Braz. J. of Develop.,Curitiba, v. 6, n. 3,p.13532-13543 mar.. 2020. ISSN 2525-8761
Ascophyllum Nodosum seaweed extract as an alternative for control of
post-harvest soft rot in strawberries
Extrato de alga marinha de Ascophyllum Nodosum como alternativa para
controle da podridão mole pós-colheita em morangos
DOI:10.34117/bjdv6n3-279
Recebimento dos originais: 30/01/2020 Aceitação para publicação: 12/03/2020
Karoline Dias Paiva
Mestre em Ciência e Tecnologia de Alimentos pelo IFSULDEMINAS Campus Machado Instituição: IFSULDEMINAS Campus Machado
Endereço: Rodovia Machado - Paraguaçu, km 3, CEP 37.750-000, Machado-MG, Brasil E-mail: kka.karol@yahoo.com.br
Fernanda Ananias Martins
Bacharel em Agronomia pelo IFSULDEMINAS Campus Machado Instituição: IFSULDEMINAS Campus Machado
Endereço: Rodovia Machado - Paraguaçu, km 3, CEP 37.750-000, Machado-MG, Brasil E-mail: nanda.ananias@hotmail.com
Tamiris Aparecida dos Santos
Bacharel em Agronomia pelo IFSULDEMINAS Campus Machado Instituição: IFSULDEMINAS Campus Machado
Endereço: Rodovia Machado - Paraguaçu, km 3, CEP 37.750-000, Machado-MG, Brasil E-mail: tamirisapsantos@gmail.com
Dalilla Carvalho Rezende
Doutora em Fitopatologia pela Escola Superior de Agricultura “Luiz de Queiroz” - Universidade de São Paulo
Instituição: IFSULDEMINAS Campus Machado
Endereço: Rodovia Machado - Paraguaçu, km 3, CEP 37.750-000, Machado-MG, Brasil E-mail: dalilla.rezende@ifsuldeminas.edu.br
Brígida Monteiro Vilas Boas
Doutora em Ciência dos Alimentos pela Universidade Federal de Lavras Instituição: IFSULDEMINAS Campus Machado
Endereço: Rodovia Machado - Paraguaçu, km 3, CEP 37.750-000, Machado-MG, Brasil E-mail: brigida.monteiro@ifsuldeminas.edu.br
Déborah Magalhães Xavier-Mis
Master´s degree, Plant Health in Louisiana State University Agricultural Center Instituição: Louisiana State University Agricultural Center, Department of Plant Pathology
and Crop Physiology
Endereço: Baton Rouge, LA 70803, USA E-mail: dxaviermis@gmail.com
Braz. J. of Develop.,Curitiba, v. 6, n. 3,p.13532-13543 mar.. 2020. ISSN 2525-8761
ABSTRACT
Strawberry post-harvest losses caused by Rhizopus stolonifer (RS) is one of the main problems affecting strawberry production in Brazil. The objective of this study was to evaluate the potential of a commercial product based on Ascophyllum nodosum (AN) seaweed extract in the inhibition of the pathogen growth and disease development in strawberries. The commercial product was tested in vitro to evaluate the influence of the seaweed extract on the mycelial growth of the pathogen. Potato-dextrose-agar (PDA) medium received different concentrations of the seaweed extract. After 24h, a disk of RS was transferred to the PDA plates containing seaweed extract. Organic strawberries were immersed for 5 minutes in a solution of the AN seaweed extract in the concentration of 40 mL.L-1. After 24h the
strawberries were inoculated by mycelial disk and incubated for 72h at 25o C. The fruits were then evaluated for disease incidence. The commercial product reduced fungal growth at the 5 mL.L-1 concentration and completely inhibited fungal incidence at 40 mL.L-. The A. nodosum seaweed extract at 40 mL L-1 was able to reduce 22.3% of soft rot incidence of strawberries in post-harvest in fruits inoculated by mycelial disks of the phytopathogen without change in humidity and at 25ºC. The results of this study suggest that AN has a potential to be used in the management of soft rot and increase the shelf life of strawberries in post-harvest.
Keywords: Biofertilizer; sustainability; phytopathology; food safety; biostimulant.
RESUMO
As perdas pós-colheita de morango causadas por Rhizopus stolonifer (RS) são um dos principais problemas que afetam a produção de morango no Brasil. O objetivo deste estudo foi avaliar o potencial de um produto comercial à base de extrato de alga marinha de Ascophyllum nodosum (AN) na inibição do crescimento de patógenos e desenvolvimento de doenças em morangos. O produto comercial foi testado in vitro para avaliar a influência do extrato de algas no crescimento micelial do patógeno. O meio de batata-dextrose-ágar (PDA) recebeu diferentes concentrações do extrato de algas marinhas. Após 24h, um disco de RS foi transferido para as placas PDA contendo extrato de algas marinhas. Morangos orgânicos foram imersos por 5 minutos em solução de extrato de alga marinha AN na concentração de 40 mL.L-1. Após 24h, os morangos foram inoculados por disco micelial e incubados por 72h a 25o C. Os frutos foram avaliados quanto à incidência de doenças. O produto comercial reduziu o crescimento de fungos na concentração de 5 mL.L-1 e inibiu completamente a incidência de fungos em 40 mL.L-. O extrato de algas marinhas de A. nodosum a 40 mL L-1 foi capaz de reduzir 22,3% da incidência de podridão mole de morangos em pós-colheita em frutos inoculados por discos miceliais do fitopatógeno sem alteração de umidade e a 25ºC. Os resultados deste estudo sugerem que a AN tem potencial para ser usada no manejo da podridão mole e aumenta o prazo de validade dos morangos na pós-colheita.
Palavras-chave: Biofertilizante; sustentabilidade; fitopatologia; segurança alimentar;
Braz. J. of Develop.,Curitiba, v. 6, n. 3,p.13532-13543 mar.. 2020. ISSN 2525-8761
1 INTRODUCTION
The horticulture industry is among the main income generators, employment and rural development in Brazilian agribusiness, especially in the South of Minas Gerais State. World demand for fruits and vegetables has been growing because of the increase of population awareness about healthy eating. However, one of the main obstacles in the production and marketing of these products is the significant post-harvest losses, which are substantially high in developing countries such as Brazil (IBRAF, 2015;FISHER et. al, 2007;GALLI et. al, 2012; HENZ et. AL, 2008; PEARSON and GOHEEN, 19900).
According to FAO (2014; 2017), 1.3 billion tons of food is wasted or lost along supply chains worldwide, which represents 30% of the total food produced annually on the planet. In Latin America, between a quarter and a third of the production of food for human consumption is lost or wasted, and 40 to 50% of this amount refers to fruits and vegetables. Some of the factors associated to post-harvest losses are: technical limitations in harvesting and storage methods, transportation, and processing activities. The lack of proper cooling facilities, infrastructure, packaging and marketing systems aggravate post-harvest losses in the region. Moreover, the occurrence of disease is the one of the most important causes of post-harvest losses.
Strawberry is an important asset of the fruit industry in Brazil. The country is among the world's largest producers, and strawberry production has been encouraged by the release of new cultivars adapted to different climates. In the South of Minas Gerais State, most of the strawberries are produced in small farms and sold locally (DIAS, 2011). In post-harvest, some diseases have caused significant losses in strawberries, especially soft rot caused by the fungus Rhizopus stolonifer. Fungi spores colonize the interior of the fruit through openings that occur during harvest and/or transportation, causing a fast decay of the whole fruit (AGRIOS, 2005). The use of agrochemicals in post-harvesting is a problem due to chemical residues, which make the fruits less attractive to consumers. The exposure of animals and the environment to the toxicity from agricultural pesticides leads to increasing restrictions on the current international market. One of the sustainable alternatives is the use of biotic or abiotic agents for post-harvest disease management (AMORIM et. al, 2016).
Ascophyllum nodosum seaweed extract has been used as a biostimulant in several crops because it is rich in nutrients which stimulate phytoalexins accumulation, increase fruit mass and productivity, as well as promote beneficial changes in the composition of plant tissues and induce pathogen resistance (BENICA et. al, 2008; CARVALHO, CASTRO, 2014).
The objectives of the current study were to evaluate the potential of A. nodosum seaweed extract to inhibit R. stolonifer in vitro and to verify the ability of the seaweed extract to increase the shelf life of strawberries.
2 MATERIAL AND METHODS
2.1 OBTAINING AND MAINTAINING THE PATHOGEN ISOLATE
The isolate of Rhizopus stolonifer used in this study was obtained from the fungi collection from the Phytopathology and Nematology Department, located in “Luiz de Queiroz” School of Agriculture - USP. The fungus was maintained in potato-dextrose-agar medium (PDA) in order to provide optimal conditions for its growth and development. 2.2 EVALUATION OF A. NODOSUM SEAWEED EXTRACT ON PATHOGEN MYCELIAL GROWTh
A commercial product was used as the source of A. nodosum seaweed extract, this being a LS (Liquid Suspension) formulation with 100% concentration of seaweed extract and brown coloration. PDA culture medium was prepared and received different dosages of the
Braz. J. of Develop.,Curitiba, v. 6, n. 3,p.13532-13543 mar.. 2020. ISSN 2525-8761 seaweed extract corresponding to the concentrations of 5, 10, 20 and 40 mL.L-1. These concentrations were related to the 5 treatments proposed to investigate possible fungal growth inhibition. The control treatment consisted of PDA medium alone.
After 24 h of preparation, the Petri dishes with the 5 treatments proposed on the solid-state media received a culture medium disc of 0.5 cm in diameter containing the mycelium of R. stolonifer. The plates were incubated in a BOD chamber at 25 °C and with 12h light/dark photoperiod. Mycelial growth was evaluated every 24 hours with a digital caliper, until the mycelium of one of the plates reached the edge of the Petri dishes.
The experiment was conducted in a randomized complete block design (DBC) containing 5 treatments and 10 replicates, each Petri dish represented one repetition. The data obtained in the evaluation of mycelial growth was submitted to analysis of variance (p≤0.05) and the means were compared by F test. Regression analysis of the variables were also done by SISVAR software(FERREIRA, 2014).
2.3 IN VIVO ASSAY TO EVALUATE THE POTENTIAL OF A. NODOSUM SEAWEED EXTRACT ON THE MANAGEMENT OF POST-HARVEST DISEASES IN STRAWBERRIES
From the results obtained in the experiments, we applied the best treatment proposed by linear regression to the strawberry fruits to evaluate how it would impact the development of soft-rot post-harvest.
Strawberries from the cultivar Camarosa were obtained from farmers from the Southern region of Minas Gerais State. The fruits used in this study were organic, and therefore cultivated without any chemical applications.
Fruits with similar size and ripeness were selected for in vitro tests. The fruits were cleaned with sodium hypochlorite 0.5% and then washed in distilled water in order to remove the excess of sanitizer. The fruits were then placed in a single layer of paper towels at room temperature for 24 h to dry. The treatments were performed according to the previous test, using a 40 mL L-1 concentration of the A. nodosum seaweed extract. After 24 h, the fruits were inoculated with a 0.5 cm diameter culture medium disc containing mycelium of R. stolonifer.
Finally, the inoculated fruits were placed inside plastic boxes closed hermetically and incubated in BOD chamber at 25ºC for 48h. Disease incidence was evaluated every 24h.
The assay was conducted in a randomized block design. The treatment included 9 replicates; each experimental unit consisted of a box containing 3 strawberries, totaling 27 strawberries per treatment; for a total of 54 strawberries in the experimental material.
2.4 IN VITRO AND IN VIVO TREATMENTS IN THE ABSENCE AND PRESENCE OF SANITIZING AGENT
In order to verify the in vitro efficiency of the A. nodosum seaweed extract in the inhibition of soft rot after the addition of the sanitizer commercially based on sodium hypochlorite at 2.5% concentration, an experiment with three treatments was designed. Thus, the pathogen was submitted to three treatments: PDA culture medium, PDA culture medium with 40 mL.L-1 seaweed extract, and PDA culture medium with seaweed extract 40 mL.L-1 and sanitizer at 0.5%.
The experiment was performed by adding mycelial disks of the R. stolonifer in Petri dishes, which were then subjected to the evaluation of mycelial growth, as previously described. Additionally, the effect of sodium hypochlorite in the in vivo experiment with the three proposed treatments, and the influence of fruit sanitation with seaweed extract were evaluated. The treatment control (T1) consisted of fruits that were sanitized and washed in distilled water.
Braz. J. of Develop.,Curitiba, v. 6, n. 3,p.13532-13543 mar.. 2020. ISSN 2525-8761 After 24 h the inoculation of the pathogen in fruits was carried out by mycelial disk of the pathogen to the Petri dish containing each treatment, as previously described. Finally, the fruits were packed in plastic boxes and placed in BOD chamber at 25 °C. The incidence of the soft rot in the fruits was evaluated every 24h during 72h.
The experiment was conducted in randomized blocks (DRB), and consisted of three treatments, identified as T1, T2 and T3, with nine replicates. Each experimental unit consisted of three strawberries, totaling 81 evaluated strawberries.
2.5 STATISTICAL ANALYSIS
The variables mycelial growth (mm) and disease incidence (%) obtained in the measurements of all treatments were submitted to the F test. Treatments significantly different at 5% probability had their means compared by the Scott-Knott test, also considering p ≤ 0.05. All analyzes were made by the SISVAR software (FERREIRA, 2014).
2.6 POST-HARVEST PHYSICAL AND CHEMICAL CHARACTERISTICS OF STRAWBERRIES TREATED WITH A. NODOSUM SEAWEED EXTRACT
These experiments included two treatments: T1 (Control) and T3 (sanitized strawberries with 40 mL.L-1 A. nodosum seaweed extract)., There were 32 strawberries per treatment. The tests were carried out in partnership with Iberpharm Laboratories of Brazil LTDA and the Laboratory of Bromatology of the Federal Institute of Teaching, Science and Technology from South of Minas Gerais - Campus Machado.
Physical and chemical analysis were done to verify possible changes of strawberry fruits in post-harvest, 24 hours after exposure to A. nodosum seaweed extract. The strawberries used in these experiments were certified to be grown with no pesticide application by the regional supplier. The selected fruits were sanitized with 0.5% sodium hypochlorite for 1 minute and then washed under running water to remove any excess of the product. Fruits were dried at room temperature for 24 hours. The seaweed extract was added by submerging the fruits in the product with concentration of 40 mL.L-1 for 5 minutes.
For color analysis, the readings of the L* value and hue angle were carried in peel and pulp of strawberries, in two points in the equatorial region, using a colorimeter with illuminant D65, 2º observation angle and CIEL*a*b* color system (MINOLTA, 1998). Fruit firmness was
determined using the texturometer with a cylindrical probe of 2 mm and the measurements were made at two opposite points in the equatorial region of the fruits. Soluble solids contents (oBrix) were determined from the pulp of the processed fruit obtained by the centrifugation of
five fruits per repetition using a manual refractometer with automatic temperature compensation at 25ºC15. The pH was determined using a pHmeter and the titratable acidity (% citric acid) was determined by titration using sodium hydroxide solution 0.2 mol/L and the phenolphthalein indicator (IAL, 2008).
The total solids content (g/100 g) was determined by the gravimetric method using heat in an oven at 105ºC15. The determination of total sugars (g/100g) was performed by the Lane-Eyon titration method. The methodology is based on the heat titration of the reducing sugar with complexed copper reagent15.
All variables were submitted to statistical analysis using the F test in the analysis of variance using the statistical program SISVAR software (FERREIRA, 2014).
3 RESULTS AND DISCUSSION
There were significant differences in mycelial growth of R. stolonifer with the use of A. nodosum seaweed extract. In control treatments, the pathogen continued growing in the
Braz. J. of Develop.,Curitiba, v. 6, n. 3,p.13532-13543 mar.. 2020. ISSN 2525-8761 Petri dishes and reached its borders in 48h. On the other hand, fungi growth was not observed in plates with seaweed extract treatment in the same period.
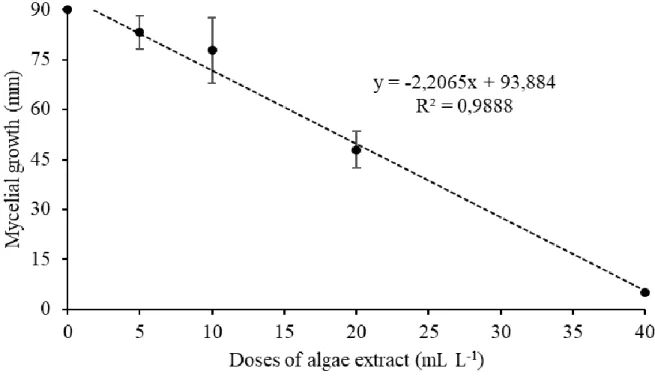
The regression graph (Figure 1) shows that the inhibition of pathogen growth is noticeable even in the lowest concentration of the product. The reduction of the mycelial diameter steadily increased until reaching the value of 49.6% in the concentration of 20 mL.L
-1. In the treatment of 40 mL.L-1 of A. nodosum seaweed extract, the inhibition of pathogen
growth was 100%, which is the same concentration observed in the linear regression model y = ax + b.
Figure 1. The daily means of the mycelial growth diameter (mm) of Rhizopus stolonifer obtained in the evaluations until the pathogen reached the border of the Petri dish of the control treatment on days 1 and 2 submitted to the respective treatments 0, 5, 10, 20 and 40 mL.L-1 of Ascophyllum nodosum seaweed extract.
Braz. J. of Develop.,Curitiba, v. 6, n. 3,p.13532-13543 mar.. 2020. ISSN 2525-8761
Figure 2. Means of the mycelial growth diameter (mm) of the fungus Rhizopus stolonifer, as a function of dosages of Ascophyllum nodosum seaweed extract at 0, 5, 10, 20 and 40 mL.L-1 on day 2 of the assay.
The results of the in vitro tests indicate the possibility of an in vivo application of A. nodosum seaweed extract to control soft rot in strawberries. Similar responses were obtained by Silva16 when evaluating the product in the post-harvest control of anthracnose in young
finger peppers (Capsicum baccatum L.). Silva (2011) observed a 50% reduction in fungal incidence when using 100 mL.L-1 concentration of the seaweed extract product. This inhibitory effect of seaweed extract on pathogens may occur due to the presence of substances produced by marine macroalgae as a defense mechanism, since they develop in an environment of extreme conditions such as salinity, low temperature and competition (PERES et. al, 2012).
Seaweed extracts have shown an effect on the growth of a wide range of phytopathogenic fungi COŞOVEANU et. al, 2010; JIMÉNEZ et. al, 2011; AMBIKA, SUJATHA, 2014, 2015). Coşoveanu(2010) showed reductions in the mean diameter of fungi colonies of Fusarium roseum, Fusarium. oxysporum, Alternaria alternata, Alternaria dauci, Alternaria longipes, Trichoderma viride, Botrytis cinerea, Aspergillus niger and Penicillium expansum. Mycelial growth reduction ranged from 50% to 90%, when in the presence of extracts of the macroalgae Alaria esculenta, Fucus veiculosos, Fucus sp. and Eklonia maxima, all available commercially.
Peres (2012) obtained results that confirm those observed in these trials, such as the suppressive effect of the of A. nodosum seaweed alcoholic extract on the growth of the fungus Colletotrichum lagenarium. However, the seaweed extract had no significant effect on growth inhibition of the fungus Aspergillus flavus. In Monilinia fructicola, A. nodosum seaweed extract presented contrasting responses to those observed in these trials. The product promoted
Braz. J. of Develop.,Curitiba, v. 6, n. 3,p.13532-13543 mar.. 2020. ISSN 2525-8761 mycelial growth, which was directly correlated to the increase in concentrations of the seaweed extract applied (OLIARI et. al, 2014).
After quantifying the A. nodosum seaweed extract concentration required to inhibit R. stolonifer fungus, the most appropriate treatment proposed by linear regression was applied to strawberry fruits to evaluate post-harvest control against soft rot. Strawberries treated with A. nodosum seaweed extract at 40 mL.L-1 and inoculated with pathogen by means of mycelial disc, remained disease-free after 24, 48 and 72 hours of inoculation.
The possible effect of sodium hypochlorite, used for fruit sanitation, as an inhibitor of the seaweed extract was tested. This experiment included three treatments: Treatment 1 (T1) - sanitized strawberry; Treatment 2 (T2) - sanitized strawberry treated with seaweed extract, and Treatment 3 (T3) - strawberry only treated with seaweed extract. Significant differences in disease incidence were observed in fruits with and without the seaweed extract (Table 1) on the third day of evaluation.
Table 1. Averages of incidence rates of soft rot on strawberries submitted or not to sanitization and treated or
not with Ascophyllum nodosum seaweed extract and inoculated with Rhizopus stolonifer by mycelial disk on the third day of evaluation.
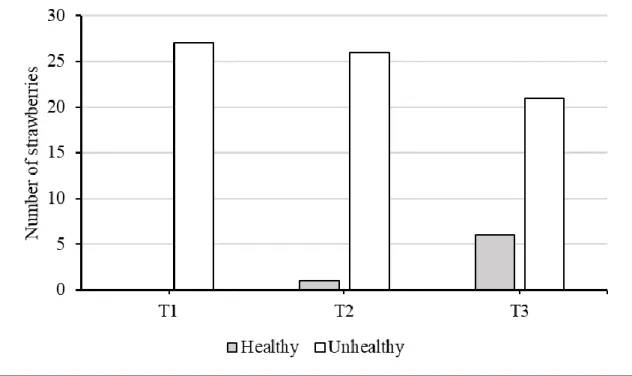
The graph represented by Figure 3 indicates the amount of healthy and contaminated strawberries on the third day of the experiment, corresponding to the incidence of disease in 100% of the fruits on T1 treatment (control).
Figure 3. Amount of strawberries contaminated in Treatment T1 - Strawberry + Sanitary Water, Treatment T2 - Strawberry + Sanitary Water + 40 mL.L-1 Algae and Treatment T3 - Strawberry + 40 mL.L-1 Algae and
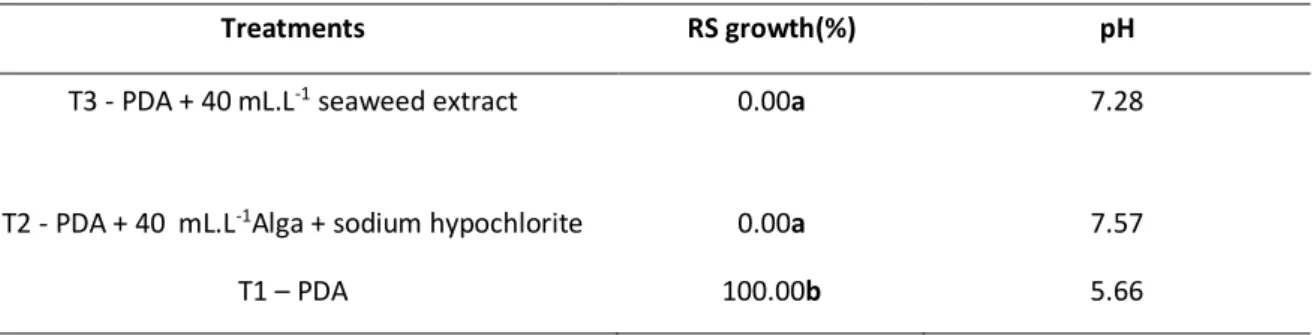
Braz. J. of Develop.,Curitiba, v. 6, n. 3,p.13532-13543 mar.. 2020. ISSN 2525-8761 The results presented in Table 2 show that the treatments of the in vivo experiment T2 and T3 do not differ statistically, corroborating with the results of the in vitro test of mycelial growth in culture medium where the A. nodosum seaweed extract was able to significantly reduce the growth of R. stolinifer.
In the in vitro assay with sodium hypochlorite pathogen growth was not observed on plates where A. nodosum seaweed extract was present, regardless of the addition or not of the sanitizer (Table 2).
To verify if there was a change in pH after the addition of the seaweed extract, pH was measured in medium containing only PDA and medium containing PDA with the seaweed extract. The measurement obtained for the PDA was 5.66, and for the other means the values were between 7.28 - 7.57 (Table 2). The addition of the A. nodosum seaweed extract increased the pH of the medium, which changed from the acid scale to the basic one, remaining very close to the ideal pH for maintenance of the seaweed extract at pH between 7,8 a 8.2 (CARVALHO, CASTRO, 2014). This basic stability was sufficient to inhibit growth of the pathogen, which development is favored at acidic pH.
For Fernandes (2017) in experiments aimed to management soft rot in tomatoes, the presence of the fungus in the fruits were associated to the high sugar content and pH (between 4.2 and 5.8) of the media, which inhibits bacteria growth, consequently favoring fungal growth.
Table 2. Averages of Petri dishes with Rhizopus stolonifer (RS) growth to verify the influence of sodium hypochlorite on pathogen development.
Treatments RS growth(%) pH
T3 - PDA + 40 mL.L-1 seaweed extract 0.00a 7.28
T2 - PDA + 40 mL.L-1Alga + sodium hypochlorite 0.00a 7.57
T1 – PDA 100.00b 5.66
Means followed by the same letter did not differ statistically by the Scott-Knott test (p≤0.05).
The physical and chemical analysis for color, firmness, soluble solids, pH, titratable acidity, total solids and total sugars did not show any significant differences between T1 and T3 in in vivo treatments. Therefore, the treatment with the product based on the seaweed extract does not interfere in the physical and chemical characteristics of the fruits (Table 3).
Table 3. Mean values of the color and firmness for the strawberry submitted to the treatment with Ascophyllum nodosum seaweed extract at the concentration of 0 mL.L-1 (control T1) and 40 mL.L-1 (T3).
The means followed by the same letter in the column do not differ statistically by the F test (p ≤ 0.05).
Concentration of Ascophyllum nodosum
seaweed extract Firmness (g) Hue angle L* value
0 mL L-1 (control - T1) 92.77 a 0.56 a 32.09 a
Braz. J. of Develop.,Curitiba, v. 6, n. 3,p.13532-13543 mar.. 2020. ISSN 2525-8761 Rezende (2010) when submitting guava fruits to the volatile compounds produced by Saccharomyces cerevisiae to control anthracnose, obtained satisfactory results in the physico-chemical quality. Parameters such as color, soluble solids content, firmness and pH were not interfered after treatment with volatile compounds. For the authors, no significant differences were observed between the control treatment and the other treatments on the three days of evaluation of the guavas, concluding that addition of the compounds did not interfere in the sensorial quality of the fruits.
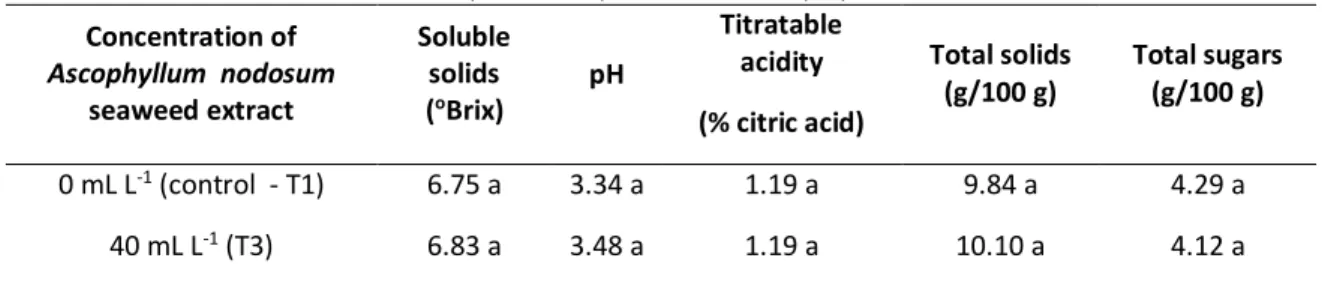
Table 4 presents the variables soluble solids, pH, titratable acidity, total solids and total sugars does not differ between treatments T1 (0 mL L-1 control) and T3 (40 mL L-1). The use of the extract did not alter the physical and chemical characteristics of the strawberry compared to the control treatment. Silva (2014) found in strawberry cultivars higher soluble solids contents with 7.58°Brix for San Andreas, 7.08°Brix for Camarosa and the lowest content for the cultivar Camino Real with 6.58°Brix. The results obtained in the present study were on average 6.75 for T1 and 6.83 for T3 within the range presented in the literature. Silva (2014), when studying soluble solids in strawberry, concluded that the plants submitted to irrigation with the A. nodosum seaweed extract showed an increase of the parameter in the fruits.
Table 4. Mean values of soluble solids, pH, titratable acidity, total solids and total sugars for the strawberry submitted to the treatment with Ascophyllum nodosum seaweed extract at the concentration of 0 mL.L-1
(control T1) and 40 mL.L-1 (T3). Concentration of Ascophyllum nodosum seaweed extract Soluble solids (oBrix) pH Titratable acidity (% citric acid) Total solids (g/100 g) Total sugars (g/100 g) 0 mL L-1 (control - T1) 6.75 a 3.34 a 1.19 a 9.84 a 4.29 a 40 mL L-1 (T3) 6.83 a 3.48 a 1.19 a 10.10 a 4.12 a
The means followed by the same letter in the column do not differ statistically by the F test (p ≤ 0.05).
The strawberries treated with A. nodosum seaweed extract presented pH above 3.30 and titratable acidity above 0.80 g/100 g of citric acid, which allows us to attest to the potential of the use of A. nodosum seaweed extract for treatment of post-harvest strawberries (MUSA, 2016). For the pH parameter, the range is between 3.34 and 3.48, which is common among strawberry cultivars such as Camino Real, San Andreas and Camarosa. According to Musa (2016) the pH of the strawberry varies between 3 and 3.9.
4 CONCLUSION
The Ascophyllum nodosum seaweed extract significantly inhibits the mycelial growth of the pathogen Rhizoupus stolonifer as its concentration is increased. The best seaweed extract concentration observed in this study was 40 mL L-1, which reached total phytopathogen inhibition.
The A. nodosum seaweed extract at 40 mL L-1 was able to reduce soft rot incidence of
strawberries in post-harvest in fruits inoculated by mycelial disks of the phytopathogen at 25ºC.
The strawberry fruits in post-harvest treated with A. nodosum seaweed extract did not have their physical and chemical characteristics altered, indicating potential of this product as a tool for postharvest management of soft rot.
Braz. J. of Develop.,Curitiba, v. 6, n. 3,p.13532-13543 mar.. 2020. ISSN 2525-8761
ACKNOWLEDGMENTS
The authors would like to thank the Federal Institute of Education, Science and Technology of South of Minas Gerais – IFSULDEMINAS, PPPI (Pró-Reitoria de Pesquisa Pós-Graduação e Inovação) and FAPEMIG (Fundação de apoio à pesquisa de Minas Gerais) for financial support.
REFERENCES
Instituto Brasileiro de Frutas (IBRAF). Acessed in 02 ago. 2015; Available from: http://www.ibraf.org.br/.
Fischer IH, Arruda MC, Almeida AM, Garcia MJ, Jeronimo EM, Pinotti RN, Bertani RM. Doenças e características físicas e químicas pós-colheita em maracujá amarelo de cultivo convencional e orgânico no centro oeste paulista. Rev. Bras. Frutic. 2007; 29(2):254-9. Galli JA, Fischer IH, Palharini MD. Doenças pré e pós-colheita em variedades de manga cultivadas em sistema orgânico. Rev. Bras. Frutic., 2012; 34(3):734-43.
Henz GP, Reis A, Silva KC, Pereira SF. Incidência de doenças de pós-colheita em frutos de morango produzidos no Distrito Federal. Boletim de Pesquisa e Desenvolvimento. 2008;45. Pearson RC, Goheen AC. Compendium of grape diseases. Aps Press; 1990.
Food Agriculture Organization (FAO). Perdas e desperdícios de alimentos na América Latina e no Caribe. 2014. Acessed in 18 jun. 2019. Available from: http://www.fao.org/americas/noticias/ver/pt/c/239394/.
Food Agriculture Organization (FAO). FAO: 30% de toda a comida produzida no mundo vai parar no lixo.2017. Acessed in 18 jun. 2019. Available from: https://nacoesunidas.org/fao-30-de-toda-a-comida-produzida-no-mundo-vai-parar-no-lixo/.
Dias, MSC. Cultivo de morango orgânico é alternativa para agricultores familiares da região central de Minas. EPAMIG, MG. 2011. Acessed in 24 mai. 2017. Available from: http://www.epamig.br/index.php?option=com_content &task=view&id=1346&Itemid=68. Agrios GN. Introduction to plant pathology. Elsevier Academic Press Publication. 2005; 922:23-37.
Amorim L, Rezende JA, Bergamin Filho A, Camargo LE. Manual de fitopatologia. Editora UFV. 2016.
Beninca, CP; Franzener, G; Assil, L; Costa, M.A; Nogueira, J. R. Indução de fitoalexinas e atividade de peroxidases em sorgo e soja tratados com extratos de Basidiocarpos de Pycnoporus Sanguineus. Arq. Inst. Biol., 2008; 75(3): 285-292.
Braz. J. of Develop.,Curitiba, v. 6, n. 3,p.13532-13543 mar.. 2020. ISSN 2525-8761 Carvalho, MEA; Castro, PRC. Extratos de algas e suas aplicações na agricultura. Piracicaba: Divisão de Biblioteca (DIBD) da Escola Superior de Agricultura “Luiz de Queiroz” (ESALQ/USP), Série Produtor Rural. 2014; 56.
Ferreira DF. Sisvar: um guia dos seus procedimentos de comparações múltiplas Bootstrap. Ciênc. agrotec. 2014;38(2):109-12.
Minolta. Precise color communication: color control from perception to instrumentation. Sakai, 1998.
Instituto Adolfo Lutz. Métodos físico-químicos para análise de alimentos. 4. ed. [1ª ed. digital]. São Paulo: Instituto Adolfo Lutz; 2008. 1020 p; Access in: 07 jan. 2020; Available in: http://www.ial.sp.gov.br.
Silva, T. P. Características Produtivas e Físico-Químicas de Frutos de Morangueiro Orgânico Cultivado com o uso de Extrato de Algas. 2011. Acessed in 24 mai. 2017. Available from: http://acervodigital.ufpr.br/bitstream/handle/1884/26514/DISSERTACAO%20THATHIA NY%20PORTO%20%20VERSAO%20FINAL.pdf?sequence=1&isAllowed=y.
Peres JC, Carvalho LR, Gonçalez E, Berian LO, Felicio JD. Evaluation of antifungal activity of seaweed extracts. Ciênc. agrotec. 2012; 36(3):294-9.
Coşoveanu AREEA, Axine O, Iacomi B. Antifungal activity of macroalgae extracts. UASVM Bucharest, 2010; 3, 442-447.
Jiménez-Escrig A, Gómez-Ordóñez E, Rupérez P. Seaweed as a source of novel nutraceuticals: sulfated polysaccharides and peptides. InAdvances in food and nutrition research. Academic Press. 2011; 1(64):325-337.
Ambika S, Sujatha K. Comparative studies on brown, red and green alga seaweed extracts for their antifungal activity against Fusarium oxysporum f. sp. udum in Pigeon pea var. CO (Rg) 7 (Cajanus cajan (L.) Mills.). JBiopest. 2014; 7(2):167.
Ambika S, Sujatha K. Antifungal activity of aqueous and ethanol extracts of seaweeds against sugarcane red rot pathogen (Colletotrichum falcatum). Sci. Res. Essay;. 2015;30;10(6):232-5.
Oliari IC, Barcelos RA, Fedrigo K, Garcia C, Marchi T, Botelho RV. Extrato de alga no controle in vitro de Monilinia fructicola. ABA-Agroecologia. 2014; 21(1):9.
Fernandes, AKC.; Cruz, CDF.; Formiga, LDAS. Análise do crescimento de fungos no fruto do tomate (Solanum lycopersicum; solanaceae). Anais da VI Jornada multidisciplinar de Biologia e Saúde e IX Mostra Científica do CESC-UEMA, 2017. v. 1. Acessed in 18 jun. 2019. Available from: https://even3.blob.core.windows.net/anais/67533.pdf.
Rezende DC. Efeito de compostos orgânicos voláteis identificados a partir de Saccharomyces cerevisiae sobre Colletotrichum gloeosporioides e Colletotrichum acutatum e no controle da antracnose em goiaba (Doctoral dissertation, Universidade de São Paulo). 2010.
Braz. J. of Develop.,Curitiba, v. 6, n. 3,p.13532-13543 mar.. 2020. ISSN 2525-8761 Silva, J F et al. Otimização da aplicação de um bio-estimulante para o aumento da produtividade e qualidade do morango. Actas Portuguesas de Horticultura. 2014; 1 (23): 380-388.
Musa CI. Caracterização físico-química de morangos de diferentes cultivares em sistemas de cultivo distintos no município de Bom Princípio/RS. 2016. Acessed in 23 jan. 2018. Availablefrom:https://www.univates.br/bdu/bitstream/10737/1594/1/2016CristianeInesMus a.pdf.