UNIVERSIDADE DE TRÁS-OS-MONTES E ALTO DOURO
ATLANTOAXIAL INSTABILITY IN DOGS
DISSERTAÇÃO DE MESTRADO EM MEDICINA VETERINÁRIA
PAULO JORGE PEREIRA AFONSO
ORIENTADOR
Professor Doutor Artur Severo Proença Varejão
iii
UNIVERSIDADE DE TRÁS-OS-MONTES E ALTO DOURO
ATLANTOAXIAL INSTABILITY IN DOGS
DISSERTAÇÃO DE MESTRADO EM MEDICINA VETERINÁRIA
PAULO JORGE PEREIRA AFONSO
ORIENTADOR
Professor Doutor Artur Severo Proença Varejão
COMPOSIÇÃO DO JÚRI
Professor Doutor Artur Severo Proença Varejão Professor Doutor Filipe da Coste Silva
Professor Doutor Mário Manuel Dinis Ginja
iv
DECLARAÇÃO
NOME: PAULO JORGE PEREIRA AFONSO
C.C.:13377976
CORREIO ELECTRÓNICO: AFONSO.P@ICLOUD.COM
DESIGNAÇÃO DO MESTRADO: MESTRADO INTEGRADO EM MEDICINA
VETERINÁRIA
TÍTULO DA DISSERTAÇÃO DE MESTRADO EM MEDICINA VETERINÁRIA:
ATLANTOAXIAL INSTABILITY IN DOGS
ORIENTADOR:
PROFESSOR DOUTOR ARTUR SEVERO PROENÇA VAREJÃO
ANO CONCLUSÃO: 2013
DECLARO QUE ESTA DISSERTAÇÃO DE MESTRADO É RESULTADO DA MINHA PESQUISA E TRABALHO PESSOAL E DAS ORIENTAÇÕES DOS MEUS SUPERVISORES. O SEU CONTEÚDO É ORIGINAL E TODAS AS FONTES CONSULTADAS ESTÃO DEVIDAMENTE MENCIONADAS NO TEXTO, E NA BIBLIOGRAFIA FINAL. DECLARO AINDA QUE ESTE TRABALHO NÃO FOI APRESENTADO EM NENHUMA OUTRA INSTITUIÇÃO PARA OBTENÇÃO DE QUALQUER GRAU ACADÉMICO.
VILA REAL, 28 DE OUTUBRO DE 2013 PAULO JORGE PEREIRA AFONSO
v
“Now this is not the end. It is not even the beginning of the end. But it is, perhaps, the end of the beginning.”
vii
ACKNOWLEDGEMENTS
A special thanks to Doctor Sofia Cerda-Gonzalez and Doctor Peter Early, for welcoming me at CUHA – Neurology and Neurosurgery Service and for supervise me during my externship, gratitude that is extended to Doctor Cameron Starr, Doctor Amanda Full, Doctor Chelsie Estey, Leslie Hopkins and Kacie Vasicek for all the valuable knowledge and good moments. Thanks to Pilar Thompson for all the work and attention enabling my externship in CUHA, and for making possible the use of Cornell cases in this work. To Linda Pedro, Iwona Popkoswski and Rachel Mays for the friendship and help.
A big recognition to my advisor Doctor Artur Varejão for guiding this work. A deep appreciation to Professor Alastair Watson for sending me his articles by mail and for all the availability and help.
My gratitude to ARS veterinária team for the teaching and knowledge, a special thanks to Doctor Alejandro Luján and Doctor Artur Font who supervised my externship, to Doctor Joan Mascort, Doctor Joseph Closa, Doctor Iolanda Naválon and Doctor Cristina Font for all the dedication in sharing knowledge. Thanks to Doctor Marta Blanchart for all the work making possible my externship at ARS veterinária. To Estel for the friendship and help.
To RRV, in special to Doctor João Ribeiro for the teachings. To Nurse Sílvia, Nurse Susana, Bruno, Filipa, Joana and Leandro.
To all those who took part in my life. To my friends.
ix
ABSTRACT
Atlantoaxial Instability (AAI) is characterized by excessive movement at the junction between the atlas and axis. This condition can be seen in any breed at any age, but mainly affects immature miniature and toy breeds. AAI is typically a congenital or developmental disease and sometimes has a traumatic origin. Abnormalities of the dens (aplasia, hypoplasia, fragmented or dorsal deviation) and absence of the transverse ligament are seen as principal causes of AAI.
When spinal cord is injured clinical signs can include neck pain and varying degrees of ataxia of all four limbs, including tetraparesis and tetraplegia in severe cases.
Treatment options embrace conservative and surgical management. Conservative treatment consisting of strict cage confinement, use of an immobilizing cervical splint, and the administration of corticosteroids is recommended for a restricted number of cases. Ventral surgical stabilization is traditionally the preferred treatment for AAI because it facilitates anatomic alignment, placement of autogenous cancellous bone and removal of the dens if necessary, however dorsal techniques are also described.
This study contains a literature review in AAI and four cases followed at Cornell University Animal Hospital: a intact male, 7 months-old, German Shepherd, a castrated male, 12 months-old, Dachshund, a spayed female, 96 months-old, Yorkshire Terrier, and a spayed female, 24 months-old, Maltese. Patients exhibited tetraplegia, tetraparesis and cervical pain as clinical signs. Preoperative MRI or/and CT scan, and postoperative CT scan were performed. All dogs were treated surgically with multiple ventral implants and bone cement. All dogs improved their neurological status. The information presented was collected during an externship in Cornell University Hospital for Animals, in the Neurology and Neurosurgery Service.
Keywords: atlantoaxial instability, dens, atlantoaxial stabilization, craniocervical
xi
LIST OF CONTENTS
ABSTRACT ... ix
LISTOFCONTENTS ... xi
LISTOFTABLES ... xiii
LISTOFFIGURES ... xv
ABBREVIATIONS,SYMBOLSANDACRONYMS ... xvii
CHAPTER 1 – LITERATURE REVIEW ... 1
1. Atlantoaxial Anatomy ... 1 1.1. Atlas ... 2 1.2. Axis ... 3 2. Atlantoaxial Articulation ... 3 2.1. Embriology ... 5 2.2. Prenatal ossification ... 6 2.3. Postnatal ossification ... 7
2.4. Fusion of the bony elements ... 9
2.5. Cartilaginous growth zones in postnatal axis ... 10
3. Atlantoaxial Instability ... 10
3.1. Definition ... 10
3.2. Epidemiology ... 11
3.3. Pathophysiology ... 11
3.3.1.Congenital and Degenerative ... 13
3.3.2.Traumatic ... 15 3.4. Clinical signs ... 16 4. Diagnosis ... 19 4.1. Physical exam ... 20 4.2. Neurological exam ... 20 4.3. Differential diagnosis ... 21
xii
4.4. Laboratory analysis ... 21
4.5. Radiographic findings ... 21
4.6. Myelography ... 22
4.7. Computerized Tomography ... 23
4.8. Magnetic Resonance Imaging ... 23
5. Prognosis ... 27
6. Treatment ... 33
6.1. Conservative management ... 34
6.2. Surgical management ... 37
6.2.1.Dorsal techniques ... 39
6.2.1.1.Dorsal wire fixation ... 40
6.2.1.2.Dorsal cross-pin fixation ... 41
6.2.2.Ventral techniques ... 42
6.2.2.1.Ventral transarticular fixation ... 44
6.2.2.2.Multiple ventral implants and bone cement ... 45
6.3. Postoperative management ... 46
6.4. Complications ... 47
CHAPTER2–AIM ... 51
CHAPTER3–MATERIALS AND METHODS ... 53
1. Case 1 ... 55
2. Case 2 ... 63
3. Case 3 ... 69
4. Case 4 ... 79
CHAPTER4–RESULTS AND DISCUSSION ... 89
CHAPTER5–CONCLUSION ... 95
xiii
LIST OF TABLES
Table 1- Prenatal and postnatal ossification centers. ... 8
Table 2- Alterations of the dens of the axis ... 14
Table 3 - Differential diagnosis for atlantoaxial subluxation ... 21
Table 4 - Ligaments and MRI planes for visualization ... 24
Table 5 - Factors affecting conservative outcome in dogs ... 28
Table 6 - Comparison of success rates of dorsal and ventral surgical treatment and conservative treatment reported in the veterinary literature ... 29
Table 7 - Factors affecting surgical outcome in dogs ... 31
Table 8- Overall rates of failure and mortality of first surgery, and need of second surgery ... 32 Table 9 - Summary of the clinical cases from Cornell University Hospital for Animal. 53
xv
LIST OF FIGURES
Figure 1 - Atlas ossification centers ... 8
Figure 2 - Axis ossification centers ... 9
Figure 3 - Final arrangement of wires in Dorsal Wire Fixation ... 41
Figure 4 - Pin position in Dorsal Cross-pin fixation ... 41
Figure 5 - Position of screws in ventral transarticular fixation ... 45
Figure 6 - Sagital T2 image ... 59
Figure 7 - Presurgery CT reconstruction showing an absent dens ... 59
Figure 8 - Postoperative CT reconstruction ... 60
Figure 9 – Recheck cervical radiographs 3 months after surgery ... 60
Figure 10 - Cervical radiographs (in extension and flexion) showing AAI ... 65
Figure 11 - Preoperative CT scan reconstruction ... 65
Figure 12 - Postoperative CT reconstruction ... 66
Figure 13 - Cervical radiographs showing a wide joint space ... 73
Figure 14 - Preoperative CT scan ... 73
Figure 15 - Preoperative CT scan ... 73
Figure 16 - Postoperative CT scan ... 74
Figure 17 - Cervical radiographs 8 weeks after surgery ... 74
Figure 18- Cervical radiographs ... 83
Figure 19 - MRI ... 83
Figure 20 - CT scan reconstruction (soft tissue window) ... 83
Figure 21 - Postoperative CT scan reconstruction ... 84
Figure 22 - Cervical radiographs 4 weeks postoperative ... 84
xvii
ABBREVIATIONS, SYMBOLS AND ACRONYMS
AA - atlantoaxial
AAI – atlantoaxial instability
AOO – atlantooccipital overlap
CBC – complete blood count
CJAs – craniocervical junction
abnormalities
CRI – continuous rate infusion
CSF – cerebrospinal fluid
CT – computed tomography
C1 – first cervical vertebra or atlas
C2 – second cervical vertebra or axis
C3 - third cervical vertebra
CUHA – Cornell University Hospital
for Animals
IV – intravenous
kg – kilogram
MRI – magnetic resonance imaging
mg – milligram
PO – per os, orally
PD – proton density
PDS - polydioxanone
PMMA – poly(methyl methacrylate)
PRN – pro re nata, when necessary
PROM – passive range of motion
rDVM – referring veterinarian
RRV – Referência Veterinária
TID – three time a day, or each every
eight hours
T1W – T1 weighted
T2W - T2 weighted
ºC – degrees Celsius
1
CHAPTER 1 – LITERATURE REVIEW
“Outside of a dog, a book is man's best friend. Inside of a dog it's too dark to read.”
Groucho Marx Clinical publications related with the subject “Atlantoaxial Instability” were searched in “PubMed”, with the keywords “Atlantoaxial Instability”, “Atlantoaxial Subluxation” “Occipitoatlantoaxial Malformations” and “Craniocervical joint abnormalities”.
1. Atlantoaxial Anatomy
The vertebral column (columna vertebralis) consists of approximately 50 irregular bones, the vertebrae, arranged in five groups: cervical (C), thoracic (T), lumbar (L), sacral (S), and coccygeal (Cc) and the vertebral formula of the dog is C7T13L7S3CC20. All vertebrae except the sacral vertebrae remain separate and articulate with contiguous vertebrae in forming movable joints. The three sacral vertebrae are fused to form a single bone, the sacrum (os sacrum). The vertebrae protect the spinal cord and roots of the spinal nerves, aid in the support of the head, and furnish attachment for the muscles governing body movements. Although the amount of movement between any two vertebrae is limited, the vertebral column as a whole possesses considerable flexibility (Miller et al., 2013; World Association of Veterinary Anatomists International Committee on Veterinary Gross Anatomical Nomenclature, 2005).
A typical vertebra consists of a body (corpus vertebrae); a vertebral arch (arcus vertebrae), consisting of right and left pedicles (pediculus arcus vertebrae) and laminae (lamina arcus vertebrae); and various processes for muscular or articular connections, which may include transverse, spinous, articular, accessory, and mamillary processes (Miller et al., 2013; World Association of Veterinary Anatomists International Committee on Veterinary Gross Anatomical Nomenclature, 2005).
In the majority of mammals there are seven cervical vertebrae (vertebrae cervicales). There is a great difference between the first, atlas, and the second, axis, and from those to the others cervical vertebrae. The third, fourth and fifth are difficult to differentiate cause their have few differences. The sixth and seventh cervical vertebrae
2 have sufficient differences between them to allow their identification (Budras, 2007; World Association of Veterinary Anatomists International Committee on Veterinary Gross Anatomical Nomenclature, 2005).
1.1. Atlas
The first cervical vertebra or atlas is unique in both structure and function. Cranially articulates with the skull and caudally with the axis. The modified articular processes that involves the exoccipital condyles, the winglike lateral expansions, the lack of spinous process and reduction of its body are the main distinctive features of the atlas (Watson et al., 1986). Two thick lateral portions (massae lateralis) unite the dorsal arch (arcus dorsalis) with the ventral arch (arcus ventralis) or body of the atlas. From those lateral masses are projected the wings (alae atlantis). The elliptical space between the dorsal arch of the atlas and the occipital bone is the spatio interarcuate atlantooccipitale. Other eminences of the atlas are the dorsal tubercle (tuberculum dorsale), on the cranial end of the dorsal arch (arcus dorsalis), and the ventral tubercle (tuberculum ventrale), wich projects from the caudal end of the body. Commonly the dorsal tubercle is bifid and the ventral tubercle can assume a conical form. The cranial articular surface (fovea articularis cranialis), that consist of two cotyloid cavities that sometimes meet ventrally, articulates with the occipital condyles of the skull forming the altantooccipital joint. The caudal articular surface of the atlas (fovea articularis caudalis) consist of two glenoid cavities that form a freely movable articulation with the axis, the second cervical vertebra. Dorsally to the body of the atlas, in the ventral arch (arcus ventralis), is the fovea of the dens (fovea dentis) wich is concave from side to side and articulates with the dens of the axis. This articular area blends with the articular areas on the caudal surface of the lateral masses. The alar foramen (foramen alar) is a canal that passes obliquely the wing of the atlas and where runs the vertebral vein and artery. The lateral vertebral foramen (foramen vertebrale laterale) perforates the craniodorsal part of the dorsal arch, where the first cervical nerve exits the vertebral canal, contrary to the others spinal nerves that go by an intervertebral foramen (König et al., 2007; Miller et al., 2013; World Association of Veterinary Anatomists International Committee on Veterinary Gross Anatomical Nomenclature, 2005). Is described a variation in the vertebral lateral foramen of a Miniature Schnauzer as a notch in the cranial border of the atlas (Richards and Watson, 1991). On the cranial origin of the transverse process is located the alar notch (incisura alaris). The depressions ventral to
3 the wings are the atlantal fossae (fossae atlantis). The vertebral vein and artery transverse the atlantal fossa. The vein goes through the alar foramen caudally and anastomoses with the internal jugular vein rostrally. Through the alar notch a venous branch runs dorsally and contributes to form the external vertebral venous plexus. After went cranially through the alar foramen the vertebral artery enters in the vertebral canal by the lateral vertebral foramen (Miller et al., 2013; World Association of Veterinary Anatomists International Committee on Veterinary Gross Anatomical Nomenclature, 2005).
1.2. Axis
The axis, or second cervical vertebra, has an elongated dorsal spinous process that overhangs the cranial and caudal articular surfaces of the vertebral body. The axis is also characterized by a cranioventral odontoid process, the dens (dens). Morphologically the dens and the cranial part of the cranial articular surface are the centrum of the atlas, which developmentally attaches to the axis (Miller et al., 2013; World Association of Veterinary Anatomists International Committee on Veterinary Gross Anatomical Nomenclature, 2005). The dens is held down in the fovea of the dens, in the dorsal surface of the ventral arch of the atlas, by the transverse ligament. The cranial articular surfaces result from the lateral cranial expansions of the vertebral body. Where the spinous process and the vertebral arch unite results the caudal articular process as ventrolateral extensions. The transverse foramen (foramen transversarium) is a hole in the pedicles of the vertebra. The second cervical nerve lefts the spinal cord by the transverse foramen formed by the cranial vertebral notches of the axis and the caudal vertebral notches of the atlas on either side. The caudal vertebral notches concur with the cranial vertebral notches of the third cervical vertebra to form the third intervertebral foramen where pass the third cervical spinal nerve and the spinal vessels (Budras, 2007; Miller et al., 2013; World Association of Veterinary Anatomists International Committee on Veterinary Gross Anatomical Nomenclature, 2005).
2. Atlantoaxial Articulation (Articulatio atlantoaxialis)
In man there are three atlantoaxial joints, two lateral and one median, in domestic mammals they are not separated, and therefore only one term is required (World Association of Veterinary Anatomists International Committee on Veterinary Gross
4 Anatomical Nomenclature, 2005). The atlantoaxial joint is a trochoid joint formed by the first two cervical vertebrae. A hollow ‘cylinder’, the atlas, rotates around a fixed articular projection, the dens of the axis, forming the atlantoaxial joint, is known as “no joint” for allowing rotational movement of the head on the column (Budras, 2007; Miller et al., 2013; Slatter, 2002). In carnivorus the joint capsule (capsula articularis) is loose and uniformly thin as goes from the dorsal part of the cranial articular surface from side to side of the axis. The joint attaches cranially to the caudal margins of the caudal articular surface and ventral arch of the atlas. The dorsal atlantoaxial membrane (membrane tectoria) is a fibrous layer of the joint capsule that extends from right to left between the dorsal arch of the atlas and the arch of the axis. The apical ligament of the dens (lig. apicis dentis) goes from the apex of the dens to the ventral part of the foramen magnum in the basioccipital bone and it represents a remnant of the notochord. There are two alar ligaments (ligg. alaria) broader and harsher than than the apical ligament. They bind on both sides of the apical ligament and go laterally to attach medial to the caudal parts of the occipital condyles. The transverse atlantal ligament (lig. transversum atlantis), is a thick ligament that connects the both side of the ventral arch of the atlas and passes over the dens, holding it ventrally against the ventral arch of the atlas. However there is some space between the ventral surface of the ligament and the dens. The elastic dorsal atlantoaxial membrane (membrana atlantoaxialis dorsalis) extends from the cranial projection of the spine of the axis to the dorsal arch of the atlas. From the cranial aspect of the spinous process of the axis to the dorsal aspect of vertebral arch of the atlas goes the dorsal atlantoaxial ligament (lig. atlantoaxiale dorsale). Lig. atlantoaxiale ventral extends from the cranial projection of the spine of the axis to the dor-sal arch of the atlas (Budras, 2007; Miller et al., 2013; World Association of Veterinary Anatomists International Committee on Veterinary Gross Anatomical Nomenclature, 2005).
The dens is held to the floor of the vertebral canal and to the occipital bone by the apical ligament of the dens, the transverse atlantal ligament and the alar ligaments. The transverse atlantal ligament is underlain by a synovial bursa and is attached to either side of the atlas. In the case of rupture of these ligaments or fracture of the dens following car accidents or strangulation, damage to the spinal cord may occur with paralysis and death as consequences (Budras, 2007).
5 There is no intervertebral disc between the atlas and the axis and the relationship between this vertebrae is maintained largely by ligaments (Wheeler, 2005).
The interacuate ligaments also known as yellow ligaments (ligg. flava) link the archs of adjacent vertebrae. In the lateral side they blend with the articular capsules. Ventrally, between this ligament and the dura is the epidural space (Miller et al., 2013; World Association of Veterinary Anatomists International Committee on Veterinary Gross Anatomical Nomenclature, 2005).
On the ventral surface of the vertebrae from the axis to the sacrum is the ventral longitudinal ligament (lig. longitudinale ventral). The dorsal longitudinal ligament (lig. longitudinal dorsale) lies on the dorsal surfaces of the bodies of the vertebrae being a part of the vertebral canal floor. It goes from the dens of the axis to the end of the vertebral canal in the coccygeal region (Miller et al., 2013; World Association of Veterinary Anatomists International Committee on Veterinary Gross Anatomical Nomenclature, 2005).
From the caudal part of the spinous process of the axis to the dorsal extremity of the spinous process of the first thoracic vertebra goes the nuchal ligament (lig. nuchae) that is composed of longitudinal yellow elastic fibers (Miller et al., 2013; World Association of Veterinary Anatomists International Committee on Veterinary Gross Anatomical Nomenclature, 2005). The nuchal ligament continues caudally as supraspinous ligament (lig. supraspinale) to the tenth thoracic spinous processs (Miller et al., 2013; World Association of Veterinary Anatomists International Committee on Veterinary Gross Anatomical Nomenclature, 2005).
2.1. Embriology
The embryonic development of the axial skeleton consists, by this order, in: somites formation, scleretomes migration from the somites, primitive segments formation, secondary segmentation (division of the primitive segments) precartilaginous vertebral bodies formation, intervertebral discs formation, vertebral bodies ossification, vertebral arches formation and vertebrae, ribs and sternum formation (Climent Peris et al., 2002).
The notochord is the forerunner of the vertebral column, and in the early embryo it is present as a solid rod of cells surrounded by a sheath of paraxial mesoderm. The paraxial mesoderm secretion in blocks to both sides of notochord and neural tube will
6 contribute to the segmentary nature of the vertebral column. This will form the somites during the organogenic period of development (Climent Peris et al., 2002; Miller et al., 2013). It is believed that the atlas is derived from the first pair of cervical somites and caudal half of the last pair of occipital somites and that the axis id derived from two and one-half pairs of cervical somites (Watson et al., 1986). Condensations within the somites form the sclerotomes that surround the notochord and partially enclose the neural tube. When chondrification takes place in the sclerotome, the notochord is almost completely obliterated within the centrum. The lateral portions of the vertebral condensation chondrify and grow dorsally to form the neural arches and ventrolaterally to form the transverse and costal process and part of the body. The spinous process of the neural arch develops after the arches meet and fuse (Climent Peris et al., 2002).
Developmentally, a typical vertebra (except the atlas and axis) is formed from three ossification centers: a body and two laminae. Postnatally epiphyses form on each end of the body and fuse with it. Epiphyseal centers appear from the second to the eighth weeks and that union is complete by the fourteenth month (Miller et al., 2013).
2.2. Prenatal ossification
Based on one study (Watson et al., 1986) with skeleton preparations of 200 Beagles of known age, ranging from embryos of 28 days of age to 10 year old adults, the atlantoaxial complex first ossification appears in the neural arch elements, then in the centra, centrum 2 before centrum 1 and intercentrum 1 is the last element to ossify prior to birth:
Neural arch: ossification starts at 33 days of age embryos. This ossification began bilaterally on the medial surface of each lamina of the cartilaginous neural arch, lateral to the spinal cord, then spreads around to complete the perichondral collar of bone. From this collar continues dorsally in the lamina, ventrally in the pedicle and ventrolaterally into the transverse process. Neural arch ossification occurs first in the axis than in the atlas.
Centrum 2: forms a large part of the body of the axis and develops earlier than centrum 1. Starts in the center of the cartilaginous body of the axis, as a small cuboidal nodule of ossification around the notochord. Ossification of centrum 2 begins at 33 days of age embryos.
7 Centrum 1: forms the dens, with exception of his first one quarter, and the cranial part of the axis body, ossifies first as a small nodule around the notochord (cranomedial part of the cartilaginous axis body, immediately caudal to the base of the dens. Ossification of centrum 1 occurs from 40 days of age embryos.
Intercentrum 1: is the last element to ossify prenatally. This center in the adult will form the body of the atlas. Intercentrum 1 ossifies as a thin median bony plate, within the ventral one-third of the cartilage of the atlas body. Ossification occurs around 45 day of age embryos.
2.3. Postnatal ossification
At birth there are present seven bony elements. The atlas has three: two bilateral neural arch elements dorsolaterally and from the intercentrum 1 ventromedially the body of the atlas. The axis has four bony elements: two bilateral neural arch elements dorsolaterally, centrum 2 in the main part of the axis body and a smaller centrum 1 in the base of the dens and the cranial part of the axis body. In postnatal life, the bony elements that are separated develop in size and shape until they achieve the adult form. Then three more separated bony elements develop in the axis: intercentrum 2, epiphysis and centrum of the proatlas (Watson et al., 1986):
Intercentrum 2: is a small bony nodule, develops in the center of a cuboidal block of cartilage, surrounded by centrum 1 cranially, centrum 2 caudally and neural arch elements laterally. Ossification of intercentrum 2 begins at 22 days of age post-partum;
Epiphysis: has origin in small granules of bone dispersed peripherally in the cartilage on the caudal end of the axis body. These granules associate forming a single fat bony disc that covers the caudal end of the centrum 2 and parts of the neural arch elements. In that study this vertebral body epiphysis was the only epiphysis detected in the atlantoaxial complex. It starts to ossify from 15 days of age post-partum;
Centrum of the proatlas: is the last bony element to develop and is a single median ossification within cartilaginous apex of the dens. Ossification occurs from 42 day of age post-partum.
8 Thus three bony elements form the atlas (Figure 1) and seven bony elements form the axis (Figure 2) (Miller et al., 2013).
Table 1- Prenatal and postnatal ossification centers (Watson et al., 1986). Prenatal
Ossification Originates
Neural arches* 33 days** Vertebral arches
Centrum 2 33 days Body of the axis
Centrum 1 40 days Caudal ¾ of the dens and cranial part of axis body
Intercentrum 1 45 days Body of the atlas
Postnatal
Ossification Originates
Intercentrum 2 22 days Body of the axis
Epiphysis 15 days Caudal part of axis body
Centrum of proatlas 42 days Cranial ¼ of the dens * Two in the atlas and two in the axis.
**Neural arch ossification occurs first in the axis than in the atlas.
Figure 1 - Atlas ossification centers (drawn by Luís Agostinho1).
1 Luís Agostinho – Luís Paulo Jerónimo Agostinho, born in 1989. Has finished his first
studies in Management, at Faculdade de Economia, in Coimbra University, and is actually studying Digital Games Design at EsACT -Instituto Politécnico de Bragança, in Mirandela. Contact: luis_agostinho_@hotmail.com.
Left half of neural arch
9
Figure 2 - Axis ossification centers (drawn by Luís Agostinho).
2.4. Fusion of the bony elements
The ten ossification centers in the atlantoaxial complex will develop and fuse to form the mature atlas and axis (Table 1), in the same study 24 Beagles of known age were studied and age f fusion determined by the examination of alizarin-clearings, cleaned bones and histological sections (Watson et al., 1986):
Atlas: the neural arches are fuse mid-dorsally from 106 days of age; intercentrum 1 fuse with the pedicles of neural arches from 115 days of age;
Axis: the neural arches fuse mid-dorsally from 30 days of age. Centrum 2 and neural arches fused between 89-105 days of age. Centrum of the proatlas fused with the rest of the dens (centrum 1) at 106 days. Intercentrum 2 fuse wit centrum 1 cranially and
Neural arch Centrum of proatlas Centrum 1 Intercentrum 2 Centrum 2 Epiphysis Ventral part of neural arch
10 centrum 2 caudally between 115-146 days. Epiphysis is fused to the caudal body from 221 to 396 days.
It is important to notice that the dens develops from two separated centers: centrum of proatlas forms the cranial one-quarter, and centrum 1 forms the caudal three-quarters of the dens and the cranial part of the axis body. From 13 months of age there were no externally visible marks indicating separated bony elements on the atlas and axis on 20 of the 24 dogs studied.
2.5. Cartilaginous growth zones in postnatal axis
There are four cartilaginous bands separating five developing ossifications. These four zones of chondrocytic proliferation and endochondral bone growth were oriented to provide longitudinal growth of the bony elements centrum 1 and centrum 2. The first growth zone is between the centrum of proatlas and centrum 1, caudal to the first cartilage band, and contributes to the cranial growth of centrum 1. Cranial to intercentrum 2 is the second cartilage band with the second growth zone in its cranial surface, contributing to the caudal growth of centrum 1. The third growth-zone is caudal to intercentrum 2, on the caudal surface of the third band, and contributes to the cranial growth of centrum 2. The fourth cartilage band is on the cranial surface of the fourth cartilage band, between the caudal end of centrum 2 and the epiphysis, and contributes to the caudal growth of centrum 2. There are two patterns of endochondral growth of the bony elements in the axis body and dens. One with well-organized parallel columns of chondrocytes form growth zones at the cranial and caudal extremities of centrum 1 and 2. The other pattern without well-organized growth-zones is present in the development of centrum of the proatlas, intercentrum 2 and in the epiphysis (Watson et al., 1986).
3. Atlantoaxial Instability
3.1. Definition
Atlantoaxial instability (AAI) is included in an “umbrella” term used to describe a group of developmental disorders of the craniocervical junction region (supraoccipital bone, C1 and C2 vertebrae), the craniocervical junction abnormalities (CJAs). These abnormalities include Chiari-like malformation, AAI, atlantooccipital overlap (AOO), and dorsal compression at C1-C2. AAI refers to excessive motion at the C1-C2 joint, usually due to a hypoplastic or absent dens (Dewey, 2013).
11
3.2. Epidemiology
Craniocervical junction abnormalities in small-breed dogs are being increasingly recognized as common and challenging disorders (Dewey et al., 2013).
AAI was first reported in dogs in 1967 and has also been documented in humans, cats, horses, and cattle (Geary et al., 1967; Havig et al., 2005; Jaggy et al., 1991; McCarthy et al., 1995; Shelton et al., 1991).
AAI is usually encountered in miniature and toy dogs, often less than 2 years of age, but has been reported in older dogs and larger dog breeds, particularly the traumatic form (Dewey et al., 2013; Platt et al., 2004). It occasionally occurs in cats (Dewey, 2013; Jaggy et al., 1991).
Most cases of congenital AAI are reported in immature small and toy-breed dogs, as miniature and toy poodle, Yorkshire Terrier, Chihuahua, Pekingese, Schnauzer and Pomeraninan breeds. Congenital AAI also occurs in large-breed dogs, Rottweiler, Doberman, Basset Hound, Standard poodle, Weimaraner and German shepherd dog. There is no sex predisposition (Aikawa et al., 2013; Beaver et al., 2000; Cerda-Gonzalez and Dewey, 2010; Denny et al., 1988; Dewey et al., 2013; Gilmore, 1984; Havig et al., 2005; Huibregtse et al., 1992; Hurov, 1979; Knipe et al., 2002; McCarthy et al., 1995; Platt et al., 2004; Read et al., 1987; Rochat and Shores, 1999; Thomas et al., 1991; Wheeler, 1992).
3.3. Pathophysiology
The functional interrelationships in the occipito-atlas-axis complex can sometimes be seen when there is a structural abnormality, or a dysfunction, in one part of the complex, which may lead to functional abnormalities in another part. AAI in the dog demonstrates this functional interdependence. Bone fractures, torn or lax ligaments, and an abnormally shortened dens may each lead to atlantoaxial subluxation; the joint becomes unstable and the axis may move abnormally relative to the atlas. AAI due to an abnormally shortened dens, which is most prevalent in small breeds of dogs, was first reported in 1967 (Geary et al., 1967; Watson et al., 1986).
Craniocervical junction abnormalities are presumed to be heritable malformations. It has become apparent that some dogs with suspected AAI have other concurrent
12 abnormalities at the craniocervical junction. Because the occipital region of the skull and the first two cervical vertebrae develop together embryologically, it makes inherent sense that multiple developmental disorders, as well as combinations of these disorders, should occur in this anatomic region, as they do in humans (Dewey et al., 2013). Both Down’s syndrome (ligamentous laxity) and erosive rheumatoid arthritis (bony destruction) can cause atlantoaxial instability (McRorie et al., 1996; Menezes and Ryken, 1992). In Standard poodle dogs is presumed an autosomal way of inheritance as underlying factor to absence or hypoplasia of the dens (Stigen et al., 2013).
In a normal dog, head and neck work as a suspension mechanism allowing movements as the ventral flexion and dorsal extension. In order to maintain stability, the atlantoaxial joint counteracts the stress from the ventral movement of the head once it is the key of all forces that act downward (Lin and Coolman, 2009).
The atlantoaxial joint is a pivot joint that permits the head and the atlas to rotate around the longitudinal axis. The dens of the axis is the center of the rotation (Lin and Coolman, 2009). The dens projects rostrally into the bony canal of the atlas and articulates with the concave fovea dentis of the atlas. The transverse atlantal ligament functions to hold the dens against the ventral arch of the atlas. The atlantoaxial joint is also stabilized by the dorsal atlantoaxial ligament between the dorsal arches of the atlas and the axis, as well as by the apical ligaments connecting the dens to the ventral part of the foramen magnum and occipital condyles (Miller et al., 2013). Thus the dens of the axis plays an important role maintaining stability of the atlantoaxial joint by its attachments to the occipital bone with the apical and bilateral alar ligaments, and its binding to the floor of C1 with the transverse ligament of the atlas. An abnormality or absence of the ens represents a substantial reduction of the atlantoaxial joint stability (Vizcaino Reves et al., 2013).
AAI refers to excessive motion at the C1-C2 joint, may result from trauma, congenital and developmental anomalies, or a combination of the three (Beaver et al., 2000; Gilmore, 1984; Havig et al., 2005; Lin and Coolman, 2009; Schulz et al., 1997). AAI is usually due to hypoplasic or absent dens (Cerda-Gonzalez and Dewey, 2010; Dewey, 2013; Watson et al., 1986). Altough fracture or separation of the dens and failure of the ligaments due to either malformation or rupture can lead to AAI (Cerda-Gonzalez and Dewey, 2010; Dewey, 2013; Watson et al., 1986; Wheeler, 2005).
13 The transverse, apical, and alar ligaments, as the atlantoaxial joint capsule and dens, may all be involved in disease, but not all structures are abnormal in every affected dog (Watson and de Lahunta, 1989). Rupture of the transverse and alar ligament of the dens may cause instability and spinal cord compression in adult dogs (Middleton et al., 2012). However, the prevalence of primary ligamentous disease in dogs is unknown due to the lack of preoperative imaging. In one MRI study three dogs with AAI had elongated and thickened apical and alar ligaments in addition to a hypo-or aplastic dens (Middleton et al., 2012).
Several anatomic factors, congenital and developmental, have been reported to predispose small-breed dogs to this condition, including abnormalities of the physes of the dens with resultant aplasia, hypoplasia, dorsal angulation, or separation of the dens from the axis (Beaver et al., 2000; Havig et al., 2005; McCarthy et al., 1995; Platt et al., 2004). Congenital absence of the transverse ligament of the dens may also contribute to atlantoaxial joint instability (Havig et al., 2005; Platt et al., 2004).
Some authors also noticed that the dorsal arch of the atlas and the spine of the axis are thin and soft in most dogs with AAI (Beaver et al., 2000).
AAI is a well-recognized cause of cervical myelopathy in dogs (Havig et al., 2005). Although the subarachnoid spaces is relatively large in the cranial cervical region the space tapers acutely at the C2-C3 interspace. A congenital malformation of the dens or a fracture through the dens or cranial body of C2 allows excessive flexion of the joint and usually results in dorsal and caudal displacement of the cranial edge of the body of the axis due to the caudal retraction by the ligamentum nuchae (Gilmore, 1984; Lin and Coolman, 2009). This results in compression of the cranial cervical spinal cord at the C1-C2 interspace from the dorsally displayed cranial portion of the axis. Associated malformations of the atlas or occipital bones may be observed in some patients like AOO, basilar invagination, dorsal compression at the level of C1-C2 and Chiari-like malformations (Dewey, 2013).
3.3.1. Congenital and Degenerative
Congenital or developmental AAI typically affects immature toy breed dogs, however may occur in any breed of dog at any age (McCarthy et al., 1995). The congenital or development anomalies include aplasia, hypoplasia, dorsal angulation or
14 separation of the dens of the axis, nonunion of the dens with the axis body, absence of the transverse ligament of the atlas and abnormal shape and size (Denny et al., 1988; McCarthy et al., 1995; Platt et al., 2004; Sanders et al., 2004; Schulz et al., 1997; Watson and de Lahunta, 1989).
Table 2- Alterations of the dens of the axis (Aikawa et al., 2013; Beaver et al., 2000; Gilmore, 1984; Havig et al., 2005; Middleton et al., 2012; Platt et al., 2004; Stigen et al., 2013).
Number
of dogs Aplasia Hypoplasia Fragmented
Dorsally deviated Normal (Havig et al., 2005) 10 5 (50%) 3 (30%) 2 (20%) 0 0 (Gilmore, 1984) 4 1 (25%) 0 3 (75%) 0 0 (Middleton et al., 2012) 3 0 3 (100%) 0 0 0 (Platt et al., 2004) 19 7 (37%) 10 (53%) 0 2 (10%) 0 (Beaver et al., 2000) 46 21 (46%) 14 (30%) 0 0 11 (24%) (Stigen et al., 2013) 2 2 (100%) 0 0 0 0 (Aikawa et al., 2013) 49 16 (33%) 16 (33%) 3 (6%) 0 14 (28%) Total 133 52 (39%) 46 (35%) 8 (6%) 2 (2%) 25 (18%)
Most dogs have either absence of the dens (39%), hypoplasia of the dens (35%), 18% have normal dens and 6% a fragmented dens, only 2% have a dorsally deviated dens (table 2). The cause of these changes is unknown in the dens. Although the dens stem from two ossification centers, the congenital absence of a center of ossification was considered unlikely as a cause of hypoplasia or aplasia of the dens. It is proposed that a vascular-related ischemia may lead to postnatal resorption of at least the middle part of the dens, resulting in dens dysplasia (Watson and Stewart, 1990).
Congenital and developmental anomalies have been reported to contribute to AAI or predispose dogs and cats to AAI following minor trauma (Gage and Smallwood, 1970; Geary et al., 1967; Johnson and Hulse, 1989; Lin and Coolman, 2009; McCarthy et al., 1995; Oliver and Smallwood, 1970; Platt et al., 2004; Shelton et al., 1991; Swain and Greene, 1975; Watson and de Lahunta, 1989).
The instability leads to a dorsal displacement of the axis in relation to the atlas causing spinal cord compression. The degree of displacement and neural damage is related on the degree of laxity on the atlantoaxial joint and can be more severe if the
15 dens is intact (Denny et al., 1988; McCarthy et al., 1995; Watson and de Lahunta, 1989).
Congenital cervical vertebrae fusion is another condition that can contribute to AAI. In this situation the load is shifted to both ends of the fused segments, and when involving C2increases the lever force on the AA joint. The authors that first described 2 cases of congenital block vertebrae as cause of AAI also hypothesized that the altered biomechanics predisposed those dogs to AAI after minor trauma (Lin and Coolman, 2009).
3.3.2. Traumatic
Traumatic AAI results from forceful overflexion of the head, which may tear the ligaments or cause a fracture of the dens or dorsal arch of the axis (Lin and Coolman, 2009).
In a retrospective study 61% (28/46 dogs) had history of minor trauma (Beaver et al., 2000). Types of trauma include falling down the stairs, falling off furniture, dog-fight injuries, being dropped while held, running into the wall, and being grabbed by the neck (Havig et al., 2005).
A traumatic episode is usually associated with automobile trauma or dog fights and can result in fracture of the dens, even a minor trauma may result in AAI. The AAI permits a dorsal displacement of the axis into the vertebral canal, which results in compression of the spinal cord (Cook and Oliver, 1981; Gilmore, 1984; Havig et al., 2005; Platt et al., 2004; Schulz et al., 1997; Sherk, 1975). Additionally axial fractures mays result in spinal cord compression with varying degrees of neurologic dysfunction and cervical pain.(Gilmore, 1984) However, considerable impact may be required to cause such injuries in a normal atlantoaxial joint (McCarthy et al., 1995).
It is hypothesized that in clinically normal dogs prior to presentation ligamentous changes occur in an adaptive process to stabilize the atlantoaxial region and this mechanism may function until the dog goes some traumatic event to the cervical spine and can no
longer compensate the instability (Middleton et al., 2012). Trauma-related onset of clinical
signs in dogs can occur where congenital anomalies appear to predispose dogs to AAI and spinal cord injury (Lorinson et al., 1988; Platt et al., 2004). In those animals we do
16 not know if the ligaments are normal or abnormal prior to trauma particularly in those with normal bony structures (Middleton et al., 2012).
3.4. Clinical signs
History: Historical complaints for dogs with AA instability typically include neck
pain and varying degrees of ataxia of all four limbs. Nonambulatory tetraparesis and tetraplegia occur in severe cases (Dewey, 2013).
The most commons signs associated with AAI are gait dysfunction, ranging from general proprioceptive ataxia to tetraplegia, and neck pain (Beaver et al., 2000; Havig et al., 2005; Platt et al., 2004).
Clinical signs result from acute or chronic spinal cord compression because of instability at the atlantoaxial joint and reflect cervical spinal cord compression. Asymmetry of signs, or preferential involvement of either thoracic or pelvic limbs, may occur (Beaver et al., 2000; Geary et al., 1967; McCarthy et al., 1995; Wheeler, 2005). Neurological signs from mild cervical hyperesthesia and general proprioceptive ataxia (of all four limbs) to tetraplegia, respiratory compromise and death (Cook and Oliver, 1981; Dewey, 2013; McCarthy et al., 1995; Thomas et al., 1991; Watson et al., 1986). Tetraplegia is rarely encountered but if present the dog must be checked for respiratory failure (Beaver et al., 2000). Some dogs may also have evidence of paraspinal pain locations other than the cervical region, suggesting the presence of syringomyelia in those areas (Dewey, 2013). hydrocephalus has been reported in dogs with AAI and can cause forebrain signs (Denny et al., 1988). Another possible cause of forebrain signs in dogs with AAI is hepatic encephalopathy, which is over-represented in toy-breed dogs (Schulz et al., 1997). Forebrain signs like disorientation an behavior change, along with vestibular deficits, have been associated with basilar artery compression by the dens (Jaggy et al., 1991). Torticollis has been described with AAI possible due to syringohydromyelia or a vestibular sign secondary to high cervical lesion (Gibson et al., 1995; Johnson and Hulse, 1989; Mayhew, 1999).
Neck pain is evident from bouts of screaming and cervical muscle spasm and the adoption of a hunched posture (Stead et al., 1993). Neck pain typically is more severe with cranial cervical spinal cord lesions than with caudal lesions. Loss of motor function does occur with compressive cervical myelopathies but is less common, due to
17 the larger volume of the vertebral canal in cervical region. Patients with cranial cervical myelopathies tipically have more thoracic limb dysfunction (Dewey, 2013). Respiratory compromise could occur with damage of the descending tracts from the medullary respiratory centers that run the spinal cord to inervate the phrenic nerve lower motor neurons (C5-C7) and the lower motor neurons of intercostal muscles in the thoracic spinal cord. Hypotension can be associated with cervical myelopathies because tonic imput to the sympathetic lower motor neurons in the thoracolumbar spinal cord that maintain normal blood pressure is provided by neurons in the medulla (rostroventrolateral medulla) that send their processes through the cervical spinal cord (Dewey, 2013).
The clinical signs normally appear in the first 2 years of age with the majority being less than 1 year at the time of onset. However the clinical signs can appear after the 2 years of age (Beaver et al., 2000; Cerda-Gonzalez and Dewey, 2010; Denny et al., 1988; Havig et al., 2005; McCarthy et al., 1995; Platt et al., 2004; Thomas et al., 1991).
However clinical manifestations of craniocervical junction abnormalities are nonspecific and complicate the identification of the most clinically relevant disorder (Cerda-Gonzalez et al., 2009).
19
4. Diagnosis
“Diagnosis is not the end, but the beginning of practice.” Martin H. Fischer In any dog with the clinical signs described, particularly in those that are young and small-breed dogs, AAI should be considered as a potential diagnosis (Wheeler, 2005).
Neurologic signs that localize a lesion to cervical spinal cord segments C1 to C5 together with radiographic imaging of the cervical vertebral column are used to make a definitive diagnosis (Havig et al., 2005).
Cervical radiographs can provide a preliminary diagnosis of AAI indicating dorsal displacement of the axis to the atlas with an increase distance between the spinous process of the axis and the dorsal arch of the atlas and abnormalities of the dens. Sometimes the radiographs should be taken with a little flexion in order to see the dorsal displacement. Fluoroscopy can be used to minimize the degree of flexion needed to identify the instability. Anesthesia may be needed (McCarthy et al., 1995; Platt et al., 2004; Wheeler, 1992). However cervical radiographs cannot show the instability and they not show the degree of spinal cord compression (Cerda-Gonzalez et al., 2009).
Ligaments could not be thoroughly assessed using CT and MRI alone. Regardless of the cause of the instability, care should be taken when imaging dogs suspected of having craniocervical junction anomalies. Mild extension, neutral or mildly flexed positions should be used for short periods in these dogs as this may provide valuable information regarding the stability of the junction. Additionally, the positioning of dogs with an AAI within a neck brace with their head and neck in extension may not be advisable if AOO is also present (Cerda-Gonzalez et al., 2009).
In dogs with head and neck pain and/or cranial cervical myelopathy an evaluation of the entire craniocervical junction using CT and/or MRI is needed, even if AAI has already been identified, due to the possibility of more than one craniocervical junction anomaly, particularly if intracranial signs are present (Cerda-Gonzalez et al., 2009).
The optimal description of an individual patient’s craniocervical junction abnormalities depends on a combination of MRI and CT, this is particularly important for surgical planning (Marino et al., 2012). The morphologic description of a particular
20 patient’s craniocervical junction abnormality is far more important than the name ascribed to that malformation (Dewey et al., 2013).
It is possible that some cases of AAI in dogs are anologues of basilar invagination. Basilar invagination is a human craniocervical junction disorder in which the atlas and/or axis telescope towards the foramen magnum (Dewey et al., 2013).
A large number of small- and toy-breed dogs with dorsal spinal cord compression at atlantoaxial level appear to have a constrictive disorder similar to AOO. That compression varies in severity, with some dogs having a mild divot in the dorsal subarachnoid space, others having severe cervical spinal cord compression. At surgery the majority of that compressive mass appears to be soft tissue, although several had an obvious bony component. It is possible that this disorder may also involve instability at the atlantoaxial junction, and possible represent a form of basilar invagination like the AAO problem. That can occur as a sole entity or in combination with Chiari-like malformation and/or AAI (Cerda-Gonzalez and Dewey, 2010; Cerda-Gonzalez et al., 2009; Dewey et al., 2013).
Soft tissue abnormalities, such as absence of the transverse ligament, thick alar ligaments and a thickened atlantoaxial joint, have been diagnosed at necropsy, due to radiographic and CT imaging incapacity in provide good evaluation of this structures (Middleton et al., 2012; Watson and de Lahunta, 1989).
4.1. Physical exam
Cervical (neck) pain and varying degrees of tetraparesis are the principal findings on physical examination. In some cases paraspinal pain can be present in other locations thant the cervical region and can be suggestive of seryngomyelia (Dewey, 2013).
4.2. Neurological exam
Neurolocalization indicates a lesion between C1 and C5 and palpation of the neck often localizes the origin of the pain to the C1-C2 region. Is not recommended to flex the neck forcibly in a patient where AAI is a possible diagnosis, as this can worsen considerably the clinical condition of the patient (Wheeler, 2005).
21
4.3. Differential diagnosis
Differential diagnosis for atlantoaxial instability includes the conditions stated in Table 3.
Table 3 - Differential diagnosis for atlantoaxial subluxation (Gough, 2007; Wheeler et al., 2004). D Degenerative disc disease, cervical spondylomyelopathy, cervical fibrotic stenosis
A Atlantooccipital dysplasia, syringo(hydro)myelia, Spinal arachnoid cyst, vertebral malformations, meningo(myelo)celes, spinal dysraphism
M Exercise intolerance, lysosomal storage disease
N Neoplasia, hypervitaminosis A
I Discospondylitis, granulomatous meningoencephalomyelitis, calcinosis circumscripta, foreign body migration, inflammatory CNS disease, polyarthritis, polymyositis
T Fracture/luxation
V Ischaemic myelopathy, haematoma, fibrocatilaginous embolism, ascending myelomalacia, vascular malformation
D – degenerative; A – anomalous ; M – metabolic; N – nutritional, neoplastic; I – inflammatory, infectious, immune-mediated, iatrogenic, idiopathic; T – traumatic, toxic; V – vascular.
4.4. Laboratory analysis
CBC, CSF and biochemistry profile analysis typically result normal in cervical myelophathies or can be indicative of prior administration of glucocorticoids. In very young patients, serum alkaline phosphatase levels may be elevated due to bone isoenzyme (Aikawa et al., 2013; Dewey, 2013).
4.5. Radiographic findings
In most cases survey radiographs allows the diagnostic of AAI. Usually general anesthesia is required and caution should be taken with endotracheal intubation (Wheeler, 2005). However radiographic appearance of the dens is not predictive of surgical or conservative treatment success or final neurologic grade and could indicate that the disease involves ligament and spinal cord damage (Beaver et al., 2000; Havig et al., 2005; Middleton et al., 2012).
22 Ventrodorsal radiographic view is used to evaluate the dens for abnormalities such as aplasia, hypoplasia, and separation or fragmentation from the axis. Lateral radiographic views, in a neutral and cautiously flexed position, are used to evaluate dorsal angulation of the dens (Gilmore, 1984; Havig et al., 2005; Lin and Coolman, 2009). In most cases, AAI is apparent on lateral radiographs of the neck, in which dorsal deviation of C2 into the vertebral canal is usually evident. Stressed views may be used to demonstrate AAI, but hey must be performed with caution. Overzealous flexion of the neck to demonstrate instability of atlantoaxial joint space may have disastrous results (Cerda-Gonzalez and Dewey, 2010; Dewey, 2013). Oblique radiographs can also provide a good evaluation of the dens (Cook and Oliver, 1981). Fluoroscopic observation whith flexion of the neck can provide the dynamic nature of the lesion allowing the diagnosis and maintaining some protetive muscle tone (Wheeler, 2005).
Some dogs exhibit a normal dens radiographically but with AAI and they may not improve with conservative management (Lorinson et al., 1988). Dorsal displacement of the body of the axis and increased space between the dorsal arch of the atlas and the dorsal vertebral column of the axis is used as confirmation of AAI diagnosis (Havig et al., 2005; Lin and Coolman, 2009).
Radiographs have traditionally been used to assess for instability and bony malformations of the atlantoaxial region, but the ligaments and the joint capsule are not visible and CT imaging has the same limitation (Middleton et al., 2012). Furthermore, it is a common error to diagnose congenital AAI on radiographs of conscious dogs where the positioning is inadequate and the region of interest is far from the center of the film (Wheeler, 2005). Reason why, in cases of CJAs, MRI followed by CT imaging of the abnormal region at the craniocervical junction is often necessary to fully characterize the abnormal anatomy (Dewey, 2013).
4.6. Myelography
Myelography is used in some cases with an acute onset or with a history of trauma to rule out other possibilities of compressive disease (Platt et al., 2004), but should not be necessary for diagnosis. Reasons that advise against performing myelography are the possibility of post-myelographic seizures, risks associated with cerebello-medullary puncture either for injection of myelographic contrast or for CSF sampling and the
23 positioning/manipulation of the animal, being the lumbar puncture preferable (Wheeler, 2005).
4.7. Computerized Tomography
Ligaments and joint capsule could not be thoroughly assessed using CT and MRI alone (Cerda-Gonzalez et al., 2009; Middleton et al., 2012). CT images clearly delineate what bony structures are causing compression at the cervicomedullary junction (Dewey et al., 2013). CT is an excellent tool to evaluate bone conformation and reveal malformations/absence of the dens and for surgical planning (Johnson and Hulse, 1989).
4.8. Magnetic Resonance Imaging
Magnetic resonance imaging is both safer for this disorder and preferable to radiographs in that concurrent craniocervical junction abnormalities and syringomyelia will also be identified if present. Magnetic resonance imaging does not provide very good bony detail (Dewey, 2013).
Magnetic resonance imaging is suitable for evaluating the ligamentous structures of the canine occipitoatlantoaxial region, allowing identification of the apical, alar, transverse, and dorsal atlantoaxial ligaments from their origin to their insertion as well the synovial joint cavities (Table 4) (Middleton et al., 2012). Diagnosis of the absence or rupture of these ligaments can have an impact on understanding of the pathophysiologic mechanisms of instability and can affect therapeutic decision-making (Middleton et al., 2012).
Absence, stretching, or disruption of the transverse ligament may be an indication for early surgical intervention to prevent neurologic disease of the spinal cord (Dickman et al., 1991). Still ligaments could not be thoroughly assessed using CT and MRI alone (Cerda-Gonzalez et al., 2009).
24
Table 4 - Ligaments and MRI planes for visualization (Middleton et al., 2012).
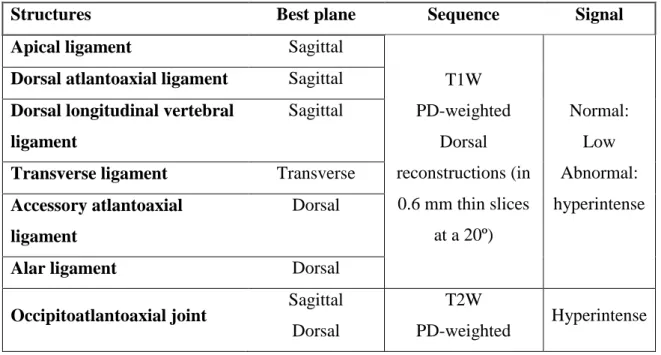
Structures Best plane Sequence Signal
Apical ligament Sagittal
T1W PD-weighted Dorsal reconstructions (in 0.6 mm thin slices at a 20º) Normal: Low Abnormal: hyperintense
Dorsal atlantoaxial ligament Sagittal
Dorsal longitudinal vertebral ligament
Sagittal
Transverse ligament Transverse
Accessory atlantoaxial ligament
Dorsal
Alar ligament Dorsal
Occipitoatlantoaxial joint Sagittal
Dorsal
T2W
PD-weighted Hyperintense
The sagittal plane best depicted the apical ligament, the dorsal atlantoaxial ligament, and the dorsal longitudinal vertebral ligament. The transverse ligament was best visualized in the transverse plane and appeared to be confluent with the dorsal longitudinal vertebral ligament dorsal to the dens in the sagittal plane. In three abnormal dogs the transverse ligament was not visible. The accessory atlantoaxial ligaments have been described in people using a 3 tesla magnet, in that study they only were visualized in 3/10 dogs and using a 20º dorsal scan. Customized dorsal imaging planes appear advantageous for identifying alar ligaments, however additional planes may likely be required in affected dogs if the spinal axis is deviated between the atlas and axis (Grabb et al., 1999; Middleton et al., 2012).
Sagittal T1W, transverse T1W, and PD-weighted, and dorsal plane reconstruction in 0.6 mm thin slices at a 20º angle were best for visualizating all ligaments that had low signal in all pulse sequences. The T2W and PD-wheighted sequences in sagittal and dorsal scan planes were ideal for visualization of the communicating occipitoatlantoaxial joint cavities due to high contrast between the hyperintense joint cavity an the hypointense bony countours. The use of fat saturation sequences may help to prevent confusion with injury. The T1W fat-satured pulse sequences may help to differentiate hyperintense synovial fluid from fat in T2W and T1W images, but did not improve visualization of ligaments themselves. Normal ligaments and membranes have a low signal in T1W and T2W pulse sequences. Abnormal ligament are recognized by
25 the extent of hyperintense MRI signal. Focal spinal cord signal changes representing either acute edema and hemorrhage or chronic gliosis and Wallerian degeneration at the second cervical vertebra can be visualized (Middleton et al., 2012).
MRI prior to treatment could help to determine that if ligamentous abnormalities or hyperintense spinal cord signal is present, and could help to determine the type of therapy required and possibly prognosis if there is extensive spinal cord malacia or seryngohydromielia (Sanders et al., 2004).
27
5. Prognosis
“Prognosis from the Greek πρόγνωση – literally foreknowing, foreseeing”
The prognosis for patients with AAI is fair to good if there are mild to moderate neurologic deficits, and guarded if the deficits are severe (e.g. tetraplegia) (Beaver et al., 2000; Cerda-Gonzalez and Dewey, 2010; Dewey, 2013).
The most important reported prognostic indicator in dogs with spinal cord injury is nociception. Loss of response to noxious stimuli is correlated to severity of spinal cord injury and a grave prognosis. Respiratory paralysis and death can occur due to the compression needed for loss of nociception in cervical spinal cord lesions. Therefore intact nociception is of little prognostic value for dogs with AAI (Oliver and Lewis, 1973).
Abnormalities of the dens are not associated with outcome. Radiographic appearance of the dens is not predictive of surgical or conservative treatment success or final neurologic grade and could indicate that the disease involves ligament and spinal cord damage (Beaver et al., 2000; Havig et al., 2005; Middleton et al., 2012). Objective inclusion criteria for radiographic evaluation of the dens and associated structures would be necessary to establish guidelines for prognoses made on the basis of specific defects (Havig et al., 2005).
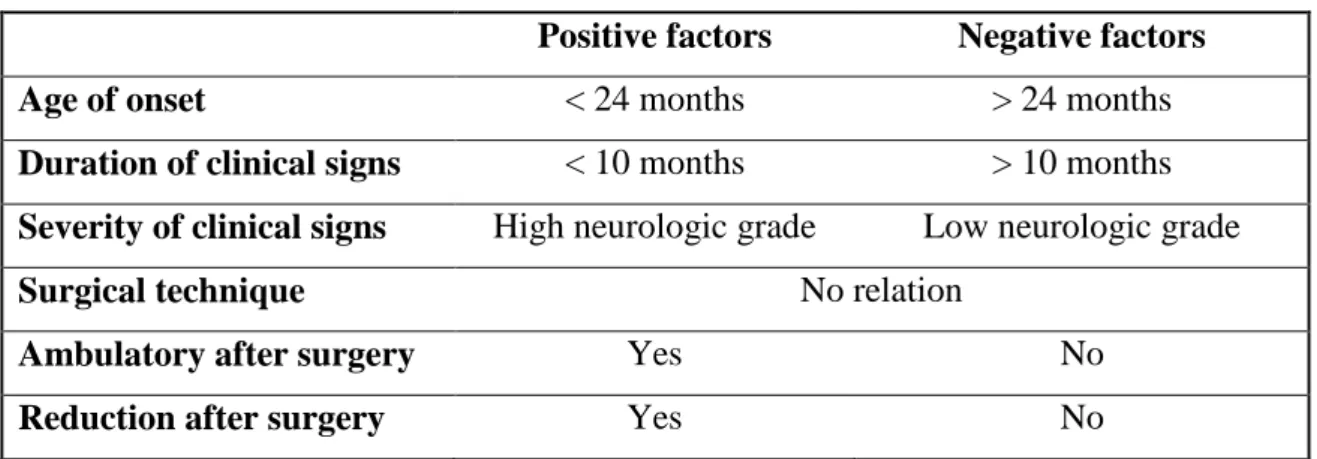
A good long-term outcome is associated with an acute duration of clinical signs. Age or a history of trauma are not correlated with long-term outcome. Final outcome (12 months after removal of the splint) for conservative treatment is not correlated with age and initial neurologic grade. Duration of clinical signs prior to admission is significantly associated with the final outcome, being the acute duration (< 1 month) of clinical signs associated with a better neurologic outcome. There is no difference in outcome on the basis of a history of trauma (Table 5). The final outcome in dogs treated with nonsurgical procedures is similar to those treated with surgical procedures to AAI. Final outcome for animals treated with cervical splint is good. In one study 10 of the 16 dogs treated with a cervical splint had a good long-term outcome (Havig et al., 2005).
28
Table 5 - Factors affecting conservative outcome in dogs (Havig et al., 2005).
Positive factors Negative factors
Duration of clinical signs < 1 month > 1 month
Age No relation
History of trauma No relation
Initial neurologic grade No relation
Surgical success rates for AAI vary in the literature from approximately 60% to 90%; however, most recent reports regarding surgical treatment of this disorder describe a surgical success rate exceeding 80% (Cerda-Gonzalez and Dewey, 2010; Dewey, 2013; Platt et al., 2004; Sanders et al., 2004).
Similar to what has been reported for nonsurgical management of AAI patients, length of clinical disease presence has been negatively associated with surgical success as well. Severity of neurologic dysfunction prior to surgery is also inversely related to outcome (Beaver et al., 2000; Cerda-Gonzalez and Dewey, 2010; Dewey, 2013), and a positive correlation has been reported between the initial and final neurologic grades in dogs treated surgically for AAI (Beaver et al., 2000).
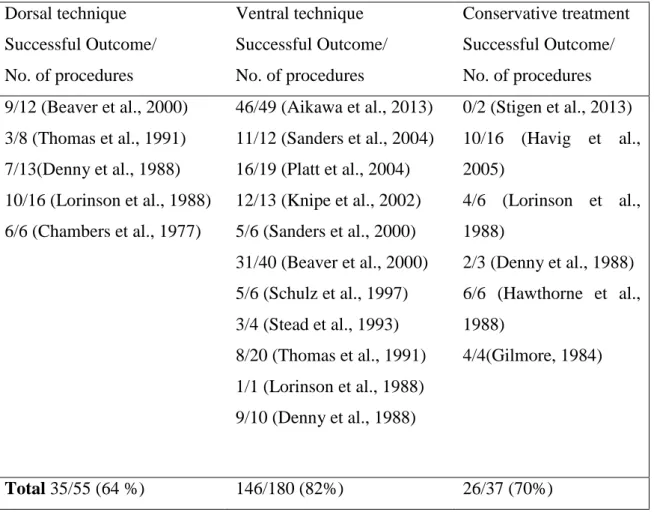
Retrospective studies of the surgical management of AAI have reported various success rates, ranging from 28% to 75% for dorsal procedures and 44% to 90% for ventral procedures (Beaver et al., 2000; Denny et al., 1988; Thomas et al., 1991). A higher success rate was reported for the ventral lag screw technique (McCarthy et al., 1995). However, when all dorsal stabilization techniques were combined and compared with all ventral stabilization techniques, no significant differences were seen in the overall outcomes with the ventral and dorsal stabilization methods (Lin and Coolman, 2009). The table 6 provides a summary of reported success rates in retrospective studies for dorsal stabilization, ventral stabilization, and conservative management.
29
Table 6 - Comparison of success rates of dorsal and ventral surgical treatment and conservative treatment reported in the veterinary literature (Aikawa et al., 2013; Beaver et al., 2000; Chambers et al., 1977; Denny et al., 1988; Gilmore, 1984; Havig et al., 2005; Hawthorne et al., 1988; Knipe et al., 2002; Lorinson et al., 1988; Platt et al., 2004; Sanders et al., 2004; Schulz et al., 1997; Stead et al., 1993; Stigen et al., 2013; Thomas et al., 1991).
Dorsal technique Successful Outcome/ No. of procedures Ventral technique Successful Outcome/ No. of procedures Conservative treatment Successful Outcome/ No. of procedures 9/12 (Beaver et al., 2000) 3/8 (Thomas et al., 1991) 7/13(Denny et al., 1988) 10/16 (Lorinson et al., 1988) 6/6 (Chambers et al., 1977) 46/49 (Aikawa et al., 2013) 11/12 (Sanders et al., 2004) 16/19 (Platt et al., 2004) 12/13 (Knipe et al., 2002) 5/6 (Sanders et al., 2000) 31/40 (Beaver et al., 2000) 5/6 (Schulz et al., 1997) 3/4 (Stead et al., 1993) 8/20 (Thomas et al., 1991) 1/1 (Lorinson et al., 1988) 9/10 (Denny et al., 1988) 0/2 (Stigen et al., 2013) 10/16 (Havig et al., 2005) 4/6 (Lorinson et al., 1988) 2/3 (Denny et al., 1988) 6/6 (Hawthorne et al., 1988) 4/4(Gilmore, 1984) Total 35/55 (64 %) 146/180 (82%) 26/37 (70%)
Based on literature review ventral surgical procedures have a higher success rate, conservative management and dorsal surgical procedures carry a lower success rate (Table 6). However final success rates of outcome for ventral procedures (85.3%) were comparable to dorsal procedures success rates (88.9%) in one study (Beaver et al., 2000). In another study overall success rate for ventral procedures was 84.2% (16/19 dogs) (Beaver et al., 2000; Denny et al., 1988; Havig et al., 2005; Lorinson et al., 1988; Platt et al., 2004; Thomas et al., 1991). Although severe neurological deficits in dogs with AAI indicate severe spinal cord damage, significant improvements in neurological signs have been observed after both surgical and conservative treatments (Beaver et al., 2000; Havig et al., 2005).