Electroencephalographic study of chlorpromazine alone or combined
with alpha-lipoic acid in a model of schizophrenia induced by
ketamine in rats
Luis Rafael Leite Sampaio
a,b, Lucas Teixeira Nunes Borges
a,b, Talita Matias Barbosa
a,
Natalia Castelo Branco Matos
a, Ricardo de Freitas Lima
a,
Mariana Nascimento de Oliveira
b, Viviane N
obrega Gularte
a,
Manoel Cl
audio Azevedo Patrocínio
c, Danielle Mac
^
edo
a, Otoni Cardoso do Vale
a,1,
Silv
^
ania Maria Mendes de Vasconcelos
a,*aDrug Research and Development Center, Department of Physiology and Pharmacology, School of Medicine, Federal University of Ceara, Fortaleza, Cear a, Brazil
b
Health Science Center, School of Nursing, University of Fortaleza, Fortaleza, Ceara, Brazil cHealth Science Center, School of Medicine, University Centre Christus, Fortaleza, Ceara, Brazil
a r t i c l e
i n f o
Article history:
Received 15 February 2016 Received in revised form 20 November 2016 Accepted 1 December 2016
Keywords:
Schizophrenia Hippocampus Ketamine
Electroencephalogram Chlorpromazine Lipoic acid
a b s t r a c t
Schizophrenia is characterized by behavioral symptoms, brain function impairments and electroen-cephalographic (EEG) changes. Dysregulation of immune responses and oxidative imbalance underpins this mental disorder. The present study aimed to investigate the effects of the typical antipsychotic chlorpromazine (CP) alone or combined with the natural antioxidant alpha-lipoic acid (ALA) on changes in the hippocampal average spectral power induced by ketamine (KET). Three days after stereotactic implantation of electrodes, male Wistar rats were divided into groups treated for 10 days with saline (control) or KET (10 mg/kg, IP). CP (1 or 5 mg/kg, IP) alone or combined with ALA (100 mg/kg, P.O.) was administered 30 min before KET or saline. Hippocampal EEG recordings were taken on the 1st, 5th and 10th days of treatment immediately after the last drug administration. KET significantly increased average spectral power of delta and gamma-high bands on the 5th and 10th days of treatment when compared to control. Gamma low-band significantly increased on the 1st, 5th and 10th days when compared to control group. This effect of KET was prevented by CP alone or combined with ALA. Indeed, the combination of ALA 100þCP1 potentiated the inhibitory effects of CP1 on gamma low-band os-cillations. In conclusion, our results showed that KET presents excitatory and time-dependent effects on hippocampal EEG bands activity. KET excitatory effects on EEG were prevented by CP alone and in some situations potentiated by its combination with ALA.
©2016 Elsevier Ltd. All rights reserved.
1. Introduction
Schizophrenia is a neuropsychiatric syndrome characterized by impairment of brain functions such as language, thought
process-ing, memory and cognition (WHO, 2015; Moore et al., 2015;
Berberian et al., 2015). The syndrome is characterized by the occurrence of symptoms divided into: positive (psychotic period, i.e. presence of abnormal behaviors such as delusions, paranoia, visual and auditory hallucinations, disordered and incoherent thoughts and loss of normal association between ideas), negative (absence of interpersonal and social behaviors often chronic and difficult to treat, such as social withdrawal, apathy and anhedonia) and cognitive symptoms (Meyer and Feldon, 2010).
Patients with schizophrenia, in addition to presenting behav-ioral symptoms, also show electroencephalographic (EEG) changes especially during crises (Dawson et al., 2015). These altered
*Corresponding author. Department of Physiology and Pharmacology, Faculty of Medicine, Federal University of Ceara, Rua Cel. Nunes de Melo 1127, CEP 60431-270, Fortaleza, Brazil.
E-mail address:silvania_vasconcelos@yahoo.com.br(S.M.M. Vasconcelos). 1 In memoriam.
Contents lists available atScienceDirect
Journal of Psychiatric Research
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / p s y c h i r e s
http://dx.doi.org/10.1016/j.jpsychires.2016.12.003
electrical activities are related to the malfunction of brain struc-tures like the hippocampus, amygdala, thalamus, temporal and frontal cortex (Kandratavicius et al., 2012).
Ketamine (KET), a non-competitive antagonist of N-methyl-D-aspartate (NMDA) glutamate receptor, is clinically used as an anesthetic when in high doses (160 mg/kg) (Yilmaz et al., 2002). However, non-anesthetic doses of KET in rodents [10 mg/kg (De Oliveira Viana Arruda et al., 2008); 20 mg/kg (Vasconcelos et al., 2015; Monte et al., 2013); 25, 50 or 75 mg/kg (Caixeta et al., 2013), and 100 mg/kg (Moghaddam et al., 2014)] induce cognitive impairment, psychosis and exacerbation of schizophrenic-like symptoms. Based on these evidences, KET is widely used as an animal model of schizophrenia (Vasconcelos et al., 2015; Monte et al., 2013; Moghaddam et al., 2014; Chatterjee et al., 2011; De Oliveira Viana Arruda et al., 2008). Several studies (Newcomer et al., 1999; Stone et al., 2006; Kim et al., 2015) showed that a single dose of KET induces a state in the brain characterized by an increase in the force and frequency of cerebral intrinsic oscillations. Spectral analysis revealed an increase in the absolute power after administration of 9 or 30 mg/kg doses of KET, with the greatest increase being achieved on delta-, beta- and gamma-bands. These changes were more prominent 10e15 min after KET administration,
which temporally correlates with the higher levels of KET and its metabolite, norketamine, in the brain (Palenícek et al., 2011).
Antipsychotics, drugs used to treat schizophrenia, are grouped into typical and atypical. Both typical and atypical antipsychotics appear to be equally effective for the treatment of positive symp-toms of schizophrenia, by inhibiting the mesolimbic dopaminergic system. However, atypical antipsychotics seems to be more effec-tive in treating the negaeffec-tive symptoms and cognieffec-tive impairment (Keefe et al., 2007). Like other typical antipsychotics, chlorproma-zine (CP) selectively blocks dopamine D2 postsynaptic receptors (Peuskens et al., 2014), which seems to be related to the occurrence of important side effects such as tardive dyskinesia and EEG ab-normalities (Momcilovic-Kostadinovic et al., 2013; Erbas and Yilmaz, 2013; Fink, 2010).
Besides from the involvement of neurotransmitters in the pathophysiology of psychiatric disorders, in the last decades the contribution of chronic inflammation in the neurobiology of these disorders has received increased attention (Fond et al., 2014). In this regard, previous studies have shown that schizophrenia is associ-ated with a dysregulation of immune responses (Drexhage et al., 2011; Miller et al., 2013; (Potvin et al., 2008) and oxidative imbal-ance (Koga et al., 2015; Mac^edo et al., 2012). Furthermore, some anti-inflammatory drugs have shown effectiveness in the treat-ment of schizophrenia (Koga et al., 2015; Mac^edo et al., 2012).
Due to its strong antioxidant and anti-inflammatory effects, the therapeutic potential of alpha-lipoic acid (ALA) has been recently studied for neuropsychiatric disorders (Silva et al., 2016; Sousa et al., 2015; Vasconcelos et al., 2015; Deslauriers et al., 2014; Silva et al., 2014, 2013; De Araújo et al., 2011). Thus, considering that schizophrenia has oxidative and inflammatory components un-derlying its pathophysiology, ALA has been investigated as a ther-apeutic alternative for schizophrenia (Vasconcelos et al., 2015; Mac^edo et al., 2012). However, the action of ALA on EEG changes present in schizophrenia has not yet been determined. Since ALA is a potential adjunct drug in the treatment of schizophrenia this needs to be clarified.
The pharmacological model of schizophrenia induced by KET promotes changes in hippocampal EEG presenting thus a trans-lational value (Kocsis et al., 2013). The hippocampus, a part of the limbic system, is located in the temporal lobe and plays a crucial role in mental functions related to behavior and memory (Zhang et al., 2016). Regarding memory, the hippocampus is involved in the storage and recall of memories and the formation of
associations, functions that are disrupted in schizophrenia (Siekmeier and vanMaanen, 2014). These hippocampal functions are important in the pathophysiology in schizophrenia.
Therefore, the present study investigated the effects of CP alone or combined with ALA in the hippocampal average spectral power changes using the pharmacological model of schizophrenia induced by KET.
2. Methods
2.1. Animals
The experiments were performed in male Wistar rats (200e300 g). The animals were kept at a room with controlled
temperature (23±1C) with a cycle of 12 h light/dark and free access to food and water. All experimental procedures were per-formed in accordance with Guide for the Care and Use of Laboratory Animals from the National Research Council. The protocols were approved by the Ethics Committee of Federal University of Ceara (No. 92/2009).
2.2. Drugs
Alpha-lipoic acid (ALA - Sigma-Aldrich, St. Louis, EUA - ALA 100 mg/kg) dissolved in 5% carboxymethyl cellulose (CMC), keta-mine hydrochloride (Konig, Brasil - KET 10 mg/kg) and chlor-€
promazine (Cristalia, Itapira, BrazileCP 1 or 5 mg/kg) were used.
The controls received saline 0.9%. All solutions were administered in a volume of 0.1 ml for each 100 g of body weight.
2.3. Outline of the study
2.3.1. Electroencephalographic study
2.3.1.1. Stereotactic surgery and electrodes implantation. The rats werefirst anesthetized with ketamine (100 mg/kg, i.p.) and xyla-zine (10 mg/kg, i.p.). During the surgical procedure bipolar and twisted electrodes of NiCr wire (diameter 150
m
m) were implanted in the hippocampus through stereotactic device (Stoelting®, EUA) at the following coordinates (mm): AP¼ 4.0, ML¼±2.6, and DV ¼ 3.5 from bregma, according to the Atlas ofPaxinos and Watson (1998). An additional screw was placed in the frontal bone cavity as the reference electrode. The electrodes werefixed to the skull with dental acrylic cement. The correct location of the implanted electrodes in the hippocampus was verified by histo-logical analysis using violet cresyl staining according toMagni et al. (2007, 2011).
2.3.2. Treatment protocol
Three days after the electrodes implantation (Magni et al.,
2007), the animals were randomly divided into nine groups
(n ¼ 6 animals/group). The treatment groups received 10 days
administration of the drugs (each one administered once a day) being divided as follows: group 1econtrol - 0.9% saline
intraper-itoneal (IP) injection; group 2 - KET 10 mg/kg, IP; group 3eALA
100 mg/kg, per os; group 4 - CP 1 mg/kg, IP; group 5eCP5 mg/kg,
IP; group 6 - CP1þKET10 with a 30 min interval between drugs; group 7 - CP5þKET10 with a 30 min interval between drugs; group
8 - ALA 100 mg/kg, per osþCP1þKET10 with a 30 min interval
between drugs; group 9 - ALA100þCP5þKET10 with a 30 min interval between drugs (Fig. 1).
2.3.3. Electroencephalographic records
The quantitative EEG was recorded continuously for 20min through a polygraph digital system (PowerLab 4/30 device) (Min et al., 2011). The signals were 20,000 amplified and filtered
(means filter), in a 1000 Hz sampling rate and recorded using version 7.3.8 of the LabChart Acquisition Software. EEG was recor-ded individually in a glass box (0.30.50.4 m), with thefloor
covered with sawdust. The acquisition cable was connected to a micro-connector on the animal's head, with the electrical activity signals being captured by a PowerLab®
system. The EEG recordings were analyzed by the LabChart 7.3.8. The data were segmented into 1024-point and the signals were converted to power spectra by the Fast Fourier Transform (FFT). The power spectra parameter was defined as the average of mean power frequency (absolute values), calculated in steps of 5min. The waves were divided into 4 bands at the following defined frequency ranges (delta: 0e4 Hz; theta:
4e8 Hz; gamma low: 30e50 Hz and gamma high 50e100 Hz
(Magni et al., 2007).
2.4. Statistical analysis
D'Agostino & Pearson omnibus normality test was used to confirm the normally distribution of data. The comparisons were performed by analysis of variance (ANOVA) using GraphPad Soft-ware 5.0 Version for Windows, GraphPad SoftSoft-ware (San Diego, CA, USA) with Bonferroni as post hoc test. Results were considered significant at p<0.05 and were presented as mean±SEM.
3. Results
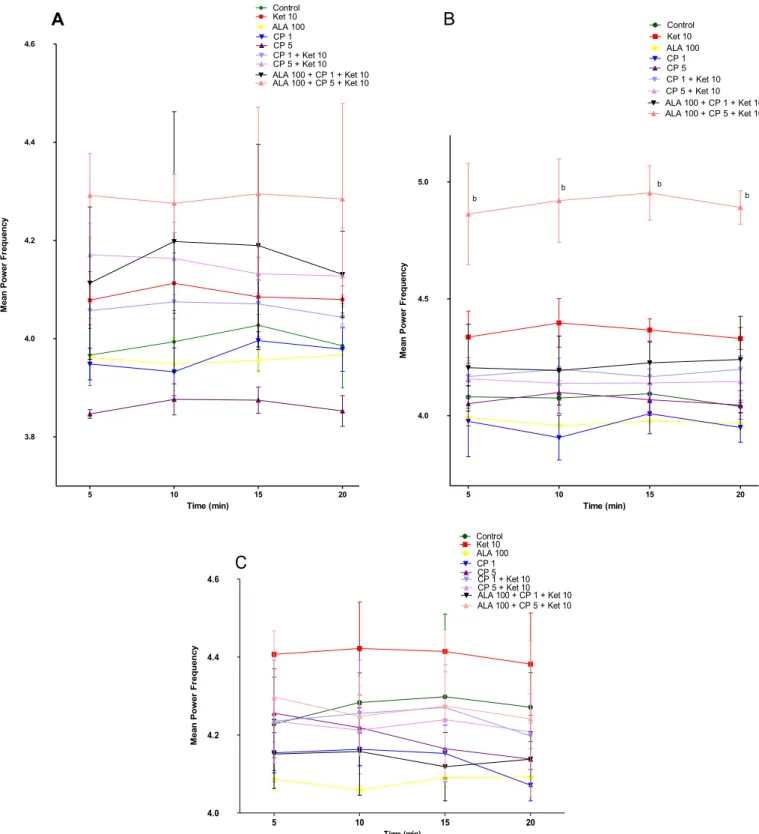
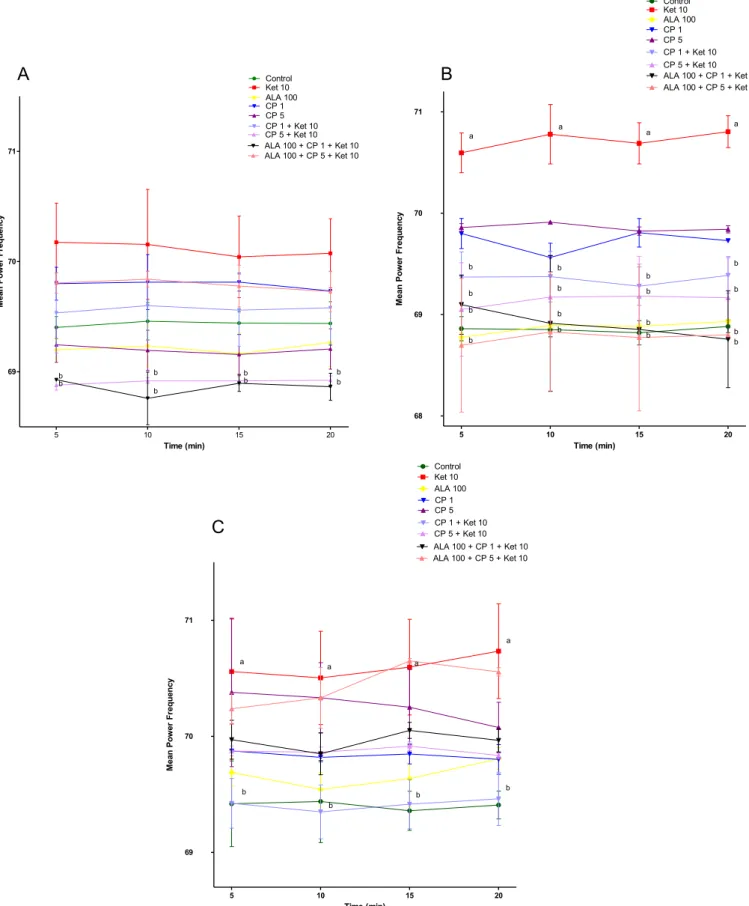
Regarding KET-induced changes we observed a significant in-crease in average spectral power of delta-band on the 5th (P<0.01) and 10th (P < 0.01) days of treatment (Fig. 2B and C) when compared to control. Regarding theta-band there was no alteration when comparing KET to control animals (Fig. 3). On the other hand, gamma low-band significantly increased on the 1st (P<0.001), 5th (P<0.001) and 10th (P<0.001) days (Fig. 4A, B, C), while gamma high-band increased only on the 5th (P<0.01) and 10th (P<0.05) days of KET administration when compared to control group (Fig. 5B and C).
Antipsychotic treatment with CP decreased the average spectral power of delta-band after the 1st day of treatment in the groups CP5þKET10 [5 min (p<0.05), 10 min (p<0.01), 15 min (p<0.05), 20 min (p< 0.01)] and ALA100þCP5 þKET10 [5 min, 10 min,
15 min (p<0.01), 20 min (p< 0.05)] when compared to KET10 group. A more pronounced decrease of delta-band was observed in the group ALA100þCP1þKET10 [5 min, 10 min, 15 min, 20 min
(p < 0.01)] when compared to control, KET10 or CP1 þ KET10
groups (P<0.05) (Fig. 2A).
The increase in average spectral power of delta-band observed on the 5th and 10th days of KET administration [5th day: 5 min, 10 min, 15 min, 20 min (p<0.001); 10th day: 5 min, 10 min, 15 min, 20 min (p<0.001)] when compared to control was prevented by CP1þKET10 [5th day: 5 min, 10 min, 15 min, 20 min (p<0.001); 10th day: 5 min, 10 min, 15 min, 20 min (p<0.01)], CP5þKET10
[5th day: 5 min, 10 min, 15 min; 20 min (p< 0.001); 10th day: 5 min, 10 min, 15 min, 20 min (p<0.01)], ALA100þCP1þKET10
[5th day: 5 min, 10 min, 15 min (p<0.01), 20 min (p<0.001); 10th day: 5 min, 10 min (p < 0.001); 15 min (p < 0.01), 20 min (p<0.001)] or ALA100þCP5þKET10 [5th day: 5 min, 10 min,
15 min, 20 min (p<0.001); 10th day: 5 min, 10 min (p<0.001); 15 min, 20 min (p<0.01)]. No significant effect on delta-band was observed after the administration of ALA100, CP1 or CP5 alone when compared to control in all three days of evaluation (Fig. 2B and C).
No alterations in the average spectral power of theta-band were observed in any of the groups and days of evaluation (Fig. 3A, B and C). An exception was the increase observed on the 5th day of administration in the ALA100þCP5þKET10 group [5 min, 10 min,
15 min, 20 min (p<0.001)] compared to KET10 (Fig. 3B). The increase in the average spectral power of gamma low-band induced by KET administration on the 1st [5 min, 10 min, 15 min, 20 min (p<0.001)], 5th [5 min, 10 min, 15 min, 20 min (p<0.001)] and 10th days [5 min, 10 min, 15 min, 20 min (p<0.001)] compared to control group was prevented by CP in all doses and days of evaluation [1st day- CP1þKET10 (5 min, 10 min, 15 min, 20 min
(p<0.001), CP5þKET10 (5 min, 10 min, 15 min, 20 min (p<0.001); 5th day - CP1þKET10 (5 min, 10 min, 15 min, 20 min (p<0.001), CP5þKET10 (5 min, 10 min, 15 min, 20 min (p<0.001) and 10th day - CP1þKET10: 5 min, 15 min (p<0.05), 20 min (p<0.01), CP5þKET10: 5 min, 10 min, 15 min, 20 min (p<0.001)] (Fig. 4A, B and C).
A similar prevention on gamma low-band oscillations was observed in the groups administered ALAþCP (1 or 5 mg/kg) in all
days of evaluation [1st day - ALA100þCP1þKET10: 5 min, 10 min, 15 min, 20 min (p<0.001), ALA100þCP5þKET10: 5 min, 10 min,
15 min, 20 min (p<0.001); 5th day - ALA100þCP1þKET10: 5 min,
10 min, 15 min, 20 min (p<0.001), ALA100þCP5þKET10: 5 min,
10 min, 15 min, 20 min (p < 0.001) and 10th day
-ALA100þCP1þKET10: 5 min, 10 min, 15 min, 20 min (p<0.001), ALA100þCP5þKET10: 5 min, 10 min, 15 min, 20 min (p<0.001)]. Of note, the decrease in the average spectral power of the low gamma-band observed in the group ALA100þCP1þKET10 on the
10th day of treatment [5 min, 10 min, 15 min and 20 min (p < 0.001)] was lower than that observed in the CP1þKET10
(Fig. 4A, B and C). CP alone increased the spectral power of gamma low-band on the 1st day [CP1: 5, 10, 15 and 20 min (p<0.001), CP5: 5, 10, 15 and 20 min (p<0.01)] and 10th days [CP1: 5, 10, 15 and 20 min (p<0.001)] when compared to control (Fig. 4A and C).
A reduction in the average spectral power of gamma high-band
was detected on the 1st day in CP5 þ KET10 group [5 min
(p < 0.001); 10 min, 15 min, 20 min (p < 0.01)] and
ALA100þCP1þKET10 [5 min (p<0.05); 10 min, 15 min, 20 min (p<0.01)] compared to KET group (Fig. 5A). On the other hand, KET administration increased gamma high-band on treatment days 5
10th day treatment EEG
StereotacƟc
5th day treatment EEG
Control
KET 10 mg/kg
ALA 100 mg/kg
CP 1 or 5 mg/kg AŌer 3 days
1st day treatment EEG
30’ CP 1 or 5 Ket 10
30’ 30’ ALA 100 CP 1 or 5 KET 10
Fig. 1.Treatment protocol with saline (Control), Ketamine 10 mg/kg (KET 10), Lipoic
5 10 15 20 3.35
3.40 3.45
Control Ket 10 ALA 100 CP 1
A
CP 5 CP 1 + Ket 10 CP 5 + Ket 10 ALA 100 + CP 1 + Ket 10 ALA 100 + CP 5 + Ket 10
b
b b
b
a,b,c
a,b b
b
b
b
Time (min)
y
c
n
e
u
q
er
F
r
e
w
o
P
n
a
e
M
a,b,c a,b,c
5 10 15 20 3.35
3.40 3.45 3.50
Control Ket 10 ALA 100 CP 1
B
CP 5 CP 1 + Ket 10 CP 5 + Ket 10 ALA 100 + CP 1 + Ket 10 ALA 100 + CP 5 + Ket 10
a a a a
b b
b
b b
b b b
b b
b
b
b b
b
b
Time (min)
y
c
n
e
u
q
er
F
r
e
w
o
P
n
a
e
M
5 10 15 20
3.35 3.40 3.45 3.50
Control Ket 10 ALA 100 CP 1
C
CP 5CP 1 + Ket 10 CP 5 + Ket 10 ALA 100 + CP 1 + Ket 10 ALA 100 + CP 5 + Ket 10
a a
a a
b b
b b
b
b
b
b b
b b b
b
b
b
b
Time (min)
yc
ne
uq
er
F
re
wo
P
na
e
M
[5 min, 10 min, 15 min, 20 min (p<0.001)] and 10 [5 min (p<0.05); 10 min, 15 min, 20 min (p<0.01)] compared to control (Fig. 5B and C). This increase induced by ketamine was prevented by CPþKET
in both doses studied on the 5th day [CP1 þ KET10: 5 min
(p < 0.05); 10 min (p < 0.01); 15 min, 20 min (p < 0.001); CP5þKET10: 5 min, 10 min, 15 min, 20 min (p<0.01)] and only by
CP1 on the 10th day of treatment [CP1 þKET10: 5 min, 10 min
(p<0.05); 15 min, 20 min (p<0.01)] (Fig. 5A, B and C).
A similar prevention was observed in the group administered ALAþCPþKET, only on the 5th day [ALA100þCP1þKET10: 5 min
(p<0.05); 10 min (p<0.001); 15 min (p<0.01); 20 min (p<0.001)
and ALA100 þ CP5 þ KET10: 5 min, 10 min, 15 min, 20 min
5 10 15 20 3.8
4.0 4.2 4.4 4.6
Control Ket 10 ALA 100 CP 1
A
CP 5 CP 1 + Ket 10 CP 5 + Ket 10 ALA 100 + CP 1 + Ket 10 ALA 100 + CP 5 + Ket 10
Time (min)
y
c
n
e
u
q
er
F
r
e
w
o
P
n
a
e
M
5 10 15 20 4.0
4.5 5.0
Control Ket 10 ALA 100 CP 1
B
CP 5 CP 1 + Ket 10 CP 5 + Ket 10 ALA 100 + CP 1 + Ket 10 ALA 100 + CP 5 + Ket 10
b
b b
b
Time (min)
y
c
n
e
u
q
er
F
r
e
w
o
P
n
a
e
M
5 10 15 20
4.0 4.2 4.4 4.6
Control Ket 10 ALA 100 CP 1
C
CP 5CP 1 + Ket 10 CP 5 + Ket 10 ALA 100 + CP 1 + Ket 10 ALA 100 + CP 5 + Ket 10
Time (min)
y
c
n
e
u
q
er
F
r
e
w
o
P
n
a
e
M
(p<0.001)] compared to KET group (Fig. 5B). No significant alter-ations were observed by the administration of ALA100, CP1 or CP5 alone when compared to control in all days of evaluation.
4. Discussion
Changes in the frequency, power and connectivity of hippo-campal oscillations have been associated with schizophrenia (Cousijn et al., 2015; Allen and Monyer, 2015). Furthermore, striking symptoms of schizophrenia such as distortion of reality (delusions and hallucinations) and cognitive deficits have been associated
with hippocampal dysfunction (Heckers and Konradi, 2010;
Tamminga et al., 2010). Based on these evidences, the hippocam-pus presents a range of brain bands frequencies as delta (Shmukler et al., 2015), theta (Liebe et al., 2012), gamma low and gamma high (Constant and Sabourdin, 2012), which are important to the study of brain function and schizophrenia, making it a prominent area in EEG studies.
Based on our quantitative analysis (mean power frequency) of hippocampal EEG in rats under the pharmacological model of schizophrenia induced by repeated administration of KET we could observe an increase in the average spectral power of delta-band only after 5 and 10 days of KET administration. These results
suggest that the action of KET on delta-band was time-dependent. This result adds more evidences to thefindings that the time of KET administration is important to induce schizophrenia-like alter-ations (Frohlich and Van Horn, 2014). Indeed, the model based on the repeated administration of KET mimics positive, negative and cognitive symptoms of schizophrenia, while a single dose of KET induces only psychotic alterations (Bubeníkova-Valesova et al., 2008). According to Zhang et al. (2012)NMDA receptor antago-nists, such as KET, can act in the thalamic nuclei and impose delta oscillations in areas such as the hippocampus. These changes in delta-band induced by KET can interfere with cognitive function since thalamus and hippocampus present core functions in learning, memory consolidation and behavioralflexibility (Cassel et al., 2013). These changes in cognition are also observed in schizophrenic patients (Shmukler et al., 2015; Lee et al., 2015).
In our results the antipsychotic CP alone or combined with ALA100 prevented the increase in the average spectral power of delta-band observed by KET repeated administration on the 5th and 10th days of treatment. Nonetheless, the combination of ALA100 did not bring additional improvements of CP effects in the frequency of delta-band oscillations. Based on these results we can raise the hypothesis that NMDA receptor antagonism on delta-band oscillation involves the participation of the dopaminergic system,
5 10 15 20
69 70 71
Control Ket 10 ALA 100 CP 1
A
CP 5 CP 1 + Ket 10 CP 5 + Ket 10 ALA 100 + CP 1 + Ket 10 ALA 100 + CP 5 + Ket 10
b
b b b
b
b
b b
Time (min)
y
c
n
e
u
q
er
F
r
e
w
o
P
n
a
e
M
5 10 15 20 68
69 70 71
Control Ket 10 ALA 100 CP 1
B
CP 5 CP 1 + Ket 10 CP 5 + Ket 10 ALA 100 + CP 1 + Ket 10 ALA 100 + CP 5 + Ket 10
a
a
a
a
b b
b
b
b b
b
b
b b
b
b b
b
b b
Time (min)
y
c
n
e
u
q
er
F
r
e
w
o
P
n
a
e
M
5 10 15 20 69
70 71
Control Ket 10 ALA 100 CP 1
C
CP 5 CP 1 + Ket 10 CP 5 + Ket 10 ALA 100 + CP 1 + Ket 10 ALA 100 + CP 5 + Ket 10
a
a a
a
b
b
b b
Time (min)
y
c
n
e
u
q
er
F
r
e
w
o
P
n
a
e
M
since CP with or without ALA100 attenuated delta-band oscillations induced by KET. In fact, KET besides being a NMDA receptor blocker is also an agonist of dopamine D2 receptors (Kapur and Seeman, 2002). Further studies are needed to better evaluate the role of dopaminergic system on delta-band oscillations induced by KET.
No change was observed in the average spectral power of hip-pocampal band after KET administration. Regarding theta-band oscillations and KET administration, contradictory results
are found in the literature, since reductions (Leung and
Desborough, 1988; Dimpfel and Spüler, 1990; Lazarewicz et al., 2010; Kittelberger et al., 2012) and increases (Mattia and Moreton, 1986; Palenícek et al., 2011) were observed. These con-flicting results are probably due to differences in the experimental protocols used in these studies, i.e. dose, animal species, time and routes of administration. In our results a curious and not explained increase in hippocampal spectral power of theta-band was observed on the 5th day of treatment in the group administered ALAþCP5þKET. Probably the evaluation of behavioral parameters
related to schizophrenia and to side effects of antipsychotic drugs, not addressed in the present study, might help explain this alteration.
In both preclinical and clinical approaches hippocampal theta-bands were correlated to memory encoding process, perhaps through coordination of group of cellsfiring, so that LTP (long-term potentiation) and LTD (long-term depression) may occur in the cellular mechanisms of learning and memory acquisition (Liebe et al., 2012; Polanía et al., 2012; Stanton and Sejnowski, 1989). In fact, schizophrenic patients present cognitive deficits that could be associated with hippocampal theta-band oscillation (Shmukler et al., 2015), although this was not confirmed in our preclinical model since, as previously mentioned, the group administered only with KET presented no alteration on theta-band.
We also observed that the administration of KET increased the average spectral power of low gamma-band since thefirst day of administration, while the changes on high gamma-band started only on the 5th day of treatment. This early change on low gamma-band induced by KET may have occurred because the relative en-ergy from low frequency oscillations is proportionally larger than the energy from gamma in many EEG/ERP/MEG recordings, so low frequency oscillations could exert a powerful influence on gamma activities (Moran and Hong, 2011). Thus, changes in the frequency of the low gamma-band may be correlated with early severe psy-chotic symptoms of schizophrenia.
Anderson et al. (2014)showed that the acute administration of KET concurrently increased gamma power and locomotor activity, and these effects were attenuated in rats chronically treated (28 days) with haloperidol (0.25 mg/kg/day), clozapine (5 mg/kg/day) or LY379268 (0.3 mg/kg/day).Olszewski et al. (2013)showed that first- (e.g. sulpiride and haloperidol) and second-generation anti-psychotics (e.g. clozapine and risperidone) presented different re-sponses on high-frequency oscillations (HFO) induced by NMDA receptor antagonist in rat nucleus accumbens. Furthermore,Jones et al. (2011)demonstrated that typical and atypical antipsychotic drugs acutely reduce cortical gamma oscillations, an effect that may be related to their clinical efficacy.
Gamma-band oscillations have been described in the hippo-campus. Gamma-band changes are related to schizophrenia, since previous studies suggest the existence of two gamma generators in the hippocampus, one residing in the dentate gyrus and the other in the CA3-CA1 axis (Sirota et al., 2003). Furthermore, there is also evidence that the oscillatory gamma activity may be related to symptoms of schizophrenia such as hallucinations, thought disor-ders and negative symptoms. Thus, the positive symptoms of schizophrenia can be correlated with an amplitude increase in the gamma-band in brain regions, while the negative symptoms have
been linked to low frequency oscillations (Constant and Sabourdin, 2012; Demiralp et al., 2007; Baldeweg et al., 1998). Similar results were observed in earlier studies demonstrating that the adminis-tration of KET induced a state in the brain characterized by an in-crease in the power and intrinsic frequency of gamma oscillations (Zhang et al., 2012; Caixeta et al., 2013; Kittelberger et al., 2012; Krystal et al., 1994).
The changes in low and high gamma-band frequency generated by KET were prevented by CP1 and 5 mg/kg, as well as by its combination with ALA100. We also observed in the analysis of low gamma-band on the 10th day of ALA100þCP1þKET10
adminis-tration a superior preventive effect of this combination when compared to CP1þKET being these results comparable to the ones
obtained in the group CP5þKET. This potentiation of CP effects by
its combination with ALA suggests that a dose reduction of the antipsychotic drug may be practicable when administered com-bined with ALA. This seems to be an important strategy to reduce the side effects of antipsychotic drugs. Taking into account the ca-pacity of ALA to revert KET-induced behavioral alterations, previ-ously demonstrated by our group (Vasconcelos et al., 2015), it would be interesting in a future study to evaluate the action of ALA alone (ALAþKET group) on changes in the average spectral power in hippocampal bands produced by KET, thus improving our un-derstanding of the protective effect of ALA.
Thus, we can infer that gamma-band activity is stimulated by glutamatergic NMDA blockage and by the resulting increases in dopamine levels in the mesolimbic dopaminergic pathway due to this blockade. In this regard we observed that CP, an antipsychotic drug that excessively blockades dopamine D2 receptors (Moreira and Guimar~aes, 2007; Danivas and Venkatasubramanian, 2013), prevented KET effects on gamma-band.
The present study has some limitations: i) the correlation be-tween changes in the spectral power and schizophrenia-like behavioral alterations (not performed here) could help explaining some EEG alterations, ii) KET is a pharmacological model of schizophrenia presenting thus limitations regarding the modeling of the ethiopatogenic mechanisms involved in this mental disorder, such as its developmental course. This may justify some inconsis-tent results observed in the present study.
In summary, our study provides an analysis of the effects of CP alone and combined with ALA on the changes induced by KET in the hippocampal electrical activity of rats. We found that acute and repeated administration of KET 10 mg/kg doses increased the average spectral power of delta-, gamma low- and gamma high-bands. This alteration in brain electrical activity was prevented by the combination of KET with CP at the doses of 1 or 5 mg/kg alone or combined with ALA. In the case of gamma low-band we observed that ALA potentiated CP1 effects bringing the result near to those obtained with CP5. This could be an indicative of anti-psychotic dose reduction when used in combination with ALA.
In conclusion, the pharmacological model of schizophrenia induced by ketamine 10 mg/kg is important for mimicking the major mechanisms of neural oscillations related to glutamatergic and dopaminergic regulation in the hippocampus. The band oscil-lations observed in this model may be related to a glutamatergic modulation of mesolimbic dopaminergic pathway as well as to a direct agonistic effect of KET at dopamine D2-like receptors, since a D2 receptor blocker, CP, prevented the alterations induced by KET. On the other hand, ALA potentiated some effects of CP adding more evidences for the therapeutic potential of this antioxidant for the treatment of psychiatric disorders.
Contributors
performed the surgical procedures and EEG recordings. LRLS, MCAP, DM, OCV, RFL, analyzed EEG recordings and performed the statistical evaluation of data. SMMV, LRLS and OCV designed the study. SMMV and LRLS wrote thefirst draft.
Conflict of interest
All the authors have read, approved and made substantial con-tributions for the manuscript, therefore, there is not conflict of interest.
Role of funding sources
CNPq and CAPES provided scholarships. FUNCAP provided the financial support of the study.
Acknowledgments
This study was supported by Grants from the Brazilian Gov-ernment Institutions National Council for Scientific and
Techno-logical Development (CNPq), Higher Education Personnel
Improvement Coordination (CAPES) and Cearense Foundation for the Support of Scientific and Technological Development (FUNCAP).
References
Allen, K., Monyer, H., 2015. Interneuron control of hippocampal oscillations. Curr. Opin. Neurobiol. 31, 81e87.http://dx.doi.org/10.1016/j.conb.2014.08.016. Anderson, P.M., Pinault, D., O'Brien, T.J., Jones, N.C., 2014. Chronic administration of
antipsychotics attenuates ongoing and ketamine-induced increases in corticalg
oscillations. Int. J. Neuropsychopharmacol. 17.
Baldeweg, T., Spence, S., Hirsch, S.R., Gruzelier, J., 1998. Gamma-band electroen-cephalographic oscillations in a patient with somatic hallucinations. Lancet (London, England) 352, 620e621. http://dx.doi.org/10.1016/S0140-6736(05) 79575-1.
Berberian, A.A., Gadelha, A., Dias, N.M., Mecca, T.P., Bressan, R.A., Lacerda, A.T., 2015. Investigation of Cognition in Schizophrenia: Psychometric Properties of In-struments for Assessing Working Memory Updating, vol. 64, pp. 238e246. http://dx.doi.org/10.1590/0047-2085000000084.
Bubeníkova-Valesova, V., Horacek, J., Vrajova, M., H€oschl, C., 2008. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci. Biobehav. Rev. 32, 1014e1023. http://dx.doi.org/10.1016/ j.neubiorev.2008.03.012.
Caixeta, F.V., Cornelio, A.M., Scheffer-Teixeira, R., Ribeiro, S., Tort, A.B.L., 2013. Ke-tamine alters oscillatory coupling in the hippocampus. Sci. Rep. 3, 2348.http:// dx.doi.org/10.1038/srep02348.
Cassel, J.-C., Pereira de Vasconcelos, A., Loureiro, M., Cholvin, T., Dalrymple-Alford, J.C., Vertes, R.P., 2013. The reuniens and rhomboid nuclei: neuro-anatomy, electrophysiological characteristics and behavioral implications. Prog. Neurobiol. 111, 34e52.http://dx.doi.org/10.1016/j.pneurobio.2013.08.006. Chatterjee, M., Ganguly, S., Srivastava, M., Palit, G., 2011. Effect of“chronic”versus
“acute”ketamine administration and its“withdrawal”effect on behavioural alterations in mice: implications for experimental psychosis. Behav. Brain Res. 216, 247e254.http://dx.doi.org/10.1016/j.bbr.2010.08.001.
Constant, I., Sabourdin, N., 2012. The EEG signal: a window on the cortical brain activity. Paediatr. Anaesth. 22, 539e552. http://dx.doi.org/10.1111/j.1460-9592.2012.03883.x.
Cousijn, H., Tunbridge, E.M., Rolinski, M., Wallis, G., Colclough, G.L., Woolrich, M.W., Nobre, A.C., Harrison, P.J., 2015. Modulation of hippocampal theta and hippocampal-prefrontal cortex function by a schizophrenia risk gene. Hum. Brain Mapp. 36, 2387e2395.http://dx.doi.org/10.1002/hbm.22778.
Danivas, V., Venkatasubramanian, G., 2013. Current perspectives on chlorpromazine equivalents: comparing apples and oranges! Indian J. Psychiatry 55, 207e208. http://dx.doi.org/10.4103/0019-5545.111475.
Dawson, N., Morris, B.J., Pratt, J.A., 2015. Functional brain connectivity phenotypes for schizophrenia drug discovery. J. Psychopharmacol. 29, 169e177. http:// dx.doi.org/10.1177/0269881114563635.
De Araújo, D.P., Lobato, R.D.F.G., Cavalcanti, J.R.L.D.P., Sampaio, L.R.L., Araújo, P.V.P., Silva, M.C.C., Neves, K.R.T., Fonteles, M.M.D.F., Sousa, F.C.F.De., Vasconcelos, S.M.M., 2011. The contributions of antioxidant activity of lipoic acid in reducing neurogenerative progression of Parkinson's disease: a review. Int. J. Neurosci. 121, 51e57.http://dx.doi.org/10.3109/00207454.2010.535934. De Oliveira Viana Arruda, M., Soares, P.M., HonA3rio Jr., J.E.R., De Sousa Lima, R.C.,~
Chaves, E.M.C., De Freitas GuimarA~£es Lobato, R., De Aguiar Rocha Martin, A.L., Sales, G.T.M., De Moraes Carvalho, K., Assreuy, A.M.S., De Brito, E.M., Vasconcelos, S.M.M., 2008. Activities of the antipsychotic drugs haloperidol and risperidone on behavioural effects induced by ketamine in mice. Sci. Pharm. 76,
673e687.http://dx.doi.org/10.3797/scipharm.0810-11.
Demiralp, T., Herrmann, C.S., Erdal, M.E., Ergenoglu, T., Keskin, Y.H., Ergen, M., Beydagi, H., 2007. DRD4 and DAT1 polymorphisms modulate human gamma band responses. Cereb. Cortex 17, 1007e1019.http://dx.doi.org/10.1093/cercor/ bhl011.
Deslauriers, J., Racine, W., Sarret, P., Grignon, S., 2014. Preventive effect ofa-lipoic acid on prepulse inhibition deficits in a juvenile two-hit model of schizo-phrenia. Neuroscience 272, 261e270. http://dx.doi.org/10.1016/ j.neuroscience.2014.04.061.
Dimpfel, W., Spüler, M., 1990. Dizocilpine (MK-801), ketamine and phencyclidine: low doses affect brainfield potentials in the freely moving rat in the same way as activation of dopaminergic transmission. Psychopharmacology (Berl) 101, 317e323.
Drexhage, R.C., Hoogenboezem, T.A., Cohen, D., Versnel, M.A., Nolen, W.A., Van Beveren, N.J.M., Drexhage, H.A., 2011. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients in-volves both pro- and anti-inflammatory forces. Int. J. Neuropsychopharmacol. 14, 746e755.http://dx.doi.org/10.1017/S1461145710001653.
Erbas, O., Yilmaz, M., 2013. The effects of typical and atypical antipsychotics on the electrical activity of the brain in a rat model. J. Clin. Exp. Investig. 4, 279e284. http://dx.doi.org/10.5799/ahinjs.01.2013.03.0284.
Fink, M., 2010. Remembering the lost neuroscience of pharmaco-EEG. Acta Psy-chiatr. Scand. 121, 161e173.http://dx.doi.org/10.1111/j.1600-0447.2009.01467.x. Fond, G., Hamdani, N., Kapczinski, F., Boukouaci, W., Drancourt, N., Dargel, A., Oliveira, J., Le Guen, E., Marlinge, E., Tamouza, R., Leboyer, M., 2014. Effective-ness and tolerance of anti-inflammatory drugs' add-on therapy in major mental disorders: a systematic qualitative review. Acta Psychiatr. Scand. 129, 163e179. http://dx.doi.org/10.1111/acps.12211.
Frohlich, J., Van Horn, J.D., 2014. Reviewing the ketamine model for schizophrenia. J. Psychopharmacol. 28, 287e302.http://dx.doi.org/10.1177/0269881113512909. Heckers, S., Konradi, C., 2010. Hippocampal pathology in schizophrenia. Curr. Top.
Behav. Neurosci. 4, 529e553.http://dx.doi.org/10.1007/7854.
Jones, N.C., Reddy, M., Anderson, P., Salzberg, M., O'Brien, T.J., Pinault, D., 2011. Acute administration of typical and atypical antipsychotics reduces EEG gamma po-wer, but only the preclinical compound LY379268 reduces the ketamine-induced rise in gamma power. Int. J. Neuropsychopharmacol. 15, 657.http:// dx.doi.org/10.1017/s1461145711000848.
Kandratavicius, L., Lopes-Aguiar, C., Bueno-Júnior, L.S., Romcy-Pereira, R.N., Hallak, J.E.C., Leite, J.P., 2012. Psychiatric comorbidities in temporal lobe epi-lepsy: possible relationships between psychotic disorders and involvement of limbic circuits. Rev. Bras. Psiquiatr. 34, 454e466. http://dx.doi.org/10.1016/ j.rbp.2012.04.007.
Kapur, S., Seeman, P., 2002. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implica-tions for models of schizophrenia. Mol. Psychiatry 7, 837e844. http:// dx.doi.org/10.1038/sj.mp.4001093.
Keefe, R.S.E., Bilder, R.M., Davis, S.M., Harvey, P.D., Palmer, B.W., Gold, J.M., Meltzer, H.Y., Green, M.F., Capuano, G., Stroup, T.S., McEvoy, J.P., Swartz, M.S., Rosenheck, R.A., Perkins, D.O., Davis, C.E., Hsiao, J.K., Lieberman, J.A., 2007. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch. Gen. Psychiatry 64, 633e647.http:// dx.doi.org/10.1001/archpsyc.64.6.633.
Kim, J.W., Lee, Y.S., Han, D.H., Min, K.J., Lee, J., Lee, K., 2015. Diagnostic utility of quantitative EEG in un-medicated schizophrenia. Neurosci. Lett. 589, 126e131. http://dx.doi.org/10.1016/j.neulet.2014.12.064.
Kittelberger, K., Hur, E.E., Sazegar, S., Keshavan, V., Kocsis, B., 2012. Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizo-phrenia. Brain Struct. Funct. 217, 395e409. http://dx.doi.org/10.1007/s00429-011-0351-8.
Kocsis, B., Brown, R.E., Mccarley, R.W., Hajos, M., 2013. Impact of ketamine on neuronal network dynamics: translational modeling of schizophrenia-relevant deficits. CNS Neurosci. Ther.http://dx.doi.org/10.1111/cns.12081.
Koga, M., Serritella, A.V., Sawa, A., Sedlak, T.W., 2015. Implications for reactive ox-ygen species in schizophrenia pathogenesis. Schizophr. Res.http://dx.doi.org/ 10.1016/j.schres.2015.06.022.
Krystal, J.H., Karper, L.P., Seibyl, J.P., Freeman, G.K., Delaney, R., Bremner, J.D., Heninger, G.R., Bowers, M.B., Charney, D.S., 1994. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 51, 199e214.
Lazarewicz, M.T., Ehrlichman, R.S., Maxwell, C.R., Gandal, M.J., Finkel, L.H., Siegel, S.J., 2010. Ketamine modulates theta and gamma oscillations. J. Cogn. Neurosci. 22, 1452e1464.http://dx.doi.org/10.1162/jocn.2009.21305. Lee, S.-H., Niznikiewicz, M., Asami, T., Otsuka, T., Salisbury, D.F., Shenton, M.E.,
McCarley, R.W., 2015. Initial and progressive gray matter abnormalities in insular gyrus and temporal pole infirst-episode schizophrenia contrasted with first-episode affective psychosis. Schizophr. Bull. http://dx.doi.org/10.1093/ schbul/sbv177.
Leung, L.W., Desborough, K.A., 1988. APV, an N-methyl-D-aspartate receptor antagonist, blocks the hippocampal theta rhythm in behaving rats. Brain Res. 463, 148e152.
Mac^edo, D.S., Medeiros, C.D., Cordeiro, R.C., Sousa, F.C., Santos, J.V., Morais, T.A., Hyphantis, T.N., McIntyre, R.S., Quevedo, J., Carvalho, A.F., 2012. Effects of alpha-lipoic acid in an animal model of mania induced by D-amphetamine. Bipolar Disord. 14, 707e718.http://dx.doi.org/10.1111/j.1399-5618.2012.01046.x. Magni, D.V., Oliveira, M.S., Furian, A.F., Fiorenza, N.G., Fighera, M.R., Ferreira, J.,
Mello, C.F., Royes, L.F.F., 2007. Creatine decreases convulsions and neurochem-ical alterations induced by glutaric acid in rats. Brain Res. 1185, 336e345.http:// dx.doi.org/10.1016/j.brainres.2007.09.023.
Magni, D.V., Souza, M.A., Oliveira, A.P.F., Furian, A.F., Oliveira, M.S., Ferreira, J., Santos, A.R.S., Mello, C.F., Royes, L.F.F., Fighera, M.R., 2011. Lipopolysaccharide enhances glutaric acid-induced seizure susceptibility in rat pups: behavioral and electroencephalographic approach. Epilepsy Res. 93, 138e148. http:// dx.doi.org/10.1016/j.eplepsyres.2010.11.007.
Mattia, A., Moreton, J.E., 1986. Electroencephalographic (EEG), EEG power spectra, and behavioral correlates in rats given phencyclidine. Neuropharmacology 25, 763e769.
Meyer, U., Feldon, J., 2010. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog. Neurobiol. 90, 285e326. http://dx.doi.org/ 10.1016/j.pneurobio.2009.10.018.
Miller, B.J., Culpepper, N., Rapaport, M.H., Buckley, P., 2013. Prenatal inflammation and neurodevelopment in schizophrenia: a review of human studies. Prog. Neuro Psychopharmacol. Biol. Psychiatry 42, 92e100.http://dx.doi.org/10.1016/ j.pnpbp.2012.03.010.
Min, B.-K., Bystritsky, A., Jung, K.-I., Fischer, K., Zhang, Y., Maeng, L.-S., Park, S.I., Chung, Y.-A., Jolesz, F.A., Yoo, S.-S., 2011. Focused ultrasound-mediated sup-pression of chemically-induced acute epileptic EEG activity. BMC Neurosci. 12, 23.http://dx.doi.org/10.1186/1471-2202-12-23.
Moghaddam, A.H., Sobarzo-Sanchez, E., Nabavi, S.F., Daglia, M., Nabavi, S.M., 2014. Evaluation of the antipsychotic effects of 2-(dimethylamino)- and 2-(methyl-amino)-7H-naphtho[1,2,3-de]quinolin-7-one derivatives in experimental model of psychosis in mice. Curr. Top. Med. Chem. 14, 229e233.
Momcilovic-Kostadinovic, D., Simonovic, P., Kolar, D., Jovic, N., 2013. Chlorproma-zine-induced status epilepticus: a case report. Srp. Arh. Celok. Lek. 141, 667e670.
Monte, A.S., de Souza, G.C., McIntyre, R.S., Soczynska, J.K., dos Santos, J.V., Cordeiro, R.C., Ribeiro, B.M.M., de Lucena, D.F., Vasconcelos, S.M.M., de Sousa, F.C.F., Carvalho, A.F., Mac^edo, D.S., 2013. Prevention and reversal of ketamine-induced schizophrenia related behavior by minocycline in mice: possible involvement of antioxidant and nitrergic pathways. J. Psychopharmacol. 27, 1032e1043. http://dx.doi.org/10.1177/ 0269881113503506.
Moore, R.C., Harmell, A.L., Harvey, P.D., Bowie, C.R., Depp, C.A., Pulver, A.E., McGrath, J.A., Patterson, T.L., Cardenas, V., Wolyniec, P., Thornquist, M.H., Luke, J.R., Palmer, B.W., Jeste, D.V., Mausbach, B.T., 2015. Improving the un-derstanding of the link between cognition and functional capacity in schizo-phrenia and bipolar disorder. Schizophr. Res. 169, 121e127.http://dx.doi.org/ 10.1016/j.schres.2015.09.017.
Moran, L.V., Hong, L.E., 2011. High vs low frequency neural oscillations in schizo-phrenia. Schizophr. Bull. 37, 659e663.http://dx.doi.org/10.1093/schbul/sbr056. Moreira, F.A., Guimar~aes, F.S., 2007. Mechanisms of antipsychotic medications:
dopaminergic hypotheses. Medicina (B. Aires) 40, 63e71.
Newcomer, J.W., Farber, N.B., Jevtovic-Todorovic, V., Selke, G., Melson, A.K., Hershey, T., Craft, S., Olney, J.W., 1999. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuro-psychopharmacology 20, 106e118. http://dx.doi.org/10.1016/S0893-133X(98) 00067-0.
Olszewski, M., Piasecka, J., Goda, S.A., Kasicki, S., Hunt, M.J., 2013. Antipsychotic compounds differentially modulate high-frequency oscillations in the rat nu-cleus accumbens: a comparison offirst- and second-generation drugs. Int. J. Neuropsychopharmacol. 16, 1009e1020. http://dx.doi.org/10.1017/ S1461145712001034.
Palenícek, T., Fujakova, M., Brunovský, M., Balíkova, M., Horacek, J., Gorman, I., Tyls, F., Tislerova, B., Sos, P., Bubeníkova-Valesova, V., H€oschl, C., Krajca, V., 2011. Electroencephalographic spectral and coherence analysis of ketamine in rats: correlation with behavioral effects and pharmacokinetics. Neuropsychobiology 63, 202e218.http://dx.doi.org/10.1159/000321803.
Paxinos, G., Watson, C., 1998. The Rat Brain in Stereotaxic Coordinates Fourth Edi-tion. Academic press.
Peuskens, J., Pani, L., Detraux, J., De Hert, M., 2014. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs 28, 421e453.http://dx.doi.org/10.1007/s40263-014-0157-3. Polanía, R., Nitsche, M.A., Korman, C., Batsikadze, G., Paulus, W., 2012. The
impor-tance of timing in segregated theta phase-coupling for cognitive performance. Curr. Biol. 22, 1314e1318.http://dx.doi.org/10.1016/j.cub.2012.05.021. Potvin, S., Stip, E., Sepehry, A. a., Gendron, A., Bah, R., Kouassi, E., 2008.
Inflam-matory cytokine alterations in schizophrenia: a systematic quantitative review. Biol. Psychiatry 63, 801e808.http://dx.doi.org/10.1016/j.biopsych.2007.09.024. Shmukler, A.B., Gurovich, I.Y., Agius, M., Zaytseva, Y., 2015. Long-term trajectories of cognitive deficits in schizophrenia: a critical overview. Eur. Psychiatry 30, 1002e1010.http://dx.doi.org/10.1016/j.eurpsy.2015.08.005.
Siekmeier, P.J., vanMaanen, D.P., 2014. Dopaminergic contributions to hippocampal pathophysiology in schizophrenia: a computational study. Neuro-psychopharmacology 39, 1713e1721.http://dx.doi.org/10.1038/npp.2014.19. Silva, M.C.C., de Sousa, C.N.S., Gomes, P.X.L., de Oliveira, G.V., Araújo, F.Y.R.,
Ximenes, N.C., da Silva, J.C., Vasconcelos, G.S., Leal, L.K.A.M., Macedo, D.,^
Vasconcelos, S.M.M., 2016. Evidence for protective effect of lipoic acid and desvenlafaxine on oxidative stress in a model depression in mice. Prog. Neu-ropsychopharmacol. Biol. Psychiatry 64, 142e148. http://dx.doi.org/10.1016/ j.pnpbp.2015.08.002.
Silva, M.C.C., de Sousa, C.N.S., Sampaio, L.R.L., Ximenes, N.C., Araújo, P.V.P., da Silva, J.C., de Oliveira, S.L., Sousa, F.C.F., Mac^edo, D.S., Vasconcelos, S.M.M., 2013. Augmentation therapy with alpha-lipoic acid and desvenlafaxine: a future target for treatment of depression? Naunyn. Schmiedeb. Arch. Pharmacol. 386, 685e695.http://dx.doi.org/10.1007/s00210-013-0867-y.
Silva, M.C.C., Sampaio, L.R.L., de Araújo, D.P., Araújo, P.V.P., Monte, A.S., Rodrigues, F.T.S., Woods, D.J., de Sousa, F.C.F., Fonteles, M.M.F., Vasconcelos, S.M.M., 2014. Central effects of lipoic acid associated with par-oxetine in mice. Am. J. Ther. 21, 85e90. http://dx.doi.org/10.1097/ MJT.0b013e318235f1a4.
Sirota, A., Csicsvari, J., Buhl, D., Buzsaki, G., 2003. Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl. Acad. Sci. U. S. A. 100, 2065e2069.http://dx.doi.org/10.1073/pnas.0437938100.
Sousa, C.N.S. de., Meneses, L.N., Vasconcelos, G.S., Silva, M.C.C., Silva, J.C. da., Mac^edo, D., de Lucena, D.F., Vasconcelos, S.M.M., 2015. Reversal of corticosterone-induced BDNF alterations by the natural antioxidant alpha-lipoic acid alone and combined with desvenlafaxine: emphasis on the neuro-trophic hypothesis of depression. Psychiatry Res. 230, 211e219. http:// dx.doi.org/10.1016/j.psychres.2015.08.042.
Stanton, P.K., Sejnowski, T.J., 1989. Associative long-term depression in the hippo-campus induced by hebbian covariance. Nature 339, 215e218.http://dx.doi.org/ 10.1038/339215a0.
Stone, J.M., Morrison, P.D., Pilowsky, L.S., 2006. Review: glutamate and dopamine dysregulation in schizophrenia a synthesis and selective review. J. Psychopharmacol. 21, 440e452.http://dx.doi.org/10.1177/0269881106073126. Tamminga, C.A., Stan, A.D., Wagner, A.D., 2010. The hippocampal formation in schizophrenia. Am. J. Psychiatry 167, 1178e1193. http://dx.doi.org/10.1176/ appi.ajp.2010.09081187.
Vasconcelos, G.S., Ximenes, N.C., de Sousa, C.N.S., Oliveira, T. de Q., Lima, L.L.L., de Lucena, D.F., Gama, C.S., Mac^edo, D., Vasconcelos, S.M.M., 2015. Alpha-lipoic acid alone and combined with clozapine reverses schizophrenia-like symptoms induced by ketamine in mice: participation of antioxidant, nitrergic and neu-rotrophic mechanisms. Schizophr. Res. 165, 163e170.http://dx.doi.org/10.1016/ j.schres.2015.04.017.
WHO, 2015. Schizophrenia [WWW Document]. URL.http://www.who.int/mental_ health/management/schizophrenia/en/(Accessed 7 February 15).
Yilmaz, A., Schulz, D., Aksoy, A., Canbeyli, R., 2002. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol. Biochem. Behav. 71, 341e344.
Zhang, J., Cheng, W., Liu, Z., Zhang, K., Lei, X., Yao, Y., Becker, B., Liu, Y., Kendrick, K.M., Lu, G., Feng, J., 2016. Neural, electrophysiological and anatomical basis of brain-network variability and its characteristic changes in mental dis-orders. Brain 139, 2307e2321.http://dx.doi.org/10.1093/brain/aww143. Zhang, Y., Yoshida, T., Katz, D.B., Lisman, J.E., 2012. NMDAR antagonist action in