J. Braz. Chem. Soc., Vol. 18, No. 6, 1220-1223, 2007. Printed in Brazil - ©2007 Sociedade Brasileira de Química 0103 - 5053 $6.00+0.00
ArticleArticleArticleArticleArticle
*e-mail: mallak@cc.iut.ac.ir
Reaction of Aromatic Carboxylic Acids with Isocyanates using Ionic Liquids as
Novel and Efficient Media
Shadpour Mallakpour* and Hamed Yousefian
Organic Polymer Chemistry Research Laboratory, Department of Chemistry, Isfahan University of Technology, Isfahan, 84156-83111, I.R. Iran
Líquidos iônicos (ILs) baseados em 1,3-dialquilimidazólio e amônea têm sido usados como meio de reação eficiente para amidação de diversos ácidos carboxílicos com isocianatos. Esse método tem larga aplicação e o procedimento é suave e eficiente quando comparado aos métodos existentes, baseados em solventes convencionais. O “projeto” adequado do (ILs) permite a obtenção de amidas com resultados de bom a excelente. Nessa reação, tanto pode ser usado um isocianato aromático, quanto um alifático.
Ionic liquids (ILs) based on 1,3-dialkylimidazolium and ammonium have been used as an efficient reaction media in the amidation of several carboxylic acids with isocyanates. The method has wide applicability, and the protocol is mild and efficient compared to the existing methods based on conventional solvents. Proper ‘design’ of the ILs allows us to obtain amides in good to excellent yields. In this reaction, both aromatic and aliphatic isocyanates could be used.
Keywords: ionic liquid, isocyanates, amides, 1,3-dialkylimidazolium, tetrabutylammonium
bromide, carboxylic acid, green media
Introduction
The chemical transformation of the carboxylic acid functional group into the corresponding carboxylic amide (peptide) is a very important reaction in bioorganic chemistry.1 The amide bond has long attracted much
interest since it is an important building unit in proteins. The high stability of the amide linkage towards hydrolysis is of crucial significance to biological systems, since it allows the construction of peptides from relatively simple amino acid precursors.2 Also amides and polyamides play
a very important role in the advance of macromolecular science and the polymer industry.3 Amides have been
prepared by a number of techniques including the reaction of amines with carboxylic acids, the condensation of amino acids, ring opening reaction of lactams, by oxidation of aromatic aldehydes,4 from activated alcohols
using manganese (IV) dioxide in a tandem oxidation process,5 from O-sulfonyloximes and Grignard reagents.6
Isocyanates are highly unsaturated organic compounds. They react readily with many diverse compounds containing
active protons such as alcohols, amines and carboxylic acids. The synthesis of amides by the reaction of a carboxylic acid with an isocyanate has received little attention.1 One of the
advantages of the isocyanation method for preparing amides lies in the good solubility of isocyanates in the reaction media compared to amines. Nevertheless, classical methods for the synthesis of amides typically have limitations and are not environmentally friendly.
The use of environmentally benign reaction media is very important in view of today’s environmentally aware stance. In connection with this, molten salts7 and room
temperature ionic liquids (RTILs)8 have received a great
deal of interest over the past decade as novel solvent systems for organic and inorganic transformations.
ILs are low melting point salts which represent a new class of non-molecular, ionic solvents. Fascinating features of these liquids are their ability to solvate a broad range of both inorganic and organic materials, low vapor pressure, recyclability, high thermal stability and ease of handling.9 In addition, another interesting aspect is the
1221 Mallakpour and Yousefian
Vol. 18, No. 6, 2007
the organic cation. Thus, ILs have been described as ‘designer solvents’. All of these properties make these systems very good candidates for use in developing environmentally benign chemical processes, and actually, nowadays, ILs are extensively diffused as greener alternatives to classical volatile organic solvents in chemical transformations.9 Particularly ILs based on
1,3-dialkylimidazolium cation have been extensively applied for organic and inorganic synthesis. Moreover, ILs are straightforward as they are easily prepared and easy to recycle.10 In view of the emerging importance of
imidazolium and ammonium based ILs as novel reaction media, we wish to investigate the application of ILs as promoters and green solvent systems for the synthesis of amides under mild conditions.
Experimental
The ILs was prepared according to the reported procedures.11
General procedure for amidation
(a) 0.4 g of dry IL (IL was dried under vacuum at 70°C for 4 h) was added to the carboxylic acid (1 mmol) and heated with stirring for 10 min until the acid was dissolved. Then isocyanate (1 mmol) was added under nitrogen atmosphere, and the mixture was stirred for the required amount of time (Table 2). At the end of reaction time (monitored by TLC), the mixture was precipitated in water, filtered off, dried and recrystallized to provide the corresponding benzamide derivatives.
(b) A pyrex reaction flask which was charged with tetrabutylammonium bromide (TBAB) (0.4 g) and benzoic acid (1 mmol) was heated at 90°C. Then the phenyl isocyanate (1 mmol) was added to the stirred molten mixture under nitrogen atmosphere, and the mixture was stirred for a period of time (Table 2). At the end of reaction time (monitored by TLC), the mixture was precipitated in water, filtered off, dried and the white solid was recrystallized from a mixture of ethanol/water (80/20) to furnish benzanilide.
(c) A mixture of NMP (0.5 ml) and benzoic acid (1 mmol) in a round-bottom flask was heated at 100°C. Phenyl isocyanate (1 mmol) was added to this mixture under nitrogen atmosphere, and the mixture was stirred for a period of time (Table 1). After completion of the reaction (checked by TLC), the mixture was precipitated in water, filtered off, dried and the white solid was recrystallized from a mixture of ethanol/water (80/20) to give benzanilide.
Results and Discussion
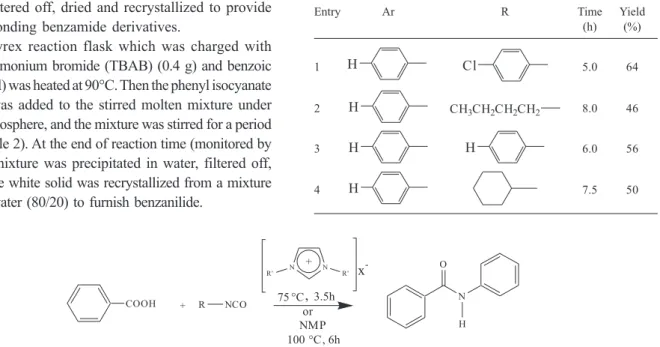
In order to get insight of the role of ILs as green and non-volatile solvents compared to the conventional organic solvents for the reactions of aromatic carboxylic acids with isocyanates, first the reaction of phenyl isocyanates with benzoic acid was performed in different conventional organic solvents such as toluene, N,N-dimethylformamide (DMF) and N-methylpyrrolidinone (NMP). When toluene and DMF were used as solvents, the reaction did not proceed effectively and by-products were observed by TLC technique. By using NMP as solvent the reaction did not show any by-products, but the moderate yields of the desired product indicate that this solvent is not very suitable for this type of reaction (Table 1, Scheme 1).
Then, the amidation reaction was carried out in ILs as solvents. First we studied the reaction of benzoic acid
Scheme 1. The reaction of benzoic acid with several isocyanates in different ILs and NMP.
Table 1. Reactions of benzoic acid with aromatic and aliphatic isocya-nates in NMP as solvent
Entry Ar R Time Yield
(h) (%)
1 H Cl 5.0 64
2 H CH3CH2CH2CH2 8.0 46
3 H H 6.0 56
1222 Reaction of Aromatic Carboxylic Acids with Isocyanates using Ionic Liquids J. Braz. Chem. Soc.
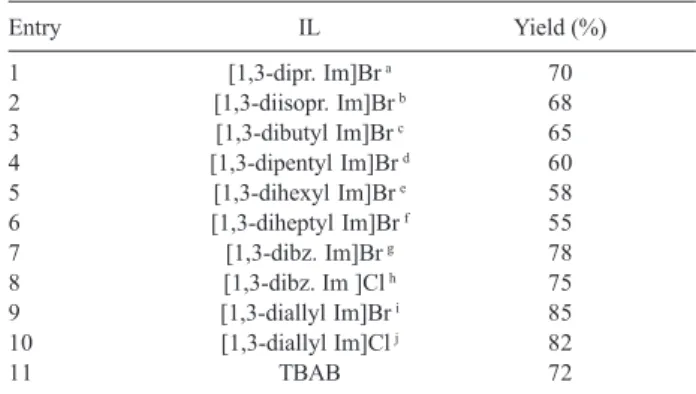
and phenyl isocyanate in details. The effect of different kinds of ILs on the yields of this reaction was also investigated (Scheme 1, Table 2). The best result was obtained with 1,3-diallylimidazolium bromide (entry 9) and this IL was selected for the other reactions.
The procedure was successfully tested by the reactions of several aromatic carboxylic acids with aromatic as well as aliphatic isocyanates in the presence of 1,3-diallylimidazolium bromide as IL (Scheme 2, Table 3). It is very interesting to mention that while aromatic isocyanates in conventional solvents do not provide good yields of corresponding amides, we obtained excellent yields of the corresponding amides in ILs without any formation of side products such as urea.1
In this work we used ILs as efficient media for the amidation of aromatic carboxylic acids with isocyanates under mild conditions. Moreover, we observed that addition of triethylamine and dibutyltin dilaurate as
catalysts did not affect the progress of the reaction and no additional compounds were necessary to activate the isocyanates. On the other hand, both, blocked isocyanates and catalysts are required for the effective preparation of amides, in other works.1 It can be concluded that ILs could
act as a promoter in this reaction without using any catalysts.
Table 2. The effect of typical ILs on reaction yields of benzoic acid with phenyl isocyanate
Entry IL Yield (%)
1 [1,3-dipr. Im]Br a 70
2 [1,3-diisopr. Im]Br b 68
3 [1,3-dibutyl Im]Br c 65
4 [1,3-dipentyl Im]Br d 60
5 [1,3-dihexyl Im]Br e 58
6 [1,3-diheptyl Im]Br f 55
7 [1,3-dibz. Im]Br g 78
8 [1,3-dibz. Im ]Cl h 75
9 [1,3-diallyl Im]Br i 85
10 [1,3-diallyl Im]Cl j 82
11 TBAB 72
a1,3-dipropylimidazolium bromide, b1,3-diisopropylimidazolium bromide, c1,3-dibutylimidazolium bromide, .d1,3-dipentylimidazolium bromide, e 1,3-dihexylimidazolium bromide, f1,3-diheptylimidazolium bromide, g 1,3-dibenzylimidazolium bromide, h1,3-dibenzylimidazolium.chloride, i 1,3-diallylimidazolium bromide, j1,3-diallylimidazolium chloride
Scheme 2. The reaction of aromatic carboxylic acids with aliphatic and aromatic isocyanates in [1,3-diallyl Im] Br.
H
Ar COOH R NCO Ar R
O
N
+ IL, 75 °C
Table 3. The reaction of aromatic carboxylic acids with aliphatic and aromatic isocyanates in ILs
No. Ar R Time (h) Yield (%) Melting point (°C)
1 H Cl 2.0 90 186-188(Lit 12:187-187)
2 Me 3.0 82 155-156(Lit 13:154-155)
3 4.0 78 145-147(Lit 14:146-148)
4 O2N 6.0 55 203-205(Lit 14:204-206)
5 H CH3CH2CH2 5.0 69 81-83(Lit 15:83-85)
6 H CH3CH2CH2CH2 4.5 71 112(Lit 16:112)
7 H H 2.5 85 157-160(Lit 5:158-161)
8 H
Cl
Cl
1.5 93 150-152(Lit 17:151-153)
9 MeO 2.0 87 158-160(Lit 14:158-160)
1223 Mallakpour and Yousefian
Vol. 18, No. 6, 2007
Conclusions
In summary, for the first time a new and efficient method was developed for the selective synthesis of benzamides derivatives in excellent isolated yields by using RTIL as a reaction medium. Importantly, the IL not only acts as a solvating medium, but also as a promoter for this reaction. The easy work-up procedures, the absence of a catalyst and using non-volatile IL as the reaction medium makes the method amenable for scale-up operations.
Acknowledgments
We wish to express our gratitude to the Research Affairs Division Isfahan University of Technology (IUT), Isfahan, for partial financial support. Further financial support from Center of Excellency in Sensors Research (IUT) is gratefully acknowledged. We also thank to Miss Z. Rafiee and Miss Kowsari for the helpful discussion.
References
1. Blagbrough, I. S.; Mackenzie, N. E.; Ortiz, C.; Scott, A. I.; Tetrahedron Lett.1986, 27, 1251. Gurtler, C; Danielmeier, K.; Tetrahedron Lett.2004, 45, 2515. Gurtler, C; Gertzmann, R.; Tetrahedron Lett.2005, 46, 6659.
2. Auzeil, N.; Largeron, M.; Fleury, M. B.; J. Chem. Soc. Perkin Trans. 2 1999, 1703.
3. Pan, J. Q.; Lau, W. W. Y.; J. Macromol. 2001, 95. 4. Gilman, N. W.; J. Chem. Soc.,Chem. Commun. 1971, 733. 5. Kanno, H.; Giblin, G. M. P.; Taylor, R. J. K.; Synthesis2003,
1055.
6. Narasaka, K.; Pure Appl. Chem.2002, 74, 143.
7. Dupont, J.; Consorti, C. S.; Spencer, J.; J. Braz. Chem. Soc. 2000,11, 337. Smietana, M.; Mioskowski, C.; Org. Lett. 2001, 3, 1037. Amantini, C.; Fringuelli, F.; Pizzo, F.; Vaccaro, L.; J. Org. Chem. 2001, 66, 6734. Selvakumar, K.; Zapf, A.; Beller, M.; Org. Lett. 2002, 4, 3031. Khalafi-Nezhad, A.; Mokhtari, B.; Tetrahedron Lett.2004, 45, 6737. Ranu, B. C.; Das, A.; Samanta, S.; J. Chem. Soc., Perkin. Trans.12002, 1520. 8. Wasserscheid, P.; Keim, W.;Angew. Chem., Int. Ed. 2000, 3772.
Welton, T.; Chem. Rev. 1999, 99, 2071. Dupont, J.; de Souza, R. F.; Suarez, P. A. Z.; Chem. Rev. 2002,102, 3667.
9. Olivier-Bourbogou, H.; Magna, L.; J. Mol. Catal. A: Chem. 2002, 182, 419.
10. Branco, L.C.; Afonso, C. A. M.; Tetrahedron 2001, 57, 4405. Gui, j.; Ban, H.; Cong, X.; Zhang, X.; Hu, Z.; Sun, Z.; J. Mol. Catal. A: Chem. 2005, 225, 27. Rosa, J. N.; Afonso, C. A. M.; Santos, A. G.; Tetrahedron 2001, 57, 4189.
11. Vygodskii, Y. S.; Lozinskaya, E. I.; Shaplov, A. S.; Lyssenko, K. A.; Antipin, M. Y.; Urman, Y. G.; Polymer2004, 45, 5031. Vygodskii, Y. S.; Lozinskaya, E. I.; Shaplov, A. S.; Macromol. Rapid Commun. 2002,23, 676. Mallakpour, S.; Kowsari, E.; J. Polym. Sci. Part A: Polym. Chem. 2005, 43, 6545.
12. Kawashima, T.; Inamato, N.; Bull. Chem. Soc. Jap.1972, 45, 3504.
13. Hendrickson, J. B.; Sajjat Hussoin, M.; J. Org. Chem. 1989, 54, 1144.
14. Auzeil, N.; Largeron, M.; Fleury, M. B.; J. Chem. Soc., Perkin. Trans.2 1999, 1703.
15. Nojima, M.; Hasegawa, Sh.; Tokura, N.; Bull. Chem. Soc. Jap. 1973, 46, 1254.
16. Yamada, Sh. I.; Kasia, Y.; Shioiri, T.; Tetrahedron Lett. 1973, 18, 1595.
17. Rinderknecht, H.; Ma, V.; Helv. Chim. Acta1964, 47, 162.