w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Spectroscopic
synthetic
optimizations
monitoring
of
silver
nanoparticles
formation
from
Megaphrynium
macrostachyum
leaf
extract
Franc¸
ois
Eya’ane
Meva
a,b,∗,
Marcelle
Loretta
Segnou
a,
Cecile
Okalla
Ebongue
c,d,
Agnes
Antoinette
Ntoumba
e,
Philippe
Belle
Ebanda
Kedi
e,
Vandi
Deli
a,
Marie-Annie
Etoh
f,
Emmanuel
Mpondo
Mpondo
a,gaDepartmentofPharmaceuticalSciences,FacultyofMedicineandPharmaceuticalSciences,UniversityofDouala,Douala,Cameroon bDepartmentofChemistry,FacultyofSciencesandEngineering,UniversityofHull,Hull,UnitedKingdom
cDepartmentofBiologicalSciences,FacultyofMedicineandPharmaceuticalSciences,UniversityofDouala,Douala,Cameroon dClinicalBiologyLaboratory,GeneralHospitalofDouala,Douala,Cameroon
eDepartmentofAnimalBiologyandPhysiology,FacultyofScience,UniversityofDouala,Douala,Cameroon fDepartmentofChemistry,FacultyofSciences,UniversityofDouala,Douala,Cameroon
gDepartmentofPharmacotoxicologyandPharmacokinetics,UniversityofYaoundeI,Yaounde,Cameroon
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received30July2015
Accepted24June2016
Availableonline20July2016
Keywords:
Silver Nanoparticles
Megaphryniummacrostachyum
UV–visiblespectroscopy
a
b
s
t
r
a
c
t
Nanobiotechnologyisoneofthemostpromisingareasinmodernnanoscienceandtechnology.
Metal-licnanoparticleshavefoundusesinmanyapplicationsindifferentfields,suchascatalysis,photonics,
electronics,medicineandagriculture.Synthesizednanoparticlesthroughchemicalandphysicalmethods
areexpensiveandhavelowbiocompatibility.Inthepresentstudy,silvernanoparticleshavebeen
syn-thesizedfromMegaphryniummacrostachyum(Benth.&Hook.f.)Milne-Redh.,Marantaceae,leafextract.
MegaphryniummacrostachyumisaplantwithlargeleavesfoundintherainforestofWestand
Cen-tralAfrica.Syntheticoptimizationsfollowingfactorssuchasincubationtime,temperature,pH,extract
andsilverion concentrationduringsilver formationarediscussed. UV–visiblespectragavesurface
plasmonresonanceforsynthesizedsilvernanoparticlesbasedMegaphryniummacrostachyumpeaksat
400–450nm.X-raydiffractionrevealedtheaveragesizeofpurecrystallitescomposedfromAgandAgCl.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen
accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Nanomaterialswithacharacteristicdimensionintherangeof 1–100nmareattheleadingedgeofnanosciencesand nanotechnol-ogy(Masarovicováetal.,2014).Inrecentyears,nanomaterialsand specificallymetalnanoparticleshavereceivedparticularinterest in diverse fields ranging frommaterial sciences to biotechnol-ogy(Huang et al., 2007).High-density thin films of silver and
copper nanoclusters have been produced in Middle-Age- and
Renaissance-eraglazedpottery, toexploittheirpeculiar optical propertiesfordecorativepurposes(Pérez-Aranteguietal.,2001). Nanometer-sizedmetallicparticlesinmeltglasseshavebeenused for centuries to produced colored glassware (Bamford, 1977). Becauseofextremelysmallsizeandhighsurfacetovolumeratio
∗ Correspondingauthor.
E-mail:mevae@daad-alumni.de(F.Eya’aneMeva).
ofnanoparticles,thephysicochemicalpropertiesof nanoparticles-containingmaterialsarequitedifferenttothoseofthebulkmaterial (El-Sayed,2001).Thus,nanomaterialshavepotentialapplications inelectronics, photonics,information storage,chemicalsensing, imaging,environmentalremediation,drugdelivery,andbiological labeling(Huangetal.,2007).
Thechemicalsynthesisofsilvernanoparticlesemployschemical reducingagentstoconvertAg+ionstoAg-nanoparticles.Oneofthe mostwidelyusedchemicalreducingagentissodiumborohydride. Thisprocessinvolvestheundesiredusedofhazardouschemicals, andthebiocompatibilityoftheresultingAg-nanoparticlesistoo lowforapplicationinbiologicalsystems(Park,2014).Thebiological methodforthesynthesisofnanoparticlesemploysuseofbiological agentslikebacteria,fungi,actinomycetes,yeastandplants provid-ingawiderange ofresourcesfor thesynthesisof nanoparticles (Rai etal.,2008; Thakkaret al.,2010).Therateofreduction of metalionsusingbiologicalagentsisfoundtobemuchfasterand alsoatambienttemperatureandpressureconditions(Raietal.,
http://dx.doi.org/10.1016/j.bjp.2016.06.002
0102-695X/©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://
2009).Theprocessofgreen synthesisrequirestheuseofwater asan environmentally friendlysolvent. Plant mediated synthe-sisofmetalnanoparticlesisgainingmore importanceowingto itssimplicity,eco-friendliness,rapidrateofsynthesisof nanopar-ticlesofattractiveand diversemorphologiesand eliminationof elaboratemaintenanceofcellcultures(VadlapudiandKaladhar, 2014).Earlierliteraturesuggestthatextractsfromvariousplants leavessuchasEucalyptushybrid,Myrtaceae(Dubeyetal.,2009), Helianthusannuus,Asteraceae(Gebruetal.,2013),Acalyphaindica, Euphorbiaceae(Krishnarajetal.,2010),Oryzasativa,Poaceae(Leela andVivekanandan,2008),Gliricidiasepium,Fabaceae(Rajeshetal., 2009),Menthapiperita,Lamiaceae(Alietal.,2011),Atrocarpus het-erophyllus,Moraceae(Thombreetal.,2012),Withaniasomnifera, Solanaceae(Rajeshetal.,2013;Gregoryetal.,2014),couldbeused fortheAg-nanoparticlessynthesis.
Megaphryniummacrostachyum(Benth.&Hook.f.)Milne-Redh. belongstofamilyMarantaceae.Theplantisfoundintherainforest ofWestandCentralAfrica(Jenningsetal.,2001).M.macrostachyum isaperennialsemi-woodyherb,rhizomatous,formingextensive clumps,withstemsto2½mhighbearingasinglelargeleaf30–60 (–90)cm long by 12–30 (–40)cm wide. The flowers, borne on thepetiolebelowtheleafarewhitishwithredorpurplecalyx. Theleavesareharvestedfromtheforestandusedfreshin wrap-ping food in order to preserve the food. In Central Africa, for instance,theyareoftenusedforwrappingcassavasticksbefore cooking(Ajayi and Ojelere,2013).No significantanti-microbial activitiesof M.macrostachyumleavesover Escherichiacoli, Kleb-siellapneumonia,Pseudomonasaeruginosa, Staphylococcusaureus andCandidaalbicansinthedosesconsideredwerefound.The phy-tochemicalscreeningoftheleavesshowspresencesofalkaloids, flavonoids,anthocyanins,saponins,reductingsugarsandgallic tan-nins(Malouekietal.,2013).
Sofar,therehavebeennoreportsonthesynthesisof nanopar-ticlesbyusingM.macrostachyum.Theleavesaresuitableforgreen synthesisandwepresentin thisresearchtheeco-friendly, sim-pleandlowcostsynthesisofsilvernanoparticlesandthesynthetic optimizationsrelatetoincubationcontacttime,temperature,pH, extractandsilver ionconcentration.Astudyofthecrystallinity andcompositionofthesilvernanoparticlesusingX-raypowder diffractionispresented.
Materialsandmethods
Materials
Silvernitrate(AgNO3)wasobtainedfromSigma–Aldrich chem-icalsGermany,H2SO498%fromMerckKGaADarmstadtGermany andNaOHfromR.P.NormapurProlaboParisandusedasreceived. De-ionizedwaterwasusedthroughoutthereactions.Freshleaves ofM.macrostachyum(Benth.&Hook.f.)Milne-Redh.,Marantaceae, wereprocuredfromlocalmarket,Douala,Cameroon,andidentified atthenationalherbariumofCameroonbyTadjouteuFulbergunder numberofdeposit10000/SRFCam.Allglasswareswerewashed withdilutenitricacid(HNO3)andde-ionizedwater,andthendried inhotairoven.M.macrostachyumleafwassurfacecleanedwith runningtapwaterfollowedbyde-ionisidedwatertoremoveall thedust and unwantedvisible particles. Aqueousextract ofM. macrostachyumwaspreparedbyboiling10gofM.macrostachyum leafin200mlde-ionizedwaterfor5minat80◦C.Theextractwas filteredtwicethroughWhatmanNo.1filterpapertoremove par-ticulatematter,getclearsolutionsand storedoneweekat4◦C. SolutionsofAgNO310−3M,10−2Mand10−1Mwerepreparedin de-ionizedwater.Mixturesolutionofplantextractandsilverion washandshakenduring1minbeforeincubation.
Fig.1. Megaphryniummacrostachyumleaf:32cmlarge,17cmwide.
Instrumentation
TheformationofAg-nanoparticleswasobservedbymeasuring theUV–visspectrumof2.5mlofthereactionsuspensionat differ-enttimeintervals.Ifabsorbancehigherthan4.5u.a.,thesample weredissolvebyafactorof½withdistilledwater.AnUV-visible Uviline9100spectrophotometeroperatedatwith1nmresolution withopticallengthof10mm.UV–visibleanalysisofthereaction mixturewasobservedfora periodof300s. XRDmeasurements werecarriedoutusingaPANalyticalEmpyreanSerie2X-ray diffrac-tometer(CuK-Alpha1[ ˚A]1.54060,K-Alpha2[ ˚A]1.54443,K-Beta[ ˚A] 1.39225)bypreparingathinfilmofsilver-macrostachyumpowder onsiliciumsubstrate.
Preparationofaqueousextract
AqueousextractofM.macrostachyumwaspreparedusing10g offreshlycollectedleaves(Fig.1).Theleavesweresurfacecleaned withrunningtapwater,followedbydistilledwaterandboiledwith 100mlofdistilledwaterat80◦Cfor5min.Theextractwasfiltered andstoredat4◦Cforfurtheruse,beingusableforoneweekdue tothegraduallossofplantextractviabilityforprolongedstorage (Eya’aneMevaetal.,2016).
Greensynthesisofsilvernanoparticles
For thesynthesisof thesilvernanoparticles,a volumeofM. macrostachyumleafextract(5,10,15ml)wasaddedto50mlof 10−3M,10−2Mor10−1MaqueousAgNO
3solutionandincubated atroomtemperatureinthedarktominimizethephotoactivation ofsilvernitrate.Thereactionsweremadeunderstaticconditions. Firsthourofreactionwasmonitoredmeasuringtheabsorbanceat 5,10,20,30,40,50and60min.Inadditionofroomtemperature (30◦C)thestudywasdoneat50and80◦Ctoinvestigatetheeffect oftemperaturefollowingthesampleof10mlextractand10−3M AgNO3during30min.DifferentpHvalues2,4,6,8,10and12were chosenforinvestigationofpHeffectinspeedofsilvernanoparticles formation.ThepHofthesolutionswasadjustedusing0.1NH2SO4 and0.1NNaOHsolutions.Thecontacttimeofincubationforall studiedsampleswasvariedfrom1hto24handthen96h.
Resultsanddiscussion
UV–visibleAg-nanoparticlesformedandincubationcontacttime
Fig.2.Silvernitrate,Megaphryniummacrostachyumleafextract,Agnanoparticles solution.
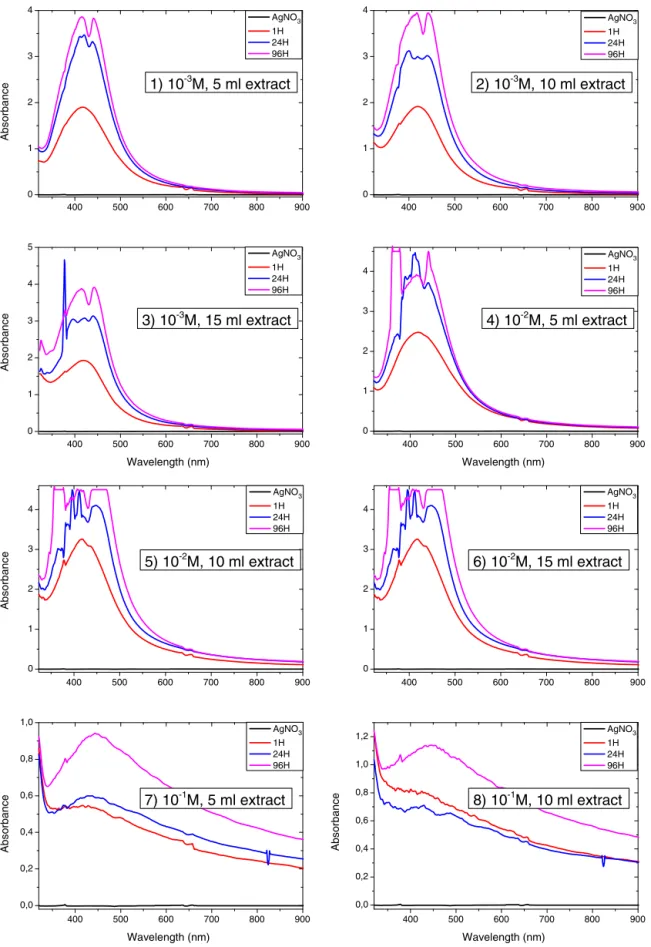
silvernanoparticlesusing50mlofAgNO3 10−3Mwith10mlof extractconcentrationduringthefirsthourisshowninFig.3.The solutioncolorchangewithinsecondstopaleyellow,andthento yellowishbrow,duetoformationofplasmonsatthecolloidsurface, indicatingthesynthesis ofsilver nanoparticles.The samesharp surfaceplasmonresonanceabsorbancebandhasbeenobtainwith differentextract concentrations(5,10, 15ml)at10−3MAgNO
3. Then,5mlofextractisenoughtoreducecompletely50mlof sil-verionat10−3Mconcentration(Fig.4).Theplasmonresonance absorbanceincreaseswhena10−2MAgNO
3solutionisusedwith differentextractsconcentration,thenmoresilverionisavailablefor
400 500 600 700 800 900 0.0
0.5 1.0 1.5 2.0
Absorbance
Wavelength (nm)
5 min 10 min 20 min 30 min 40 min 50 min 60 min
5 10 20 30 40 50 60
Fig.3.Sixtyminutesofreactionbetween10mlofMegaphryniummacrostarchyum
leavesextractand50mlAgNO310−3M.
reduction.At10−1Mconcentrationofthesilverionswiththe dif-ferentM.macrostachyumextractconcentrationsthenanoparticles areaggregatebecauseofthedeficiencyofmoleculesofleafextract toactascappingagents.Thebarrierpotentialdevelopedasaresult ofthecompetitionbetweenweakVanderWaalsforcesofattraction andelectrostaticrepulsionisbroken(Prathnaetal.,2011).As pos-tulatedbyMie’stheory,sphericalnanoparticlesresultsinasingle surfaceplasmonresonance(SPR)bandintheabsorptionspectra. Ontheotherhand,anisotropicparticlesprovidetwoormoreSPR bandsdependingontheparticleshape(Mie,1908).Inthepresent study,reactionmixturesconfirmsingleSPRbandsdisclosing spher-icalshapeofAg-nanoparticleswhichtendtobecomeanisotropic withtime.TheplantleafextractfromM.macrostachyumactas reductantaswellascappingagent,thereforemediatethe synthe-sisaswellasstabilizationofthesilvernanoparticles.Thesharp surfaceplasmonresonancebandincreaseswithsilverion concen-trationas observedfor oliveleafextract (Khalilet al.,2013).It
900 800 700 600 500 400 0.0 0.5 1.0 1.5 2.0
900 800 700 600 500 400 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5
900 800 700 600 500 400 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4
A
b
so
rb
an
ce
AgNO3
5 ml 10 ml 15 ml
Wavelength (nm)
AgNO3
5 ml 10 ml 15 ml
A
b
so
rb
an
ce
Wavelength (nm)
AgNO3
5 ml 10 ml 15 ml
1) 10-3M AgNO3 2) 10-2M AgNO3
3) 10-1M AgNO
3
900 800 700 600 500 400 0 1 2 3 4
900 800 700 600 500 400 0 1 2 3 4
900 800 700 600 500 400 0 1 2 3 4 5
900 800 700 600 500 400 0 1 2 3 4
A
b
so
rb
an
ce
AgNO3
1H 24H 96H
AgNO3
1H 24H 96H
Absor
b
ance
Wavelength (nm)
AgNO3
1H 24H 96H
Wavelength (nm)
AgNO3
1H 24H 96H
1) 10
-3M,
5 ml
extra
ct
2)
10
-3M, 10 ml extract
3) 10
-3M, 1
5 ml
extract
4)
10
-2M,
5 ml
extra
ct
900 800 700 600 500 400 0 1 2 3 4
900 800 700 600 500 400 0 1 2 3 4
900 800 700 600 500 400 0,0 0,2 0,4 0,6 0,8 1,0
900 800 700 600 500 400 0,0 0,2 0,4 0,6 0,8 1,0 1,2
Abso
rb
an
ce
AgNO3
1H 24H 96H
AgNO3
1H 24H 96H
5) 10
-2M, 1
0 ml
extract
6) 10
-2M,
15 ml e
xtrac
t
7) 10
-1M, 5
ml extr
act
8) 10
-1M,
10 ml e
xtrac
t
Abs
o
rban
ce
Wavelength (nm)
AgNO3
1H 24H 96H
Abs
o
rban
ce
Wavelength (nm)
AgNO3
1H 24H 96H
Fig.5. UV–visspectraofdifferentquantitiesofextractandofsilvernitrateinfunctionoftime.
canbeseenthattheabsorbancebandmaximaofAg-nanoparticles usingM.macrostachyumisintherange400–450nmdueto sur-faceplasmonresonance(Mulvaney,1996)ofAg-nanoparticles.The conductionelectronsundergooscillationduetothestrong interac-tionoflightwiththesilvernanoparticles(Thombreetal.,2012).As
900 800 700 600 500 400 0,0 0,5 1,0 1,5 2,0 2,5 3,0 3,5
Absorbance
Wavelength (nm)
30 °C 50 °C 80 °C
30 °C 50 °C 80 °C
Fig.6. UV–visspectraoftemperaturevariationfrom30to80◦Cduringsilver
nanosynthesis.
ofAg-nanoparticlesoccurredrapidlywithinfewminutes indicat-ingthat M.macrostachyumspeedsupthebiosynthesis of silver nanoparticles.Suchofrapidreductionwasobservedforcarobleaf extractwith2minreductiontime(Awwadetal.,2013).
Thereaction between Ag+ and the reducing material in the extractwasfollowedfor96HandUV–visiblemeasurementswere madeat1h,24hand96h.Fig.5showstheUV–visiblespectraof Ag-nanoparticlesasafunctionoftimeafteradditionofdifferent quantitiesofM.macrostachyumleafextract.Reductionaswellas nucleationandgrowingsizeofnanoparticlesincreasesfrom24h to96hbutpoly-dispersionsoccur.Thereactiontimeresultedin gradualincreasingofabsorbancebands.Thecolorintensityofthe solutionchangefromlightyellowtodeep-brownattheendofthe reactionbecauseofincreasingamountofsilver nanoparticlesas wellasaggregation.
Effectoftemperature
Fig.6 showsUV–visiblespectraoftheAg-nanoparticles pre-pared at 30, 50 and 80◦C. It can be seen that the absorbance increaseswithincreasingtemperature.Thisexperimentsuggests thattherateofnanoparticlesynthesisatroomtemperaturecanbe acceleratedbyincreasingtemperatureofthereactionmixture.On theotherhandtheparticlestendtobepolydispersedat80◦C.
EffectofpH
TheUV–visiblefollowingthepHduringtheformationofsilver nanoparticlesfrompH2to12isshowninFig.7.Thevariationof coloris inFig.8.It canbeseenthatPlasmonabsorbancebands increaseswithincreasingpHfrom2to12,whichcanbeduetothe
Ab
sorbanc
e
0 1 2 3 4
2
400 4
6 8 10 12
500
Wavelength (nm)
600 700 800 AgN pH2 pH4 pH6 pH8 pH1 pH12
900 O3
0 2
Fig.7. UV–visspectraofthevariationofpH.
Fig.8. ColorsofAgnanoparticlessolutionatpH2,4,6and8,10,12.
increaseinproductionofcolloidalsilvernanoparticlesand reduc-tionrate.TheabsorbancedoesnotdecreaseatpHhigherthan8 suchasobserved for oliveoilleafextracts (Khalil etal., 2013). Furthermore,itisobservedthatthebrowncolorofthe nanopar-ticlesappearedshortlyaftermixingtheAgNO3 withtheextract atpH4–12.AsobservedinPinuseldaricabarkextract,pHaffects theamountofnanoparticleproductionandtheirstabilityandis acriticalfactor ofcontrolofsizeandmorphologyof nanoparti-cles(IravaniandZolfaghari,2013).Furthermore,pHinfluencedthe rateofthereductionreaction.Thereactionmixtureturnedbrown whensilverwasreduced,andthereactionmixturecoloring accel-eratedwhenincreasingpH.AtpH10,thesharpsurfaceplasmon resonancebandindicatesthatamonodispersesuspensionoccurs. Inpreviousstudies,itwasshownthatthesizeandshapeof biosyn-thesizednanoparticlescouldbemanipulatedbyvarying thepH ofthereaction mixtures(Khalilet al.,2013).Amajorinfluence ofthereactionpHisitsabilitytochangetheelectricalchargesof biomoleculeswhichmightaffecttheircappingandstabilizing abil-itiesandsubsequentlythegrowthofthenanoparticles(Khaliletal., 2013).Then,highpHenvironmentenhancedthereducingand sta-bilizingcapabilityoftheantioxidantsintheM.macrostachyumleaf extractasituationfoundforoliveleafextracts.Thenumberofnuclei increaseswithelevatedpHmaybeduetopromotedreactivityof theM.macrostachyumleafextractsreductants.Gardea-Torresdey etal.(2003)foundthatpHisanimportantfactorinthe biosyn-thesisofcolloidalgoldusingalfalfabiomassandconcludedthat thesizeofnanoparticlesvariedwiththechangeinpH.Mocketal. (2002)alsohavereachedsimilarconclusionsandreportedthatpH isresponsiblefortheformationofnanoparticlesofvariousshapes andsizeasdifferentplantextractsandeventheextractscoming fromdifferentpartsofthesameplantmayhavedifferentpH val-ueswhichfurtherneedoptimizationfortheefficientsynthesisof nanoparticles.
X-raydiffraction
20 30 40 50 60 70 80 100
150 200 250 300 350
(311) (220)
(222) (311) (220)
(200) (111)
(200)
(111)
In
te
n
sity
c
oun
ts
(a
.u
.)
2 theta (Degree) (2
Fig.9. XRDpatternofthenanoparticlesfromMegaphryniummacrostarchyum,
representAgnanocrystallitesand䊉representAgClnanocrystallites.
TheaveragecrystallitesizeofthesynthesizedNPwas deter-minedusingtheDebye–Scherrerequation:
D
v
= Kˇcos
where Dv is the average crystalline size; K is a dimensionless shapefactor,withavalueclosetounity(0.9);isthewavelength of Cu K␣; ˇ is thefull width at half-maximum of the diffrac-tionpeaks;andisBragg’sangle.Noothercharacteristicpeaks werefoundintheXRDspectra,indicatingthehighpurityofthe as-preparedAg@AgClnanoparticles.Tocalculatetheaverage crys-tallineparticlesizeofthesynthesizedAg@AgClnanoparticles,we preferredthemostintensepeaksofAgandAgCl(Eya’aneMeva etal.,2016).Wehaveselectedthe(111)and(200)latticeplanes ofAgandAgCltocalculatetheaveragecrystallineparticlesizeof Ag@AgClNPs.Thecalculatedaveragecrystallineparticlesizeofthe Ag@AgCl-Megaphryniumwasfoundtobe33.7nmand44.2nmfor AgandAgCl,respectively.Theintenseandnarrowdiffractionpeaks revealedthecrystallinenatureofthesynthesized nanoparticles (Wangetal.,2010).
Patternidentificationshowstheformationofpurecrystalsof Ag@AgCl.Asimilarobservationwasmadeusingleafextractsof CorchorusolitorusandIpomeabatatasorflowersextractofAlbizia julibrissin(Eya’aneMevaetal.,2016;Awwadetal.,2015).
Conclusions
Wehavedescribedasimplegreenmethodforsilver nanoparti-clessynthesisusingthereducingpropertiesofM.macrostachyum leafaqueousextract. TheextractofM.macrostachyumleavesin contactwithsilverionsiscapableofproducingsilver nanoparti-cleswithin5min.Theextractactasreductantandstabilizerand thenanoparticlescanbepreparedeasily,rapidlyand ina cost-effectivelymanner.Itwasfoundthatsilvernanoparticlessynthetic rateincreasewithextractandsilverionconcentration,incubation contacttimeand temperature.UV–visiblemeasurementsshows thatthesynthesisispromotedathighpHwithpH10beenmore favorable.PowderX-raydiffractionstudiesconfirmthepurenature ofthecrystallitescomposedwithAgandAgClnanocrystalliteswith size33.7nmand44.2nmforAgandAgCl.
Authors’contributions
SML, AAN, and PBEKcontributed in collectingplant sample and identification,confection ofherbarium,runningpartofthe
laboratorywork.EMFandEMMcarryanalysisofthedata,run lab-oratorywork,providechemicalsanddraftedthepaper.Allauthors contributedtodiscussthespectroscopyandpowder diffraction. EMFandEMMdesignedthestudy,supervisedthelaboratorywork andcontributedtocriticalreadingofthemanuscript.Alltheauthors havereadthefinalmanuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
TheauthorsthanktheMultidisciplinaryLaboratoryofthe Fac-ulty of Medicine and Pharmaceutical Sciences, Department of PharmaceuticalSciencesfortechnicalandfinancialsupport. Sup-portofWordUniversityServiceunderAPA2668forprovidingthe equipmentsusedisappreciate.TheauthorsthanktheAssociation ofCommonwealthUniversityforthegenerousAcademic Fellow-shipCMCF-2015-3.SincerethanksareexpressedtoProf.DavidJ. Evans(UniversityofHull)forhiscontinuoussupportofourwork andhelpfuldiscussions.
References
Ajayi,I.A.,Ojelere,O.O.,2013.Phytochemicalscreening,proximateanalysisand
antimicrobial activityof aqueous extractof Megaphryniummacrostachyum seeds.Int.J.Eng.Res.Technol.2,2123–2131.
Ali,D.M.,Thajuddin,N.,Jeganathan,K.,Gunasekaran,M.,2011.Plantextract
medi-atedsynthesisofsilverandgoldnanoparticlesanditsantibacterialactivity againstclinicallyisolatedpathogens.ColloidsSurf.B85,360–365.
Awwad,A.M.,Salem,N.M.,Abdeen,A.O.,2013.Greensynthesisofsilver
nanopar-ticlesusingcarobleafextractanditsantibacterialactivity.Int.J.Ind.Chem.4, 1–6.
Awwad,A.M.,Salem,N.M.,Ibrahim,Q.M.,Abdeen,A.O.,2015.Phytochemical
fabri-cationandcharacterizationofsilver/silverchloridenanoparticlesusingAlbizia julibrissinflowersextract.Adv.MatterLett.6,726–730.
Bamford,C.R.,1977.ColourGenerationandControlinGlass.Elsevier,Amsterdam,
Netherlands.
Dubey,M.,Bhadauria,S.,Kushwah,B.S.,2009.Grennsynthesisofnanosilverparticles
fromextractofEucalyptushybrid(Safeda)leaf.Dig.J.Nanomater.Biostruct.4, 537–543.
El-Sayed,M.A.,2001.Someinterestingpropertiesofmetalsconfinedintimeand
nanometerspaceofdifferentshapes.AccountsChem.Res.34,257–264.
Eya’aneMeva,F.,Segnou,M.L.,OkallaEbongue,C.,Ntoumba,A.A.,DjiopangYadou,
S.,EssombeMalolo,F.A.,LidwineNgah,L.,HarounaMassai,EmmanuelMpondo
Mpondo,E.,2016.Unexploredvegetalgreensynthesisofsilvernanoparticles:a
preliminarystudywithCorchorusolitorusLinnandIpomeabatatas(L.)Lam.Afr. J.Biotechnol.15,341–349.
Gardea-Torresdey,J.L.,Gomez,E.,Peralta-Videa,J.R.,Parsons,J.G.,Troiani,H.,
Jose-Yacaman,M.,2003.Alfalfasprouts:anaturalsourceforthesynthesisofsilver
nanoparticles.Langmuir19,1357–1361.
Gebru,H.,Taddesse,A.,Kaushal,J.,Yadav,O.P.,2013.Greensynthesisofsilver
nanoparticlesandtheirantibacterialactivity.J.Surf.Sci.Technol.29,47–66.
Gregory,M.,Selvakesavan,R.K.,Franklin,G.,Sarmento,B.,Dias,A.C.P.,2014.Green
synthesisofsilvernanoparticlesusingWithaniasomniferaextractandtheir incorporationintoacreamwithantibacterialactivity.PlantaMed.80,SL26.
Huang,C.C.,Yang,Z.,Lee,K.H.,Chang,H.T.,2007.Synthesisofhighlyfluorescentgold
nanoparticlesforsensingmercury(II).Angew.Chem.Int.Ed.46,6824–6828.
Iravani,S.,Zolfaghari,B.,2013.GreensynthesisofsilvernanoparticlesusingPinus
eldaricabarkextract.BioMedRes.Int.,http://dx.doi.org/10.1155/2013/639725.
Jennings,S.B.,Brown,N.D.,Boshier,D.H.,Whitmore,T.C.,Lopes,J.A.,2001.
Ecol-ogyprovidesapragmaticsolutiontothemaintenanceofgeneticdiversityin sustainablymanagedtropicalrainforest.ForestEcol.Manage.154,1–10.
Khalil,M.M.H.,Ismail,E.H.,El-Baghdady,K.Z.,Mohamed,D.,2013.Greensynthesis
ofsilvernanoparticlesusingoliveleafextractanditsantibacterialactivity.Arab. J.Chem.7,1131–1139.
Krishnaraj,C.,Jagan,E.G.,Rajasekar,S.,Selvakumar,P.,Kalaichelvan,P.T.,Mohan,
N.,2010.SynthesisofsilvernanoparticlesusingAcalypticaindicaleafextracts
anditsantibacterialactivityagainstwaterbornepathogens.ColloidsSurf.B76, 50–56.
Leela,A.,Vivekanandan,M.,2008.Tappingtheunexploitedplantresourcesforthe
synthesisofsilvernanoparticles.Afr.J.Biotechnol.7,3162–3165.
Maloueki,U.,Musuyu,M.,Mbomba,N.B.A.,Ndimbo,K.S.P.,Kapetshi,K.J.,Kabena,
N.O.,2013.Activitésantimicrobiennesetantioxydantesdesextraitsaqueux
Masarovicová,E.,Králóvá,K.,Zinjarde,S.S.,2014.Metalnanoparticlesinplants, for-mationandaction.HandbookofPlantandCropPhysiology,vol.33.,3rded.CRC Press,Taylor&FrancisGroup,pp.684–719.
Mie,G.,1908.BeiträgezurOptiktrüberMedien,speziellkolloidalerMetallösungen.
Ann.Phys.330,345–377.
Mock,J.J.,Barbic,M.,Smith,D.R.,Schultz,D.A.,Schultz,S.,2002.Shapeeffectsin
plasmonresonanceofindividualcolloidalsilvernanoparticles.J.Chem.Phys. 116,6755–6759.
Mulvaney,P.,1996.Surfaceplasmonspectroscopyofnanosizedmetalparticles.
Langmuir12,788–800.
Pérez-Arantegui,J.,Molera,J.,Larrea,A.,Pradell,T.,Vendrell-Saz,M.,Borgia,I.,
Brunetti,B.G.,Cariati,F.,Fermo,P.,Mellini,M.,2001.Lusterpotteryfromthe
thirteenthcenturytothesixteenthcentury:ananostructuredthinmetallicfilm. J.Am.Ceram.Soc.84,442–446.
Park,Y.,2014.Newparadigmshiftforthegreensynthesisofantibacterialsilver
nanoparticlesutilizingplantsextracts.Toxicol.Res.30,169–178.
Prathna, T.C., Chandrasekaran, N., Raichur, M.A., Mukherjee, A., 2011.
Biomimeticsynthesisofsilvernanoparticlesbycitruslimon(lemon) aque-ousextractandtheoreticalpredictionofparticlesize.ColloidsSurf.B82, 152–159.
Rai,M.,Yadav,A.,Gade,A.,2008.Currenttrendsinphytosynthesisofmetal
nanopar-ticles.Crit.Rev.Biotechnol.28,277–284.
Rai,M.,Yadav,A.,Gade,A.,2009.Silvernanoparticlesasanewgenerationof
antimi-crobials.Biotechnol.Adv.27,76–83.
Rajesh, R.W.,Lakkakula, J.R., Niranjan, K.S.,Mendhulkar, V.D.,Sahebrao, K.B.,
2009.PhytosynthesisofsilvernanoparticlesusingGliciridasepium(Jacq).Curr.
Nanosci.5,117–122.
Rajesh,P.,Swati,W.,Sandesh,M.,Sangita,J.,Kulkarni,S.,2013.Greensynthesisof
silvernanoparticlesbyWithaniasomniferaandevaluationofitsantimicrobial potential.J.Empir.Biol.1,38–48.
Thakkar, K.N.,Mhatre,S.S., Parikh,R.Y., 2010. Biologicalsynthesisofmetallic
nanoparticles.Nanomedicine-UK6,257–262.
Thombre,R.,Parekh,F.,Lekshminarayanan,P.,Francis,G.,2012.Studieson
antibac-terialandantifungalactivityofsilvernanoparticlessynthesizedusingArtocarpus heterophyllusleafextract.Biotechnol.Bioinf.Bioeng.2,632–637.
Vadlapudi,V.,Kaladhar,D.S.V.G.K.,2014.Review:greensynthesisofsilverandgold
nanoparticles.MiddleEastJ.Sci.Res.19,834–842.
Wang,P.,Huang,B.,Lou,Z.,Zhang,X.,Qin,X.,Dai,Y.,Zheng,Z.,Wang,X.,2010.