D
EPARTAMENTO DE
ROLE OF A TRIPARTITE EFFLUX PUMP IN
THE SYMBIOSIS BETWEEN
MELILOTI
M
ESTRADO EM
F
ACULDADE DE
C
IÊNCIAS
EPARTAMENTO DE
B
IOLOGIA
V
EGETAL
ROLE OF A TRIPARTITE EFFLUX PUMP IN
THE SYMBIOSIS BETWEEN SINORHIZOBIUM
AND LEGUMINOUS PLANTS
Andreia Filipa Tomás Marques
ESTRADO EM
M
ICROBIOLOGIA
A
PLICADA
2011
ROLE OF A TRIPARTITE EFFLUX PUMP IN
SINORHIZOBIUM
D
EPARTAMENTO DE
ROLE OF A TRIPARTITE EFFLUX PUMP IN
THE SYMBIOSIS BETWEEN
MELILOTI
Dissertação orientada por
e Prof. D
M
ESTRADO EM
F
ACULDADE DE
C
IÊNCIAS
EPARTAMENTO DE
B
IOLOGIA
V
EGETAL
ROLE OF A TRIPARTITE EFFLUX PUMP IN
THE SYMBIOSIS BETWEEN SINORHIZOBIUM
AND LEGUMINOUS PLANTS
Dissertação orientada por Prof. Doutora Leonilde Moreira (IST)
e Prof. Doutora Sandra Chaves (FCUL)
Andreia Filipa Tomás Marques
ESTRADO EM
M
ICROBIOLOGIA
A
PLICADA
2011
ii
ROLE OF A TRIPARTITE EFFLUX PUMP IN
SINORHIZOBIUM
AND LEGUMINOUS PLANTS
iii
ROLE OF A TRIPARTITE EFFLUX PUMP IN
THE SYMBIOSIS BETWEEN SINORHIZOBIUM
MELILOTI AND LEGUMINOUS PLANTS
Andreia Filipa Tomás Marques
M
ASTER
T
HESIS
2011
This thesis was fully performed at the Biological Sciences Research Group of
Instituto Superior Técnico of the Technical University of Lisbon under the direct
supervision of Prof. Dra. Leonilde Moreira.
Prof. Dra. Sandra Chaves was the internal designated supervisor in the scope of
the Master in Applied Microbiology of the Faculty of Sciences of the University of
Lisbon.
iv
Acknowledgements
This master thesis reflects the support of many people who influenced my life and this work, and who I would like to thank.
I am very thankful to Prof. Dra. Leonilde Moreira from IST for giving me the opportunity to develop this research work under her careful guidance and for all her attention in the very beginning of my scientific path. I acknowledge her patience, encouragement and cheerfulness and all the help in the writing of this thesis.
Second, I would like to acknowledge as well the Ph.D. student Mário Santos as my lab-supervisor for all his patience for teaching me the basics of the scientific research and for transmitting to me part of his knowledge on Sinorhizobium meliloti.
Thanks also to the group members, Dr. Sofia Ferreira and Inês Silva, for accompany me from the beginning, helping me to clarify doubts and sharing their knowledge with me.
Heartfelt thanks to Prof. Dr. Sandra Chaves from FCUL, who was designated to be my internal supervisor in the scope of the Master in Applied Microbiology, and from the start proved to be always available.
Furthermore I would like to thank all members of BSRG by their welcoming, and last but not least, my “thank you” to my family and friends for all their constant support.
This work was supported by Fundação para a Ciência e a Tecnologia (FCT), Portugal (project PTDC/BIA-MIC/113733/2009).
v
Resumo
Sinorizhobium meliloti é uma bactéria fixadora de azoto que estabelece uma relação
simbiótica com plantas leguminosas do género Medicago. Durante o processo simbiótico as raízes da planta são infectadas, ocorrendo uma troca de sinais entre os dois simbiontes e iniciando-se o desenvolvimento de um órgão único, o nódulo, aonde após colonização pelo microssimbionte ocorre a fixação do azoto. Durante este processo, as bactérias estão sujeitas a diferentes tipos de stress que afectam o estabelecimento da simbiose e impõem limitações durante a fixação de azoto, entre eles o stress oxidativo, osmótico e pH do solo.
SMc03167, SMc03168 e SMc03169 são três genes presentes no genoma de S. meliloti que ainda não foram caracterizados, mas cuja expressão surge alterada na literatura sob algumas condições de stress. Está previsto que os genes SMc03167 e SMc03168 codifiquem para um transportador do tipo MFS (Major Facilitor Superfamily) que constituem uma família de bombas de efluxo responsáveis pela resistência a vários antibióticos e compostos tóxicos. O gene SMc03167 partilha semelhanças com membros da subfamília ErmB/QacA e TetB, enquanto o gene SMc03168 apresenta um domínio conservado da proteína HlyD. Observou-se que a expressão destes genes de S. meliloti se encontrava induzida num mutante tolC em resposta a condições de stress que afectam a estabilidade da parede celular. O gene SMc03169, adjacente aos genes SMc03167 e SMc03168, é um possível regulador de transcrição da família TetR, provavelmente envolvido no controlo da transcrição desses transportadores e na resposta a condições de stress. Assim, decidiu-se analisar o papel destes três genes em S. meliloti e procedeu-se à construção de mutantes de eliminação. Uma vez que não se obteve um mutante eliminação para o gene SMc03168, é apenas apresentada uma caracterização dos mutantes obtidos para os genes SMc03167 e SMc03169.
Várias experiências foram feitas na tentativa de descobrir qual o papel desempenhado pelos genes SMc03167 e SMc03169, nomeadamente na resposta a vários stresses e avaliar o seu envolvimento no estabelecimento da simbiose. Para se atingir estes objectivos, comparou-se o crescimento dos mutantes em relação à estirpe selvagem em meio complexo TY e meio mínimo GMS, analisou-se o seu comportamento a diferentes valores de pH, mediu-se a sua sensibilidade a alguns agentes antimicrobianos, comparou-se a actividade de efluxo ao composto tóxico brometo de etídio e caracterizou-se o seu fenótipo simbiótico em plantas da espécie Medicago
sativa.
Não se observaram alterações no crescimento dos mutantes SMc03167 e SMc03169 quando comparados à estirpe selvagem em meio complexo TY. No entanto, em meio GMS (pH 7.0) o mutante SMc03169 apresentou uma menor formação de biomassa final. Apesar desta diferença observada, a morfologia das células vistas ao microscópio era semelhante entre as três estirpes e uma análise das proteínas totais não revelou diferenças óbvias na expressão proteica.
vi
A expressão do gene SMc03169 aparece alterada na literatura em resposta a pH ácido. Para se investigar a influência do pH no crescimento deste mutante, vários pH foram testados. A pH 4.0 e 5.0 não se observou crescimento quer no mutante quer na estirpe selvagem apesar das células se manterem viáveis. A pH 6.0 e 7.0 não havia alterações no crescimento para qualquer das estirpes. Estabeleceu-se o pH 5.5 como o pH mínimo tolerado para o crescimento de S.meliloti, tendo-se observado que para este valor de pH o crescimento do mutante SMc03169 era
muito inferior ao da estirpe selvagem. Estudos de complementação do mutante pela introdução de um plasmídeo que expressava o gene SMc03169 sob o controlo do seu próprio promotor, restabeleceram a capacidade de crescer a pH 5.5. Este resultado sugere um possível envolvimento deste gene na adaptação de S. meliloti ao stress imposto pelo ácido.
Uma vez que os genes SMc03167 e SMc03169 codificam para um possível transportador e regulador da transcrição, respectivamente, que poderão estar envolvidos na resistência a compostos tóxicos, testou-se o comportamento dos mutantes na presença de concentrações tóxicas de vários compostos químicos, através da medição dos halos de inibição do crescimento. Testaram-se três compostos antimicrobianos derivados de plantas: a berberina, o ácido
p-coumárico e a genisteína; os agentes de stress oxidativo H2O2 e o hidroperóxido de cumeno; e
ainda o detergente SDS. O mutante SMc03167 apresentou halos de inibição idênticos aos halos da estirpe selvagem para todos os compostos e concentrações estudadas. O mutante SMc03169 revelou um comportamento semelhante, excepto para os compostos hidroperóxido de cumeno e SDS onde os halos de inibição foram superiores aos halos obtidos na estirpe selvagem, revelando a sua maior sensibilidade a estes agentes de stress.
A capacidade de excreção do composto tóxico brometo de etídio foi comparada entre as três estirpes, tendo-se usado como controlo uma estirpe mutante para o gene tolC que exibe defeitos na parede celular e, como tal, é incapaz de excretar este composto tóxico. O mutante tolC tornou-se fluorescente devido à acumulação de brometo de etídio, mesmo a baixas concentrações. Os mutantes para os genes SMc03167 e SMc03169 apresentaram uma baixa fluorescência comparável à da estirpe selvagem devido ao transporte deste composto, mostrando que a eliminação de ambos os genes não afectou o efluxo deste composto.
As propriedades simbióticas dos genes em estudo foram testadas através de ensaios de simbiose com plantas da espécie M. sativa, nomeadamente pela contagem dos nódulos induzidos durante cinco semanas. Os nódulos brancos foram os primeiros a aparecer e depois evoluíram para nódulos maiores e que apresentavam uma cor rosa. A estirpe selvagem apresentou o maior número de nódulos ao longo das 5 semanas, estando o mutante SMc03167 logo a seguir com uma diferença média de um nódulo a menos. Esta pequena diferença mostra que este mutante é tão competitivo como a estirpe selvagem. As plantas inoculadas com estas duas estirpes apresentavam-se verdes e saudáveis, com raízes longas e ramificadas. O mutante SMc03169 apresentou uma nodulação muito inferior à da estirpe selvagem, originando uma média de 50% nódulos a menos. Apesar desta diferença no número de nódulos ser significativa, não houve qualquer diferença a nível morfológico dos mesmos. Contudo, as plantas inoculadas eram mais pequenas, encontravam-se menos saudáveis e com raízes pouco ramificadas, o que revela uma
vii
menor eficiência na nodulação em M. sativa e consequente fixação de azoto e desenvolvimento da planta. No final do ensaio simbiótico, alguns nódulos brancos e rosa foram recolhidos das plantas, esmagados e os CFUs bacterianos calculados. Ainda que se tenham observado valores diferentes para cada nódulo esmagado, concluiu-se que os nódulos rosa estavam efectivamente colonizados por bactérias.Apesar de não se ter identificado o tipo de substrato para o transportador MFS SMc03167/SMc03168, este trabalho identificou um novo gene com relevância para o estabelecimento da simbiose. É pois de todo o interesse caracterizar a proteína SMc03169 de forma a identificar a sua importância quer na resistência a pH ácido quer na interacção com plantas de forma a aumentar a nossa compreensão desta importante simbiose.
Palavras-Chave: Sinorhizobium meliloti, Medicago sativa, Major Facilitor Superfamily (MFS), simbiose, fixação do azoto.
viii
Abstract
Sinorizhobium meliloti is a bacterium capable of establishing a symbiotic nitrogen fixation
relationship with leguminous plants. The symbiotic process involves root infection mediated by exchange of signals between the two symbionts, which then culminates by development of a root nodule. During this process, bacteria are exposed to various stresses. The expression of SMc03167 and SMc03168 genes, encoding a transporter of the Major Facilitor Superfamily (MFS), was found to be induced in a S. meliloti TolC mutant in response to cell wall perturbations and oxidative stress. Similarly, the adjacent SMc03169 gene, encoding a putative transcriptional regulator TetR, was predicted to be involved in the transcriptional control of the previous genes and perhaps also having a role in stress response. In this work, we characterized the two deletion mutants for ORFs SMc03167 and SMc03169. When compared with the wild-type, the SMc03167 mutant showed no growth defects in complex and minimal medium, and its capacity for ethidium bromide extrusion and resistance phenotype to antimicrobial and oxidative stress agents was not affected. Moreover, the symbiotic assays with Medicago sativa revealed that SMc03167 mutant was as competitive as wild-type for nodulation. Contrastingly, the SMc03169 mutant showed a reduced biomass formation in minimal medium, although no changes were observed in the morphology of the cells. In addition, its sensitivity to low pH was altered which suggests a possible involvement on S. meliloti tolerance to acid conditions. In trans complementation of the SMc03169 mutant rescued its ability to grow at low pH. Simultaneously, the SMc03169 mutant was more sensitive to the cell envelope-disrupting agent SDS and oxidative stress agent cumene hydroperoxide. Plants inoculated with SMc03169 mutant showed a reduced number of nodules and did look not quite as healthy as plants inoculated with the wild-type strain. Together, our data suggests that SMc03169 gene plays a role in cell’s adaptation to acid environments and in symbiosis establishment. Further studies are needed to understand the exact role of this transcriptional regulator in S. meliloti-plant interaction.
Keywords: Sinorhizobium meliloti, Medicago sativa, Major Facilitor Superfamily (MFS), symbiosis, nitrogen-fixation.
ix
Table of Contents
Acknowledgements ... iv
Resumo ... v
Abstract ... viii
1.
Introduction ... 1
1.1.
The importance of Biological Nitrogen Fixation ... 1
1.2.
Symbiosis between Rhizobia and Legumes ... 1
1.3.
The genome of Sinorhizobium meliloti 1021 ... 4
1.3.1.
Transport systems ... 4
1.3.2.
Adaption of S. meliloti to stress conditions ... 5
1.4.
Role of multidrug efflux systems in symbiosis ... 8
2.
Objectives ... 10
3.
Experimental Approach ... 10
4.
Materials and Methods ... 11
4.1.
Bacterial strains and plasmids ... 11
4.2.
Bacteria growth conditions ... 12
4.3.
Plant growth conditions ... 12
4.4.
Extraction of genomic and plasmid DNA ... 12
4.5.
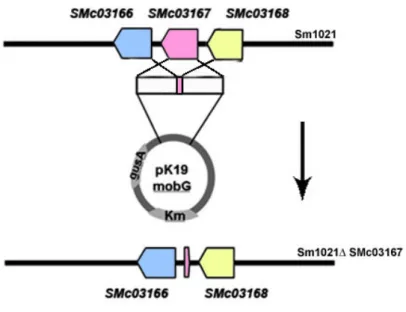
Construction of S. meliloti deletion mutants ... 13
4.6.
Mutant genotype confirmation ... 14
4.7.
Growth curves ... 15
4.8.
Polyacrylamide gel electrophoresis ... 15
4.9.
Zone inhibition assays ... 15
4.10.
Ethidium bromide supplemented agar screening method... 15
5.
Results and Discussion ... 16
5.1.
Sequence analysis of ORFs SMc03167, SMc03168 and SMc03169 ... 16
5.2.
Construction of deletion mutant strains ... 20
5.3.
Assessment of phenotypic properties ... 24
5.3.1.
Growth of S. meliloti deletion mutants in rich and minimal medium ... 24
5.3.2.
Growth of S. meliloti SMc03169 mutant at pH 5.5 ... 27
5.3.3.
Sensitivity of the mutants to antimicrobials of plant origin and other stress
agents.... ... 27
5.3.4.
Efflux activity of the mutants in the presence of a toxic compound ... 28
5.3.5.
Symbiotic properties of the mutants ... 29
6.
Conclusions ... 32
1
1. Introduction
1.1. The importance of Biological Nitrogen Fixation
Nitrogen is one of the most abundant elements on Earth and it is also one of the most
limiting for biological growth of plants because it is found in its inaccessible dinitrogen (N2) form for
them. Since the reduced availability of nitrogen in the soil represents a problem for agriculture, various industrial fertilizers rich in nitrogen are used in order to achieve maximum productivity. The production of fertilizers uses large amount of fossil fuel, which is costly and consumes many natural
resources. Furthermore, the carbon dioxide (CO2) released in the process of combustion of fossil
fuels and the nitric oxide released during decomposition of fertilizers contribute to the increased greenhouse effect. The use of nitrogenous fertilizers has also resulted in unacceptable levels of water pollution (increasing concentrations of toxic nitrates in drinking water supplies) and the eutrophication of lakes and rivers, beyond the wastes of energy and economic resources (Zahran, 1999). It is then needed to reduce dependence on chemical fertilizers and find alternative methods that provide the nitrogen necessary for the plant.
The largest input of nitrogen in the biosphere comes from a process called Biological
Nitrogen Fixation (BNF) by which chemically inert N2 present in the atmosphere is enzymatically
reduced to the metabolically usable form ammonia (NH3) through the action of nitrogenase. The
ability to catalyze the conversion of N2 to NH3 has evolved only among microbes, including archea,
cyanobacteria, azobacteria, Frankia and rhizobia (Gibson et al., 2008).
Rhizobia and legumes have evolved a mutualistic endosymbiosis of major ecological importance that contributes between 25-30% of available nitrogen. Crop legumes can fix about
23-176 kg N ha-1 year-1 and some tree legumes fix as much as 600 kg N ha-1 year-1, depending on the
plant species and also on the rhizobia species (Lindström et al., 2010; Zahran, 1999). Rhizobium spp. and legume symbiosis also represents a significant research model for symbiosis and the signal exchange between rhizobia and their host plants has been elucidated in some detail.
1.2. Symbiosis between Rhizobia and Legumes
Rhizobia are gram-negative bacteria with the ability to establish a N2-fixing symbiosis on
legume roots. Although require a plant host to fix nitrogen, they can survive in soil over periods of several years even in the absence of their legume hosts. Rhizobia form a group which falls into two classes of the proteobacteria, α-proteobacteria and β-proteobacteria. Most belong to families within the α-proteobacteria (order Rhizobiales) and are closely related to nonsymbiotic soil bacteria. However, symbiotic nitrogen-fixing β-proteobacteria (β-rhizobia) also have been reported, and the evolution of new diverse strains of rhizobia is attributed to the horizontal transfer of symbiosis genes into different types of bacteria (Becker et al., 2009).
2
Rhizobia establish a symbiosis with legume plants, leading to the formation of root structures called nodules, containing many nitrogen-fixing bacteria. By supplying its host with nitrogen, rhizobia can enhance host photosynthesis and increase their own access to the plant carbon sources, enabling the synthesis of energy-rich storage molecules like polyhydroxybutyrate (PHB) which increases bacterial survival and reproduction after they return to the soil (Prell & Poole, 2006). This type of infection develops as a consequence of several different stages of interaction between bacteria and a specific host-legume pairing. Signaling between rhizobia and legumes is one of the best examples of signal exchange identified in interactions between bacteria and their eukaryotic hosts. The legumes secrete signals, usually phenolics (often flavonoids or isoflavonoids), which can passively diffuse across the bacterial membrane. The bacteria recognize these signals using a positively acting transcriptor factor, usually encoded by nodD gene. NodD belongs to the LysR family of DNA-binding transcription factors, which have an N-terminal ligand-binding domain that regulates the activity of the associated C-terminal DNA-ligand-binding domain. The NodD is thought to function as a flavonoid receptor and induces the expression of the nod genes whose products are involved in Nodulation Factors (Nod Factors) biosynthesis. Nod Factors are oligomers of usually four or five 1,4-linked N-acetyl-glucosamine residues that carry an N-linked acyl group. Different legumes secret different types of signals and rhizobia have different NodD proteins that recognize these root-exudate signals, allowing a highly specific relationship. For instance, the bacterium Sinorhizobium meliloti is compatible with Medicago (alfalfa), Trigonella andMelilotus species, whereas Rhizobium etli is compatible only with Phaseolus species (bean). Nod
Factors can induce plant responses like root-hair deformation, alterations in the root hair
cytoskeleton, calcium oscillations (called calcium-spiking) at low concentrations as little as 10-13 M
and production of reactive oxygen species in root hairs (Downie, 2010).
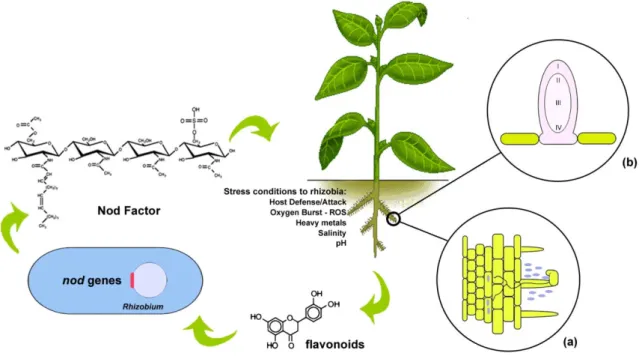
The formation of nodules capable of nitrogen fixation requires two different bacterial programs: one to activate plant nodule morphogenesis and the other leading to nodule infection. In many legumes such as peas, clovers, beans, soybean, Medicago spp. and Lottus spp., infection is usually initiated by bacteria attached near root hair tips. Nod Factors promote the deformation of root hairs growth on itself trapping intimately the bacteria, which are then able to invade the root through a host-derived structure called the infection thread (Downie, 2010). This structure continues its growth via deposition of new cell wall material while carries bacteria from the root surface into the host cell cytoplasm by a process that resembles endocytosis (Fig. 1.1a). Simultaneous, Nod Factor stimulates root cortex cells to reinitiate mitosis, occurring a
differentiation of quiescent (G0) root cortical cells into an actively dividing meristem (Capela et al.,
2006; Gibson et al., 2008). These dividing meristem cells form the nodule primordium. A subset of these meristem cells enter in a modified cell cycle program that results in endoreduplication and produces greatly enlarged cells, that will receive the invading rhizobia for the purpose of nitrogen fixation. The bacteria are budded off the end of the infection threads before the synthesis of the plant cell wall surrounding the infection thread and the release into the plant cell. As the bacteria differentiate into bacteroids, they also undergo endoreduplication to yield a chromosome count of 24 compared to one or two for free-living bacteria. The plant infected cells become large polyploid
3
cell harboring thousands of organelle-like structures known as symbiosomes, where many genes required for nitrogen fixation are expressed and nitrogen is fixed (Oldroyd et al., 2011; Saeki, 2011).Fig. 1.1 - Signal exchange and root hair invasion in Rhizobium-plant symbiosis. Flavonoids produced by the host plant
induce rhizobial nod genes and leads to production of Nod Factors. (a) Infection thread passes the root cortex toward a cluster of dividing cells that will become a nodule primordium. (b) An indeterminate nodule originates from the root inner cortex and has a persistent meristem (Zone I). The nodule also contains an invasion zone (Zone II) and a nitrogen-fixing zone (Zone III). In older nodules, a senescent zone (Zone IV) develops, in which both plant and bacterial cells degenerate.
Generally, nodules fall into two morphological classes based on their pattern of meristem growth: determinate, with a meristem derived from outer cortical cells (e.g. Glycine max, Lotus
japonicus, Vicia faba), and indeterminate, with a meristem derived from inner cortical cells (e.g. Medicago sativa, Medicago truncatula, Pisum sativum and Trifolium species). Determinate and
indeterminate nodules also differ in the relative persistence of meristem proliferation. Determinate nodules lacks a persistent meristem, are usually round, and display a relatively homogenous population of developing plant and bacterial cells at any given time point. In contrast, indeterminate nodules are elongated and create a persistent meristem that continually gives rise to new nodule cells that are subsequently infected by rhizobia residing in the nodule (Fig. 1.1b). As a result, each developmental stage required to establish the symbiosis is present within a single mature indeterminate nodule and in a spatially organized manner from the young meristem at the nodule tip to the older senescent tissue near the root (Gibson et al., 2008). The environment within the nodule is very unusual and forms a rather special case of an interaction that involves quite specific nutrient uptake systems and a specialized electron transport chain that operates at low oxygen concentrations.
4
1.3. The genome of Sinorhizobium meliloti 1021
Since the genome of S. meliloti is well characterized, this rhizobia specie is considered as ideal candidate to study. S. meliloti genome contains a circular chromosome (3.65 Mb) and two megaplasmids, pSymA (1.35 Mb) and pSymB (1.68 Mb) (Galibert et al., 2001) with a 6204 predicted protein-encoding genes: 3341 on the chromosome (Capela et al., 2001), 1293 on pSymA (Barnett et al., 2001), and 1570 on pSymB (Finan et al., 2001).
At the time of S. meliloti genome sequence determination, there was no experimentally prove function for a vast majority of the predicted genes and 40% could not be placed into a functional category. Moreover, 8% were orphan genes, defined as those not found in any other sequenced genome. Becker et al. (2009) published a S. meliloti genome annotation update which incorporates information published from 2001 to 2008. With improved prediction tools, they identified 86 new putative genes, removed 66 previously predicted orphan genes and adjust the start positions of 360 coding regions. As a result, more than 71 % of genes have now a predicted function.
During evolution, Sinorhizobium’s genome has been acquiring new functions through acquisition of new transport and regulatory genes (Galibert et al., 2001). The megaplasmid pSymA carries the most genes required for nodulation and nitrogen fixation (nod, nif, and fix genes), carbon metabolism, transport and stress responses, whereas pSymB reveals a high number of genes involved in polysaccharide biosynthesis.
In this section it will be described S. meliloti genes and proteins with relevance to this work.
1.3.1. Transport systems
Two membranes separate the cytoplasm from the external medium in gram-negative bacteria like rhizobia. The two membranes are permeable only to small uncharged molecules and neutral lipophilic molecules. All other molecules require dedicated transport systems. The exchange of substances between cytoplasm and periplasm is regulated by highly specific transport systems, whereas the exchange between periplasm and environment occurs via porins which may be unspecific or specific for groups of substances (Saier, 2000). Rhizobia face unfavorable conditions both in the soil environment and during symbiosis establishment due to plant defense activation. Transporters allow them to survive in most cases. A large number of ABC transporters are encoded by pSymB. Using 2-D gel electrophoresis followed by peptide mass fingerprint, Djordjevic et al. (2003) identified 23,9% of the proteins encoded by the 430 ABC transport system-encoding genes reported by Finan et al. (2001).
After ABC transporters, the Major Facilitor Superfamily (MFS) class is the second largest family of membrane transporters found. Members of this superfamily are typically single-polypeptide secondary carriers, comprising 10-14 transmembrane α-helices, which are able to
5
transport small solutes such as sugars or toxins in response to chemiosmotic ion gradients. MFS can also be multidrug efflux pumps involved in the resistance to various antibiotics and toxic compounds. In S. meliloti, tep1 gene product shows similarities with members of the MFS transporters and is a transmembrane protein which can confer chloramphenicol resistance by expelling the antibiotic outside from bacteria. Moreover, a deletion mutant for this protein showed an improved nodulation of alfalfa (Dillewijn et al., 2009).Several others transporters are present in the S. meliloti genome and some of them play an important role in the physiology and biochemistry of nodule bacteria. Sugars are not utilized by bacteroids, instead amino acids are an important carbon source. Therefore, no protein involved in sugar transport has been detected in nodule bacteria, but several transporters for amino compounds are present. These transporters are encoded by genes SMc01946 (leucine); SMc02118, SMc00140 and SMb20428 (amino acids); SMb21196 (oligopeptides); and SMc04439 (glycine betaine). Multiple transporters for iron (FbpA and SitA) and phosphate (PhoD and SMc02146) are also present (Djordjevic et al., 2003).
1.3.2. Adaption of S. meliloti to stress conditions
The process of nitrogen fixation is dependent on the physiological state of the host plant and the availability of nitrogen in the soil, directly affecting its symbiotic partner. In addition, S. meliloti must adapt to the stress situation imposed by plant defenses during the infection process and to the nodule environment. Exopolysaccharides (EPS), capsular polysaccharides (KPS), lipopolysaccharides (LPS) and cyclic-β-glucans are important factors for rhizobial invasion of plant tissues, avoiding plant defense responses and helping bacteroid maintenance in symbiosomes (Batut et al., 2011). However, several environmental conditions are also limiting factors to the growth and activity of both plants and bacteria, like water stress, salinity, soil pH, temperature, heavy metals, nutrient deficiency and mineral toxicity (Fig. 1.1). Response and adaptation to environmental stresses by both organisms constitute a complex phenomenon involving many physiological and biochemical processes that reflect changes in gene expression and in the activities of enzymes and transport proteins. Since the genes we are studying are predicted to be involved in various stress conditions, the present knowledge on the mechanisms used by S. meliloti to adapt to some of these environmental stress conditions is described below.
Oxidative stress
The key enzyme of nitrogen fixation, the nitrogenase, is subjected of an irreversible inactivation by oxygen. Therefore, nodules have a diffusion barrier in the cortex to limit oxygen passage and also leghemoglobin, a plant oxygen carrier that delivers the necessary oxygen to the bacteroids. However, a high respiration rate is required to support the nitrogen fixation process, and this leads to the generation of large amounts of reactive oxygen species (ROS) such as
6
superoxide radicals (O2−) and hydrogen peroxide (H2O2), which can also inactivate the nitrogenase.
H2O2 accumulation was observed in some infection threads but never inside bacteroids, indicating
an efficient H2O2-scavenging system (Jamet et al., 2005). S. meliloti encodes a set of enzymes
against ROS, including superoxide dismutases, catalases and alkylhydroperoxidases, but this oxidative stress is a complex process not yet fully understood. In fact, some genes required for defense against ROS in the free-living state are dispensable for symbiosis, as well as different responses may be required only at different stages to allow bacteria to further proceed in symbiosis.
S. meliloti has two monofunctional catalases encoded by katA and katC genes, a bifunctional
catalase-peroxidase encoded by katB, superoxide dismutases encoded by sodA and sodB and other uncharacterized ROS scavenging enzymes. Mutation of any of the three kat genes does not
affect H2O2 sensitivity, but a katA/katC and a katB/katC double mutants are deficient in nodule
formation and nitrogen fixation (Jamet et al., 2003; Saeki, 2011), whereas a disruption of the global
regulator of H2O2 protection, oxyR, makes the free-living strain extremely sensitive to H2O2 but
does not affect symbiosis (Jamet et al., 2005).
Iron and manganese are important metals for oxidative stress protection. Iron is used as a
cofactor of defense enzymes such as catalase, although in the Fe2+ state it can produce hydroxyl
radicals from peroxides through Fenton chemistry. Manganese ions help by scavenging both H2O2
and O2-, as part of low-molecular-weight complexes with cellular ligands such as phosphate,
lactate, or bicarbonate. A mutation of sitA, a periplasmic binding-protein of the putative iron/manganese ABC transporter SitABCD reveals a high sensitivity to ROS, leading to a severe decrease in SodB protein level and activity (Davies & Walker, 2007a).
Other gene important in either symbiosis or oxidative stress protection is cycK. This gene is part of the cycHJKL operon and is involved in cytochrome c-type biosynthesis. In S. meliloti, c-type cytochromes are required for nitrate reduction in free-living form and nitrogen fixation in root nodules (Davies & Walker, 2007b).
Osmotic stress
Nearly 40% of the world’s land surface has potential salinity problems (Zahran, 1999). Most legumes are sensitive to relatively low levels of salinity and rhizobia are sensitive to salinity both at the free-living stage and during the symbiotic process. M. sativa is salt tolerant, but the Rhizobium spp. and legume symbiosis is highly sensitive to salt or osmotic stress, since these conditions may inhibit the initial steps of the symbiotic interaction like root colonization, nodule infection and nodule development and also have a negative effect on nitrogen fixation. Nogales et al. (2002) observed that Rhizobium tropici mutants by the adaptation to high salinity showed deficiencies in their symbiotic capacity. Soybean root hairs also displayed little curling or deformation when inoculated with Bradyrhizobium japonicum in the presence of 170 mM NaCl, and nodulation was completely suppressed by 210 mM NaCl (Tu, 1981).
7
S. meliloti may use distinct mechanisms for osmotic adaptation upon salt stress, such as the
intracellular accumulation of low-molecular-weight organic solutes called osmolytes, which are described as compatible solutes because they do not interfere with the metabolism. Some exogenous osmolytes identified in bacteria are: sugars (e.g. sucrose, trehalose), polyols (e.g. sorbitol, mannitol), amino acids and derivates (e.g. proline, glutamate, glutamine), pipecolic acid, ectoine, glycine betaine and derivatives (Costa et al., 1998; Fernandez-Aunión et al., 2010). The osmoregulation strategy in S. meliloti is atypical compared to other bacteria, since only the osmoprotectant glycine betaine is accumulated within stressed cells, while the others are catabolized (Fernandez-Aunión et al., 2010). BetS was identified as a major glycine betaine transporter required for early osmotic adjustment in S. meliloti to high salt concentrations (Rüberg
et al., 2003). Besides the protection of glycine betaine, the primary response to a hyperosmotic
stress is the stimulation of potassium (K+) uptake. S. meliloti has four potassium uptake systems:
Kup1, Kup2, Trk, and Kdp. Two of these K+ transport systems, Trk and Kup1, were reported as
required for bacterial growth even under osmotically balanced conditions and the absence of both systems leads to a reduced infectivity and competitiveness of the bacteria in alfalfa roots (Domínguez-Ferreras et al., 2009).
Acid tolerance
Approximately ¼ of the world’s agriculture soils are acidic and there is an increasing concern about soil acidification (Tiwari et al., 1996a). Soil acidity affects both the growth and survival of
Rhizobium and its host plants. Most leguminous plants require a neutral or slightly acidic soil for
growth, especially when they depend on symbiotic nitrogen fixation. When in the soil, rhizobia face an acidic environment due to the presence of protons and organic acids excreted by the plant. S.
meliloti has been described as the most sensitive rhizobia to low pH. It presents poor survival at pH
values below 6.0, whereas some strains of Mesorhizobium loti have shown a high degree of acid tolerance in laboratory, being capable of growing at pH values as low as 4.0 (Rickert et al., 2000).
Most bacteria maintain a relatively constant internal pH (pHi) over a range of external pH
(pHe). S. meliloti maintains a slightly alkaline pHi even when the pHe is acidic. This control of pH
presumably involves one or more mechanisms for regulating the influx and efflux of protons across the cytoplasmic membrane. Little is known about such mechanisms or how the cells sense
changes in the pHe, but it has been proposed that S. meliloti may have a signal transduction
system for pH environment sensing and response (Tiwari et al., 1996a). Four genes have been reported as essential for S. meliloti growth at acid pH: a gene pair (actS/actR), an ATPase (actP), and another gene involved in lipid metabolism (actA). actR/actS encode the response regulator ActR which is activated by its corresponding sensor histidine kinase ActS, whose loss leads to sensitivity to low pH. ActR also regulates the expression of some genes involved in nitrate assimilation as well as the nitrogen fixation regulator genes fixK and nifA (Hellweg et al., 2009; Reeve et al., 2002).
8
Copper homeostasis is another mechanism for the acid tolerance of root nodule bacteria which prevents this heavy metal from becoming overtly toxic in acidic conditions. Higher free copper concentrations will occur at lower pH, resulting in greater influx into the cell and the means for exporting excess copper are essential to the cell (Reeve et al., 2002). ActP is a P-type ATPase that belongs to the CPx family with considerable similarity to putative cation-transporting P-type ATPases from numerous organisms, which recognizes and transport heavy metals like copper (Reeve et al., 2002). In the same way, ActA is a lipid-metabolizing protein which, if inactivated, results in an heavy metals-sensitive phenotype (Tiwari et al., 1996b).Beyond these genes, S. meliloti has a range of acid-responsive genes, which are not themselves critical to growth at low pH. One of these (phrR) is itself a regulator gene induced by
acid pH and a range of stresses including high concentrations of Zn2+, Cu2+, H202 or ethanol, but
not controlled by the ActS/ActR system (Reeve et al., 1998). Another, a putative transmembrane protein lpiA, responds specifically to acidity and may be an antiporter related to nhaB, which is
involved in Na+ transport in other bacteria (Reeve et al., 2006).
1.4. Role of multidrug efflux systems in symbiosis
An important group of inner membrane efflux pumps interacts with the outer membrane channel proteins to form complexes that transverse the inner membrane, periplasm, and outer membrane. In E. coli, TolC is the major outer membrane channel, which plays an important role in the excretion of a wide range of molecules, including antibiotics, bile salts, organic solvents, enterobactin, several antibacterial peptides, and also a large protein toxin, α-hemolysin (Horiyama
et al., 2010). TolC protein folds into a remarkably similar three-dimensional homotrimeric structure
with an outer membrane-embedded β-barrel domain and a large α-helical domain extending into the periplasm (Misra & Bavro, 2009). TolC associates with different inner-membrane protein systems such as the Resistance Nodulation Division (RND) family transporter AcrB or the MFS transporter ErmB, both driven by proton influx, or the ABC transporter MacB driven by ATP hydrolysis. The cognate periplasmic Membrane Fusion Protein (MFP) such as AcrA, ErmA and MacA, respectively, stabilize these transporter-channel interactions (Deininger et al., 2011). AcrAB/TolC is a well known tripartite efflux pump involved in the resistance to various compounds, such as acriflavine, colicin, antibiotics and lipophilic molecules in E. coli (Kang & Gross, 2005; Misra & Bavro, 2009).
TolC protein of S. meliloti was identified as important for nitrogen fixation symbiosis. Cosme
et al. (2008) observed that a tolC gene insertion mutant of S. meliloti induced none or only very few
nodules in M. sativa roots and these nodules did not fix nitrogen. This mutation also affects the bacterial resistance to antimicrobial plant agents such as coumaric acid, genistein and berberine and cells became more susceptible to osmotic and oxidative stress. In order to understand the contribution of TolC protein from S. meliloti to the physiology of the cell, Santos et al. (2010) compared the transcriptome of TolC mutant with the one of wild-type S. meliloti 1021. Data showed
9
an increased expression in the tolC mutant of genes that encode for proteins involved in stress response, central metabolic pathways and many transporters. Genes whose expression was found decreased are involved in nitrogen metabolism, transport and cell division. In the analysis of the genes with increased expression and involved in transport processes, it was observed that genes SMc03167 and SMc03168 were more than 40-fold upregulated when the tolC mutant transcriptome was compared against the wild-type. In the wild-type strain, these genes encode a MFS-like transporter system that, together with TolC protein, forms a tripartite efflux pump probably responsible to excrete toxic compounds and/or not yet identified cellular metabolites. Although, there is no biochemical data on SMc03167 and SMc03168 proteins, several experiments showing their altered expression under several conditions is found in the literature. As an example, Rüberget al. (2003) monitored the S. meliloti 1021 gene expression in response to an osmotic upshift and
found that the expression of SMc03167 and SMc03168 was repressed. Furthermore, Hellweg et al. (2009) carried a global transcriptional analysis of S. meliloti 1021 genome to identify genes responding to a pH shift from 7.0 to pH 5.75. The response to acidic pH involved whole sets of genes associated with various cellular functions and, among these genes, SMc03167, SMc03168 and SMc03169 were transiently up-regulated during a short period of time following the pH shift. In another report, Capela et al. (2005) analyzed the S. meliloti transcriptome in response to the overexpression of nodD1 and the plant flavonoid luteolin. They identified new genes regulated by both luteolin and NodD1 and among them were SMc03167 and SMc03168 with a 16-fold increase in the presence of luteolin. SMc03168 gene was also found to have a luteolin-induction by Barnett
et al. (2004), showing to be repressed by NodD1 in the absence of luteolin, whereas it was
expressed upon luteolin addition. Moreover, a SMc03167 null mutant showed to be as competitive as wild-type for nodulation. Data also showed that SMc03169, a divergently transcribed transcription regulator, was induced 3-fold by luteolin. Although its role remains to be determined (Capela et al., 2005), SMc03169 protein belongs to a family of repressors (TetR) and might be responsible for the induction of these genes.
10
2. Objectives
Many S. meliloti genes have no confirmed function and some of them may have a role in symbiosis. Among these uncharacterized genes are SMc03167, SMc03168 and SMc03169 which are expected to be related to bacterial resistance to toxic compounds. The aim of this thesis was to further elucidate the role of the two transporter encoding genes (SMc03167/SMc03168), as well as the adjacent gene SMc03169 encoding a putative transcriptional regulator, to determine under which conditions are they expressed and evaluate their possible involvement in the establishment of symbiosis with Medicago plants.
3. Experimental Approach
In order to fulfill these objectives the following experiments were performed:
1. In silico analysis of the genes and their predicted functions; 2. Construction of deletion mutant strains;
3. Comparison of the growth curves in complex and minimal medium between wild-type and mutants;
4. Analysis of the behavior at low pH between wild-type and SMc03169 mutant;
5. Measurement of the sensitivity towards antimicrobials and other stresses between wild-type and mutants;
6. Comparison of the efflux activity of a toxic compound ethidium bromide between wild-type and mutants;
7. Characterization of the symbiotic phenotype with the leguminous plant M. sativa, by performing symbiosis assays between wild-type and mutants.
With this set of experiments we expected to get a better knowledge on the role of this efflux system and on the mechanisms underlying the establishment of the symbiosis between S. meliloti and M. sativa.
11
4. Materials and Methods
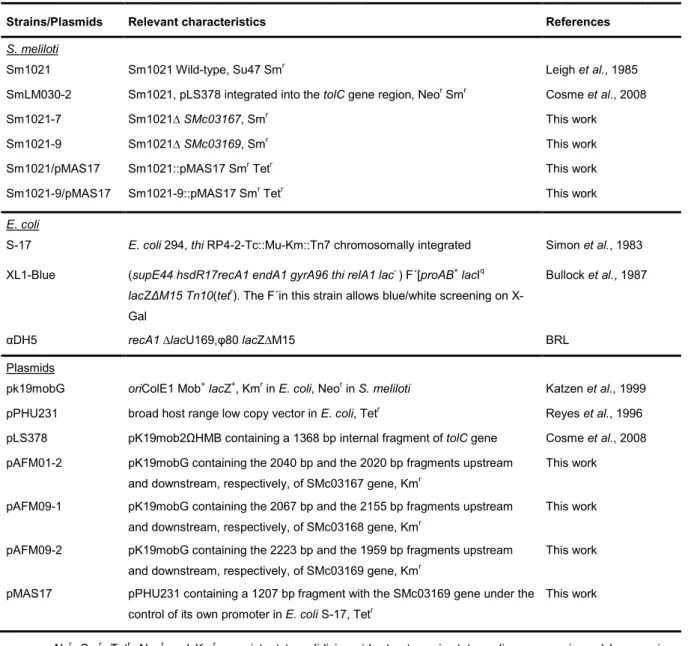
4.1. Bacterial strains and plasmids
Bacterial strains and plasmids used in this study are listed in Table 4.1. Fresh cultures were obtained by transferring a portion of the frozen cellular material at -80ºC to plates of selective solid medium (described in 4.2), followed by incubation at proper temperature, until visible cell growth. These cultures were then maintained at 4ºC until further use.
Table 4.1. Strains and plasmids used in this study.
Strains/Plasmids Relevant characteristics References
S. meliloti
Sm1021 Sm1021 Wild-type, Su47 Smr Leigh et al., 1985
SmLM030-2 Sm1021, pLS378 integrated into the tolC gene region, Neor Smr Cosme et al., 2008
Sm1021-7 Sm1021∆ SMc03167, Smr This work
Sm1021-9 Sm1021∆ SMc03169, Smr This work
Sm1021/pMAS17 Sm1021::pMAS17 Smr Tetr This work
Sm1021-9/pMAS17 Sm1021-9::pMAS17 Smr Tetr This work
E. coli
S-17 E. coli 294, thi RP4-2-Tc::Mu-Km::Tn7 chromosomally integrated Simon et al., 1983
XL1-Blue (supE44 hsdR17recA1 endA1 gyrA96 thi relA1 lac- ) F´[proAB+ lacIq
lacZ∆M15 Tn10(tetr). The F´in this strain allows blue/white screening on X-Gal
Bullock et al., 1987
αDH5 recA1 ∆lacU169,φ80 lacZ∆M15 BRL
Plasmids
pk19mobG oriColE1 Mob+ lacZ+, Kmr in E. coli, Neor in S. meliloti Katzen et al., 1999
pPHU231 broad host range low copy vector in E. coli, Tetr Reyes et al., 1996
pLS378 pK19mob2ΩHMB containing a 1368 bp internal fragment of tolC gene Cosme et al., 2008
pAFM01-2 pK19mobG containing the 2040 bp and the 2020 bp fragments upstream
and downstream, respectively, of SMc03167 gene, Kmr
This work
pAFM09-1 pK19mobG containing the 2067 bp and the 2155 bp fragments upstream
and downstream, respectively, of SMc03168 gene, Kmr
This work
pAFM09-2 pK19mobG containing the 2223 bp and the 1959 bp fragments upstream
and downstream, respectively, of SMc03169 gene, Kmr
This work
pMAS17 pPHU231 containing a 1207 bp fragment with the SMc03169 gene under the
control of its own promoter in E. coli S-17, Tetr
This work
Nxr, Smr, Tetr, Neor and Kmr = resistant to nalidixic acid, streptomycin, tetracycline, neomycin and kanamycin,
12
4.2. Bacteria growth conditions
E. coli strains were grown in Luria Broth (LB) medium (Sambrook et al., 1989) at 37ºC with
orbital agitation (250 rpm). S. meliloti strains were grown in Triptone-Yeast (TY) medium (Beringer, 1974) or Glutamate–Mannitol–Salts (GMS) medium (York & Walker, 1997) at 30ºC with orbital agitation (180 rpm). Solid medium was prepared by adding 20.0 g/L Agar.
Media was supplemented with antibiotics when required at the following concentrations (µg/ml): for S. meliloti 1021 (a streptomycin-resistant strain), streptomycin 600, neomycin 120 and tetracycline 10; for E. coli, kanamycin 50 and tetracycline 10. With the exception of tetracycline, the antibiotics were dissolved in water and sterilized by filtration with 0.2 µm pore size filter. Tetracycline was dissolved in 70% ethanol. Stock solutions at 10 mg/ml were prepared for p-coumaric acid, berberine (5,6-dihydro-9,10-dimethoxybenzo-1,3-benzodioxoloquinolizimium) and genistein (4,5,7-trihydroxyisoflavone). p-coumaric acid was dissolved using 95% ethanol, berberine was dissolved in water and genistein was dissolved in DMSO. 5-Bromo-4-chloro-3-indoxyl-β-D-glucuronide CHA salt (X-Gluc) was dissolved in N,N-dimethylformamide at 80 mg/ml stock solution. Ethidium bromide was dissolved in water at 5 mg/ml stock solution and luteolin was dissolved in 100% ethanol at 4 mg/ml stock solution.
4.3. Plant growth conditions
M. sativa seeds were surface sterilized with concentrated sulfuric acid for 10 min and
washed several times with large volumes of sterile water. Seeds were kept at 4ºC over 2 days, and then allowed to germinate on 1.5% agar plates at 30ºC, in the dark for 24h. Seeds were then grown on BNM medium (Rolfe et al., 1980) and inoculated with cultures of S. meliloti strains. Each plate contained three plantlets which were inoculated with 200 µl of S. meliloti cell suspension (OD600nm=0.0001; 10
4
cells). Plants were grown under a 16h light: 8h dark cycle for 5 weeks. The nodulation process was followed by counting the number of white (immature) and pink (mature) nodules of each plant on a weekly basis.
Bacteria were isolated from several nodules and colony forming units (CFU) counted. Briefly, the nodules were harvested from the roots of M. sativa, surface-sterilized with 70% ethanol for 1 min, washed with sterile water, and crushed in TY medium. These bacterial suspensions were diluted and plated on TY plates containing streptomycin.
4.4. Extraction of genomic and plasmid DNA
Genomic DNA extraction from S. meliloti 1021 was carried out using glass beads to lyse cells, followed by purification with phenol/chloroform and precipitation with isopropanol as described in Sambrook & Russell (2001). For restriction analysis, plasmid DNA from E. coli was
13
extracted using the minipreps method described by Sambrook & Russell (2001). When higher purity was required, plasmid extraction was carried out using the QIAprep® Spin Miniprep Kit (Qiagen) following the procedures recommended by the manufacturer.4.5. Construction of S. meliloti deletion mutants
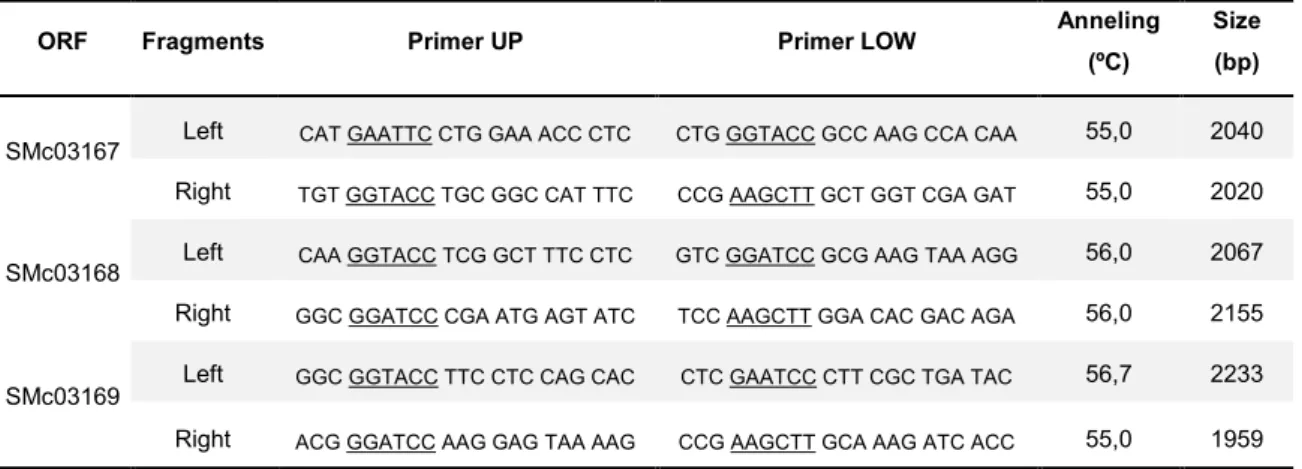
For the construction of the SMc03167, SMc03168 and SMc03169 deletion mutants, the flanking regions of each gene were cloned into the suicide vector pK19mobG. The primers used to amplify the region upstream and downstream of the ORFs under study are present in Table 4.2. Amplification reactions were carried out using an initial step of denaturation for 3 min at 94ºC, followed by 30 cycles of 45 s at 94ºC for denaturation, 30 s at an optimized annealing temperature for primer annealing (Table 4.2), 2.5 min at 72ºC for polymerization; and a final elongation step at 72ºC for 10 min. All PCR products were visualized after electrophoresis in a 0.8% agarose gel and staining with GelRed (Biotium).
Table 4.2. Primers used to amplify the flanking regions of the SMc03167, SMc03168 and SMc03169 ORFs. ORF Fragments Primer UP Primer LOW Anneling
(ºC)
Size (bp)
SMc03167 Left CAT GAATTC CTG GAA ACC CTC CTG GGTACC GCC AAG CCA CAA 55,0 2040
Right TGT GGTACC TGC GGC CAT TTC CCG AAGCTT GCT GGT CGA GAT 55,0 2020
SMc03168 Left CAA GGTACC TCG GCT TTC CTC GTC GGATCC GCG AAG TAA AGG 56,0 2067
Right GGC GGATCC CGA ATG AGT ATC TCC AAGCTT GGA CAC GAC AGA 56,0 2155
SMc03169
Left GGC GGTACC TTC CTC CAG CAC CTC GAATCC CTT CGC TGA TAC 56,7 2233
Right ACG GGATCC AAG GAG TAA AAG CCG AAGCTT GCA AAG ATC ACC 55,0 1959
The plasmids that contained the correct construction, pAFM01-2, pAFM09-1 and pAFM09-2 (Table 4.1), were introduced in electrocompetent E. coli S-17 cells by electrotransformation. A volume of 5 µl of plasmid DNA was mixed with an aliquot of 100 µl of electrocompetent E. coli cells and the suspensions were transferred into an appropriate cuvette. The electrotansformation apparatus (400 Ω, 25 µFD, 2.5 kV) was applied to the cuvette. After electrotransformation, cell suspensions were recovered from the cuvette with 1 ml of liquid LB media and incubated for 1h at 37ºC with orbital agitation (250 rpm). These cultures were plated onto the surface of LB plates supplemented with 40 µl/plate X-Gal (25 mg/ml) and 10 µl/plate IPTG (25 mg/ml) followed by incubation overnight at 37ºC. White colonies were selected.
After transformation, the plasmids were mobilized to S. meliloti 1021 by conjugation and colonies were selected in the presence of neomycin for the first crossover. For this, bacterial strains E. coli S-17 and S. meliloti strains were grown in selective liquid media until the exponential
14
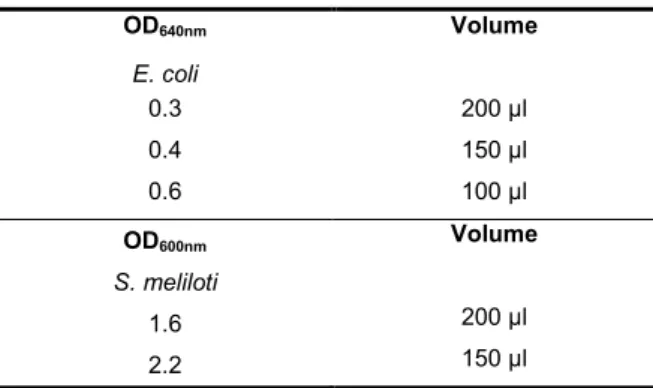
each strain (Table 4.3) was then harverested and mixed in the same tube, cells were then washed by centrifugation for 1 min at 13000 rpm, at room temperature in a table-top microcentrifuge and ressuspended in TY medium. Cells were harvested by a second centrifugation. The resulting pellet was re-suspended in residual supernatant and spot-inoculated on the surface of a TY plate followed by overnight incubation at 30ºC. The bacterial layer on the surface of the plate was re-suspended in TY medium, dilutions were prepared and 50 µl were plated on appropriate selective medium, thus enabling the positive selection of transconjugants.Table 4.3. Co-relation between OD and volume used in
conjugation. OD640nm Volume E. coli 0.3 200 µl 0.4 150 µl 0.6 100 µl OD600nm Volume S. meliloti 1.6 200 µl 2.2 150 µl
Strains with the first recombination event were grown in TY medium at 30ºC with orbital agitation (180 rpm) during 8 days. Colonies where the second crossover occurred were selected by blue/white screen in the presence of X-Gluc (80 mg/ml).
In order to complement the SMc03169 deletion mutant, a 1207 bp fragment containing SMc03169 under the control of its own promoter was amplified by PCR using primers P03169-up (5’ - GGCGAATTCTCCACCAATCAT) / P03169-low (5’ – GGCGAATTCGCTTTTACTCCT) and Sm1021 genomic DNA as a template. The amplified fragment was inserted into the EcoRI site of broad host range vector pPHU231 and the resulting plasmid was named pMAS17 (Table 4.1).
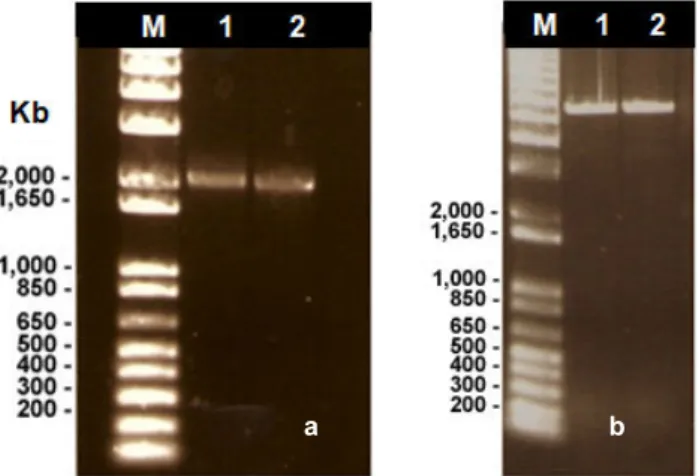
4.6. Mutant genotype confirmation
The confirmation of the deletion of each of the genes under study from the constructed mutants was done by PCR amplification using primers for the gene that was eliminated. PCR reactions were done for each candidate, differing only in the DNA template used (DNA from the mutant or from the wild-type strain). To prepare the template DNA for PCR, cells were harvested from a plate and ressuspended in 50 µl of sterile water, boiled for 5 min and then centrifuged for 1 min at 13000 rpm. The supernatant was then used for PCR as DNA template.
15
4.7. Growth curves
Growth behavior was examined as follows: 50 ml of liquid TY or GMS media supplemented with appropriated antibiotics were inoculated with the strains under study (Sm1021 or the deletion mutants), and incubated overnight at 30ºC, with orbital agitation (180 rpm). Then, 50 ml of liquid TY
or GMS medium were inoculated with previously grown culture to an initial OD600nm=0.1 and
incubated under the same conditions. Growth was followed spectrophotometrically by measuring OD at 600nm for approximately 72h. To confirm the differences observed in GMS medium, colony forming units (CFUs) counting was also performed.
4.8. Polyacrylamide gel electrophoresis
To search for changes in the strains protein expression patterns, an appropriate culture
volume was harvested (V=1.2/DO600nm) at different times to prepare cell extracts. Cells were then
centrifuged for 2 min at 13200 rpm at room temperature and the pellets were solubilized in SDS sample buffer (100 mM Tris-HCl pH 6.8; 4% (w/v) SDS; 20% (v/v) glycerol; 0.2 M β-mercaptoethanol; 0.2% (w/v) bromophenol blue) to yield a preparation of total cellular proteins. Proteins from cell extracts were visualized by vertical SDS-PAGE in 12.5% acrylamide (Sambrook & Russell, 2001) stained with Coomassie Brilliant Blue R-250 (40% Methanol, 10% Glacial acetic acid, 0.05% Brilliant Blue R-250).
4.9. Zone inhibition assays
Cultures of strains under study were grown overnight and approximately 106 cells were
plated in TY or GMS agar. Sterile paper disks with 6 mm diameter were placed on the surface of the agar. A total of 10 µl of p-coumaric acid (750, 1000 and 1500 µg/ml), berberine (2000, 2500
and 3000 µg/ml), genistein (2000 and 3000 µg/ml), SDS (10 and 20% [wt/vol]), H2O2 (10, 50 and
100 mM and 1 M), cumene hydroperoxide (5% [wt/vol]), and DOC (10% [wt/vol]) was pipetted onto separate disks. The plates were incubated for 48h at 30ºC and the diameters (in millimeters) of the inhibition zones were measured.
4.10. Ethidium bromide supplemented agar screening method
To analyze the extrusion capacity of the mutants to the toxic compound ethidium bromide, cultures of strains under study were swabbed onto TY plates containing ethidium bromide concentrations ranging from 0.5 to 1.5 mg/L, following the procedure described by Couto et al. (2008). The plates were incubated at 30ºC for 24h and fluorescence was viewed under UV light.
16
5. Results and Discussion
5.1. Sequence analysis of ORFs SMc03167, SMc03168 and SMc03169
ORFs SMc03167, SMc03168 and SMc03169 are present on the chromosome of S. meliloti 1021 and are expected to be related to bacterial resistance to toxic compounds. DNA sequence analysis suggests that the two first genes may encode a transporting system that together with TolC protein forms a tripartite efflux system and the last one a transcriptor factor. SMc03167 and SMc03168 ORFs are located in the negative strand, between positions 3127155 to 3128744 and 3128773 to 3129921, respectively. SMc03169 ORFs is encoded in the positive strand between positions 3130227 to 3130937 (Fig. 5.1). These three ORFs are predicted to encode proteins of 529, 382 and 236 amino acids, respectively. In silico analysis of the amino acid sequence encoded by these three genes using various online tools available on the web was carried out.
Fig. 5.1 - Genetic organization of the SMc03167, SMc03168 and SMc03169 ORFs (information retrieved from the S. meliloti
genome website http://iant.toulouse.inra.fr/bacteria/annotation/cgi/rhime.cgi).
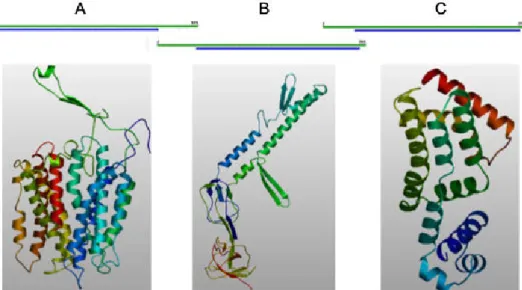
ORF SMc03167 is predicted to contain typical domains of MFS transporters of the subfamilies ErmB/QacA and TetB (Fig. 5.2A). Several members of the MFS family are multidrug efflux pumps involved in the resistance to various antibiotics and toxic compounds. Examples are ErmB from E. coli, involved in the excretion of toxic compounds (Lomovskaya & Lewis, 1992) and QacA, a multidrug efflux protein from Staphylococcus aureus, being important in the maintenance and dissemination of important Methicillin-resistant Staphylococcus aureus (MRSA) strains (Costa
et al., 2010). In order to predict the cellular location of the SMc03167 protein, a TMHMM module
available on the Expasy server was used. SMc03167 protein has a signal peptide and is predicted to be integrated into the inner membrane, with 13 transmembrane helices (Fig. 5.3A).
The protein encoded by SMc03168 contains a conserved domain of HlyD protein (Fig. 5.2B), which makes the connection between the inner membrane and outer membrane protein TolC. This protein has a signal peptide and a transmembrane helix in the N-terminal and should be inserted in the inner membrane, facing the periplasm (Fig. 5.3B).
SMc03169 encodes a protein which shares homology with transcription regulators from the TetR family and is predicted to have an N-terminal DNA-binding site of helix-turn-helix (HTH) type (Fig. 5.2C). TetR family is a family of repressors which are involved in the transcriptional control of multidrug efflux pumps, pathways for the biosynthesis of antibiotics, response to osmotic stress and toxic chemicals (Ramos et al., 2005). This protein is more likely cytoplasmic, showing no transmembrane helix domains (Fig. 5.3C).
17
Fig. 5.2 - Conserved motifs present in SMc03167 (A), SMc03168 (B) and SMc03169 (C) proteins. These motifs were
obtained using the InterProScan tool available in www.expasy.ch.
Fig. 5.3 - Predicted number of transmembrane helices of SMc03167 (A), SMc03168 (B) and SMc03169 (C), using the
TMHMM software from www.expasy.ch.
A
B
C
A B
18
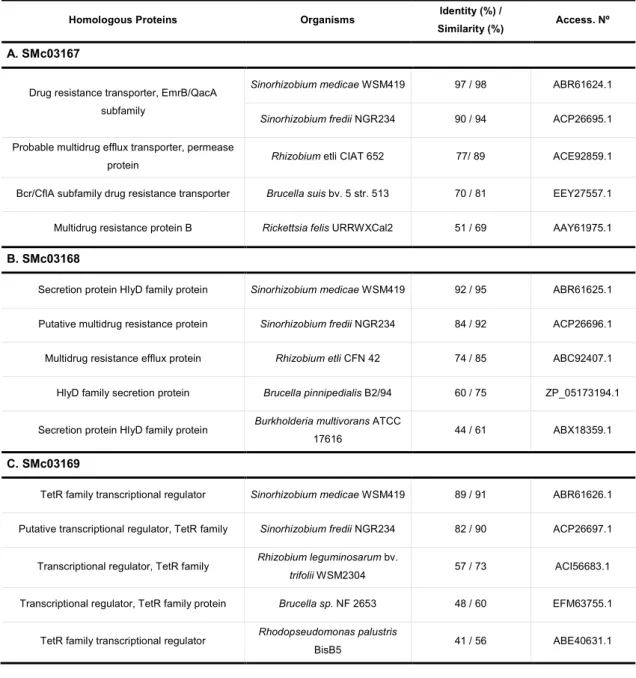
Simultaneous with the tool used before, a basic alignment search involving the BLASTP tool from the National Center for Biotechnology Information database (NCBI) was employed, confirming the homology between our proteins and proteins from databases. Again, SMc03167 shares homology with multidrug efflux pumps from other organisms (Table. 5.1A); SMc03168 is homologous with the other component from efflux pumps like HlyD (Table. 5.1B), an adaptor component of the Hly transporter in which the N-terminal end interacts with the substrate in the cytoplasm (Delepelaire, 2004); and SMc03169 is a probable transcription regulator from TetR family (Table. 5.1C), generally involved in the repression of genes that control susceptibility to hydrophobic compounds and detergents. As expected, the analysis of these protein sequences showed a highest similarity between members of the Rhizobiaceae order, such S. medicae, S.fredii and R. etli (above 75% identity).
Table 5.1 - Similarity searches for SMc03167 (A), SMc03168 (B) and SMc03169 (C) at the amino acid level using the
BLASTP algorithm.
Homologous Proteins Organisms Identity (%) /
Similarity (%) Access. Nº
A. SMc03167
Drug resistance transporter, EmrB/QacA subfamily
Sinorhizobium medicae WSM419 97 / 98 ABR61624.1
Sinorhizobium fredii NGR234 90 / 94 ACP26695.1 Probable multidrug efflux transporter, permease
protein Rhizobium etli CIAT 652 77/ 89 ACE92859.1
Bcr/CflA subfamily drug resistance transporter Brucella suis bv. 5 str. 513 70 / 81 EEY27557.1
Multidrug resistance protein B Rickettsia felis URRWXCal2 51 / 69 AAY61975.1
B. SMc03168
Secretion protein HlyD family protein Sinorhizobium medicae WSM419 92 / 95 ABR61625.1
Putative multidrug resistance protein Sinorhizobium fredii NGR234 84 / 92 ACP26696.1
Multidrug resistance efflux protein Rhizobium etli CFN 42 74 / 85 ABC92407.1
HlyD family secretion protein Brucella pinnipedialis B2/94 60 / 75 ZP_05173194.1
Secretion protein HlyD family protein Burkholderia multivorans ATCC
17616 44 / 61 ABX18359.1
C. SMc03169
TetR family transcriptional regulator Sinorhizobium medicae WSM419 89 / 91 ABR61626.1
Putative transcriptional regulator, TetR family Sinorhizobium fredii NGR234 82 / 90 ACP26697.1
Transcriptional regulator, TetR family Rhizobium leguminosarum bv.
trifolii WSM2304 57 / 73 ACI56683.1
Transcriptional regulator, TetR family protein Brucella sp. NF 2653 48 / 60 EFM63755.1
TetR family transcriptional regulator Rhodopseudomonas palustris
To get further insights into proteins could be involved, the
genes of S. meliloti genome were analyzed possible to infer much about their
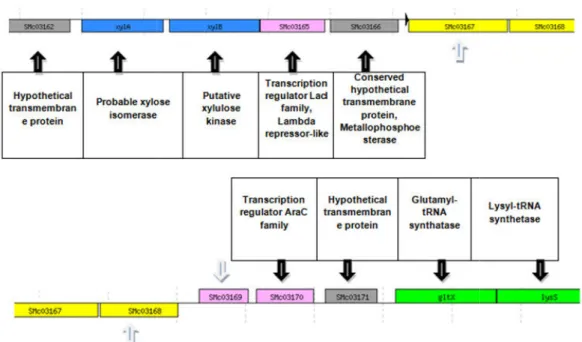
Fig. 5.4 - Genetic organization and putative role of the adjacent genes of the ORFs SMc03167, SMc03168 and SMc03169.
To evaluate whether SMc03167, SMc03168 and SMc03169 within the S. meliloti encoding
S. meliloti genome using the MultA
show in Fig. 5.5.
Fig. 5.5 - Phylogram tree comprising the SMc03167 (A), SMc03168 (B) and SMc03169 (C)
proteins of S. meliloti 1021. The sequences were aligned by Mult
http://iant.toulouse.inra.fr/bacteria/annotation/cgi/rhime.cgi) and sorted by Tree View. clusters.
The cluster obtained for SMc03167 gene relate drug or other small molecules efflux. The SMc03167
A
further insights into the function or process in which these putative
the surrounding genetic organization and putative role of the adjacent were analyzed (Fig. 5.4). However, from the neighbor genes it
much about their function since they are flanked by non-characterized genes.
Genetic organization and putative role of the adjacent genes of the ORFs SMc03167, SMc03168 and SMc03169.
SMc03167, SMc03168 and SMc03169 proteins had other homolog encoding genes with similar functions, their sequences were aligned
using the MultAlin server. The phylograms obtained from these alignments are
tree comprising the SMc03167 (A), SMc03168 (B) and SMc03169 (C) proteins 1021. The sequences were aligned by MultAlin server from the S. meliloti http://iant.toulouse.inra.fr/bacteria/annotation/cgi/rhime.cgi) and sorted by Tree View. The square
obtained for SMc03167 gene related with MFS transport systems involved in ecules efflux. The SMc03167 must related homologues are SMc00563, a
B C
19
putative efflux system surrounding genetic organization and putative role of the adjacent rom the neighbor genes it was notcharacterized genes.
Genetic organization and putative role of the adjacent genes of the ORFs SMc03167, SMc03168 and SMc03169.
proteins had other homologues were aligned against obtained from these alignments are
proteins and homologous
S. meliloti genome website
quares are the considered
with MFS transport systems involved in homologues are SMc00563, a