www.rpped.com.br
REVISTA
PAULISTA
DE
PEDIATRIA
REVIEW
ARTICLE
Safety
of
human
papillomavirus
6,
11,
16
and
18
(recombinant):
systematic
review
and
meta-analysis
Pedro
Luiz
Spinelli
Coelho
a,∗,
Gustavo
Lacerda
da
Silva
Calestini
a,
Fernando
Salgueiro
Alvo
a,
Jefferson
Michel
de
Moura
Freitas
a,
Paula
Marcela
Vilela
Castro
a,
Tulio
Konstantyner
b,caCentroUniversitárioLusíada,Santos,SP,Brazil
bUniversidadedeSantoAmaro,SãoPaulo,SP,Brazil
cLondonSchoolofHygiene&TropicalMedicine,Londres,England
Received29October2014;accepted27February2015 Availableonline12September2015
KEYWORDS
Papillomavirus vaccines; Adverseeffects; Adolescent; Meta-analysis; Safety
Abstract
Objective: Toidentifyandquantifytheadverseeffectsassociatedwiththerecombinanthuman papillomavirus(types6,11,16and18)vaccineinadolescents.
Datasource: SystematicreviewofrandomizedclinicaltrialsfromPubMed,SciELOandLilacs databases.Articlesinvestigatingthesafetyofthevaccineinsubjectsunder18yearsand com-paringtherecombinanthumanpapillomavirustypes6,11,16and18vaccinewithacontrolgroup wereincluded.Meta-analyseswereperformedfortheoutcomesofpain,erythema,swellingand fever,usingclinicaltrialswithmaximumJadadscore.
Datasynthesis: Fourteenstudieswereincluded.Themostcommonadverseeffectsrelatedto thehumanpapillomavirusvaccinewereeffectswithnoseverity(pain,erythema,edema,and fever).Fivestudieswereusedforthemeta-analyses:pain---riskdifference(RD)=11%(p<0.001); edema---RD=8%(p<0.001);erythema---RD=5%(p<0.001);fever---RD=2%(p<0.003).
Conclusions: Therecombinanthumanpapillomavirustypes6,11,16and18vaccinewassafe andwell tolerated. Themain adverse effectsrelated to vaccinationwere pain, erythema, edemaandfever.Thelowfrequencyofsevereadverseeffectsencouragestheadministration ofthevaccineinthepopulationatrisk.
©2015SociedadedePediatriadeS˜aoPaulo.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-license(https://creativecommons.org/licenses/by/4.0/).
DOIoforiginalarticle:http://dx.doi.org/10.1016/j.rpped.2015.02.006
∗Correspondingauthor.
E-mail:plsc.fcms@gmail.com(P.L.S.Coelho).
2359-3482/©2015SociedadedePediatriadeS˜aoPaulo.PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCC
PALAVRAS-CHAVE
Vacinascontra papillomavirus; Efeitosadversos; Adolescente; Metanálise; Seguranc¸a
Seguranc¸adavacinapapillomavirushumano6,11,16e18(recombinante):revisão sistemáticaemetanálise
Resumo
Objetivo: Identificar e quantificar os efeitos adversos associados à vacina papillomavirus humano6,11,16e18(recombinante)emadolescentes.
Fontesdedados: Revisãosistemáticadeensaiosclínicosrandomizadosnasbasesdedadosdo PubMed,SciELO eLilacs.Foramincluídos artigosqueabordavamaseguranc¸adavacina em menoresde18anosequecomparavamavacinapapillomavirushumano6,11,16e18 (recom-binante) comgrupo controle. Foram feitasmetanálises para osdesfechos de dor,eritema, edemaefebrecomousodeensaiosclínicoscomescoredeJadadmáximo.
Síntesedosdados: Foramincluídos14estudos.Osefeitosadversosmaiscomunsrelacionados àvacina foramintercorrênciassemgravidade(dor, eritema,edema efebre).Cincoestudos foramusadosparaasmetanálises,incluindoosdesfechos:Dor---Diferenc¸adeRisco(DR)=11% (p<0,001);Edema---DR=8%(p<0,001);Eritema---DR=5%(p<0,001);Febre---DR=2%(p<0,003).
Conclusões: Avacinapapillomavirushumano6,11,16e18(recombinante)mostrou-sesegura ebemtolerada.Osprincipaisefeitosadversosrelacionadosàvacinac¸ãoforamdor,eritema, edemaefebre.Abaixafrequênciadeefeitosadversosgravesencorajaaaplicac¸ãodavacina napopulac¸ãoderisco.
©2015SociedadedePediatriadeS˜aoPaulo.PublicadoporElsevierEditoraLtda.Esteéumartigo OpenAccesssobalicençaCCBY(https://creativecommons.org/licenses/by/4.0/deed.pt).
Introduction
Cervicalcanceristhesecondmostcommontypeofcancer thataffectswomenworldwide,withanincidenceof approx-imately500,000casesand270,000deathseachyear.1,2The
disease is often detected at advanced stages due to the lowerefficiencyofscreeningstrategies intheinitialstage andtreatmentoptionsthatarenotalwayseffective.3---6At
least80%ofdeathsfromcervicalcanceroccurin develop-ingcountries, mostof them in thepoorest regions of the world,suchasSouthernAsia,Sub-SaharanAfricaandparts ofLatinAmerica.Inthoseareas,whichreceiveonly5%of theresourcesforcancerintheworld,cervicalinvolvement isresponsiblefor15%ofallcancerdeaths.7
Infection by human papillomavirus (HPV) is a common occurrence,andtheprobabilityofacquiringitthroughout anindividual’slifetimeishigherthan50%.8Approximately
35---40typesofHPVcaninfectthegenitalepithelium.The infectionmaybetransientandnotclinicallydetectable,but canalsocausegenitalwartsandavarietyofpre-malignant andmalignantanogenitallesionsinbothgenders.9---14
Stud-iesshow that the peakincidence of HPVinfection occurs 5---10 years afterthe first sexualintercourse (between 15 and25yearsold),15---19andinfectionpersistencebyan
onco-genic HPV type is crucial in the pathogenesis of cervical cancer.2,20---22 Thus,itbecomespossibletopreventthe
dis-ease onsetthroughvaccination beforethe start of sexual activity.19,23---26
ThecurrentlyavailablevaccinesagainstHPVdifferinthe numberofgenotypes,inthewaytheyaremanufacturedand theadjuvanttheycontain.Bothvaccinescurrentlyavailable foruse,bivalentandquadrivalent,arehighlyimmunogenic and preventthe primary infection against HPV genotypes and CIN 2/3 adenocarcinoma (CIN --- cervical intraepithe-lialneoplasia,which referstosquamousepithelial lesions
inthelowergenitaltract,whichareprecursorsofinvasive cancer,presentingastissueimpairment,fromcytoplasmic alterationstoseveredysplasia).Studiesindicateavery sim-ilarsafety profile for severe and mild adverse effects for eachoneofthevaccines.27,28
The introduction of newvaccinesrequires safety stud-ies. Concerns about the adverse effects is considered a barrier to vaccination and one of the reasons for low adherence to the recommendations for human papillo-mavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine administration.29,30 The opinion of health
profes-sionalsregardingitssafetyisyettobeunanimous.Several debates have been carried out withpersistent controver-sies about the advantages and disadvantages of its use. Therefore, the knowledge of the possible local and sys-temic adverse effects can subsidize adherence strategies andguidehealthcareactionsforthepopulationatrisk.
Therefore,theobjectiveofthisstudyistoidentifyand quantifythe adverse effects associatedwiththe adminis-trationofthehumanpapillomavirusquadrivalent(types6, 11,16,18)recombinantvaccine,asatooltodeterminethe safetyofitsuseinadolescents.
Method
the first stage of article selection, the Decs/Mesh health descriptor‘‘papillomavirusvaccines/adverse effects’’was used. The study design filter ‘‘RCTs’’ was added to the obtainedresults.Subsequently,theidentifiedarticleswere analyzedbyreadingthetitlesandabstracts.
Atthisstage,theexclusioncriteriaofthearticleswere: concurrentuseofHPVvaccine withothervaccines;useof thevaccineinpatientswhoalreadyhadcervicaldiseases; patientspositiveforHIV;patientswhohadalreadyreceived theHPVvaccine; studiesontheacceptanceamongfamily members of the administrationof HPV vaccine in adoles-cents;studiescarriedoutexclusivelywithpatientsaged>18 years;repeatedstudiesandsystematicreviews.
Allotherstudieswerereadinfull,analyzedbyfive inde-pendentinvestigatorsandclassifiedaccordingtotheJadad score.31 Inthisclassification,onepointwasattributedfor
eachof thefollowing items: descriptionof article as ran-domizedtrialanddescriptionofarticleasdoubleblind;one additionalpoint for each of thearticles of whichmethod wasdescribed,andincaseitwasappropriate;thepointfor randomizationand blindingwas subtractedif themethod usedforthose wasinappropriate;andan additionalpoint fordescriptionoflosses.
Afterthisstep,theresearchersmetatapaneldiscussion oneligibilitycriteria.Therefore,articlesthatevaluatedthe safetyof HPVbivalent andquadrivalent vaccines,in both genders,withJadadscore31≥3wereincluded.Theprocess
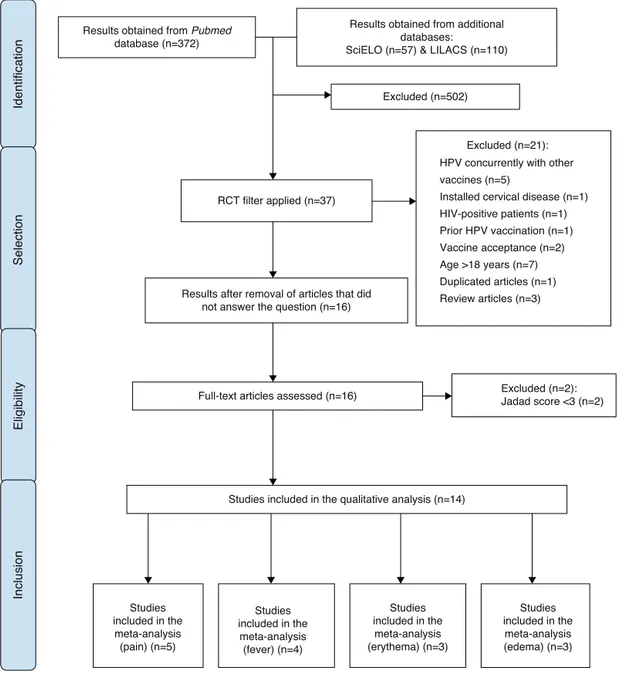
ofarticleselectionisshowninFig.1.
Initially, all adverse effects identified in the selected RCTs were listed. The selected articles were described according to the results shown in each of the RCTs, dis-closingprevalencedataandcharacteristicsoftheidentified adverse effects. Subsequently,four meta-analyses offour different outcomes evaluatedin RCTs (pain, edema, ery-thema,fever)wereperformed,whichincludedarticleswith Jadadscore=531 andcomparedtheir occurrencebetween
thegroupthatreceivedtheHPVquadrivalentvaccineand theplacebogroup.
ThestatisticalpackageReviewManager5.2softwarewas used.Theresultswereexpressedasriskdifference(RD)with fixedconfidenceintervalof95%andastatisticalsignificance levelwithamaximump=0.05(5%).Heterogeneitywas calcu-latedusingthestatisticalMantel---Haenszelchi-squaretest, andexpressedasI2,andthosewithvalue>50%(I2>50%)were consideredheterogeneous. Asymmetrieswererepresented inthefunnelplot.
Results
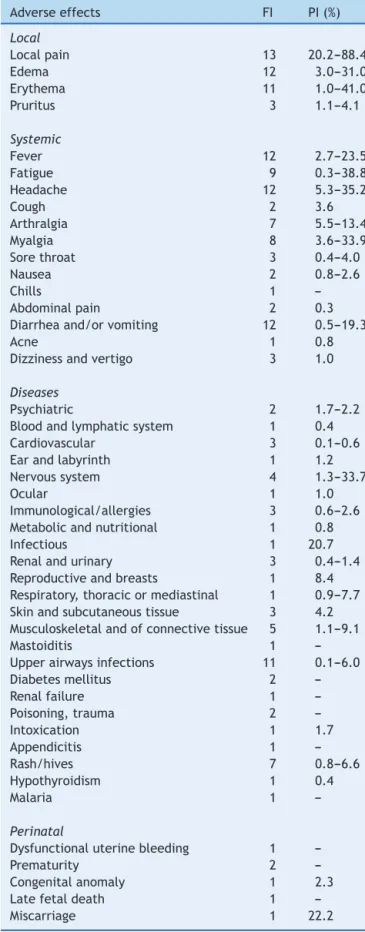
A total of 14 RCTs were included in this study, none of themfromtheSciELOor LILACSdatabases.Table1 shows theadverse effects associatedwith the administrationof the human papillomavirus quadrivalent (types 6, 11, 16, 18)recombinantvaccine,withoutnecessarilyestablishinga causallinkbetweenthevaccineandtheeffects,indicating howmanyofthesestudiesidentifiedthemandthevariations betweenprevalencerates.
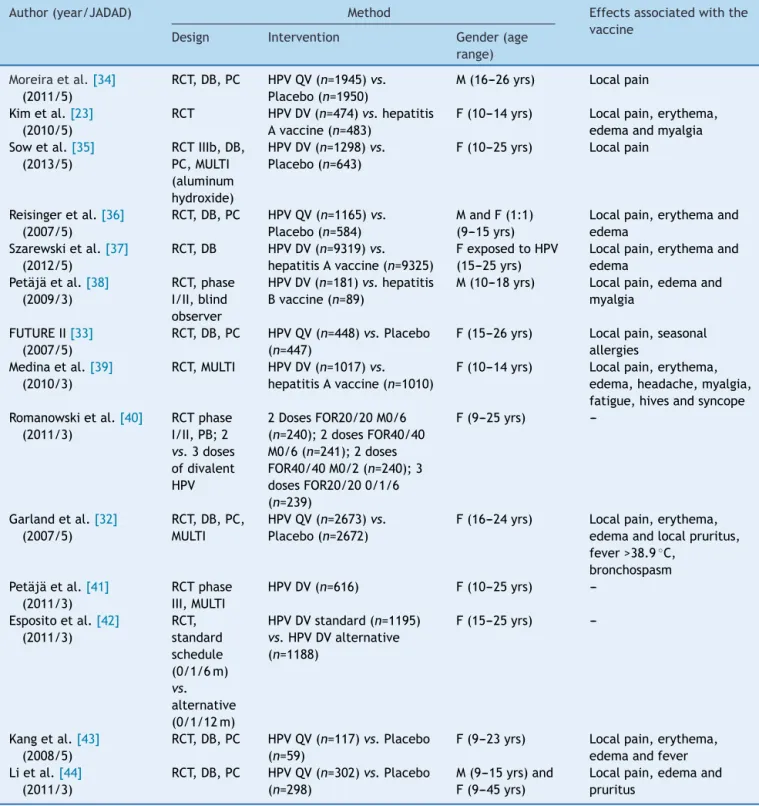
Table2describesthecharacteristicsoftheselected stud-iesduring thesearch process,23,32---43 including authorship,
year of publication,classification according tothe Jadad
Table 1 Frequency ofidentification (FI) andprevalence interval(PI)ofadverseeffectsassociatedwiththeHPV vac-cine and identified in 14 selected randomized controlled trials.
Adverseeffects FI PI(%)
Local
Localpain 13 20.2---88.4
Edema 12 3.0---31.0
Erythema 11 1.0---41.0
Pruritus 3 1.1---4.1
Systemic
Fever 12 2.7---23.5
Fatigue 9 0.3---38.8
Headache 12 5.3---35.2
Cough 2 3.6
Arthralgia 7 5.5---13.4
Myalgia 8 3.6---33.9
Sorethroat 3 0.4---4.0
Nausea 2 0.8---2.6
Chills 1
---Abdominalpain 2 0.3
Diarrheaand/orvomiting 12 0.5---19.3
Acne 1 0.8
Dizzinessandvertigo 3 1.0
Diseases
Psychiatric 2 1.7---2.2
Bloodandlymphaticsystem 1 0.4
Cardiovascular 3 0.1---0.6
Earandlabyrinth 1 1.2
Nervoussystem 4 1.3---33.7
Ocular 1 1.0
Immunological/allergies 3 0.6---2.6 Metabolicandnutritional 1 0.8
Infectious 1 20.7
Renalandurinary 3 0.4---1.4 Reproductiveandbreasts 1 8.4 Respiratory,thoracicormediastinal 1 0.9---7.7 Skinandsubcutaneoustissue 3 4.2 Musculoskeletalandofconnectivetissue 5 1.1---9.1
Mastoiditis 1
---Upperairwaysinfections 11 0.1---6.0
Diabetesmellitus 2
---Renalfailure 1
---Poisoning,trauma 2
---Intoxication 1 1.7
Appendicitis 1
---Rash/hives 7 0.8---6.6
Hypothyroidism 1 0.4
Malaria 1
---Perinatal
Dysfunctionaluterinebleeding 1
---Prematurity 2
---Congenitalanomaly 1 2.3
Latefetaldeath 1
---Miscarriage 1 22.2
Results obtained from Pubmed
database (n=372)
RCT filter applied (n=37)
Excluded (n=21):
HPV concurrently with other vaccines (n=5)
Installed cervical disease (n=1) HIV-positive patients (n=1)
Prior HPV vaccination (n=1) Vaccine acceptance (n=2)
Age >18 years (n=7) Duplicated articles (n=1)
Review articles (n=3)
Identification
Selection
Results after removal of articles that did not answer the question (n=16)
Full-text articles assessed (n=16)
Eligibility
Excluded (n=2): Jadad score <3 (n=2)
Studies included in the qualitative analysis (n=14)
Inclusion
Studies included in the meta-analysis (pain) (n=5)
Studies included in the
meta-analysis (fever) (n=4)
Studies included in the
meta-analysis (erythema) (n=3)
Studies included in the meta-analysis (edema) (n=3) Excluded (n=502)
Results obtained from additional databases:
SciELO (n=57) & LILACS (n=110)
Figure1 Articleselectionprocess.
score,31methodologyusedandtheadverseeffectsthatwere
statisticallyrelatedtothevaccine.
Among the analyzed studies, therewas only one case ofsevereadverseeventrelatedtothevaccine,whichwas bronchospasm.32 Theothersshowednoreportsof
vaccine-relatedsevereadverseeffectsordeaths.Theincidenceof adverseeffectswashigherafterthefirstdoseofthevaccine schedule,withareductionintheiroccurrenceatsubsequent doses.23,33---36,43
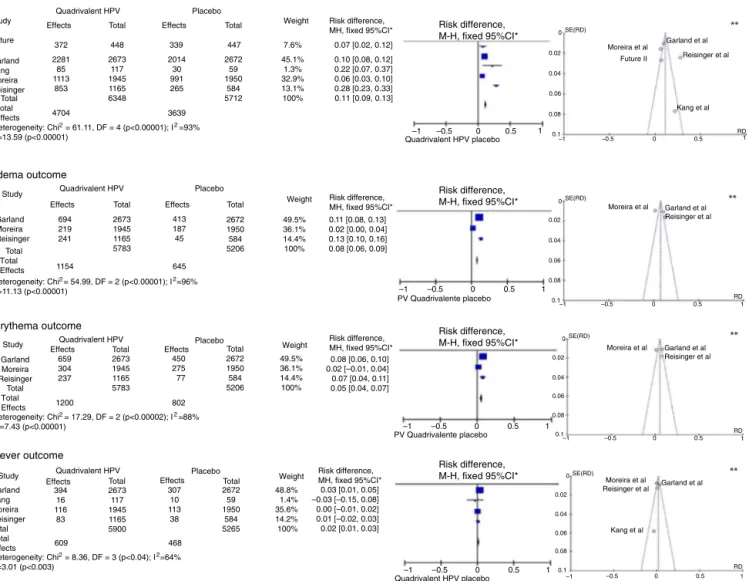
The selection of outcomes for the meta-analyses was carried out according to the frequency of appearance of adverse effects assessed in the selected publications, comparingtheiroccurrencebetweenvaccinatedand unvac-cinatedsubjectsagainstHPV,emphasizingthelocaleffects (pain,erythemaandedema)and,assystemiceffect,fever. ThisanalysiswasperformedwithfivestudieswithaJadad score31 of 5,32---34,36,43without gender or agegroup
restric-tionforthequadrivalentvaccine,withfourofthembeing
multicenterstudies32---34,36 andonehavingbeencarriedout
inSouthKorea.43Themeta-analysisresultsforthe‘‘pain’’,
‘‘edema’’,‘‘erythema’’and‘‘fever’’outcomesareshown inFig.2.
For the ‘‘pain’’ outcome, the meta-analysis assessed five RCTs, totaling 12,060 participants, 6348 vaccinated for HPV and 5712 placebo-controlled. Of the vaccinated ones,4704hadpainat theinjectionsite,while only3639 reportedthesameoutcome withtheplacebo,resultingin anRD=11%(95%CI:9---13%;I2=93%),therefore being signifi-cantlymorecommonaftertheadministrationofthevaccine (p<0.001).
The‘‘edema’’outcomewasanalyzedinthree multicen-terarticles.32,34,36 These included 5783 patients,of which
Table2 Characteristicsof14randomizedclinicaltrialsselectedbytheusedsearchcriteria.
Author(year/JADAD) Method Effectsassociatedwiththe
vaccine Design Intervention Gender(age
range)
Moreiraetal.[34] (2011/5)
RCT,DB,PC HPVQV(n=1945)vs.
Placebo(n=1950)
M(16---26yrs) Localpain
Kimetal.[23] (2010/5)
RCT HPVDV(n=474)vs.hepatitis Avaccine(n=483)
F(10---14yrs) Localpain,erythema, edemaandmyalgia Sowetal.[35]
(2013/5)
RCTIIIb,DB, PC,MULTI (aluminum hydroxide)
HPVDV(n=1298)vs.
Placebo(n=643)
F(10---25yrs) Localpain
Reisingeretal.[36] (2007/5)
RCT,DB,PC HPVQV(n=1165)vs.
Placebo(n=584)
MandF(1:1) (9---15yrs)
Localpain,erythemaand edema
Szarewskietal.[37] (2012/5)
RCT,DB HPVDV(n=9319)vs.
hepatitisAvaccine(n=9325)
FexposedtoHPV (15---25yrs)
Localpain,erythemaand edema
Petäjäetal.[38] (2009/3)
RCT,phase I/II,blind observer
HPVDV(n=181)vs.hepatitis Bvaccine(n=89)
M(10---18yrs) Localpain,edemaand myalgia
FUTUREII[33] (2007/5)
RCT,DB,PC HPVQV(n=448)vs.Placebo (n=447)
F(15---26yrs) Localpain,seasonal allergies
Medinaetal.[39] (2010/3)
RCT,MULTI HPVDV(n=1017)vs.
hepatitisAvaccine(n=1010)
F(10---14yrs) Localpain,erythema, edema,headache,myalgia, fatigue,hivesandsyncope Romanowskietal.[40]
(2011/3)
RCTphase I/II,PB;2
vs.3doses ofdivalent HPV
2DosesFOR20/20M0/6 (n=240);2dosesFOR40/40 M0/6(n=241);2doses FOR40/40M0/2(n=240);3 dosesFOR20/200/1/6 (n=239)
F(9---25yrs)
---Garlandetal.[32] (2007/5)
RCT,DB,PC, MULTI
HPVQV(n=2673)vs.
Placebo(n=2672)
F(16---24yrs) Localpain,erythema, edemaandlocalpruritus, fever>38.9◦C,
bronchospasm Petäjäetal.[41]
(2011/3)
RCTphase III,MULTI
HPVDV(n=616) F(10---25yrs)
---Espositoetal.[42] (2011/3)
RCT, standard schedule (0/1/6m)
vs.
alternative (0/1/12m)
HPVDVstandard(n=1195)
vs.HPVDValternative (n=1188)
F(15---25yrs)
---Kangetal.[43] (2008/5)
RCT,DB,PC HPVQV(n=117)vs.Placebo (n=59)
F(9---23yrs) Localpain,erythema, edemaandfever Lietal.[44]
(2011/3)
RCT,DB,PC HPVQV(n=302)vs.Placebo (n=298)
M(9---15yrs)and F(9---45yrs)
Localpain,edemaand pruritus
RCT,randomizedcontrolledtrial;DB,double-blind;PB,partiallyblind;PC,placebo-controlled;MULTI,multicenter;HPV,human papil-lomavirus;M,male;F,female;FOR,formulation;m,month;DV,divalent;QV,quadrivalent;yrs.,years.
Themeta-analysisofthe‘‘erythema’’outcomeincluded the same articles used for edema. Of all patients vacci-natedfor HPV, 1200 developed erythema at theinjection site,while802haderythemaintheplacebogroup,withan RD=5%(95%CI:4---7%,p<0.001,I2=88%).
In turn, the meta-analysis of the ‘‘fever’’ outcome involvedfourarticles.Ofthe5900vaccinatedpatients,609 developedfever,while468ofthe5265intheplacebogroup
hadthesameoutcome.TheRD=2%(95%CI1---3%,I2=64%)was significant(p<0.003).
Discussion
Pain outcome Edema outcome Erythema outcome Fever outcome Study Quadrivalent HPV Quadrivalent HPV Quadrivalent HPV Quadrivalent HPV Effects Effects Effects Effects 694 219 241 659 304 237 1200 609 468 802 2673 1945 1165 5783 413 187 45 2672 1950 584 5206 49.5% 36.1% 14.4% 100% Total Total Total Total 2673 1945 1165 5783 450 275 77 339 448 3639 447 2672 59 1950 584 5712 45.1% 1.3% 32.9% 13.1% 100%
0.07 [0.02, 0.12] 7.6%
0.10 [0.08, 0.12] 0.22 [0.07, 0.37] 0.06 [0.03, 0.10] 0.28 [0.23, 0.33] 0.11 [0.09, 0.13]
0.11 [0.08, 0.13] 0.02 [0.00, 0.04] 0.13 [0.10, 0.16] 0.08 [0.06, 0.09]
0.08 [0.06, 0.10] 0.02 [–0.01, 0.04] 0.07 [0.04, 0.11] 0.05 [0.04, 0.07]
0.03 [0.01, 0.05] –0.03 [–0.15, 0.08] 0.00 [–0.01, 0.02] 0.01 [–0.02, 0.03] 0.02 [0.01, 0.03] 2673 117 1945 1165 6348 Effects Effects Effects Effects Placebo Placebo Placebo Placebo Total Total Total Total 2672 1950 584 5206 49.5% 36.1% 14.4% 100% Weight Weight Weight Weight 2014 30 991 265 372 4704 2281 85 1113 853 Future II Garland Kang Moreira Reisinger Total Total Effects Study Study Study 1154 645 Garland Moreira Reisinger Garland Moreira Reisinger Garland Kang Moreira Reisinger Total Total Effects Total Total Effects Total Total Effects
Heterogeneity: Chi2 = 61.11, DF = 4 (p<0.00001); I2 =93% Z=13.59 (p<0.00001)
Heterogeneity: Chi2 = 54.99, DF = 2 (p<0.00001); I2 =96% Z=11.13 (p<0.00001)
Heterogeneity: Chi2 = 17.29, DF = 2 (p<0.00002); I2 =88% Z=7.43 (p<0.00001)
Heterogeneity: Chi2 = 8.36, DF = 3 (p<0.04); I2 =64% Z=3.01 (p<0.003) 394 16 116 83 2673 117 1945 1165 5900 307 10 113 38 2672 59 1950 584 5265 48.8% 1.4% 35.6% 14.2% 100% Risk difference, M-H, fixed 95%CI*
Risk difference, M-H, fixed 95%CI*
Risk difference, M-H, fixed 95%CI*
Risk difference, M-H, fixed 95%CI*
Risk difference, MH, fixed 95%CI*
Risk difference, MH, fixed 95%CI* Risk difference, MH, fixed 95%CI* Risk difference, MH, fixed 95%CI*
–1 –0.5
Quadrivalent HPV placebo0 0.5 1 –1 –0.5
PV Quadrivalente placebo
0 0.5 1
–1 –0.5 PV Quadrivalente placebo
0 0.5 1
–1 –0.5 Quadrivalent HPV placebo
0 0.5 1
SE(RD) 0 0.02 0.04 0.06 0.08 0.1 SE(RD) 0 0.02 0.04 0.06 0.08
0.1–1 –0.5 0 0.5 1 1 –1 –0.5 0 0.5
–1 –0.5 0 0.5 1
1 –1 –0.5
Moreira et al
Reisinger et al Garland et al Moreira et al
Moreira et al Moreira et al Future II
Garland et al Reisinger et al Garland et al Reisinger et al
Reisinger et al
Kang et al
Kang et al
0 0.5 SE(RD) SE(RD) RD RD RD RD 0 0.02 0.04 0.06 0.08 0.1 0 0.02 0.04 0.06 0.08 0.1 ** ** ** ** Garland et al
Figure2 Meta-analysisofoutcomes‘‘pain’’,‘‘edema’’,‘‘erythema’’and‘‘fever’’fromtheresultsprovidedbytheselected RCTs.*M-H,Mantel-Haenszel;CI,confidenceinterval;intheforestplots,thehorizontalaxisrepresentstheCIofriskdifference. Thepointsrepresenttheriskdifferenceineachstudy.Thedotslocatedtotherightofthemedianlineindicatehigherincidenceof theoutcomeinthegroupthatreceivedthevaccine;thesizeofthedotsrepresentstherelativeweightofeachstudyinthefinal outcome.Thediamondindicatesthefinaloutcomeofthemeta-analysis;**funnelplots(horizontalaxis=magnitudeoftheeffect; verticalaxis=samplesize)illustratetheheterogeneitybetweenstudies.
schedule of female adolescents,and also the future pos-sibility of expanding its target audience, the study of the safety profile of the human papillomavirus quadriva-lent (types 6,11, 16, 18) recombinant vaccine is crucial. Moreover, considering its good efficacy demonstrated in severalstudies,32,33,39,41theclearassociationbetween
cer-vical cancer and chronic HPV infection and the need for the administration of more than one dose to meet the purpose, the knowledge of possible adverse reactions is important to ensure adherence to the proposed vacci-nation schedule and, consequently, the success of this strategytopreventthisinfectiousdiseaseaswellascervical cancer.
Inthiscontext,theclinicaltrialsselectedforthisstudy suggesttheexistenceofseverallocalandsystemicadverse effects, severe or not, chronic disease onset during the studyperiodandnewrelevantmedicalconditionsreported by the affected patients.However, most of theseclinical
phenomenacannotbedefinedasadverseeffectsassociated with the administration of the human papillomavirus quadrivalent(types6,11,16,18)recombinantvaccine,for theiroccurrencehasnotbeencomparedtocontrolgroups, andthecausalassociationhasnotbeenestablished.
Intwoarticles, differentimmunizationschedules were tested and compared and no significant differences were found regarding the occurrence of pain or other adverse effects between the groups.40,42 The remaining 12
Painwasidentifiedin11ofthe12articlesandthiswasthe mostcommonadverseeffect,alwaysassociatedwithhuman papillomavirusquadrivalent(types6,11,16,18) recombi-nantvaccinewhencomparedtotheplacebogroup,32---36,43,44
tothecontrolgroupwithhepatitisAvaccine23,37,39or
hep-atitis B vaccine.38 Edema was present in ten articles. Of
these,eightidentifiededemaasaneffectthatwasdirectly relatedtoHPVvaccination.Erythemawasdescribedinnine articles,butonlysixshowedadirectassociationwith vacci-nation.Prurituswasanadverseeffectthatwasrarelyfound amongtheassessedarticles,beingpresentinonlythreeof them.
Given the magnitude of the identified local adverse effects, the performance of the meta-analysisaimed not onlytoconfirm,butalsotoquantifythem,usingstatistical methodsthatallowajointassessmentoftrialsinvestigating the association of adverse effects with the human papil-lomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine.Themeta-analysesperformedinthisstudyshowed a higher probability of vaccinated individuals to develop localeffects,withsignificantdifferenceofrisk, especially regardinglocalpain,andtoalesserextent,erythemaand edema.
Moreiraetal.34 suggest thattheadjuvantAS04 maybe
implicated ina higher incidenceof local adverse effects; however,itsroleisofutmostimportancetoenhancethe vac-cineimmunogenicity.As thisfindingwasexclusivelyfound inthetrialcarriedoutbytheseauthors,furtherstudiesare requiredtodeterminethecausalassociation,whichislikely tochangetheuseofthisadjuvantinthevaccine manufac-turingprocess.
Among the wide variety of systemic adverse effects identifiedinthisreview,fewwereactuallyrelatedto immu-nization,suchasfeverandmyalgia. Tenofthe12articles listed fever as an adverse effect, but only two of them wererelatedtovaccinationwhencomparedtocontrols.The riskof fever afterthe administrationofthe human papil-lomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccineobservedinthemeta-analysiswasgreaterthanthat intheplacebogroup.Thisfactshouldbeawarningtohealth care professionals whorecommend care after prescribing thevaccinetotheirpatients.However,itisnoteworthythat althoughthisriskdifferenceissignificant,itisnotsufficient tocontraindicateitsuse,basedonthebenefitofprotection againstviralinfectionandcervicalcancer.
Other mentioned systemic effects, such as headache, rash,myalgia,hives,syncope,fatigue,39andallergies33were
systemic adverse effects also related to vaccination, but werenotincludedin themeta-analyses,astheywerenot reproduced in other studies. The other systemic adverse effects,whichdifferedbetweenarticles,wereunrelatedto thevaccinationandshowednodifferencebetweenthestudy groupsregardingtheiroccurrence,positivelycontributingto itssafetyprofile.Someof themdidnothaveanexplicitly reportedfrequencyofoccurrence,makingitdifficultto ana-lyzethem.Thisobservationleadsustoconsidertheneedfor additionallong-termstudies,andwithlargersamplesizes, sothatrareoutcomescanbeassessed.32,39
Althoughtheyarefrequent,moresevereadverseeffects relatedtovaccinationwerenotdescribedand,forthemost part,theywerenotacauseoflossesinthecompletionof themultiplevaccinationschedule.Itwasalsoobservedthat
theincidenceofadverseeffectsishigherafterthefirstdose oftheschedule,withdecreasingoccurrenceinsubsequent doses.23,34---36,43
Consideringthe prevalence ofHPV infection in adoles-cence and the incidence and morbimortality of cervical cancer, the risk/benefit ratio of the vaccine shows to be completelyacceptable,confirmingitsgoodtolerabilityand safety proposed by other authors,23,32---36,39---44 which
con-tributestotheeffectivenessofpublichealthpolicies. Despite the identification of effects in observational studies,whichcancontributetotheconstructionof knowl-edge, the present study chose the exclusive selection of RCTswithcomparisontoa controlgroup,especiallythose usingplacebo,asstudieswiththisdesignallowestablishing aclearassociationofadverseeffectswithvaccinationand ruleouttheundesirableeffectofconfoundingfactors,often presentinobservationaldesigns.Thus,thefindingshereare theresultoftheknowledgegeneratedbythemaximumlevel ofscientificevidenceavailablein theliterature.However, itisnoteworthythattheRCTsthatevaluatethesafetyand reactogenicityofthe vaccine includealimitednumber of subjectswhencomparedwiththegeneralpopulationof indi-vidualseligibleforvaccination.Thischaracteristicrestricts theidentificationofrareorunknownadverseeffects. There-fore,the resultsshown herein shouldbe interpretedwith caution.
This study did not differentiate between genders, becausealthough themassvaccinationwasrecommended forfemaleadolescents,themalepopulationisalso suscep-tibletoinfectionanddiseasescausedbyHPV.Additionally, themalepopulationcanplayakeyroleindisease transmis-sion,and oneshouldconsiderthe possibilityof thisbeing a possible target of vaccination campaigns in the future. Reisingeretal.36suggestthatthefemalepopulationreports
moreadverseeffectsthanthemalepopulation,butno for-malcomparisonwasmadebetweengendersforthisfinding. Moreover,theselectedarticlesshowedgreatvariabilityin the assessed age group, including schoolchildren, adoles-cents,youngadultsandadults.
This fact also contributed to differences between the studypopulationsand,possibly,totheexternalvalidityof the obtained results. Therefore, the selection of articles could not limit the age group, as there wasno standard methodologybetweenstudiesregardingtheageofthestudy population.
The meta-analyses showed moderate and high hetero-geneity values (I2>50%), which reduces the degree of confidence in theresults shown here.Overall, the results followed the trend of a large sample population study.32
Somevariablesmaybeattributedasthecauseofthe hetero-geneity,especiallyage,genderandsamplesize,suggesting thattheoutcomesstudiedinthemeta-analysescannotbe dependentonthevaccineonly,butalsoonthe abovemen-tionedfactors.Ontheotherhand,theconfidenceintervals ofthecalculatedriskdifferenceswerenarrow,duetothe similarityoftheresultsidentifiedineachtrial selectedto comprisethemeta-analyses,reinforcingtheplausibilityof theobservedmagnitudeeffect.
Anothermethodological divergence occurred regarding thecontrol groupsof each study.Some studiesperformed comparisonswithacontrolgroupthatusedplacebo32---36,43,44
Thesearticlesweremaintainedinthisstudy,because hep-atitis A and Bvaccines have a well-established use, with awell-definedsafetyprofile,andcanbeagoodreference. However,themeta-analysisusedonlyarticlesthatcompared thehumanpapillomavirusquadrivalent(types6,11,16,18) recombinantvaccinewithplacebo,sothatthesamplewould be ashomogeneousaspossible. Moreover, informationon theintensityof theassessedadverseeffectshasnotbeen demonstratedinalltheselectedstudies,whichmightimpair theevaluationoftheeffectseverity.Ourstudy,therefore, didnotconsiderdifferencesinseveritywhenalloutcomes wereanalyzedtogether.
Althoughitwasnotthefocusofthisstudy,itis notewor-thythat,whenthebivalentandquadrivalentvaccinesare compared,theresultssuggestthatbothhavesimilarsafety profile andadverse effects,witha predominance of local effects.However,thisfindingcanonlybeestablishedby per-formingfurtherstudies,ofwhichobjectiveistocomparethe occurrenceofadverseeffectsbetweenthevaccines.
Inthiscontext,theresultsshownheresuggestthatthe useofhumanpapillomavirusquadrivalent(types6,11,16, 18) recombinant vaccine is potentially safe and well tol-erated. The main adverse effects related to vaccination are local effects, such aspain, erythema and edema. As for systemic effects, fever was associated with vaccina-tion. Bothgroups of adverse effects werenot considered severe.Finally,itisconcludedthatthehighimmunogenicity andsafetyprofileofthehumanpapillomavirusquadrivalent (types6,11,16,18)recombinantvaccinedeterminethatits usehasanadvantageousrisk/benefitratioandisafavorable strategy topreventthis viralinfectionaswell ascervical cancer,whichsupportsthepersistentencouragementfrom healthprofessionalstoprovideHPVvaccinationtotherisk population.
Funding
Thisstudydidnotreceivefunding.
Conflicts
of
interest
Theauthorhasnoconflictsofinteresttodeclare.
References
1.ParkinDM,BrayF,FerlayJ,PisaniP.Globalcancerstatistics, 2002.CACancerJClin.2005;55:74---108.
2.Cutts FT, Franceschi S, Goldie S, et al. Human papillo-mavirusandHPVvaccines:areview.BullWorldHealthOrgan. 2007;85:719---26.
3.HPVInformationCentre[páginanaInternet].Human papillo-mavirusandrelatedcancersinAfrica---summaryreport;2010. Available at http://www.hpvcentre.net/statistics/reports/ XWX.pdf[accessed26.01.15].
4.GakidouE,Nordhagen S, ObermeyerZ. Coverageof cervical cancerscreeningin57countries:lowaveragelevelsandlarge inequalities.PLoSMed.2008;5:e132.
5.DennyL,QuinnM,SankaranarayananR.Screeningforcervical cancerindevelopingcountries.Vaccine.2006;24Suppl.3:S71---7 [Chapter8].
6.Louie KS, de Sanjose S, Mayaud P. Epidemiology and pre-vention of human papillomavirus and cervical cancer in
sub-Saharan Africa: a comprehensive review. Trop Med Int Health.2009;14:1287---302.
7.BoyleP,LevinB.Worldcancerreport2008.Lyon:IARC;2008.
8.WorldHealthOrganization.Humanpapillomavirus(HPV) infec-tionandcervicalcancer.WorldHealthOrganization;2004.
9.WileyDJ,DouglasJ,BeutnerK,etal.Externalgenitalwarts: diagnosis,treatment,andprevention.ClinInfectDis.2002;35 Suppl.2:S210---24.
10.LuB,WuY,NielsonCM,etal.Factorsassociatedwithacquisition andclearanceofhumanpapillomavirusinfectioninacohortof USmen:aprospectivestudy.JInfectDis.2009;199:362---71.
11.Aho M, Vesterinen E, Meyer B, Purola E, Paavonen J. Natural history of vaginal intraepithelial neoplasia. Cancer. 1991;68:195---7.
12.Schiffman M, KjaerSK. Naturalhistory ofanogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr.2003;31:14---9[Chapter2].
13.SrodonM,StolerMH,BaberGB,KurmanRJ.Thedistributionof lowandhigh-riskHPVtypesinvulvarandvaginalintraepithelial neoplasia(VINandVaIN).AmJSurgPathol.2006;30:1513---8.
14.HamplM,SarajuuriH,WentzensenN,BenderHG,KueppersV. Effectofhumanpapillomavirusvaccinesonvulvar,vaginal,and analintraepitheliallesionsandvulvarcancer.ObstetGynecol. 2006;108:1361---8.
15.Woodman CB, Collins S, Winter H, et al. Natural history of cervicalhumanpapillomavirusinfectioninyoungwomen:a lon-gitudinalcohortstudy.Lancet.2001;357:1831---6.
16.CollinsS,MazloomzadehS,WinterH,etal.Highincidenceof cervicalhumanpapillomavirusinfectioninwomenduringtheir firstsexualrelationship.BJOG.2002;109:96---8.
17.WinerRL,LeeSK,HughesJP,AdamDE,KiviatNB,KoutskyLA. Genitalhumanpapillomavirusinfection:incidenceandrisk fac-torsinacohortoffemaleuniversitystudents.AmJEpidemiol. 2003;157:218---26.
18.De Sanjosé S, Diaz M, Castellsagué X, et al. Worldwide prevalenceandgenotypedistributionofcervicalhuman papil-lomavirusDNAinwomenwithnormalcytology:ameta-analysis. LancetInfectDis.2007;7:453---9.
19.Keam SJ,HarperDM. Humanpapillomavirustypes16and 18 vaccines(recombinant,AS04adjuvanted,adsorbed)[Cervarix]. Drugs.2008;68:359---72.
20.SchiffmanM,CastlePE,JeronimoJ,RodriguezAC,Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890---907.
21.BoschFX,LorinczA,Mu˜nozN,MeijerCJ,ShahKV.Thecausal relationbetweenhumanpapillomavirusandcervicalcancer.J ClinPathol.2002;55:244---65.
22.Walboomers JM,Jacobs MV, ManosMM, et al. Human papil-lomavirus is a necessary cause of invasive cervical cancer worldwide.JPathol.1999;189:12---9.
23.Kim YJ, Kim KT, Kim JH, et al. Vaccination with a human papillomavirus (HPV)-16/18 AS04-adjuvanted cervical cancer vaccine inKoreangirls aged 10---14years.JKorean MedSci. 2010;25:1197---204.
24.InsingaRP,DasbachEJ,MyersER.Thehealthandeconomic bur-denofgenitalwartsinasetofprivatehealthplansintheUnited States.ClinInfectDis.2003;36:1397---403.
25.MountSL,PapilloJL.Astudyof10,296pediatricand adoles-centPapanicolaousmeardiagnosesinnorthernNewEngland. Pediatrics.1999;103:539---45.
26.Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseasesamongAmericanyouth:incidenceandprevalence esti-mates,2000.PerspectSexReprodHealth.2004;36:6---10.
27.World Health Organization [página naInternet]. Information
sheetobservedrateofvaccinereactionshumanpapillomavirus
vaccine; 2014. Available at: http://www.who.int/vaccine
safety/initiative/tools/HPVVaccineratesinformationsheet.
28.DescampsD,HardtK,SpiessensB,etal.Safetyofhuman papil-lomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancerprevention:apooledanalysisof11clinicaltrials.Hum Vaccines.2009;5:332---40.
29.TrimK,NagjiN,ElitL,RoyK.Parentalknowledge,attitudes, andbehaviourstowardshumanpapillomavirusvaccinationfor theirchildren:asystematicreviewfrom2001to2011.Obstet GynecolInt.2012;2012:1---12.
30.LazTH,RahmanM,BerensonAB.Anupdateonhuman papil-lomavirus vaccine uptake among 11---17year old girls inthe UnitedStates:nationalhealthinterviewsurvey,2010.Vaccine. 2012;30:3534---40.
31.Jadad AR, Moore RA, Carroll D,et al. Assessing thequality ofreportsofrandomizedclinicaltrials:isblindingnecessary. ControlClinTrials.1996;17:1---12.
32.Garland SM,Hernandez-Avila M, Wheeler CM, et al. Quadri-valent vaccine against human papillomavirus to prevent anogenitaldiseases.NEnglJMed.2007;356:1928---43.
33.TheFUTUREIIStudyGroup.Quadrivalentvaccineagainsthuman papillomavirustopreventhigh-gradecervicallesions.NEnglJ Med.2007;356:1915---27.
34.MoreiraEDJr,PalefskyJM,GiulianoAR,etal.Safetyand reac-togenicityofaquadrivalenthumanpapillomavirus(types6,11, 16,18)L1viral-like-particlevaccineinolderadolescentsand youngadults.HumVaccines.2011;7:768---75.
35.SowPS,Watson-JonesD,KiviatN,etal.Safetyand immuno-genicity of human papillomavirus-16/18 AS04-adjuvanted vaccine: a randomizedtrialin 10---25-year-oldHIV seronega-tive African girls and young women. J Infect Dis. 2013;207: 1753---63.
36.ReisingerKS,BlockSL,Lazcano-PonceE,etal.Safetyand per-sistentimmunogenicityofaquadrivalenthumanpapillomavirus types6,11,16,18L1virus-likeparticlevaccinein preadoles-centsand adolescents:arandomizedcontrolledtrial.Pediatr InfectDisJ.2007;26:201---9.
37.SzarewskiA,PoppeWA,SkinnerSR,etal.Efficacyofthehuman papillomavirus(HPV)-16/18AS04-adjuvantedvaccineinwomen aged15---25yearswithandwithoutserologicalevidenceof pre-viousexposuretoHPV-16/18.IntJCancer.2012;131:106---16.
38.Petäjä T, Keränen H, Karppa T, et al. Immunogenicity and safetyofhumanpapillomavirus(HPV)-16/18AS04-adjuvanted vaccineinhealthyboysaged 10---18years.JAdolescHealth. 2009;44:33---40.
39.Medina DM, Valencia A, de Velasquez A, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine: a randomized,controlled trialin adolescentgirls. JAdolesc Health.2010;46:414---21.
40.RomanowskiB,SchwarzTF,FergusonLM,etal.Immunogenicity andsafetyoftheHPV-16/18AS04-adjuvantedvaccine adminis-teredasa2-doseschedulecomparedwiththelicensed3-dose schedule: results from a randomized study. Hum Vaccines. 2011;7:1374---86.
41.PetäjäT,PedersenC,PoderA,etal.Long-termpersistenceof systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women.IntJCancer.2011;129:2147---57.
42.EspositoS,BirlutiuV, JarcuskaP,et al. Immunogenicityand safetyofhumanpapillomavirus-16/18AS04-adjuvantedvaccine administeredaccordingtoanalternativedosingschedule com-paredwiththestandarddosingscheduleinhealthywomenaged 15to25years:resultsfromarandomizedstudy.PediatrInfect DisJ.2011;30:e49---55.
43.KangS,KimKH,KimYT,etal.Safetyandimmunogenicityofa vaccinetargetinghumanpapillomavirustypes6,11,16and18: arandomized,placebo-controlledtrialin176Koreansubjects. IntJGynecolCancer.2007;18:1013---9.