Residual 2,4-D in plant tissue culture discarded media: a neglected source of
environmental pollution
2,4-D residual em meios descartados para cultura de tecidos de plantas: uma
fonte negligenciada de poluição ambiental
DOI:10.34117/bjdv6n5-457
Recebimento dos originais: 10/04/2020 Aceitação para publicação: 22/05/2020
Dora dos Santos Costa
Doutoranda em Biotecnologia Vegetal e Bioprocessos pela Universidade Federal do Rio de Janeiro Instituição: Universidade Federal do Rio de Janeiro
Endereço: Avenida Carlos Chagas Filho, 373 – Ilha do Fundão, Rio de Janeiro – RJ, Brasil E-mail: dora.cost@hotmail.com
Carla Caroline Amaral da Silva
Mestranda em Produção Vegetal pela Universidade Estadual do Norte Fluminense Darcy Ribeiro Instituição: Universidade Estadual do Norte Fluminense Darcy Ribeiro
Endereço: Avenida Alberto Lamego, 2000 - Parque Califórnia, Campos dos Goytacazes – RJ, Brasil
E-mail: carla.carolineas@gmail.com
Antonio Jorge Ribeiro da Silva
Doutor em Química pela Universidade Federal do Rio de Janeiro e Pós-doutor pela Tierärztliche Hochschule Hannover
Instituição: Universidade Federal do Rio de Janeiro
Endereço: Avenida Carlos Chagas Filho, 373 – Ilha do Fundão, Rio de Janeiro – RJ, Brasil E-mail: ajorge@ippn.ufrj.br
Norma Albarello
Doutorado em Biologia (Biociências Nucleares) pela Universidade do Estado do Rio de Janeiro Instituição: Universidade do Estado do Rio de Janeiro
Endereço: Rua São Francisco Xavier, 524 – Maracanã, Rio de Janeiro – RJ, Brasil E-mail: albarellon@gmail.com
Ida Carolina Neves Direito
Doutora em Biotecnologia Vegetal pela Universidade Federal do Rio de Janeiro Instituição: Centro Universitário Estadual da Zona Oeste
Endereço: Avenida Manuel Caldeira de Alvarenga, 1203 – Campo Grande, Rio de Janeiro – RJ, Brasil
E-mail: idacarolinadireito@gmail.com
Cristiane Pimentel Victório
Doutora em Ciências Biológicas (Biofísica) pela Universidade Federal do Rio de Janeiro Instituição: Centro Universitário Estadual da Zona Oeste
Endereço: Avenida Manuel Caldeira de Alvarenga, 1203 – Campo Grande, Rio de Janeiro – RJ, Brasil
ABSTRACT
The herbicide 2,4-D (2,4-dichlorophenoxyacetic acid) is a plant growth regulator for callus induction and somatic embryogenesis in tissue culture protocols. Research has shown that exposure to 2,4-D causes a number of environmental and health problems. Routine laboratory work involves the use of 2,4-D in the preparation of culture media, thus generating culture media with 2,4-D residues, the disposal of which is often improper. Therefore, this study aimed to determine the residual content of 2,4-D in MS (Murashige and Skoog), agrowth medium used in plant tissue cultures, after callus development. MS media were used from callus cultures of bananeira (Musa sp.) and basil (Ocimum basilicum L.). Callus cultures were supplemented with 2,4-D at concentrations of 1.0 and 0.5 mg.L- 1, respectively. MS media were also evaluated in the absence of plant culture at the 0.2; 0.5 and 1.0 mg.L-1 concentrations of 2,4-D under light and dark conditions for a period of one month. Banana callus cultures consumed about 79% ± 0.27% of 2,4-D in the culture medium after two months. After three months of culture, it was not possible to detect 2,4-D in basil callus culture since the values found were below the detection limit (LOD ≤ 0.096 mg.L-1). Light does not appear to influence the degradation of 2,4-D in the culture medium. Results suggest that the concentration of 2,4-D residues depends on the species cultured. Improper disposal of these media might be a source of ignored and, hence, environmental contamination, depending on 2,4-D concentration and volume of mediawaste.
Keywords: 2,4-D; Chemical residue; Plant tissue culture; Chemical pollution; Phytorregulator. RESUMO
O 2,4-D (ácido 2,4-diclorofenoxiacético) é um regulador do crescimento de plantas para indução de calos e embriogênese somática em protocolos de cultura de tecidos. Pesquisas mostram que a exposição ao 2,4-D causa uma série de problemas ambientais e de saúde. A rotina laboratorial envolve o uso de 2,4-D na preparação dos meios de cultura, cujo descarte é frequentemente inadequado. O objetivo deste trabalho foi determinar o conteúdo residual de 2,4-D no meio de cultura MS (Murashige e Skoog), utilizado para cultura de tecidos vegetais, após o desenvolvimento deculturas de calos de bananeira (Musa sp.) e manjericão (Ocimum basilicum L.). Meios de cultura foram suplementados com 2,4-D em concentrações de 1.0 e 0.5 mg.L-1, respectivamente. Meios MS também foram avaliados na ausência de culturas nas concentrações de 0.2; 0.5 and 1.0 mg.L-1 de 2,4-D na ausência e presença de luz durante um mês. As culturas de calo de bananeira consumiram cerca de 79% ± 0.27% de 2,4-D após dois meses de cultivo. Após três meses decultivo não foi possível detectar 2,4-D nos meios de cultura de calos de manjericão, pois os valores encontrados estavam abaixo do limite de detecção (LOD ≤ 0.096 mg.L-1). A luz não parece influenciar a degradação do 2,4-D no meio de cultura. Os resultados sugerem que as concentrações residuais de 2,4-D no meio de cultivo dependem da espécie cultivada. O descarte inadequado de meios de cultivo oriundos da cultura de tecidos vegetais pode ser uma fonte de contaminação ambiental negligenciada em função do quantitativo de 2,4-D presente e do volume de resíduo gerado.
Palavras chave: 2,4-D; Resíduo químico; Cultura de tecidos vegetais; Poluição química;
Fitorregulador.
1 INTRODUCTION
Among synthetic plant growth regulators used, such as auxins, 2,4-D (2,4- dichlorophenoxyacetic acid) stands out for its wide use in plant tissue culture laboratories, mainly for callus induction (BOIX et al., 2012; FARHADI et al., 2017) and somatic embryogenesis
suspension culture to obtain secondary metabolites of industrial and pharmaceutical interest (IKEUCHI et al., 2013; DIAS et al., 2016; NARAYANI et al., 2017; PARRAY et al., 2018).
Callus culture is defined as a culture of disorganized cell masses produced from a tissue or a single differentiated totipotent plant cell, the development of which is mediated by a balance between auxin and cytokine growth regulators (IKEUCHI et al., 2013). This system has been used in different plant tissue culture strategies and has been intensively applied to provide bioactive substances.
Although 2,4-D is a plant growth regulator classified as an auxin and used for tissue culture at low concentrations that vary from 0.0045 mg.L-1 to 10 mg.L-1 (BEYL, 1999; CONGER, 2018), it is also recognized as a toxic substance. Reports show that 2,4-D causes damage to the central nervous system, kidneys and liver (IGBINOSA et al., 2013). It also affects the reproductive system, deregulates the endocrine system and causes some types of cancer (CARNEIRO et al., 2015; JEFFRIES et al., 2016).
Studies also indicate that 2,4-D can cause some types of cancer because it is a possible source of dioxins, which are highly carcinogenic substances, but this hypothesis remains inconclusive (GARABRANT & PHILBERT, 2002; BURNS & SWAEN, 2012; SURA et al., 2012;
POCHETTINO et al., 2016; CHEN et al., 2018; NPIC, 2018). 2,4-D was developed in 1940 during World War II as a defoliant agent (SONG, 2014). Since then, 2,4-D has been one of the most widely used herbicides in the world (CHEN et al., 2018), and it is a member of the chlorophenoxyacetic family (MEYER & SCRIBNER, 2009).
According to Resolution No. 357 of the National Environment Council of March 17, 2005 of the Brazilian Ministry of the Environment (MMA, 2005), waters containing more than 0.004 mg.L-1 of 2,4-D are not suitable for consumption, while, for comparison purposes,the U.S. EPA Maximum Contaminant Level is set to 0.07 mg.L-1 (EPA, 2018). The values described as tolerable maximums
for water consumption could coincide with 2,4-D concentrations normally used for plant tissue culture (BEYL, 1999; VICTÓRIO et al., 2010; BOIX et al., 2012, CONGER, 2018). Since 2,4-D is typically discarded as commom refuse, it can bring considerable environmental risk when residues exhibit excess concentration or have not been properly treated (SKIBA et al., 2017). In liquid media of plant tissue culture, this means that the concentration of 2,4-D therein could be sufficient to be characterized as inappropriate for consumption. More specifically, it can easily be imagined that 2,4- D residues could leach into water bodies, thus compromising water security and causing environmental damage.
Procedures for disposing of culture media residues containing 2,4-D are not regulated in Brazil. As such, the final destination of plant tissue culture media is, usually, sanitary landfills, which only
serve to confine, but not treat, this waste (SILVA et al., 2010). Another possible destination is incineration (SILVA et al., 2010), but this pathway can also be pollutant by inhalation of toxic air.
Thus, the objective of this study was to evaluate the amount of residual 2,4-D in MS medium (MURASHIGE & SKOOG, 1962) after in vitro plant tissue culture to analyse the potential risk of environmental contamination from these residues in the very likely event that such residues reach a state of contamination through leaching or other routes of disposal eventually harmful to humans and the environment.
2 METHODOLOGY
2.1 PLANT CULTURES GROWN WITH 2,4-D AND EVALUATION OF CULTURE MEDIA MS media (MURASHIGE & SKOOG, 1962) were supplemented with vitamins (0.1 mg.L-1 Thiamine-HCl; 0.5 mg.L-1 Nicotinic acid; 0.5 mg.L-1 Pyridoxine-HCl), myo-inositol (100 mg.L-1), sucrose
(30 g.L-1) and solidified with 6 g.L-1 of agar. In all culture media, 2,4-D was added before autoclaving at
concentrations of 1.0 mg.L-1 for banana callus culture (Musa sp., Silver dwarf cultivar) and 0.5 mg.L-1 for
basil callus culture (O. basilicum L.). Culture media were autoclaved for 15 minutes at 1 atm.
For banana plantlets culture, the experimental design consisted of 5 or more explants per flask (4 flasks, n= 20 explants). Subcultures were performed every 2 months. For basal banana callus culture, the experimental design consisted of 5 explants (thin layers of the meristematic tissue of axillary buds obtained from banana plantlets) per flask (4 flasks, n= 20 explants. Subculture took place over the course of 2 months.
For basil plantlets culture, the experimental design consisted of 5 seeds per flask (4 flasks, n= 20 explants) cultured by 35 days. For basil callus culture, the experimental design consisted of 5 explants (thin tissue of the nodal segments obtained from basil plantlets) per flask (4 flasks, n= 20 explants). Subculture took place over the course of 3 months.
Both banana and basil callus culture media were analyzed after that time were maintained under standard physical conditions with photoperiod of 16/8 hours (light/ darkness). The cultures were illuminated by Empalux fluorescent lamps (T10 20W 6/400K FT20416XGR03), daylight type, under 5.616 µmol.m-2.s-1 of light intensity and temperature of 25±2°C.
MS medium was also analyzed without 2,4-D (MS0) and with 2,4-D at concentrations of 0.2; 0.5 and 1.0 mg.L-1. These media were prepared and maintained under the same conditions as those of callus cultivation in the presence or absence of light. To accomplish this, flasks containing 2,4-D MS medium (0.2; 0.5 and 1.0 mg.L-1) were maintained with 5 replicates for each concentration (n=
5) during 30 days after initiation of the experiment. Samples of all flasks were separated to determine 2,4-D concentration by HPLC (High-performance Liquid Chromatography).
2.2 PREPARATION OF CULTURE MEDIA SAMPLES FOR HPLC ANALYSIS
Aliquots of the residual media from each treatmentwere stored in sterile test tubes at 4°C until the time of processing for analysis by HPLC. Sample preparation of all culture media for analysis consisted of acidification with concentrated HCl (2.5 μL of acid for each illiliter of medium), followed by autoclaving at 121°C at 1 atm for 15 minutes. After cooling the media, the samples were filtered (0.22 μm pore filters) into vials and held at -20°C until analysis.
2.3 HPLC ANALYSIS OF THE CULTURE MEDIUM
For quantification of 2,4-D in basil and banana callus culture media, a Zorbax Eclipse XDB- C8 chromatographic column 150 mm long by 4.6 mm in diameter in stationary phase (reversed phase) was used with irregular granules 5 μm in diameter. An Eclipse XDB-C8 pre-column 12,5 mm long by 4.6 mm in diameter with irregular granules 5 μm in diameter on the Agilent 1260 Infinity Series chromatograph was also used. Detection of 2,4-D was performed at 225 nm. Data acquisition and processing were performed using the Agilent ChemStation program. The calibration curve was performed with 2,4-D (> 98% purity, Sigma-Aldrich) diluted in HPLC standard methanol (puritygrade > 99.9%, TediaBrasil Ltda.) at concentrations of 5; 2; 1; 0.5; 0.1; 0.05; 0.01; 0.005; 0.001; 0.0005 and 0.0001 mg.L-1.
The mobile phase consisted of ultrapure water acidified with 0.1% (v/v) phosphoric acid and acetonitrile, also acidified with 0.1% (v/v) phosphoric acid. In the first 3 min, the acetonitrile concentration was 30%. From 3 to 10 min, it ramped up from 30 to 100%, and from 10 to 15 min, it was maintained at 30%. The time of the chromatographic run was 15 min for each sample with 2,4- D detected at the retention time (RT) of 8 min. The oven temperature was 45°C, and the flow rate at each injection in this method was 1 mL.min-1. The injection volumes used were 80 μL. The regression equation obtained for the calibration curve was y = 101,1x + 13.133. The linearity was R2= 0.9967
with a limit of detection (LOD)= 0.096 mg.L-1 and a limit of quantification (LOQ)= 0.322 mg.L-1.
3 STATISTICAL ANALYSIS
BioEstat 5.0 (AYRES et al., 2007) was used to quantify the residual 2,4-D present in the MS culture medium used for banana and basil callus culture, as well as quantifyresidual 2,4-D in the MS medium maintained under the presence or absence of light, but without callus cultures. ANOVA was used, adopting a value of p< 0.01 as significant.
4 RESULTS AND DISCUSSION
4.1 DETECTION AND QUANTIFICATION OF 2,4-D IN MEDIA AFTER CULTURE OF BANANA AND BASIL CALL
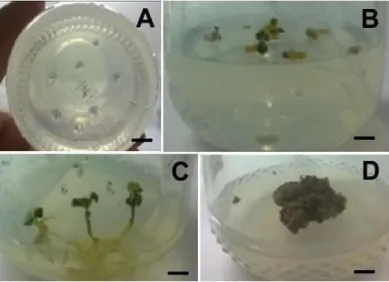
The banana (Figure 1A – 1B) and basil (Figure 2A – 2C) cultures are present below.
Banana subcultures for plantlet maintenance were carried out every 2 months (Figure 1A), since after this period the leaves were yellowish due to the lack of nutrients in the medium. This subculture of banana seedlings was made in order to provide explants to callus culture. Friable calli were observed after 15th day of culturing (Figure 1B).
Figure 1 - In vitro banana plantlets after 2 months of culture in MS medium. A) Silver-dwarf banana subculture, bar =
2.0 cm. Banana callus of the silver-dwarf variety (arrow) in MS medium with 1.0 mg.L-1 2,4-D, 2 months in vitro, bar = 1.73 cm
Basil seeds germinated between the 4th and 5th day after seeding, under light. The percentage of germination and disinfestation was 100%, that is, there was no contamination, and the seedlings were kept for 35 days in culturing (Figure 2A – 2C). The calli were observed from the 14th day of cultivation onwards, and had a compact appearance (Figure 2D), requiring subculture every 3 (three) months. The calli had an average of the longest length of 1.5 ± 1.1 cm.
Figure 2 - In vitro germination of basil (Ocimum basilicum) seeds in MS medium without 2,4-D. A) On the day the seeds
were introduced, bar = 0.86 cm; B) 21st day after sowing, bar = 1.13 cm; C) 35th day after sowing, bar = 1.13 cm; D) 14th day of callus culture onwards, bar = 1.13 cm.
As expected, the concentrations were below those originally present in the culture media (Table 1). The analysis of variance of the concentration values obtained before and after the cultivation of banana calli presented significant differences (p= 0.0015). The banana callus culture consumed about 79% ± 0.27% of 2,4-D added to the medium. Evaluating the results of Table 1, it was not possible to quantify 2,4-D in the basil callus culture medium since the values found were below the LOD (≤0.096 mg.L-1). The analysis of variance of the concentration values obtained before and after the cultivation of basil calli presented significant differences (p<0.0001). These results indicate that the cultivation of basil callus resulted in the consumption of 2,4-D up to the LOD.
Table 1 - Mean amounts of 2,4-D added to the MS medium and detected by HPLC after banana and basil callus cultures.
2,4-D concentration (mg.L-1) Callus culture Before culture (prepared concentration) After culture (determined concentration) # Culture period
Banana (Musa sp.) 1.0 0.21 ± 0.23 2 months
Basil (Ocimum
basilicum)
0.5 ND 3 months
Routine lab work, in which 2,4-D is added to the culture medium, generates a considerable amount of waste. For example, one study reported that one laboratory generated about 100 kg of 2,4- D residues in the tissue culture process of plants over a period of three years (SINSKI et al., 2012). However, it is difficult to estimate the amount of residues generated by research institutions by propagating in vitro plants using 2,4-D as auxin. By 2015, 139 biofactories were registered as using plant tissue culture techniques for plant propagation in Brazil (CARVALHO et al., 2016). The amount of 2,4-D released in procedures of this type is indeterminant. Nevertheless, from the values reported by Sinski et al. (2012), it can be estimated that the values released into the sewer or trash can be extremely high, considering the activities of laboratories around the world.
In addition to the general data on discarded and accumulated culture media presented by Sinski et al. (2012), no data were found in the literature related to the amount of 2,4-D after plant tissue culture of a specific plant species. One exception is a study by Costa (2018) (unpublished data) who reported that the concentration of 2,4-D used for callus culture of mussambê (Cleome rosea Vahl ex DC.) was 0.2 mg.L-1 at the time of medium preparation, but was reduced 100 times after three months of subculture. Our study did demonstrate that 2,4-D is consumed extensively by the vegetal tissue, but we also showed that residues of this agent is variable, depending on the type of plant.
Thus, it is safe to say that 2,4-D from plant tissue culture media residue is widely overlooked in the context of safe disposal. This is aggravated by reports of improper practices involving its daily use and handling in the preparation of culture media, washing glass material after use and especially, as noted, in the act of throwing in the trash. In Brazil, most laboratory culture media residues are usually discarded without following any chemical waste disposal protocol. Such practices above are not consistent with the hazardous nature of 2,4-D and therefore pose a risk to human and environmental health.
4.2 DETECTION AND QUANTIFICATION OF 2,4-D IN MS MEDIA IN THE PRESENCE AND ABSENCE OF LIGHT
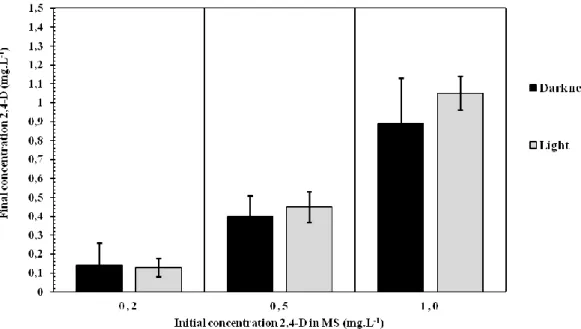
The analysis of variance between the detection and quantification experiments of 2,4-D in MS medium in the presence and absence of light did not show significant differences (p<0.01) in the 2,4- D concentrations analysed (Figure 3). Therefore, the quantification of 2,4-D at the end of 30 days after the start of the experiment showed that light did not influence the degradation of 2,4-D in MS medium used for plant tissue culture under the conditions observed (data not shown).
Figure 3 - Concentration of 2,4-D in MS medium without plant culture after 30 days in the presence and absence of light.
In the literature, many studies have reported on thephotodegradation of 2,4-D under field conditions, but generally with higher efficiency of degradation by catalysts such as TiO2 in laboratory
experiments (ALVAREZ et al., 2007; MANTILLA et al., 2009; YU et al., 2014). Photocatalysis has shown good results in terms of the complete degradation of the chemical structure of 2,4-D, a technology which, if effective, is cost-effective and relatively simple to operate (BIAN et al., 2013). However, to the best of our knowledge, no studies have, thus far, reported on the photodegradation of the substance when it is present in culture media.
5 CONCLUSION
Based on our results, the amount of residual 2,4-D in MS medium varies according to plant species, culture time and period of development in vitro. Therefore, we conclude that Musa sp. and
O. basilicum are plants that consume auxin during the calogenesis process extensively; nevertheless,
2,4-D persists, albeit at lower concentration, in MS medium after banana callus tissue culture. Our results are related to the mass of culture medium used, not that portion absorbed by plant tissue.
Further analyses are required to verify the concentration of 2,4-D in callus cells separately after culturing since cell debris is also released into the environment and may be harmful.
Artificial fluorescent light in the culture room had no significant effect on the degradation of 2,4-D, particularly in the MS culture medium. Thus, the degradation can be attributed exclusively to the plants used in the experiments. Consequently, the appropriate governmental policymakers should
take notice and establish strategies for the safe disposal of laboratory materials contaminated by 2,4- D waste, as well as raise awareness of proper disposal of such wasteby the appropriate means.
ACKNOWLEDGEMENTS
We are grateful to Campo Biotecnologia Vegetal (Cruz das Almas-BA/BR) for the donation of banana plantlets and Ari Miranda da Silva (UFRJ) who assisted in the use of HPLC.
We also would like to thank to the technicians of Labplan (UERJ), Cecília Azevedo de Souza and Lívia da Silva Cordeiro who donate the mussambê culture media.
FUNDING
This work was supported by the Agência da Bacia do Rio Paraíba do Sul - AGEVAP (Call Notice 002/2017, process 03.019.001.17) and the Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ, process E-26 010.001536/2016).
REFERENCES
ANVISA- AGÊNCIA NACIONAL DE VIGILÂNCIA SANITÁRIA. Parecer Técnico de
Reavaliação Nº 07, de 2015/GGTOX/ANVISA. Reavalia os riscos à saúde humana do ingrediente ativo ácido 2,4-diclorofenoxiacético (2,4-D). Available in:< http://portal.anvisa.gov.br/ >Access in: June, 06th,2019.
ALVAREZ M, LÓPEZ T, ODRIOZOLA JA, CENTENO MA, DOMÍNGUEZ MI, MONTES M, GONZÁLEZ RD. 2007. 2,4-Dichlorophenoxyacetic acid (2,4-D) photodegradation using an Mn+/ZrO2photocatalyst: XPS, UV–vis, XRD characterization. Appl Catal B 73: 34–41.
AYRES M, AYRES JR M, AYRES DL, SANTOS AA. 2007. Bioestat 5.0 aplicações estatísticas nas áreas das ciências biológicas e médicas. Belém: IDSM, 364p.
BEARD JD, ENGEL LS, RICHARDSON DB, GAMMON MD, BAIRD C, UMBACH DM, ALLEN KD, STANWYCK CL, KELLER J, SANDLER DP, SCHMIDT S, KAMEL F. 2017. Military service, deployments, and exposures in relation to amyotrophic lateral sclerosis survival. Plos One 12:1-22.
DI (Eds.). Plant tissue culture concepts and laboratory exercises. Boca Raton: CRC, p. 21-38.
BIAN X, CHEN J, JI R. 2013. Degradation of 2,4-dichlorophenoxyacetic acid (2,4-D) by novel photocatalytic material of tourmaline-coated TiO2 nanoparticles: kinetic study and model. Materials
6:1530-1542.
BOIX YF, ARRUDA RCO, DEFAVERI ACA, SATO A, LAGE CLS, VICTÓRIO CP. 2013. Callus in Rosmarinus officinalis L. (Lamiaceae): a morphoanatomical, histochemical and volatile analysis. Plant Biosyst 147: 751-757.
BURNS CJ, SWAEN GMH. 2012. Review of 2,4-dichlorophenoxyacetic acid (2,4-D) biomonitoring and epidemiology. Crit Rev Toxicol 42: 768-786.
CARNEIRO FF, AUGUSTO LGS, RIGOTTO RM, FRIEDRICH K, BÚRIGO AC. (Org.). Dossiê ABRASCO: um alerta sobre os impactos dos agrotóxicos na saúde. 2015. Rio de Janeiro: EPSJV; São
Paulo: Expressão Popular, 624 p.
CARVALHO ACPP, RODRIGUES AAJ, SANTOS ED. 2016. Documentos 157. Panorama da produção de mudas micropropagadas no Brasil (2008-2015). Fortaleza: Embrapa Agroindústria Tropical, 36p.
CHEN X, ZHANG H, WAN Y, LI Y. 2018. Determination of 2,4-Dichlorofenoxiacetic acid (2,4-D) in rat serum for pharmacokinetic studies with a simple HPLC method. Plos One 13: 1-10.
CONGER BV. 2018. Cloning agricultural plants via in vitro techniques. Boca Raton: CRS Press, 280p.
COSTA DS. 2018. Ensaios de avaliação da remediação de resíduos de 2,4-D em meios de cultura de tecidos vegetais. Dissertação (Mestrado), Programa de Pós-graduação em em Ciência e Tecnologia Ambiental) - Centro Universitário Estadual da Zona Oeste, Rio de Janeiro, 2018, 134p.
DIAS MI, SOUSA MJ, ALVES RC, FERREIRA ICFR. 2016. Explorating plant tissue culture to improve the production of phenolic compounds: A review. Ind Crop Prod 82: 9-22.
DOPICO M, GÓMEZ A. 2015. Review of the current state and main sources of dioxins around the world. J Air Waste Manage 65: 1033-1049.
EPA – United States Environmental Protection Agency. 2018 Edition of the Drinking Water
Standards and Health Advisories Tables.Avaiable in:
https://www.epa.gov/sites/production/files/2018-03/documents/dwtable2018.pdf> Access: August, 13th, 2019.
FARHADI N, PANAHANDEH J, AZAR AM, SALTE SA. 2017. Effects of explant type, growth regulators and light intensity on callus induction and plant regeneration in four ecotypes of Persian shallot (Allium hirtifolium). Sci Hortic 218: 80-86.
GARABRANT DH, PHILBERT MA. 2002. Review of 2,4-Dichlorophenoxyacetic acid (2,4-D) epidemiology and toxicology. Crit Rev Toxicol 32: 233–257.
IGBINOSA EO, ODJADJARE EE, CHIGOR VN, IGBINOSA IH, EMOGHENE AO, EKHAISE FO, IGIEHON NO, IDEMUDIA OG. 2013, Toxicological profile of chlorophenols and their derivatives in the environment: The public health perspective. Sci World J 2013: 1-11.
IKEUCHI M, SUGIMOTO K, IWASE A. 2013. Plant callus: mechanisms of induction and repression. Plant Cell 25: 3159-3173.
JEFFRIES MD, GANNON TW, BROSNAN JT, BREEDEN GK. 2016. Comparing dislodgeable 2,4- D residues across athletic field turfgrassspecies and time. Plos One 11: 1-13.
KABIR A, ZENDEHDEL R, TAYEFEH-RAHIMIAN R. 2018. Dioxin exposure in the manufacture of pesticide production as a risk factor for death from prostate cancer: A meta-analysis. Iran J Public Health 47: 148-155.
MANTILLA A, TZOMPANTZI F, FERNÁNDEZ JL, DÍAZ GÓNGORA JAI, MENDOZA G, GÓMEZ R. 2009. Photodegradation of 2,4-dichlorophenoxyacetic acid using znalfe layered double hydroxides as photocatalysts. Catal Today 148:119–123.
MEKKY H, AL-SABAHI J, ABDEL-KREEM MFM. 2018. Potentiating biosynthesis of the anticancer alkaloids vincristine and vinblastine in callus cultures of Catharanthus roseus. S Afr J Bot
MEYER TM, SCRIBNER EA. 2009. The evolution of analytical technology and its impact on water- quality studies for selected herbicides and their degradation products in water. In: AHUJA S (Ed.). Handbook of Water Purity and Quality. Amsterdam: Academic Press, p. 289-313.
MMA - MINISTÉRIO DO MEIO AMBIENTE. Resolução N˚ 357, de 17 de março de 2005. Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e dá outras providências. Avaiable in: http://www.mma.gov.br/port/conama/res/res05/res35705.pdf >Acess in: July, 16th. 2016.
MURASHIGE T, SKOOG F. 1962. A revised medium for rapid growth and bio assays with tobacco
tissue cultures. Physiol Plantarum 15: 473-497.
NARAYANI M, CHADHA A, SRIVASTAVA S. 2017. Callus and cell suspension culture of Viola
odorata as in vitro production platforms of known and novel cyclotides. Plant Cell Tiss Org 130:
289–299.
NPIC- Nacional Pesticide Information Center. 2,4-D. Avaiable
in:<http://npic.orst.edu/ingred/24d.html>Acess in: October, 14th. 2018.
PARRAY J, KAMILI AN, JAN S, MIR MY, SHAMEEM N, GANAI BA, ABD_ALLAH EF, HASHEM A, ALQARAWI AA. 2018. Manipulation of plant growth regulators on phytochemical constituents and DNA protection potential of the medicinal plant Arnebiabenthamii. Biomed Res Int 2018: p. 1-8.
POCHETTINO AA, HAPON MB, BIOLATTO SM, MADARIAGA MJ, JAHN GA, KONJUH CN. 2016. Effects of 2,4-dichlorophenoxyacetic acid on the ventral prostate of rats during the peri- pubertal, pubertal and adult stage. Drug Chem Toxicol 39: 392-399.
PRATAP A, PRAJAPATI U, SINGH CM, GUPTA S, RATHORE M, MALVIYA N, TOMART R, GUPTA AK, TRIPATHI S, SINGH NP. 2018. Potential, constraints and applications of in vitro methods improving grain legumes. Plant Breeding 137: 235-249.
SILVA AF, SOARES TRS, AFONSO JC. 2010. Gestão de resíduos de laboratório: Uma abordagem para o ensino médio. Química Nova na Escola 32: 37-42.
SIMÕES C, BIZARRI CHB, CORDEIRO LS, CASTRO TC, COUTADA LCM, SILVA
AJR, ALBARELLO N, MANSUR E. 2009. Anthocyanin production in callus cultures of Cleome
rosea: modulation by culture conditions and characterization of pigments by means of HPLC-
DAD/ESIMS. Plant Physiol Bioch 47: 895-903.
SKIBA E, KOBYŁECKA J, WOLF WM. 2017. Influence of 2,4-D and MCPA herbicides on uptake and translocation of heavy metals in wheat (Triticum aestivum L.). Environ Pollut 220: 2017.
SONG Y. 2014. Insight into the mode of action of 2,4-dichlorophenoxyacetic acid (2,4-D) as an herbicide. J Integr Plant Biol 56: 106-113.
SURA S, WAISER M, TUMBER V, FARENHORST A. 2012. Effects of herbicide mixture on microbial communities in prairie wetland ecosystems: a whole wetland approach. Sci of Total Environ 435-436: 34-43.
VICTÓRIO CP, HENRIQUES AB, TAVARES ES, ESQUIBEL MA, LAGE CLS. 2010.
Standardized production of Phyllanthus tenellus Roxb. by plant tissue culture. Rev Ciênc Agron 41: 272-278.
VON STACKELBERG K. 2013. A systematic review of carcinogenic outcomes and potential mechanisms from exposure to 2,4-D and MCPA in the environment. J Toxicol 2013: 1-53.
WÓJCIKOWSKA B, GAJ MD. 2017. Expression profiling of auxin response factor genes during somatic embryogenesis induction in Arabidopsis. Plant Cell Rep 36: 843-858.
YU C, WANG H, LIU X, QUAN X, CHEN S, ZHANG J, ZHANG P. 2014. Photodegradation of 2,4-D induced by NO2− in aqueous solutions: The role of NO2. J Environ Sci 26: 1383–1387.
ZHANG M, BUEKENS A, LI X. 2017. Dioxins from biomass combustion: an overview. Waste Biomass Valori 8: 1-20.