Jean-Marc Chobert
Milk protein tailoring to improve functional
and biological properties
Author’s address:
UR 1268, INRA Biopolymères Interactions Assemblages-équipe Fonctions et Interactions des Protéines, Nantes, France.
Correspondence: Jean-Marc Chobert
UR 1268, INRA Biopolymères Interactions Assemblages-équipe Fonctions et Interactions des Protéines, rue de la Géraudière, B.P. 71627, 44316 Nantes Cedex 03, France. Tel.: +33 2 4067 5230
e-mail: chobert@nantes.inra.fr
Article info:
Received: 07 November 2012 Accepted: 14 December 2012
ABSTRACT
Proteins are involved in every aspects of life: structure, motion, catalysis, recognition and regulation. Today’s highly sophisticated science of the modifications of proteins has ancient roots. The tailoring of proteins for food and medical uses precedes the beginning of what is called biochemistry. Chemical modification of proteins was pursued early in the twentieth century as an analytical procedure for side-chain amino acids. Later, methods were developed for specific inactivation of biologically active proteins and titration of their essential groups. Enzymatic modifications were mainly developed in the seventies when many more enzymes became economically available. Protein engineering has become a valuable tool for creating or improving proteins for practical use and has provided new insights into protein structure and function. The actual and potential use of milk proteins as food ingredients has been a popular topic for research over the past 40 years. With today’s sophisticated analytical, biochemical and biological research tools, the presence of compounds with biological activity has been demonstrated. Improvements in separation techniques and enzyme technology have enabled efficient and economic isolation and modification of milk proteins, which has made possible their use as functional foods, dietary supplements, nutraceuticals and medical foods. In this review, some chemical and enzymatic modifications of milk proteins are described, with particular focus on their functional and biological properties.
Key words: milk protein, modification, functional properties, biological properties
INTRODUCTION
The actual and potential use of milk proteins as food ingredients has been a popular topic for research over the past 40 years. Milk and dairy products have numerous advantages over competitors when used as ingredients: they are colorless, have a bland taste, are rather stable to processing, are free of toxins and have constituents that can be easily fractionated. As ingredients, dairy products are used mainly for their physico-chemical properties.
The effective utilization of proteins in food systems is dependent on tailoring the protein’s functional characteristics to meet the complex needs of the manufactured food products. Many food proteins require modification to improve such functional properties as solubility, foaming and emulsifying activity. Reviews on classical food protein
modifications for improved functionality are available in the literature (Means & Feeney, 1971; Feeney & Whitaker, 1977, 1982, 1986).
Functional properties of proteins are closely related to their size, structural conformation, and level and distribution of ionic charges. Chemical treatments that could cause alteration of these properties include reactions that either introduce a new functional group to the protein or remove a component part from the protein. Consequently, reactions such as acylation, phosphorylation, esterification, glycation, limited hydrolysis, and deamidation have been used to impart improved functional properties to the dairy proteins.
I. CHEMICAL MODIFICATION OF MILK
PROTEINS
Primary structure of proteins can be chemically modified in order to improve their functional properties. This approach has been used with success to study the structure-function relationships (enzymatic function, biological function, physico-chemical and functional properties). Deliberate
chemical modification of food proteins can result in alteration of the nutritive value, formation of potentially toxic amino acid derivatives, and contamination by toxic chemicals.
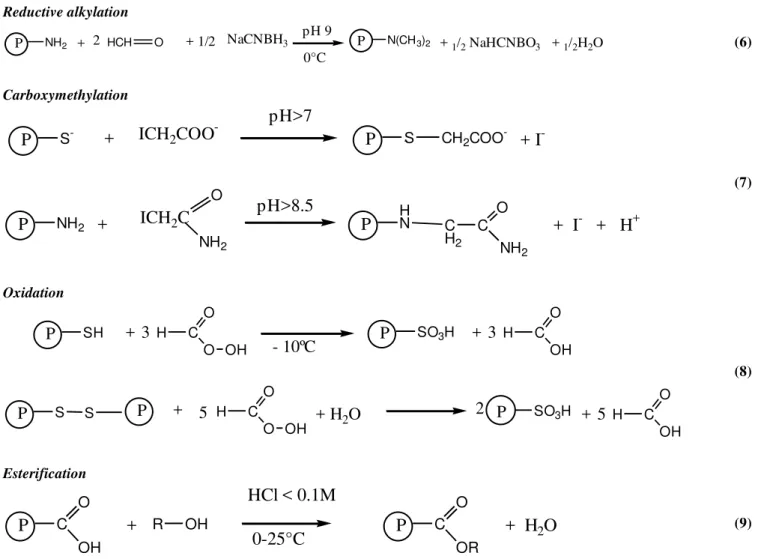
Alteration of amino acid residues can be obtained by heating at acid or alkaline pH. Main classes of reactions used to chemically modify the side chain of amino acids are acylation, alkylation, oxidation and reduction (Figure 1). Some of them are described in this chapter.
Acetylation
Succinylation
Carbamylation
Guanidination
Amidation
Figure 1.Main chemical modifications of food proteins (1 – 5).
P
NH2+
C CH3 O
C O
O
CH3
pH>7
P
NH C
CH3 O
+
CH3COO-+ H
+P
NH2+
P
NH C H2 C O H2C C
H2C C O O
O
pH
7.5-9.5
C
H2 COO
-+ H
+P
NH2+
P
NH C
NH2 O
pH>7
HN C OP
NH2+
P
NH C
NH2 NH2+
pH>9.5
C NH2
+
H2N
RO
+ ROH
P
COO-P
CpH-5
R NH2+
R N C N H+ R
O
NHR
P
CO
NH2
+
R NH2isopeptide
(1)
(2)
(3)
(4)
Reductive alkylation
Carboxymethylation
Oxidation
Esterification
Figure 1.Main chemical modifications of food proteins (6 – 9).
A. ESTERIFICATION
The esterification reaction is an important tool for modifying food proteins. Esterification with different alcohols leads to the blocking of free carboxyl groups raising thus the net positive charge, making the modified proteins more basic (Wilcox, 1967; Mattarella et al., 1983; Halpin & Richardson, 1985). The basicity of the modified protein depends on the degree of esterification and on the original content of basic amino acid residues on the protein molecules. This modification has shown its impact on the folding and peptic hydrolysis of -lactoglobulin (BLG) (Briand et al., 1995). While BLG is resistant to peptic hydrolysis in aqueous and physiological conditions, an ethylated BLG derivative was very susceptible to pepsin
hydrolysis in aqueous conditions. The esterified BLG exhibited 22 new sites of pepsin cleavage (Briand et al.,
1995; Chobert et al., 1995) as compared to the peptic peptides obtained when hydrolysis of native BLG was performed in hydro-ethanolic conditions (Dalgalarrondo et al., 1995). Hence, the reaction might be a good tool for the increase of peptic digestibility of food proteins or producing specific new unconventional peptides exhibiting novel activities. Moreover, the high isoelectric points of the esterified proteins might endow them with new physicochemical and functional properties (Mattarella & Richardson, 1983; Halpin & Richardson, 1985; Chobert et al., 1990).
Since the initial work of Fraenkel-Conrat & Olcott (1945), protein esterification has been described in a number P NH2 +
pH 9
P N(CH3)2 HCH O
2 + 1/2 NaCNBH3
0°C
+ 1/2 NaHCNBO3 + 1/2H2O
P
S-+
ICH
2COO
-
pH>7
P
S- CH2COO-+ I
-P
NH2+
ICH
2C
NH2 O
pH>8.5
P
HN CH2 C O
NH2
+ I
-+ H
+P
SH+
H CP
SO3HO
O OH
- 10ºC
3
+
H CO
OH
3
P
S H CO
O OH
5
+
+ H
2
O
2
P
SO3H+
H CO
OH
5
S
P
P
CO
OH
+
R OHHCl < 0.1M
0-25°C
P
CO
OR
+ H
2O
(6)
(7)
(8)
of studies (Mattarella et al., 1983; Chobert et al., 1990, 1995; Bertrand-Harb et al., 1991; Briand et al., 1994, 1995). The conventional procedure involves three steps. The first step is the mixing of reactants (protein, alcohol and acid). The second step is the esterification reaction itself, which generally ranges in length from one to several days, at 4°C. The last step is reaction termination and product recovery. The length of the reaction, which may reach 10-12 days, could limit the reproducibility and the applicability of this reaction. Additionally, the method used to recover the product at the end of the reaction can lead to side-reactions. In some procedures (e.g. Halpin & Richardson, 1985; Bertrand-Harb et al., 1991), the reaction medium at the end of the reaction time was combined with an equal volume of water, then dialyzed against water for several days, which can lead to de-esterification (Bertrand-Harb et al., 1991). Another way to recover the reaction product was by drying under vacuum (Briand et al., 1995).
Protons are essential catalysts of the esterification process, which will not proceed to any extent if they are absent in the reaction medium. Consequently, determining the optimum amount of protein catalyst required for the reaction is extremely important (Sitohy et al., 2000).
1. Factors influencing esterification. Study of BLG as a model
a. Influence of time-course of reaction. The reaction time-course (8, 16, 24, 48, 72 and 96 h) was followed in an esterification process using BLG (3% protein concentration) dispersed in 95% ethanol in the presence of 0.7 N HCl. Esterification extent increased gradually with the length of reaction to be 11, 14, 23, 28, 32 and 36%, respectively. It is obvious that under the conditions used, the esterification did not reach its maximum even after 96 h of reaction. Consequently, in order to increase the extent of modification after shorter reaction times, conditions should be modified drastically. For example, protein and acid concentrations can be increased. The length of reaction is alcohol-dependent since it was observed that esterification with methanol reached its maximum within 24 h by using less concentrated solutions.
b. Influence of temperature. Comparative experiments (2% protein, 0.7 N HCl final concentration) were carried out esterifying BLG with 99.7% ethanol at three different temperatures (4, 10 and 20°C). The reaction was stopped after 8 h. The extent of esterification increased with the increase of temperature, being 2, 4 and 8% at 4, 10 and 20°C,
respectively. However, the products obtained in higher reaction temperatures developed violet coloration after drying showing that high temperatures may induce side-reactions. Wilcox (1967) stated that higher temperatures might give insoluble esterified products. Consequently, although higher temperatures may increase the reaction rates they should not be used in order to avoid undesired side-reactions.
c. Influence of the type of alcohols. The comparison of the different alcohols used for esterification showed that methanol is the most reactive and can esterify most of the carboxyl groups of BLG, leading to a maximum esterification extent of 97% when traces or 1% water is present in the starting alcohol. The final amount of water in the reaction medium depends also on its quantity contained in the added acid.
The difference between reactivity of alcohols agrees with the results of Fraenkel-Conrat & Olcott (1945) and Halpin & Richardson (1985) who found that methanol was most reactive as an esterifying agent followed by ethanol then n-butanol. Matarella et al. (1983) found that in methanol the esterification reaction progressed quicker than in ethanol, while there was no evidence for esterification in propanol, butanol or pentanol even after one week reaction time. The reason for such a difference according to the alcohols used is due to their different polarity and structural hindrance, which is especially evident in iso-propanol.
2. Application to -lactalbumin (ALA)
SDS-PAGE patterns of esterified ALA did not differ from those observed for native protein, indicating no sign of hydrolysis or polymerization. Hence, the conditions used for esterification did not affect the molecular size of the modified protein.
3. Application to -casein (BCN)
Extent of esterification of -casein (BCN) increased gradually parallel to the increase in the molar ratio of HCl (Sitohy et al., 2001a). As observed with BLG, methanol achieved the highest rates of esterification. However, propanol was more efficient than ethanol, which was the opposite of the results obtained with BLG. This discrepancy might be due to conformational differences between these two proteins.
The relatively low extents of esterification obtained using ethanol and propanol could be due to the following factors: 1. Low polarity of the reaction medium; 2. Inadequate protein concentration; 3. Insufficient amount of catalyst HCl.
In order to increase the polarity of the medium, ethanol and propanol were used at a concentration of 95% during esterification of 5% BCN using molar ratio of 30, 40 and 50. The extent of esterification decreased considerably (about 50% decrease) when compared with the values obtained in the presence of >99.5% alcohol. Although water might be needed to enhance the polarity of the reaction medium, it should not exceed a certain limit.
The role of protein concentration was examined by esterifying BCN for 6 h with 99.7% ethanol and 99.5% propanol using a molar ratio of 70 and protein concentrations of 3, 4 and 5%. The respective extents of esterification were 26, 45 and 56% with ethanol and 36, 49 and 54% with propanol indicating that a protein concentration of 5% was the most efficient as was observed with BLG.
In order to examine the effectiveness of the third important factor playing a role in esterification yield, BCN (5%) was esterified using higher molar ratio (60, 70 and 80). The modified product was recovered either by centrifugation or by filtration under vacuum. The extent of esterification could be increased up to 59 and 56% in the presence of ethanol and propanol, respectively, when using a molar ratio of 80 and when the resulting product was recovered by centrifugation. These values were slightly lower (51 and 53%) when the modified product was recovered by filtration. Since centrifugation is more time consuming than filtration under vacuum, and leaves the reactants in contact for an additional period, this may be one of the causes of a higher
esterification yield. However, the use of filtration under vacuum may have the advantage of reducing the time and energy costs associated with freeze-drying (3–4 days).
4. Factors influencing pepsinolysis of ester derivatives of BLG
5. Improvement of functional properties of milk proteins by esterification
Milk proteins have good inherent functionalities as well as high nutritional values. Hence, they are used as ingredients in a wide range of food products (Huffman 1996; McCrae et al., 1999). Due to its amphiphilic properties, BCN is one of the most surface-active milk proteins (Mitchell et al., 1970; Benjamin et al., 1975). However, because of the acid isoionic points of milk proteins, their functional properties are worst in that range of pH, when compared to the alkaline range. Adsorption of BLG to fat globules increases with increased pH, exhibiting good emulsifying activity in the alkaline pH range (Yamauchi et al., 1980, Nagasawa et al., 1996, Hattori et al., 1997). Adsorption of BLG on the oil/water interfacial layer is strongly reduced with decreasing pH reaching 61.6 and 12.9% at pH 9 and 3, respectively. This can be attributed to pH-dependent structural changes since BLG molecules are more rigid at acid pH (Shimizu et al., 1981, 1985; McCrae et al., 1999). Generally, the most important factors determining the emulsifying properties of whey proteins are protein concentration, pH, ionic strength and history of processing and storage conditions. Emulsifying activity depends also on the flexibility of protein molecules since flexible molecules such as caseins can spread over the oil-water interface in contrast to more rigid proteins such as the whey globulins (Hunt & Dalgleish, 1994). When BLG adsorbs at an oil-water interface, it denatures partially exposing its highly reactive free sulfhydryl groups (Corredig & Dalgleish, 1995), which can then interact with similar groups of neighboring molecules forming polymers. The second major whey protein, ALA, was found to inhibit BLG polymerization (Dickinson & Matsumura, 1991, Monahan et al., 1993). Hence, the ability of a protein molecule to form and stabilize oil droplets is closely linked to the structure and conformation of the molecule (Das & Kinsella, 1990).
Since good solubility and emulsification properties at acidic pHs are required for some food applications such as acid soft drinks and acid foods, modification of proteins improving their functional properties in this pH range may be required. Esterification of proteins was proved to block the protein negative charges increasing thus the net positive charge and raising the protein isoionic points. This should render the modified proteins more soluble and likely much more tensio-active in the acidic range of pH. The isoionic point of BLG was raised from 5.2 in its native state to 6.2, 8.7 and 9.8 after its esterification with butanol, ethanol and methanol, respectively (Mattarella & Richardson, 1983;
Halpin & Richardson, 1985). Similar features were observed during esterification of BCN (Chobert et al., 1990). The magnitude of the change in the isoionic point values depends on the degree of esterification (Chobert et al., 1990; Bertrand-Harb et al., 1991; Sitohy et al., 2000). Alternatively, methyl and ethyl esterification of BLG resulted in randomized protein structures (Mattarella et al., 1983). Generally, partial denaturation of proteins results in increased surface activity compared to the native globular forms (Mitchell et al., 1970). Hence, esterified BLGs were more efficient in lowering the interfacial tension at the oil/water interface as a result of increased surface hydrophobicity (Halpin & Richardson, 1985). It was reported also that emulsifying activity of esterified BLGs is worse than that of the native form (Mattarella & Richardson, 1983; Chobert et al., 1990). This discrepancy might be due to differences in pH or any other limiting factors used in each study. In previous studies the change of emulsifying activity of esterified proteins was followed by measurement of turbidity according to the method of Pearce & Kinsella (1978). Since the oil droplet size may better reflect emulsifying activity, Sitohy et al. (2001b) measured the change of this parameter in order to elucidate the changes in emulsifying activity and stability of different esterified milk proteins in the acidic and neutral ranges of pH. This yielded information on the suitability of using esterified proteins as ingredients in acid foods or to fortify acid soft drinks. Moreover, it was reported that oil droplet size influences the organoleptic perception of food emulsions and that the interfacial layer surrounding the oil droplet may be a determinant factor controlling the stability of the emulsion towards flocculation and coalescence (Mine, 1998; Aluko & Mine, 1999; Le Denmat et al., 1999).
groups. The overall protein solubility is a result of the interplay between these two effects. The first effect is more efficient in the case of the most esterified derivatives and when the grafted ester group is of a less hydrophobic nature (e.g. methyl). The second effect is more prominent in case of derivatives with a low degree of esterification and when the grafted ester groups are more hydrophobic (e.g. propyl). This may explain the reduced solubility reported in case of some esterified proteins at acid pHs (Mattarella & Richardson, 1983; Chobert et al., 1990).
ALA methyl ester was more soluble in the acidic range of pH and less soluble in the alkaline range of pH as compared to unmodified protein. The low methylated sample (38%) showed two regions with a minimum of solubility (pH 5.6 and 6.8) compared to pH 7.2 and 8.8 for the 52% methylated derivative. Similarly, the highly ethylated ALA sample (36%) showed improved solubility in acid conditions and decreased solubility in the alkaline range of pH. The solubility of the low ethylated derivative (11%) was improved whatever the pH. The solubility curves of ALA propyl esters were similar to that of ethyl derivatives. However, the reduction of solubility of propyl-esterified derivatives was more pronounced when compared to ALA ethyl esters showing solubility levels of 47-50% and 67-70%, respectively. As observed with BLG ester derivatives, the nature of the grafted ester group is an important factor for the solubility of the modified ALA. Comparison of solubility of ALA propyl esters with that of BLG propyl esters shows a different behavior for these two families of esters. While grafted propyl ester groups decrease solubility of BLG in the acidic pH range, they improve the solubility of ALA. Consequently, the conformation of the esterified protein might play an important role on the solubility of the protein. Hence, it is not only the nature of the grafted ester group that determines the solubility of the modified protein but also the nature of the protein itself.
As observed with BLG and ALA, the isoelectric points of esterified BCN shifted towards the alkaline pH range. The magnitude of this shift was proportional to the extent of esterification. BCN methyl esters being the more esterified samples (60, 88 and 100% esterification) showed higher magnitudes of isoelectric point shift compared with other types of esters. The decrease of solubility in the alkaline pH range (7-9) was more evident for BCN methyl esters, giving a minimum of solubility as low as 20% for the highly esterified samples (88 and 100% esterified). The minimum of solubility of BCN esters is mainly observed for one pH value or one pH
range in contrast to two minima previously observed with BLG and ALA. This may be because the flexible structure of BCN makes the alcoholic groups more accessible to nucleophilic attack, giving rise to more homogeneous esterified populations. The hydrophobic core of BLG is certainly more resistant to the nucleophilic attack of the alcoholic groups at the beginning of the reaction, becoming only accessible with the progress of esterification or at higher protein concentrations giving rise to different molecular populations.
Comparison of methyl and ethyl esters of BCN shows a more important shift of isoelectric point for the methyl derivative due to the higher extent of modification. Moreover, the solubility of BCN methyl esters is smaller in the alkaline range of pH (7-9) due also to the high extent of modification. However, although ethyl and propyl esters of BCN displayed similar extents of substitution (~20 - ~60%) and had a similar shift of isoelectric points, the propyl ester derivatives were less soluble in the alkaline pH range than the ethyl ester derivatives probably because of the more hydrophobic nature of grafted propyl groups. It can be concluded that the solubility was improved only between pH 4 and 5. This property might find some practical application when using esterified proteins as additives for beverages instead of protein hydrolysates that often have a bitter taste.
Comparison of the solubility of all three esterified proteins showed that the magnitude of the shift in the isoelectric points decreased in the following order:
BLG > ALA > BCN.
Emulsifying properties of esterified milk proteins were also studied in the pH-range of 3-7 by measuring the oil-droplet size using laser light scattering. Emulsifying activity and stability of esterified milk proteins in the acidic pH range of 3-5 were generally higher compared to the corresponding native proteins. This improvement was associated with the degree of solubility, the degree of esterification, the type of ester group grafted and the nature of the used protein. The highest emulsifying activity improvement was observed for methyl ester derivatives at pH 5 where the native proteins have poor emulsifying properties (Sitohy et al., 2001b).
6. Change in biological properties. Interactions between esterified milk proteins and nucleic acids. Inhibition of virus
change the result of the reaction seen by electrophoresis, even at excessive ratios of basic amino acids in proteins:phosphate groups in DNA as high as 100:1. Addition of esterified proteins greatly reduced the intensity of the bands corresponding to the newly synthesized DNA, at ratios as low as 1:1 and 5:1 in case of BLG and methylated-ALA, respectively. The inhibitory effect of esterified proteins was directly proportional to their extent of esterification and strongly related to their DNA-binding capacity. Generally, inhibition of PCR with esterified proteins was similar to what can be observed with histones. However, stronger inhibition was observed with highly esterified proteins when using a higher ratio of basic:acid residues (1:1) when compared to 0.5:1 ratio in case of histones. Highly esterified BCN did not exert any inhibitory effect because of its relatively lower pI when compared to that of other esterified milk proteins and due to its lower positive net charge at the pH used for PCR. During a second PCR run, only the addition of new DNA template was able to reinitiate the reaction, giving rise to new synthesized DNA. Addition of Taq DNA polymerase did not enhance DNA synthesis, showing that inhibition was performed only by binding of DNA template and not by the inhibition of the polymerase.
Herpes simplex virus (HSV) causes a wide spectrum of clinical manifestations in the central nervous system of infants (encephalitis with or without disseminated visceral infection) and adults. Herpes simplex virus type 1 (HSV-1) is a large enveloped DNA virus replicating in the nuclei of mammalian cells. It is a significant human pathogen causing cutaneous lesions primarily in the oral mucosa but also in other sites. More serious manifestations of HSV-1 infection include encephalitis and blinding keratitis. Nucleoside analogues of guanine e.g. acyclovir (ACV) have been used for therapeutic use against HSV.
Antiviral activity of methylated ALA, methylated and ethylated BLGs was evaluated against sensitive and resistant strains of HSV-1 and compared to that of acyclovir and polylysine (4-15 kDa) using fixed or suspended Vero cell lines. Esterified proteins and their peptic hydrolysates displayed protective action against sensitive HSV-1, which was relatively lower than that induced by acyclovir or polylysine. Higher activity of polylysine was maintained against a resistant strain of HSV-1 while acyclovir lost much of its activity. The mean 50% inhibitory concentration (IC50)
was about 0.8-0.9 µg/ml for polylysine against two strains of HSV-1 when using two concentrations of virus (50 and 100% cytopathic effect, CPE). IC50 values of acyclovir against the
sensitive strain of HSV-1 were of 3 and 15 µg/ml when using the low and high concentrations of virus, respectively. When using the lower concentration of virus (50% CPE), IC50
values for esterified milk proteins ranged from 20 to 95 µg/ml, depending on the nature of the ester group, the degree of esterification, and the nature of the protein. Esterified milk proteins were active against both sensitive and resistant strains of HSV-1. By using the real-time PCR technique, it was shown that methylated ALA inhibited the HSV-1 replication (Sitohy et al., 2007).
The enteroviruses, RNA viruses of the Picornaviridae
family, are ubiquitous pathogens, which include more than 70 different serotypes (3 of which are polio serotypes) infecting people of all ages. Enteroviruses can produce severe and sometimes fatal illnesses such as meningitis, encephalitis, myocarditis, neonatal sepsis and polio. Enteroviruses belong to the group of enteric viruses, which are excreted in feces of infected individuals and may directly contaminate drinking water supplies. Numerous studies described the presence of enteroviruses in raw and in inadequately treated drinking water, waste waters and sludges. Enteroviruses create a public health risk as they can be transmitted via the fecal-oral route through contaminated water, initiating severe infections in humans.
Poliovirus is a small positive strand RNA virus replicating in the human oropharynx and extending to the central nervous system in 1 to 4% of cases.
Adenoviruses present as great public health hazard as do enteroviruses. Both contaminate water and cause epidemics. Adenoviruses are an important cause of morbidity and mortality in the immuno-compromised hosts. There are no approved antiviral agents with proven efficiency for treatment of severe adenovirus diseases.
Coxsackie B viruses (CVB) have been associated with such pathologies as aseptic meningitis, non-specific febrile illness and myocarditis (Morens & Pallansch, 1995). Most CVB infections are asymptomatic, but different organs may be affected (e.g. myocardium, pancreas). Some acute and persistent CVB infections are particularly severe in infants (Woodruff, 1980).
The antiviral activity of esterified whey proteins (IC50 =
medium after 24 h of incubation with new medium containing the same amount of esterified proteins enhanced the anti Coxsackie virus effect. Difference between start of infection of the reporter Vero cells and the time of addition of esterified proteins decreased significantly their antiviral efficacies. The poliovirus titer reduced gradually in response to the increase of the concentrations of esterified proteins. The relative amounts of viral RNA were less affected by the esterified proteins concentration than was the virus infectious titer, indicating that inhibitory proteins may slow down viral multiplication yielding reduced amounts of viral RNA (Sitohy et al., 2008).
The extent of inhibition of poliovirus and Coxsackie virus was related to the degree of cationisation of esterified whey proteins as well as to the size of the backbone protein. Hence, the results obtained in this study agree well with previous results obtained during studies of antiviral action of other cationic proteins.
In this preliminary study, the mechanism of antiviral activity of esterified whey proteins could be due to:
1- Saturating binding to viral RNA by purely coulombic interactions, inhibiting its replication and transcription;
2- Hydrophobic interactions with viral capsid proteins; 3- Perturbation of viral RNA–protein interactions, hence inhibition of the translation of viral proteins;
4- Interference with/saturation of viral entry sites on the cellular membranes.
However, whichever effect is prevalent esterified whey proteins may present several advantages over other cationic proteins described by other authors: they are available in large quantities and simplicity of their modification favors them as cheap and efficient antiviral agents that may be used with success for the disinfection of infected solid surfaces and liquids. Their susceptibility to proteases can be an additional asset allowing their rapid recycling in the environment (Chobert & Haertlé, 2003).
In the last 100 years there have been 3 major influenza pandemics: Spanish Flu in 1918, Asian Flu in 1957 and Hong Kong Flu in 1968. These claimed the lives of approximately 50 million, 2 million and 1 million people, respectively. Added to this is the annual death toll of 250,000 to 500,000 people worldwide with a further 3 to 4 million people suffering severe illness. In 1997 the alarming emergence of a new, highly pathogenic subtype, H5N1, which has a 50% mortality rate, provided a major impetus for renewed influenza research. Re-emergence of fatal human influenza A
subtype H5N1 disease was reviewed (Peiris et al., 2004). At the same time another subtype, H1N1, has emerged (Neumann et al., 2009). This latter subtype causes a relatively mild infection in humans, however it is highly transmittable between people and a new influenza pandemic was declared by the World Health Organization. Development of effective new drugs for the treatment and/or prevention of epidemics and pandemics of influenza is a major sanitary objective.
Addition of methylated BLG (Met-BLG) in the medium of MDCK cell lines infected with influenza virus subtype H1N1 reduced hemagglutination activity (HA) in a concentration dependent manner. Antiviral activity of Met-BLG depended on its concentration, viral load, and duration of infection. Using 17 µg/ml of Met-BLG inhibited 50% of HA of H1N1 grown in MDCK cells at 1 MOI after 24 h of incubation at 37°C and in 5% CO2. Extension of incubation
time enhanced antiviral action since the same concentration of Met-BLG inhibited about 61% of viral activity after 48 h. This viral inhibition was accompanied by a protection of MDCK cells as observed by using neutral red or by direct microscope examination. Reduction of viral RNA replication upon the addition of Met-BLG (50 µg/ml) was observed by real time-PCR showing a reduction of viral log value of about 0.9. When viral stock solution was mixed with 25 µg/ml Met -BLG in absence of cell lines, the morphology and viability of virus particles were significantly affected as observed by electron microscopy, and the number of intact virus particles was reduced by roughly 65% (Sitohy et al., 2010).
B. GLYCATION OF BLG USING MILD CONDITIONS
The non-enzymatic browning or Maillard reactions are of great importance in food manufacturing. This reaction was first described by the French biochemist Louis Camille Maillard at the beginning of the 20th century (Maillard,
1912). The browning reaction can be defined as the sequence of events that begins with the reaction of the amino group of amino acids, peptides, or proteins with a glycosidic hydroxyl group of sugars; the sequence terminates with the formation of brown nitrogenous polymers or melanoidins (Ellis, 1959; O'Brien & Morrissey, 1989; Ames, 1990; Friedman, 1996; Chuyen, 1998). This is also one of the principal pathways of final degradation of organic matter in nature.
accompanied by a reduction of the nutritive value of different foods and by the formation of toxic compounds harmful for human health (Ledl & Schleicher 1990). Results of non-enzymatic browning can be either desirable or undesirable. The brown crust formation on bread is desirable; the brown discoloration of evaporated and sterilized milk is undesirable. For products in which the browning reaction is favorable, the resulting color and flavor characteristics are generally experienced as pleasant. In other products, color and flavor may become quite unpleasant.
The reaction velocity and pattern are influenced by the nature of the amino acid or protein and carbohydrate involved in the reaction. Generally, lysyl residue is the most reactive amino acid because of the free -amino group. Reaction of the -amino group of lysyl residues of proteins with reducing sugar results in the so-called “Amadori product” via the formation of a Schiff’s base and the Amadori rearrangement (Ledl & Schleicher, 1990; Friedman, 1996). Through this reaction, the conjugation of the sugar to the protein does not require chemical catalysis, but just heating in order to accelerate the spontaneous reaction. A well-controlled Maillard reaction can thus be a good method for protein processing in the food industry. Since lysine is the limiting essential amino acid in many food proteins, its destruction can reduce the nutritional value of proteins. Foods that are rich in reducing sugars are very reactive and this explains why lysine in milk is destroyed more easily than in other foods (de Man, 1999).
Study and characterization of the Maillard reaction products could allow one to control the formation of Advanced Maillard Products (AMP), responsible in part for noxious effects in diabetes and in age-related cardiovascular diseases (Al Abed et al., 1999). This knowledge could lead to a better control of these reactions in order to modify food proteins and their functions. Maillard reactions are one of the simplest ways to modify food proteins since they take place when a protein and a sugar are just heated together (Chuyen, 1998).
BLG is the major whey protein. It is present in the milk of various ruminant species (Godovac-Zimmermann et al., 1990a, b; Sawyer & Holt, 1993; Ochirkhuyag et al., 1998). This protein constitutes a major waste product of the cheese industry. At the end of the 90’s, its uses increased as a food additive thanks to its good nutritional properties (Smithers et al., 1996). Consequently, the improvement of BLG functional properties may be of considerable interest to industry.
To improve their functional and physicochemical
properties, dairy proteins have been modified by several methods (Haertlé & Chobert, 1999), such as phosphorylation (Sitohy et al., 1995c), esterification (Chobert et al., 1995, Sitohy et al., 2001b), alkylation (Kitabatake et al., 1985), and reductive amidation (Mattarella et al., 1983); (see Section I.). However, most of these methods, using toxic chemicals, cannot be used in food processing.
BLG showed better emulsifying properties after glycation with glucose-6-P (Aoki et al., 1997) and better heat stability and solubility after glycation with glucose, mannose or galactose (Nacka et al., 1988). Study of the Maillard reactions between BLG and lactose revealed the presence of
-lactulosyllysine (Jones et al., 1998), a unique lactosylation site in the early step of the reaction (Léonil et al., 1997; Fogliano et al., 1998) and heterogeneity of protein glycoforms (Morgan et al., 1997).
1. Reaction conditions. Glycation in the presence of different sugars
Protein polymerization and glycation site specificity were investigated according to the nature of sugar used for modification of BLG (Chevalier et al., 2001c). Among the six common sugars used, arabinose and ribose induced the highest degree of modification. Glucose, galactose and rhamnose were less reactive and lactose generated the lowest degree of modification. Proteins substituted with ribose or arabinose formed polymers stabilized by sugar-induced covalent bonds. When other sugars were used, part of the aggregated proteins were stabilized only by hydrophobic interaction and disulfide bonds. According to mass spectrometry analysis, leucine 1 (N-terminal amino acid), lysine 14 and lysine 47 were modified in the presence of galactose, glucose or lactose. Lysines 69, 75 and 135 were modified only in the case of protein glycated with glucose. Lysine 100 was modified only when protein was glycated with lactose. No glycation site could be detected for proteins glycated with ribose or arabinose due to the higher degree of modification, which inhibited the tryptic hydrolysis used before mass spectrometry analysis.
2. Change in protein structure
polymerization of BLG after heating. When BLG was heated in the presence of sugars, larger structural modifications were observed depending on the sugar used (Chevalier et al., 2002). Conformational modification of BLG was related to the degree of glycation. The more reactive the sugar was, the more denatured was the glycated protein. According to peptic hydrolysis data, near- and far- UV Circular Dichroism spectra and micro-calorimetry analysis, proteins modified with ribose or arabinose (the most reactive sugars) showed important conformational changes. In contrast, proteins modified with lactose or rhamnose (the less reactive sugars) had similar three-dimensional structures to native BLG. According to Chevalier et al. (2001a,c), glycation of BLG induced polymerization of protein monomers. Moreover, as observed by calorimetry analysis, Maillard glycation increased the temperature of denaturation of proteins glycated with galactose, glucose, lactose or rhamnose. These results correlated well with those obtained in a previous study of the functional properties of glycated BLG (Chevalier et al., 2001c; see below), which demonstrated the importance of the sugar used on the improvement of emulsifying and foaming properties of the derivatives.
3. Change in functional properties
The whey produced during cheese and casein manufacturing contains approximately 20% of all milk proteins. It represents a rich and varied mixture of secreted proteins with wide-ranging chemical, physical and functional properties (Smithers et al., 1996). Due to their beneficial functional properties, whey proteins are used as ingredients in many industrial food products (Cheftel & Lorient, 1982). According to Kinsella & Whitehead (1989), functional properties of foods can be explained by the relation of the intrinsic properties of the proteins (amino acid composition and disposition, flexibility, net charge, molecular size, conformation, hydrophobicity, etc.), and various extrinsic factors (method of preparation and storage, temperature, pH, modification process, etc.).
Numerous attempts were made to improve the functional properties of whey proteins through physical, chemical and/or enzymatic treatments (Haertlé & Chobert, 1999). Many studies were carried out with BLG, the major whey protein. Focusing on the improvement of solubility, heat stability, foaming properties and emulsifying properties, this protein has been conjugated with ester (Mattarella & Richardson, 1983; Sitohy et al., 2001b), gluconic or melibionic acids (Kitabatake et al., 1985), carbohydrates
(Waniska & Kinsella, 1988; Bertrand-Harb et al., 1990) and phosphoric acid (Sitohy et al., 1995a-c).
However, most of these methods utilize toxic chemicals and are not permitted for potential industrial applications. Some attempts were made to improve the functional properties of BLG by conjugation with glucose-6-phosphate (Aoki et al. 1997).
The functional properties (solubility, heat stability, emulsifying and foaming properties) of BLG after glycation of the protein with several sugars (arabinose, galactose, glucose, lactose, rhamnose or ribose) were studied (Chevalier et al., 2001d). Protein samples were heated in the presence or in the absence (heated control) of different sugars for three days at 60°C. Subsequent glycation induced a modification of the solubility profile, shifting the minimum solubility towards more acidic pH. Native BLG was soluble over the whole pH range studied. After heating, a 50% decrease of solubility was observed in the pH range 4.0 - 5.5 with a minimum observed at pH 5, which is near the pI of the protein. After heating BLG in the presence of arabinose or ribose, the resulting glycated derivatives exhibited 35% solubility at pH 4, which is lower than the solubility obtained with heated BLG and BLG glycated with the other sugars. Major conformational modification induced by the glycation could explain such a decrease. However, due to the shift of their isoelectric point, these derivatives showed an increased solubility at pH 5 as compared with heated BLG. In the case of proteins modified with galactose, glucose, lactose or rhamnose, 75% solubility was observed at pH 4.5, showing a protective effect of glycation by these sugars against the decrease of solubility due to heating.
Glycated proteins exhibited a better thermal stability when heated at pH 5 as compared to native or heated control.
Glycation of BLG with arabinose or ribose (the most reactive sugars) improved its emulsifying properties. Solubility is a determining factor for a protein’s ability to form an emulsion (Kinsella & Whitehead, 1989). Despite the fact that native BLG is highly soluble in the pH range 2-10, its emulsifying activity index was low at acid pHs and increased with increasing pH (Shimizu et al., 1985), suggesting that structural changes occur in BLG at acidic pH that influence its emulsifying properties. Such effects close to the pI of BLG could be at the origin of the decrease of emulsion stability (Klemaszewski et al., 1992) observed in the d3.2 values.
structure of the protein. In contrast, in an aqueous system, an important structural change was observed at the protein-protein interface (Morgan et al., 1999a-c). This suggests that protein modified with galactose, glucose, lactose or rhamnose could present structural changes as compared with the native protein. Polymerization observed with glycated BLG (Bouhallab et al., 1999; Chevalier et al., 2001a) could be involved in these changes.
Proteins modified with galactose, glucose, lactose or rhamnose were less glycated. Consequently, they may have conserved a globular three-dimensional structure close to the conformation of the native protein, accounting for the increase in d3.2 at pH 5.
Foaming properties were better when BLG was glycated with glucose or galactose (moderately reactive sugars). These results suggest that the nature of the sugar is an essential factor for improving the functional properties of glycated proteins by processes of Maillard reaction.
4. Change in biological properties
Since many attempts are made to control food storage and to preserve food from oxidation and microorganism contamination, it is interesting to subject protein to oxido-reductive modification in order to see whether new biological properties could be induced. The Maillard reaction is one of major reactions modifying proteins in food and in nature. The study of how it influences biological properties of derived proteins and peptides is of particular interest. The consequences of this reaction on the biological properties of the modified products have been largely studied mainly on model systems, which consist in heating a single amino acid with a reducing sugar. The Maillard reaction induced: (i) anti-oxidative activity of glucose-glycine (Anese et al., 1994), lysine (Yen & Hsieh, 1995) and xylose-arginine reacting systems (Beckel & Waller, 1983); (ii) anti-microbial activity of xylose-arginine and glucose-histidine systems (Einarsson et al., 1983; Einarsson, 1987a, b) and of glucose-glycine system (Stecchiniet al., 1993); (iii) cytotoxic activity of glucose-lysine and fructose-lysine systems (Jing & Kitts, 2000); (iv) clastogenic activity of ribose-lysine (Vagnarelli et al., 1991) and of glucose-lysine systems (Kitts et al., 1993). Proteins modified by the Maillard reaction can also present some of these properties. For example, lysozyme modified with dextran by glycation revealed a significant anti-microbial activity against both Gram-negative and Gram-positive bacteria (Nakamura et al., 1991); glycation of casein with glucose or lactose resulted in enhancement of
antioxidant activity when compared with native casein (McGookin & Augustin, 1991).
Maillard reaction occurs during many industrial and domestic thermal treatments of foods. It is widely used because of its role in creating colors, flavors, textures and other functional properties in foodstuffs. Proteins glycated without the use of conventional chemical reagents have demonstrated to improve techno-functional properties as heat stability, emulsifying and foaming properties. A study was carried out to determine the extent in which this reaction can convey antioxidant, anti-microbial or cytotoxic activities to BLG, and to its tryptic and peptic hydrolysates. BLG was modified with six different sugars in solution at 60°C. Antiradical properties were estimated using a radical scavenging activity test. Anti-microbial activities against different bacterial strains were studied with a diffusion disk method. Cytotoxic tests were performed using two cell lines and the MTT [3, (4,5-dimethylthiazoyl-2-yl) 2,5 (diphenyl-tetrazolium bromide)] rapid colorimetric assay. Glycation induced a radical scavenging activity to BLG which intensity depended on the sugar used for modification. Proteins modified with ribose and arabinose showed the highest radical scavenging activities depicted by about 80 and 60% of DPPH (2,2-diphenyl-1-picrylhydrazyl) absorption decrease at 515 nm. No anti-microbial effect of any glycated form of BLG against Escherichia coli, Bacillus subtilis, Listeria innocua and Streptococcus mutans was observed. MTT test showed no enhancement of cytotoxicity by modified proteins and peptides against COS-7 and HL-60 cells. Thus, glycated proteins could be used in formulated food as functional ingredients with a radical scavenging activity able to delay deterioration due to oxidation. This use could be even more advisable considering the lack of toxicity to eukaryotic and prokaryotic cell cultures demonstrated in this work (Chevalier et al., 2001b).
II. ENZYMATIC PROTEIN PROCESSING
rapidly than the corresponding non-enzymatic reactions, resulting in reduction of energy costs and increased processing efficiency. Enzymatic methods are consequently more attractive to the food processor. Moreover, most enzymes can be produced in large quantities, each having appropriate physical, chemical and catalytic properties. The cost of enzyme production is reasonable if one uses microbial fermentation or biotechnological processes.
Besides the proteases, which have been investigated extensively and which are the only modifying enzymes currently in use commercially, there are transglutaminase, protein kinase, and peptidoglutaminase. These enzymes have only been reported for use in food protein modification on a laboratory scale. Feeney & Whitaker (1977, 1982, 1986) addressed possible approaches applicable to the modification of proteins and discussed the impact of these potential modifications on the structure and properties of the proteins in great detail.
A. LIMITED PROTEOLYSIS AND FUNCTIONAL PROPERTIES
A large number of papers describe the effect of proteolytic breakdown of food proteins on their functional properties. They comprise animal proteins such as casein (Chobert et al., 1987, 1988a, b; Haque & Mozaffar, 1992; Agboola & Dalgleish, 1996; Slattery & FitzGerald, 1998; Smyth & FitzGerald, 1998), soybean proteins (Puski, 1975; Adler-Nissen & Olsen, 1979; Mohri & Matsushita, 1984; Adler-Nissen, 1986; Kim, et al., 1990), wheat gluten and gluten fractions (Popineau & Pineau, 1993), among others.
Hydrolysis of peptide bonds causes several changes in proteins: (i) the NH3+ and COO– content of the protein
increases, increasing its solubility; (ii) the molecular mass of the protein decreases; (iii) the globular structure of the protein is altered, exposing the previously hidden hydrophobic groups.
Proteolytic modification has special importance for the improvement of solubility of proteins. This effect becomes significant even after very limited proteolysis. Hydrolysis of casein to degree of hydrolysis (DH) of 2 and 6.7% with
Staphylococcus aureus V8 protease increased the isoelectric solubility to 25 and 50%, respectively (Chobert et al., 1988a). However, it should be noted that the solubility profiles were not identical, due to a shift of the isoelectric point of the modified proteins. Solubility of a protein hydrolysate depends on the enzyme used (Adler-Nissen, 1986).
Protamex™ (a Bacillus proteinase complex) hydrolysates of sodium caseinate (DH 9 and 15%) displayed 85-90% solubility between pH 4.0 and 5.0 (Slattery & FitzGerald, 1998).
Emulsifying properties of proteins are sensitive to proteolytic modification (Mietsch et al., 1989). Limited hydrolysis (DH 2 and 6.7%) of casein decreased the emulsifying activity (EA) at all pH (Chobert et al., 1987, 1988a), whereas the emulsion stability (ES) at DH = 2% was higher than that of native casein (Chobert et al., 1987). The EA of casein was reported to decrease with increasing net charge and with the decreasing hydrophobicity due to proteolysis (Mahmoud et al., 1992). Since the Staphylococcus aureus V8 protease is highly specific for glutamate residues, and as these residues are uniformly distributed in the sequence of caseins, a poor EA was not expected at these DH values. This could be due to some properties of the obtained peptides, which had the glutamyl residue at the C-terminal end. In the case of using trypsin for hydrolysis of dairy proteins (Chobert et al., 1988b), it was reported that a limited hydrolysis of casein (DH of 4.3, 8.9, and 9.9%) improved its EA, showing the importance of the specificity of the enzyme used. However ES of such hydrolysates was lower than that of unmodified casein. This could be attributed to the fact that the obtained peptides were not amphiphilic enough to impart a high stability to the emulsion. Hydrolysis of casein with trypsin, chymotrypsin and Rhozyme-41 resulted in an improvement in EA (Haque & Mozaffar, 1992). Protamex hydrolysates (DH 0.5 and 1.0%) of casein showed higher EA at pH 2.0 as compared with unmodified casein (Slattery & FitzGerald, 1998). However, at higher DH of 9.0 and 15%, hydrolysates exhibited lower EA compared to caseinate.
Generally, the pH of protein solutions during emulsification affects their emulsifying properties via electrostatic effects. The emulsifying capacity of protein hydrolysates is usually low at the isoelectric point. Addition of salt improves the emulsifying properties at pI (Turgeon et al., 1992). Several studies have shown no correlation between emulsifying capacity and high solubility (Chobert et al., 1988a, b; Turgeon et al., 1992).
the pH range of 3 to 9. Furthermore, the stabilities of emulsions formed with the domain peptide fraction were greater as indicated by the lack of separation of an oil phase after standing at ambient temperature for one week, whereas emulsions formed with the native protein separated (Huang et al., 1996).
Because of the lower structural stability and altered molecular characteristics of the domain peptides, their interactions and thus their gelling properties were significantly different from those of the native protein. Solutions of a limited proteolysate of BLG formed gels at 60°C, whereas the untreated protein required temperatures of 70-80°C for gelation (Chen et al., 1994). The domain peptides, even in the presence of other whey proteins, alter the gelation properties of BLG. Thus, limited proteolysis of whey protein isolate exhibited gelation characteristics that were different from those of untreated whey protein isolate. For example, 10% solutions of enzyme-treated whey protein isolate at pH 7.0 exhibited a gelation point at 77°C, while untreated whey protein isolate only gelled after holding at 80°C for 1.4 min. The enzyme-treated whey protein isolate solution formed an opaque particulate gel at 80°C whereas the whey protein isolate gel was clear and fine stranded. Values for hardness, cohesiveness, gumminess and chewiness were significantly greater for enzyme-treated whey protein isolate gels (Huang et al., 1999).
Enzymatic hydrolysis modifies the foaming properties of casein. Protamex hydrolysates of sodium caseinate (DH 0.5 and 1.0%) displayed increased foam expansion at pH 2, 8 and 10 as compared with unhydrolyzed caseinate (Slattery & FitzGerald, 1998). Hydrophobic peptides resulting from plasmin hydrolysis of BCN improved foam formation and stabilization at pH 4.0 (Caessens et al., 1999).
A commercial range of hydrolysates of whey proteins with DH ranging from 8 to 45% was used to make emulsions with soybean oil (Singh & Dalgleish, 1998). As estimated by the particle sizes, the maximum emulsifying capacity was obtained from hydrolysates with a 10 or 20% DH. Higher hydrolysis resulted in peptides that were too short to act as effective emulsifiers, and, at lower proteolysis, the somewhat reduced solubility of the hydrolysates slightly decreased their emulsifying power. All of the emulsions were unstable when they were subjected to heat treatment at high temperatures (122°C for 15 min), but emulsions prepared from the less hydrolyzed peptide mixtures were stable to heat treatment at 90°C for 30 min.
One of the applications of enzymes in the preparation of
food gels is the production of cheeses. During this process, chymosin hydrolyses a specific bond of -casein, resulting in the destabilization of the micelle structure followed by aggregation and formation of an insoluble coagulum.
Enzymatic gelation of partially heat-denatured whey proteins by trypsin, papain, pronase, pepsin, and a preparation of Streptomycesgriseus has been studied (Sato et al., 1995). Only peptic hydrolysate did not form a gel. The strength of the gel depended on the enzyme used and increased with increasing DH. Hydrolysis of whey protein concentrate with a glutamic acid specific protease from
Bacillus licheniformis at pH 8 and 8% protein concentration has been shown to produce plastein aggregates (Budtz & Nielsen, 1992). The viscosity of the solution increased dramatically during hydrolysis and reached a maximum at 6% DH. Incubation of sodium caseinate with pepsin or papain resulted in a 55 to 77% reduction in apparent viscosity (Hooker et al., 1982).
B. ALLERGENICITY
It should be highlighted that the onset of food allergies is rising significantly amongst the world populations. It is concerning particularly infant group of the European Community population. In some cases, very severe allergic reactions against consumed foods and to dairy proteins in particular, lead to anaphylaxis. In this context well described and well-studied influence of the fermentative processing of foods, and in particular the dairy food systems, could help to circumvent or limit the severity of some adverse allergenic reactions. This could be achieved thanks to pre-prandial proteolysis occurring in fermented dairy (and other food) systems changing the allergen presentation or cleaving the allergenic protein epitopes. Hence, it could be expected that well managed fermentative transformations of dairy (and other food) products with appropriate lactic acid bacteria (LAB) strains, could produce hypoallergenic products at least for some classes of allergic patients.
milk digestibility and enhancing nutritional quality of yogurt. It is accepted that proteolytic system of LAB degrades proteins and hence, changes the texture, the taste and the aromas of fermented products.
Allergy to cow milk (CMA) concerns ~2.5% of children below 3 years of age, hence the feeding of an infant allergic to cow milk casein may create serious trouble. Most studies revealed that caseins and BLG are the main allergens in cow milk (Cocco et al., 2003, Gaudin et al., 2008). Different attempts have been made to reduce the allergenicity of dairy proteins, and various technological processes have been applied. Attempts to modify the protein components of cow milk in an effort to reduce their allergenic potential have included the application of heat treatment, enzymatic treatment with a variety of enzymes and some combination of these processes, such as heating and glycation (Taheri Kafrani et al., 2009). Heating has only a small effect on the antigenic/allergenic potential of cow’s milk proteins, even though some authors have shown a reduction in whey protein antigenicity. Furthermore, heating processes can only modify conformational epitopes, which lose their binding capacity to specific IgEs. However, linear epitopes remain unaffected by structural changes and maintain their allergenicities after heating. Milk proteins contain both types of epitopes and, even though a slight reduction of antigenicity can be observed in case of whey proteins, only very insignificant changes in binding properties were reported for caseins (Restani et al., 2009).
Another method for reduction of antigenic/allergenic properties of milk proteins is bacterial fermentation. During microbial fermentations proteolytic enzymes can be produced and they can degrade milk protein allergens. Proteolytic systems of lactobacilli are complex and are composed of proteinases and peptidases with different sub-cellular locations. Cellular proteases grown on milk showed similar hydrolytic activities towards S- and β-caseins. Proteolysis is
often followed by a reduction of the number of epitopes and consequently by a decrease in allergenicity of hydrolyzed proteins or could contribute to preventing allergenic problems frequent in children under 3 years of age due to poor digestion of milk proteins. Protein hydrolysates are also included in specific formulations, as well as hypoallergenic infant formulas to reduce their antigenicity as compared with intact protein (Bertrand-Harb et al., 2003; Cocco et al., 2003; Peñas et al., 2006; Pescuma et al., 2009, 2010, 2011; El -Ghaish et al., 2010a,b, 2011).
It was already reported that some LAB reduce the
antigenic response of milk proteins (Wróblewska et al., 1995). The reduction of milk protein antigenicity depends on the species of LAB and on conditions of fermentation (Kleber et al., 2006, Bu et al., 2010).
Lactic acid fermentation was applied to reduce the BLG antigenicity of sweet whey and skim milk, and furthermore, its products were reported to have beneficial effects on the consumer including the activation of the immune system (Shida et al., 1998). BLG is in about 80% of all cases the main cause of milk allergies in children and infants. BLG is the major whey protein in milk and in dairy products and it is of particular interest since it is the only whey protein of cow’s milk absent in human milk. Kleber et al. (2006) studied the ability of some LAB strains to reduce the antigenicity of BLG in skim milk and in sweet whey by indirect competitive ELISA, using polyclonal antibodies. They observed the reduction of antigenicity of BLG exceeding 70% in sweet whey and higher than 90% in skim milk compared to the initial value. In addition, the synergic reduction of the antigenicity was observed when co-cultures of LAB were used. The proteases produced by different LAB are more or less specific and the using of co-cultures yields higher hydrolysis of native allergens as well as of their epitopes. Fermentation of sterilized cow’s milk using a mixture of meso- and thermophilic LAB resulted in almost complete disappearance of antigenicity of ALA and BLG (ELISA, rabbit antibodies) (Jedrychowski & Wróblewska, 1999). Different studies revealed that the combination of
Lactobacilli strains with Streptococcus thermophilus during fermentation of milk and whey resulted in higher immunoreactivity reduction of native allergenic proteins. It was found in a recent study (Bu et al., 2010) that combined strains of Lactobacillus helveticus and Streptococcus thermophilus were the most effective in the reduction of the antigenicity of ALA and BLG in skim milk as compared with
Lactobacillus helveticus and Streptococcus thermophilus
alone. ALA antigenicity was reduced by 87% with combined strains fermentation by Lactobacillus helveticus and
Streptococcus thermophilus. However, ALA antigenicity was inhibited by 71% and 49% with Lactobacillus helveticus and
Streptococcusthermophilus alone, respectively.
found that at the beginning of the fermentation, antigenicity of BLG decreased gradually but at longer fermentation time it slightly increased, what indicates that there was no linear correlation between the degree of hydrolysis of milk protein and the antigenicity of whey proteins during the fermentation (Bu et al., 2010). Ehn et al. (2005) used different strains of
Lactobacillus helveticus to hydrolyze milk whey proteins and observed 80% hydrolysis of BLG. However, this did not affect the epitope recognition by IgE as judged by the unchanged inhibition pattern (ELISA, human serum) after this treatment. This suggests that the extracellular proteolytic activity in the fermentation process did not degrade enough the IgE epitopes. It is also possible that the degradation was only partial, leaving peptides long enough to bind the antibodies, or it is possible that better access to some buried internal epitopes compensated any hydrolytic reduction of external epitopes. It is also possible that the preheating temperature might have a significant impact on the changes of the antigenicity of whey proteins. Kleber et al. (2006) studied the effect of different LAB on the antigenicity of BLG in skim milk heated 40 min at 90°C. They found that the antigenicity of BLG decreased by 84–98% as compared with unfermented skim milk. Jedrychowski & Wróblewska (1999) claimed that after the fermentation with LAB the antigenicity of sterilized milk (110°C, 10 min) was reduced by over 99%. However, when prick test with whey samples from fermented whole milk were done on allergic to milk patients their allergic reactions were only slightly attenuated. Further in vitro and in vivo studies are necessary to understand better the mechanism of allergenicity of dairy proteins and the methods to decrease it. Additionally, it would be interesting to study the combination of various methods, such as fermentation and hydrolysis with animal and plant proteases, heat treatment, high pressure, and microwave combined with enzymatic proteolysis (El Mecherfi et al., 2011).
C. LIBERATION OF BIOLOGICALLY FUNCTIONAL PEPTIDES
Milk contains components that provide critical nutritional elements, immunological protection, and biologically active substances to both neonates and adults (Meisel & Schlimme, 1990). In general, the major protein fractions in bovine milk include ALA, BLG, caseins (S1, S2, , and ),
immunoglobulins, lactoferrin, proteose-peptide fractions (heat-stable, acid soluble phosphoglycoproteins), and minor
whey proteins such as transferrin and serum albumin. From these, bioactive peptides may be generated in vivo through gastrointestinal processes. These peptides, encoded within the sequence of native protein precursors, may also be generated
in vitro by enzymatic hydrolysis. The number of such peptides identified in caseins and whey proteins continues to grow (Gobetti et al., 2002). Lastly, physiologically active peptides have been chemically synthesized to confirm the biological properties associated with a specific amino acid sequence. Many bioactive peptides serve in multifunctional capacities and often share common structural features based on a defined, bio-specific role.
1. Milk defense peptides - antimicrobial peptides
Jones & Simms (1930) reported the first dairy protein derived antibacterial factor, named lactenin, which slowed the growth of streptococci. Other antimicrobial milk proteins, such as lactoferrin, were described early in the literature in the 70’s. Discovery of basic glycopeptides with bactericidal activity against various strains of Staphylococcusaureus and
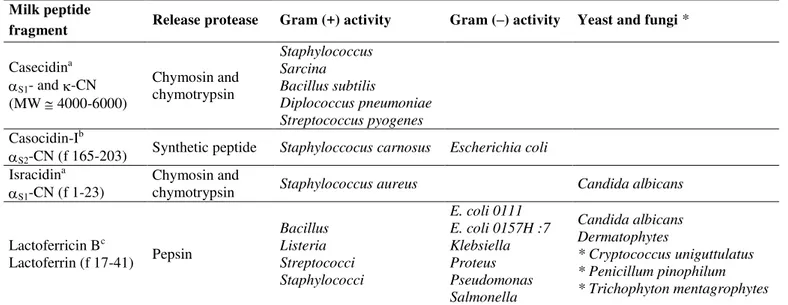
Streptococcus have been also detailed at this time. Later, there has been a new interest in using bioactive peptides for application within the health care industry (Table 1 and references therein).
Casecidin, obtained by chymosin digestion of casein at neutral pH, was among the first defense peptides actually purified and exhibited activity in vitro against
Staphylococcus, Sarcina, Bacillus subtilis, Diplococcus pneumoniae, and Steptococcus pyogenes. Casocidin I from bovine milk, a cationic S2-CN derived peptide, inhibited
growth of Escherichia coli and Staphylococcus carnosus. Isracidin, an N-terminal segment of S1-CN-B, protected
mice against Staphylococcus aureus and Candida albicans. Hydrolysate of bovine lactoferrin was active against both Gram positive (Bacillus, Listeria, and Streptococcus) and Gram negative (E. coli, Klebsiella, Salmonella, Proteus, and
Pseudomonas) microorganisms in vitro. A bactericidal peptide generated by peptic hydrolysis of lactoferrin (named lactoferricin-B) was active against positive and Gram-negative bacteria.