular immune signalling proceeds [68]. In this context, it is
of particular note that Artocarpus lectins have a distinct repertoire of biological
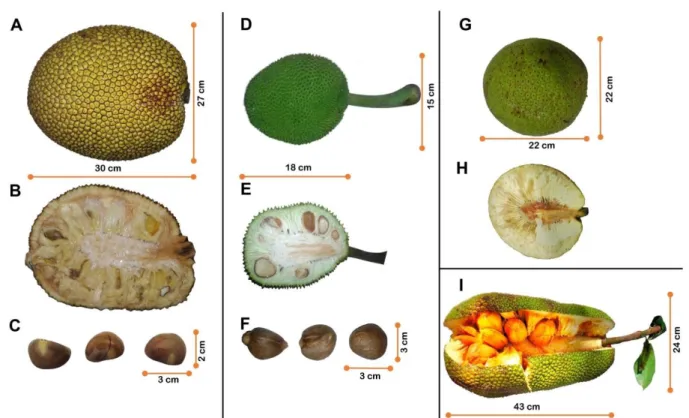
Figure 1.Artocarpus species.
(A) Jackfruit. (B) Cut section of jackfruit. (C) Jackfruit seeds. (D) Breadfruit (var.
seminifera). (E) Cut section of breadfruit (var. seminifera). (F) Breadfruit (var.
seminifera) seeds. (G) Breadfruit (var. apyrena). (H) Cut section of breadfruit (var.
Figure 2. Sequence alignment using Clustal W and structural superposition of
Artocarpus lectins.
(A) Sequence alignment of galactose-binding lectins: Jacalin (PDB 1UGW), Frutalin
(PDB 5BN6) and CGB (PDB 4AKB). (B) Sequence alignment of mannose-binding
lectins: ArtinM (PDB 1J4S), Frutapin (PDB 5KRP) and CMB (PDB 4AKD). (C)
Structural superposition of galactose-binding lectins with their carbohydrate
interacting residues in complex with D-galactose: jacalin (cyan), frutalin (red) and
CGB (mangeta). (D) Structural superposition of mannose-binding lectins with the
carbohydrate interacting residues highlighted: ArtinM (green), frutapin (yellow) and
Table 1. Biological activities and biochemical studies on Artocarpus seed
lectins.
Lectin Study Focus [Reference]
Jacalin
Jacalin crystals in complex with methyl-α-galactose: structural basis of carbohydrate recognition [69].
Jacalin specifically triggers cell signaling in CD4+ T lymphocytes [70]. Crystal structure of the jacalin-T-antigen complex and a comparative study of lectin-T-antigen complexes [71].
Structural basis for the unusual carbohydrate-binding specificity of jacalin towards galactose and mannose [41].
The multiple binding profile of Jacalin [72].
Further studies on CD4-mediated signal transduction and HIV-impaired CD4+ T cell activation by jacalin [73].
Structural basis for the energetics of jacalin-sugar interactions: promiscuity versus specificity [74]
Jacalin induces human B-lymphocyte apoptosis through glycosylation-dependent interaction with CD45 [75].
Specific interaction of jacalin with phycocyanin: the lectin as a carrier for targeted delivery of a fluorescent phycobiliprotein [76].
Jacalin can interact with desmoglein-1 and abrogates the pathogenicity of pemphigus foliaceus autoantibodies in vivo[77].
Piezoelectric biosensoring studies on jacalin interaction with human IgA1 and bovine IgG1[78].
Synthesis and characterization of jacalin-gold nanoparticles conjugates as specific markers for cancer cells [79].
Spectroscopic investigation on the interaction of ruthenium complexes with jacalin [80].
Interaction of sugar stabilized silver nanoparticles with jacalin [81].
Proinflammatory activity of jacalin: jacalin-activated macrophages exhibit an antitumor phenotype [82].
Interaction of cadmium sulfide quantum dots with jacalin for specific recognition of cancer cells [83].
activity against drug resistant bacteria via cell surface glycan recognition [84].
Jacalin capped platinum nanoparticles confer persistent immunity against multiple Aeromonas infection in zebrafish [85].
Frutalin
Preliminary survey of lectins in breadfruit extracts [43,86] Isolation and partial characterization of frutalin [17]. Unfolding and refolding studies of frutalin [45]
Modulation of neutrophil (PMN) functions by frutalin [87].
The mechanisms involved in the mitogenic effect of frutalin on human lymphocytes [88].
Expression of frutalin in the yeast Pichia pastoris [89]. Functional expression of frutalin in Escherichiacoli [90].
A comparative study of recombinant and native frutalin binding to human prostate tissues [91].
The specificity of frutalin lectin using biomembrane models [92].
Cytotoxic effects of native and recombinant frutalin on Hela cervical cancer cells [93].
Gastroprotective potential of frutalin against ethanol-induced gastric lesions [94].
Enhanced heterologous protein production in Pichia pastoris under increased air pressure [95].
Crystallization and preliminary X-ray diffraction studies of frutalin [44] Frutalin reduces acute and neuropathic nociceptive behaviours in rodent models of orofacial pain [96].
A panel of glycoproteins as candidate biomarkers for early diagnosis and treatment evaluation of B-cell acute lymphoblastic leukemia [97]. Label-free proteome analysis of plasma from patients with breast cancer: stage-specific protein expression [98].
Neuropharmacological characterization of frutalin in mice: Evidence of an antidepressant-like effect mediated by the NMDA receptor/NO/cGMP pathway [99].
Crystallization and preliminary structural studies [101].
Structures and binding specificity of galactose- and mannose-binding lectins from chempedak [50].
Conformational analysis of CGB under different urea concentrations [102].
ArtinM
Crystal structures of ArtinM and its complex with methyl-alpha-D-mannose: implications to the generation of carbohydrate specificity [58]. Carbohydrate-binding properties of ArtinM [103].
Structural basis for the carbohydrate specificities of ArtinM [59]. Neutrophil haptotaxis by ArtinM [104].
Further studies on neutrophil activation induced by ArtinM [105].
Release of inflammatory mediators and enhancement of effector functions by neutrophil activation induced by ArtinM [106].
Effect of ArtinM on human T lymphocyte activation and cell death [107]. Corneal epithelial wound healing induced by ArtinM [108].
Protection against Paracoccidioides brasiliensis infection conferred by
the prophylactic administration of native and recombinant ArtinM [109]. ArtinM induces recruitment of rat mast cells from the bone marrow to the peritoneal cavity [110].
ArtinM plays a potent adjuvant and immunostimulatory role in immunization against Neospora caninum[111].
ArtinM binds to mast cells inducing cell activation and mediator release [112].
Characterization and optimization of ArtinM lectin expression in
Escherichia coli[113].
Elucidation of carbohydrate molecular interaction mechanism of recombinant and native ArtinM [114]
Recognition of TLR2 N-glycans: critical role in ArtinM immunomodulatory activity [115].
IL-17 induction by ArtinM is due to stimulation of IL-23 and IL-1 release and/or interaction with CD3 in CD4+ T cells [116]
murine model for treatment of Toxoplasma gondii infection [117]
Yeast expressed ArtinM shares structure, carbohydrate recognition, and biological effects with native ArtinM [118]
Neutrophils Contribute to the Protection Conferred by ArtinM against Intracellular Pathogens: A Study on Leishmania major [119]
Recombinant ArtinM activates mast cells [120].
Inhibition of hepatocarcinogenesis by ArtinM via anti-proliferative and pro-apoptotic mechanisms [121].
Systemic effects in naïve mice injected with immunomodulatory lectin ArtinM [122].
Artinm mediates murine T cell activation and induces cell death in jurkat human leukemic T cells [123].
Frutapin Cloning, expression and molecular insights of Frutapin [62]. Frutackin,
References
[1] Zerega NJC, Ragone D, Motley TJ. (2005) Systematics and Species Limits of Breadfruit (<I>Artocarpus</I>, Moraceae). Syst. Bot. [Internet]. 30, 603–615. Available from:
http://www.ingentaselect.com/rpsv/cgi-bin/cgi?ini=xref&body=linker&reqdoi=10.1600/0363644054782134
[2] Lopes MM de A, Souza KO de, Silva E de O. (2018) Cempedak - Artocarpus champeden. In: Rodrigues S, Silva E de O, Brito ES de, editors. Exotic Fruits Reference Guide. London: Wolff, Andre Gerhard; 2018. p. 121–126.
[3] Ragone D. (2018) Breadfruit - Artocarpus altilis (Parkinson) Fosberg. In: Rodrigues S, Silva E de O, Brito E de S, editors. Exotic Fruits Reference Guide. London: Wolff, Andre Gerhard; 2018. p. 53–59.
[4] Peumans WJ, Van Damme EJ. (1995) Lectins as plant defense proteins. Plant Physiol. 109, 347–352.
[5] Kabir S. (1998) Jacalin: a jackfruit (Artocarpus heterophyllus) seed-derived lectin of versatile applications in immunobiological research. J. Immunol. Methods. 212, 193–211.
[6] Ajayi IA. (2011) Use of Jackfruit (Artocarpus heterophyllus) Seeds in Health. In: Preedy VR, Watson RR, Patel VB, editors. Nuts & Seeds in Health and
Disease Prevention. London: Elsevier; 2011. p. 677–682.
[7] Zhang L, Tu Z cai, Xie X, Wang H, Wang H, Wang Z xing, et al. (2017)
Jackfruit (Artocarpus heterophyllus Lam.) peel: A better source of antioxidants and a-glucosidase inhibitors than pulp, flake and seed, and phytochemical profile by HPLC-QTOF-MS/MS. Food Chem. 234, 303–313.
[8] Ajayi IA. (2008) Comparative study of the chemical composition and mineral element content of Artocarpus heterophyllus and Treculia africana seeds and seed oils. Bioresour. Technol. 99, 5125–5129.
[9] Rahman AKMM, Huq E, Mian AJ, Chesson A. (1995) Microscopic and chemical changes occurring during the ripening of two forms of jackfruit (Artocarpus heterophyllus L.). Food Chem. 52, 405–410.
[10] Jagtap UB, Bapat VA. (2010) Artocarpus: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 129, 142–146. [11] Xu S-Y, Liu J-P, Huang X, Du L-P, Shi F-L, Dong R, et al. (2018)
Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT [Internet]. 90, 577–582. Available from:
https://www.sciencedirect.com/science/article/pii/S0023643818300070 [12] Govindaraj D, Rajan M, Hatamleh AA, Munusamy MA. (2018) From waste to
high-value product: Jackfruit peel derived pectin/apatite bionanocomposites for bone healing applications. Int. J. Biol. Macromol. [Internet]. 106, 293–301. Available from:
[13] Zhu H, Zhang Y, Tian J, Chu Z. (2018) Effect of a new shell material—Jackfruit seed starch on novel flavor microcapsules containing vanilla oil. Ind. Crops Prod. [Internet]. 112, 47–52. Available from:
https://www.sciencedirect.com/science/article/pii/S0926669017307458
[14] Swami SB, Thakor NJ, Haldankar PM, Kalse SB. (2012) Jackfruit and Its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 11, 565–576.
[15] Zerega N, Wiesner-Hanks T, Ragone D, Irish B, Scheffler B, Simpson S, et al. (2015) Diversity in the breadfruit complex (Artocarpus, Moraceae): genetic characterization of critical germplasm. Tree Genet. Genomes. 11, 1–26.
[16] Jones AMP, Baker R, Ragone D, Murch SJ. (2013) Identification of pro-vitamin A carotenoid-rich cultivars of breadfruit (Artocarpus, Moraceae). J. Food
Compos. Anal. [Internet]. 31, 51–61. Available from: http://dx.doi.org/10.1016/j.jfca.2013.03.003
[17] Moreira R de A, Castelo-Branco CC, Monteiro AC de O, Tavares RO, Beltramini LM. (1998) Isolation and partial characterization of a lectin from Artocarpus incisa L. seeds. Phytochemistry. 47, 1183–1188.
[18] Murch SJ, Ragone D, Shi WL, Alan AR, Saxena PK. (2008) In vitro conservation and sustained production of breadfruit (Artocarpus altilis, Moraceae): Modern technologies for a traditional tropical crop.
Naturwissenschaften. 95, 99–107.
[19] Jones a. MP, Ragone D, Tavana NG, Bernotas DW, Murch SJ. (2011) Beyond the Bounty: Breadfruit (Artocarpus altilis) for food security and novel foods in the 21st century. Ethnobot. Res. Appl. 9, 129–149.
[20] Sairam S, Urooj A. (2014) Safety Evaluation of Artocarpus altilis as
Pharmaceutical Agent in Wistar Rats. J. Toxicol. [Internet]. 2014, 980404. Available from:
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3996309&tool=pmce ntrez&rendertype=abstract
[21] Siddesha JM, Angaswamy N, Vishwanath BS. (2011) Phytochemical screening and evaluation of invitro angiotensin-converting enzyme inhibitory activity of Artocarpus altilis leaf. Nat. Prod. Res. 25, 1931–1940.
[22] Indrowati M, Pratiwi R, Rumiyati, Astuti P. (2017) Levels of blood glucose and insulin expression of beta-cells in streptozotocin-induced diabetic rats treated with ethanolic extract of Artocarpus altilis leaves and GABA. Pakistan J. Biol. Sci. [Internet]. 20, 28–35. Available from:
http://dx.doi.org/10.3923/pjbs.2017.28.35
[23] Subhadrabandhu S. (2001) Species with possible development potential for homegarden use. In: Under-Utilized Tropical Fruits of Thailand. Bangkok, Thailand: RAP Publication; 2001. p. 16–17.
308–312.
[25] Widyawaruyanti A, Subehan, Kalauni SK, Awale S, Nindatu M, Zaini NC, et al. (2007) New prenylated flavones from Artocarpus champeden, and their
antimalarial activity in vitro. J. Nat. Med. 61, 410–413.
[26] Sundarraj AA, Thottiam Vasudevan R, Sriramulu G. (2018) Optimized extraction and characterization of pectin from jackfruit (Artocarpus integer) wastes using response surface methodology. Int. J. Biol. Macromol. [Internet].
106, 698–703. Available from: https://doi.org/10.1016/j.ijbiomac.2017.08.065 [27] Farooq U, Malviya R, Sharma PK. (2014) Extraction and Characterization of
Artocarpus integer Gum as Pharmaceutical Excipient. Polim. Med. 44, 69–74. [28] Dang L, Van Damme EJM. (2015) Toxic proteins in plants. Phytochemistry
[Internet]. 117, 51–64. Available from:
http://dx.doi.org/10.1016/j.phytochem.2015.05.020
[29] Singh H, Sarathi SP. (2012) Insight of Lectins- A review. Int. J. Sci. Eng. Res.
3, 1–9.
[30] Bourne Y, Zamboni V, Barre A, Peumans WJ, Van Damme EJM, Rougé P. (1999) Helianthus tuberosus lectin reveals a widespread scaffold for mannose- binding lectins. Structure. 7, 1473–1482.
[31] Wright CS. (1997) New folds of plant lectins. Curr. Opin. Struct. Biol. 7, 631– 636.
[32] Peumans WJ, Hause B, Van Damme EJM. (2000) The galactose-binding and mannose-binding jacalin-related lectins are located in different sub-cellular compartments. FEBS Lett. 477, 186–192.
[33] Damme EJM Van, Hause B, Hu J, Barre A, Rouge P, Proost P, et al. (2002) Two Distinct Jacalin-Related Lectins with a Different Specificity and Subcellular Location Are Major Vegetative Storage Proteins in the Bark of the Black
Mulberry Tree 1. Plant Physiol. 130, 757–769.
[34] Pinedo M, Orts F, Carvalho A de O, Regente M, Soares JR, Gomes VM, et al. (2015) Molecular characterization of Helja, an extracellular jacalin-related protein from Helianthus annuus: Insights into the relationship of this protein with unconventionally secreted lectins. J. Plant Physiol. [Internet]. 183, 144– 153. Available from: http://dx.doi.org/10.1016/j.jplph.2015.06.004
[35] Jiang S-Y, Ma Z, Ramachandran S. (2010) Evolutionary history and stress regulation of the lectin superfamily in higher plants. BMC Evol. Biol. [Internet].
10, 79. Available from:
http://bmcevolbiol.biomedcentral.com/articles/10.1186/1471-2148-10-79 [36] Chatterjee B, Vaith P, Chatterjee S, Karduck D, Uhlenbruck G. (1979)
Comparative studies of new marker lectins for alkali-labile and alkali-stable carbohydrate chains in glycoproteins. Int. J. Biochem. 10, 321–327.
Fraction. Biol. Plant. 23, 186–192.
[38] Sankaranarayanan R, Sekar K, Banerjee R, Sharma V, Surolia A, Vijayan M. (1996) A novel mode of carbohydrate recognition in jacalin, a Moraceae plant lectin with a β-prism fold. Nat. Struct. Biol. 3, 596–603.
[39] Abhinav K V., Sharma K, Surolia A, Vijayan M. (2017) Distortion of the ligand molecule as a strategy for modulating binding affinity: Further studies involving complexes of jacalin with β-substituted disaccharides. IUBMB Life [Internet].
69, 72–78. Available from: http://doi.wiley.com/10.1002/iub.1593
[40] Abhinav K V., Sharma K, Surolia A, Vijayan M. (2016) Effect of linkage on the location of reducing and nonreducing sugars bound to jacalin. IUBMB Life [Internet]. 68, 971–979. Available from: http://doi.wiley.com/10.1002/iub.1572 [41] Bourne Y, Astoul CH, Zamboni V, Peumans WJ, Menu-Bouaouiche L, Van
Damme EJM, et al. (2002) Structural basis for the unusual carbohydrate-binding specificity of jacalin towards galactose and mannose. Biochem. J. 364, 173–180.
[42] Sharma A, Sekar K, Vijayan M. (2009) Structure, dynamics, and interactions of jacalin. Insights from molecular dynamics simulations examined in conjunction with results of X-ray studies. Proteins. 77, 760–777.
[43] Moreira R de A, Oliveira JTA de. (1983) Lectins from the genus Artocarpus. Biol. Plant. 25, 343–348.
[44] Monteiro-Moreira ACO, D’Muniz Pereira H, Vieira-Neto AE, Moreno FBMB, Lobo MDP, Sousa FD, et al. (2015) Crystallization and preliminary X-ray diffraction studies of frutalin, an α-D-galactose-specific lectin from Artocarpus incisa seeds. Acta Crystallogr. Sect. F Struct. Biol. Commun. [Internet]. F71, 1282–1285. Available from:
http://scripts.iucr.org/cgi-bin/paper?S2053230X15015186
[45] Campana PT, Moraes DI, Monteiro-Moreira ACO, Beltramini LM. (2002) Unfolding and refolding studies of frutalin, a tetrameric D-galactose binding lectin. Eur. J. Biochem. 269, 753–758.
[46] Vieira-neto AE. (2015) Caracterização estrutural da frutalina, uma lectina α -D-galactose ligante de sementes de artocarpus incisa e análise das suas bases moleculares de ligação à D-galactose.
[47] De-Simone SG, Netto CC, Silva FP. (2006) Simple affinity chromatographic procedure to purify β-galactoside binding lectins. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 838, 135–138.
[48] Hashim O, Gende G, Jaafar M. (1992) Lectin extracts of champedak seeds demonstrate selective stimulation of T lymphocyte proliferation. Biochem. Int.
27, 139–143.