ABSTRACT
Modulation of host cell signaling pathways as a
therapeutic approach in periodontal disease
João Antonio Chaves de SOUZA1, Carlos ROSSA JUNIOR2, Gustavo Pompermaier GARLET3, Andressa Vilas Boas
NOGUEIRA1, Joni Augusto CIRELLI2
1- DDS, MSc, PhD student, Department of Diagnosis and Surgery, School of Dentistry, UNESP-Univ. Estadual Paulista, Araraquara, SP, Brazil. 2- DDS, MSc, PhD, Professor, Department of Diagnosis and Surgery, School of Dentistry, UNESP-Univ. Estadual Paulista, Araraquara, SP, Brazil. 3- DDS, MSc, PhD, Professor, Department of Biological Sciences, Bauru School of Dentistry, University of São Paulo, Bauru, SP, Brazil.
Corresponding address: Joni Augusto Cirelli - Rua Humaitá, 1680 - Centro - 14801-903 - Araraquara, SP - Brazil - Phone: +55-16-3301-6375 - e-mail: cirelli@foar.unesp.br
R
ecently, new treatment approaches have been developed to target the host component of periodontal disease. This review aims at providing updated information on host-modulating therapies, focusing on treatment strategies for inhibiting signal transduction !" #$ STAT pathways are being developed to manage rheumatoid arthritis, periodontal disease %& be inhibited at cell signaling level, interfering on transcription factors activation and ' & & & ( ) After overcoming these limitations, adjunctive host modulating drugs will provide new therapeutic strategies for periodontal treatment.Key words: Periodontitis. Signal transduction. Janus kinases. STAT transcription factors. Mitogen-activated protein kinases. NF-kappa B.
1- ETIOLOGY AND PATHOGENESIS OF
PERIODONTAL DISEASES
The hallmark of inflammatory/infectious conditions is the increased production of cytokines, * The synthesis and expression of these mediators occur in a transitory and strictly controlled way, seeking resolution of the problem and re-establishment of the homeostasis. Regulation of cytokine gene expression is controlled by various mechanisms, including transcriptional, transcriptional, translational and post-translational regulations. In the event of defective negative regulation of cytokine gene expression, the exaggerated and uncontrolled expression of cytokines and proteases can have deleterious consequences to the host, including cancer, autoimmune and chronic diseases2. Periodontal
disease is one of these chronic pathologies, and it is ) & adults10. The pathologic mechanisms of periodontal
disease are still not completely understood. Current knowledge concerning the pathogenesis of periodontitis suggests that it is a mixed infection ) * mediators. These mediators trigger a cascade of events that, in some individuals, culminate in the irreversible degradation of connective and bone tissues, and consequent periodontal attachment loss6,57. Periodontal diseases have
& and, consequently, the susceptibility to periodontal disease1,40. The association of periodontal disease
with increased levels of cytokines and & & mechanisms for the negative regulation of these genes may be defective or dysfunctional; and this, in turn, may be one mechanism of increased susceptibility to periodontal disease.
Many studies have shown that the biological activity of a variety of cytokines may be directly relevant to periodontal destruction2,29. The
interaction between cytokines and their antagonists will ultimately determine the severity and extent of tissue destruction, which may occur either as a direct effect of increased level of cytokines, or as indirect consequences of cytokine expression57.
There is evidence of changes in the expression of these immune regulatory molecules found in diseased sites, compared to health clinic conditions. < =>?*@ >?*D & *G H%*GKO % @ ( *Q H>*QK with higher periodontal disease severity, the opposite is considered true for T helper 2 type cytokines, such as IL-4 and IL-108,19,21. The balance
between the expression of T helper 1 and T helper 2 type mediators is thought to be a relevant factor in the outcome of disease, possibly regulating the *$* ( ' metalloproteinases (MMPs)/tissue inhibitors of metalloproteinases (TIMPs) and receptor activator & *!" HV?K$ (OPG). Imbalances between these mediators in the periodontal tissues are a major cause of periodontal destruction22-23,46.
2- TRADITIONAL THERAPY AND
NEW THERAPEUTIC STRATEGIES ON
PERIODONTAL DISEASES
The therapeutic approach to managing periodontal diseases has been traditionally targeted & $ ) ) & ) Z& maintenance82. More recently, modulation of the
host component of periodontal disease has been studied as an alternative therapeutic approach. Manipulation of the immune response to suppress undesirable reactions is an established therapeutic & autoimmune diseases but also in cancer25,38. The
purpose of host modulatory therapy is to restore * * V & demonstrated that modulation of the host immune/ inflammatory response resulted in significant
\& ) in the treatment of chronic periodontitis71. The
adjunctive use of host modulatory therapy can slow down the progression of the disease, especially in susceptible patients at increased risk for whom conventional therapeutic approaches are not effective. Importantly, modulation of host response can prevent and/or minimize the destruction
There are several strategies for modulation of host response. Studies suggest that certain local and systemic pharmacologic agents, that block specific inflammatory mediators, appear to attenuate disease progression90. Modulating
biochemical agents and drugs have been postulated to be of therapeutic value as an adjunctive therapy to the management of chronic periodontitis28,42,65.
The most studied drugs are antibiotics used in non-antimicrobial doses (e.g. tetracycline derivatives, which inhibit collagenolytic activities and activity of neutrophils and osteoclasts), anti-inflammatory agents and bone-sparing drugs. % * * * & H^>_K weak organic acids, which prevent prostanoid formation by blocking the cyclooxygenase pathway of arachidonic acid metabolism. The bone-sparing drugs (e.g., bisphosphonates) bind to the hydroxyapatite crystals of bone and prevent their dissolution by interfering with osteoclasts activity, thus reducing alveolar bone loss.
> ' H`K >?*D >?*@ %*G * ( demonstrated successful protective effects against bone resorption18,44,54. The use of anti-cytokine
therapies (by anti-cytokine drugs and soluble cytokine blockers) has been proven effective to block the negative effects of cytokines, slowing down the disease process. However, most of these & ) & side effects, including hemorrhage, gastrointestinal problems, and renal and hepatic impairment that preclude their use. Another important consideration is the protective role of immune response and the potential hazards of its negative modulation, especially aggravation of infection as demonstrated by studies using animals with defective immune response and clinical studies in severely immunocompromised patients11,16-17.
pathway may affect expression of more than cytokine network. This is important considering the frequently redundant and interchangeable ( also implies that the impact of therapies targeting ( be somewhat limited because of compensatory mechanisms30. As promising and attractive as
these strategies may be, intracellular signaling pathways have some important characteristics that should be considered, including the fact that they may be essential to various cellular processes, such as proliferation, apoptosis and survival. This suggests that modulation of some cell signaling pathways may have negative effects; however another hallmark of cytokine cell signaling is that activation of these pathways by cytokines and their agonists is usually a very rapid and transient event (minutes) that results in somewhat long-lasting consequences (modulation of cytokine gene expression). It is, thus, conceivable that a short-term modulation of cell signaling pathways may have an impact on cytokine gene expression without having much negative consequences on other fundamental cellular processes. For this reason, it is important to understand the role of individual & destruction in periodontal disease. Because these mediators, including many cytokines, chemokines, cell-adhesion molecules, acute-phase proteins and anti-apoptotic proteins, their inhibition will probably prove more progress than current treatment strategies. This review aims at providing updated information on host-modulating therapies, focusing on control mechanisms to inhibiting the most important signal transduction pathways related to
3- CELL SIGNALING PATHWAYS IN
PERIODONTAL DISEASE
) && the teeth area adjacent to gingival margin, an inflammatory process is initiated, triggering a dynamic cascade of events. The main purpose of these events is the combat of microbial invaders & * % * the external antigenic stimuli by host leukocytes of the innate immune response, e.g. macrophages, neutrophils, dendritic cells, natural killer cells and others. This recognition of external “danger” then triggers a signal that travels through the cytoplasm and reaches the nucleus, and ultimately the pattern of gene expression is altered by transcriptional and post-transcriptional mechanisms. The ability of the
determine the sequence of the process. Within 3-4 &) robust to initiate connective destruction. If the & &) resolve the aggression and eliminate the “danger” signals, then an adaptive immune response is initiated, involving lymphocytes that will produce immunoglobulins, cytokines and also try to clear out the aggression by direct cytotoxic mechanisms. Regardless of the type of host response (innate or acquired immunity), all cellular events depend on the activation of multiple signal transduction pathways, which may be affected by various factors both microbial- and host-derived, including lipopolysaccharide (LPS), proteases, cytokines and other enzymes47,80. Signal transduction depends on
receptor-ligand interactions which usually involves ) associated with these receptors. The most common ) & ) & kinases, which induces a conformational change on the tridimensional structure of the protein. & ) to transfer energy and modulate their biological activity, since it does not involve de novo gene expression (i.e., the signaling intermediates are usually constitutively expressed by the cells and “ready to go into action”, as soon as they are )K ^ & varying number of signaling intermediates that are activated (phosphorylated) sequentially and relay the energy to one or more protein substrates. These substrates can exert their biological effect as transcriptional factors, transcriptional repressors, by V ) % ' ( during periodontal disease progression results from the activation of intracellular signaling pathways, which are determined by the nature of extracellular stimuli. In periodontal disease the most important pathways include the mitogen activated protein ( HK & ( " H*!"K and janus tyrosine kinase-signal transducer and activator of transcription (JAK/STAT)3,20.
3.1- MAPK pathway
MAPKs are an evolutionarily conserved family of protein kinases that mediate fundamental biological processes and cellular responses to different extracellular stimuli through multiple receptors41.
MAPKs are involved in signal transduction of extracellular hormones, growth factors, cytokines, bacterial antigens and environmental stresses and play a crucial role in many aspects of immune 63,67. The three
extracellular-regulated kinases (ERK-1/-2), c-Jun N-terminal activated kinases (JNK) and p38. ERK kinases are traditionally considered as being primarily activated by mitogens and growth factors while inducers of * ( by JNKs and p3860, although this general concept
does not apply to all cell types and to all external stimuli. However, there is evidence of cross-activation and interaction between various levels of the main MAPKs pathways (ERK and p38)64,86.
The MAPK cascade consists of a series of three-tired protein kinases, a MAPK and two upstream components, MAPK kinase (MAPKK) and MAPKK kinase (MAPKKK). Activation of the MAPKs results ) that mediate gene transcription (Figure 1). The multiple interactions between the different MAPK cascades serve to integrate the responses and activate separate sets of genes43,63. All three MAPK
families are expressed in periodontal disease20.
During initial interaction of pathogens with the host’s immune system, pathogen-associated molecular patterns (PAMPs), such as LPS, peptidoglycans, lipoteichoic acid, or bacterial CpG-DNA can trigger cells of the innate immune
system52. Cytokines produced in response to this
initial activation by PAMPs can also activate signal transduction pathways by autocrine or paracrine mechanisms. These microbial products and ( H >?*@ %*GK & Toll receptors, IL-1 receptor (TIR) family or the TNF receptor family. Activation of these receptors triggers MAPK pathway (Figure 1), leading to *!" known to be relevant for cytokine gene expression43.
% $*!" ( & & arthritis81.
p38 activation leads to increased expression of various cytokine genes by modulation of both transcriptional and post-transcriptional mechanisms. The contribution of each mechanism to the global change of gene expression varies with the cell type and nature of external stimulation, but among the genes that are at least partially modulated by post-transcriptional mechanisms ) V* & %*G >?* >?* 6, IL-2 and cyclooxygenase (COX)-233,61,91. Other
genes are primarily regulated by transcriptional
& >?*@ V? ( and metalloproteinases7,70,72.
The evidence indicating a prominent role of H & G K cytokine gene expression and signaling, make them * & > G developed and preclinical and clinical data suggest
' * 36, as
)& ( inhibitors interfere with phosphorylation or bind in the ATP binding site. Several compounds such as SD-282, SC-409, SB (SmithKline Beecham) -242235, AW-814141 and other capable of inhibit p38 have been studied in murine models of rheumatoid arthritis and/or periodontal disease and have prevented progression of the disease and bone resorption4,12,49,51,69. The promising results obtained
in both in vitro and in preclinical studies generated interest of pharmaceutical companies to develop protein kinase inhibitors. The p38 inhibitor BIRB-796 (Boehringer Ingelheim Pharmaceuticals Inc., V) % ^K * in a phase II study in rheumatoid arthritis but shown
limited results15,92. Studies to evaluate the safety
) & arthritis are currently underway76 % )
of these compounds in arthritis appears limited and
) 79. VX-745
was discontinued because in animal test revealed adverse neurological effects. Although no adverse effects were reported in human, gastrointestinal symptoms were described31,87.
Inhibitors of JNK and ERK have also shown efficacy in inhibiting the production of 32,89 (Figure 4). So far,
no human trials have been initiated with these inhibitors. In murine model of rheumatoid arthritis, the JNK inhibitor SP600125 (Celgene Corporation, San Diego, California, USA), besides the reduction %*G >*Q >?*D `* also inhibit joint destruction in a rat adjuvant
arthritis model32 ^) V
available but there is limited information about their
& 83.
Recently, a potent and selective inhibitor for ERK, FR180204, has been proven effective against mouse collagen-induced arthritis. This compound
Figure 2-&%"'*<='*&%">*?IOQVOW
suppresses the activation of T cells, which play a important role in progress of the disease56.
The MAPK inhibitors are capable of reducing * ( & ) & rheumatoid arthritis and periodontal disease27,37,59,62.
In several cases, however, the clinical studies have been stopped87. MAPKs play several physiological
roles and suppression of these functions may lead to a number of problems. While many inhibitors ) have prevented the development of some of these compounds. Therefore, most of these compounds have subsequently been discontinued. One of the underlying reasons for these unacceptable side effects might be the cross-reactivities against other kinases or other cellular signaling molecules14.
1)lj%SDWKZD\
*!" ) ) that binds to a 10 base pairs (bp) DNA element in kappa immunoglobulin light-chain enhancer in B
cells74 % *!"
has been shown to be involved in many different pathways and has a central role in regulating the expression of a wide variety of genes that control both innate and adaptive immune responses. Activated *!" & &
\ 26. Activation
*!" & * quantities in tissues with periodontal disease such ?^ %*G >?*@ ` inducible nitric oxide synthase (iNOS)5,81. In vitro
studies have established that both Porphyromonas gingivalis and other periodontal pathogenic bacteria
*!" &78.
% *!" & a diversity of biologically active molecules is the consequence of the activation of other signaling pathways, including MAPKs and TLR pathways. A & & *!" pathways will provide a platform for developing ) & recent study in patients with chronic periodontitis and healthy controls showed that activation of NF-!" H$DK ) & & *!" in managing periodontitis3. In animal models of
& *!" inhibitors seems to be effective53.
% *!" ) V?* HDK *!"@ H+ @K *!" H+ p100), c-REL and REL-b24. These subunits, except
REL-b, form homodimers and heterodimers to & *!" %
Figure 3-{Q$$< { > ?{W >
is a heterodimer of p50 and p65. They bind to the *!" * gene transcription13,45 % ( & *!"
*!" H>!"K >!"G >!" >!"24 >!"
bind to functional NF-kB dimers in cytoplasm in absence of stimuli and prevent their nuclear translocation. Signaling through IL-1 receptor (IL-1R) or toll-like receptor (TLR) can activate a cascade that involves the recruitment of MYD88 (myeloid differentiation primary response gene 88) and IRAK (interleukin-1-receptor associated kinase). Activation of IRAK results in the phosphorylation of TNF-receptor associated factor 6 (TRAF6), leading ** ( 1 (TAK1) activation, which, in turn, is required for >!" ( ' H>K > >!"G & &Z& >!"G *!" >!"G % *!" protein then translocates to the nucleus where it ) _ multiples genes, including cytokines, chemokines, ' mediators (Figure 2). Many strategies to prevent *!" & >!" ( >!" ' >!" recombinant protein or by gene therapy9. The IKK
inhibitor, BMS (Bristol-Myers Squibb)-345541, was evaluated in the collagen-induced arthritis model
and joint destruction50 (Figure 4). Inhibition of the
( >!" *!" ` ( *!" activity are being developed by pharmaceutical industries, and are based either on targeting the _* *!" ( & *!"
Despite the potential use of this pathway in development of therapeutic interventions for &$ *!" participates in normal physiological process. % ( *!" & unwanted side effects as liver failure related to hepatocyte apoptosis48.
3.3- JAK/STAT pathway
Many cytokines and growth factors (interferons, interleukins, epidermal growth factor, growth hormone, erythropoietin and others) exert their biological functions through JAK-STAT signal transduction pathway55,73. Classically, interferons
and interleukins, cytokines with key roles in regulating the immune response, activate enzymes called Janus kinases (JAK1, JAK2, JAK3 and Tyk2), which are associated with the cytoplasmic portion of the transmembrane receptors34. Activated JAKs,
phosphorylate the cytoplasmic domain of the receptor leading the activation of its substrates, especially the proteins known as STATs (STAT1-4, 5a, 5b, and 6). Upon phosphorylation, STATs may
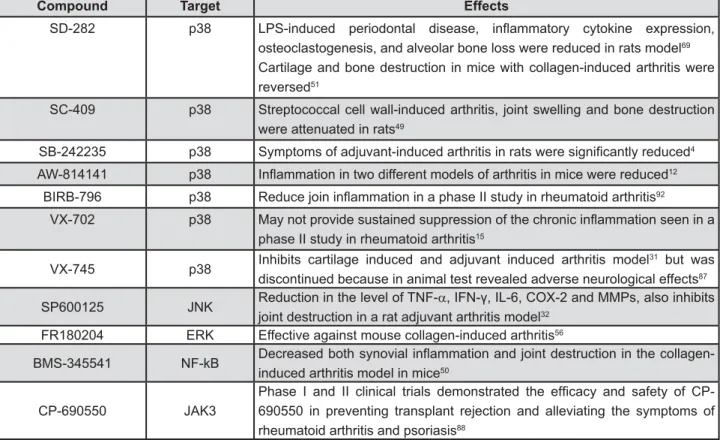
Figure 4- Pharmacological compounds with potential host-modulation actions
Compound Target Effects
SD-282 p38 |" ! } > ] !
osteoclastogenesis, and alveolar bone loss were reduced in rats model69
Cartilage and bone destruction in mice with collagen-induced arthritis were reversed51
SC-409 p38 Streptococcal cell wall-induced arthritis, joint swelling and bone destruction were attenuated in rats49
SB-242235 p38 " < 4
AW-814141 p38 =}< < 12
BIRB-796 p38 Z } == 92
VX-702 p38 }
phase II study in rheumatoid arthritis15
VX-745 p38 Inhibits cartilage induced and adjuvant induced arthritis model
31 but was
discontinued because in animal test revealed adverse neurological effects87
SP600125 JNK Reduction in the level of TNF-D!=%&"!=|"V! "! joint destruction in a rat adjuvant arthritis model32
FR180204 ERK Effective against mouse collagen-induced arthritis56
BMS-345541 NF-kB _ } "
induced arthritis model in mice50
CP-690550 JAK3
form homo- or hetero-dimers; which enables them to enter to the nucleus where they can regulate gene transcription55 (Figure 3). Although individual
STAT proteins may be activated by multiple ligands, certain cytokines preferentially activate particular ^%% >*Q ^%%@ & JAK1/JAK2 and IL-6 activates STAT3 through JAK1. This pathway is crucial to many responses like hematopoiesis, oncogenesis and immune/ inflammation regulation. However, abnormal activity of JAK/STAT pathway is associated with a wide variety of human malignancies such as cancer. Regulatory mechanisms controlling the duration of the signal include the downregulation of the receptor/ligand complex, degradation of signaling intermediates, inactivation of positive regulators by dephosphorylation (receptor, JAK or STAT) or
) &77.
The JAK-STAT pathway is the signaling target of many cytokines which are thought to have ) & (IFN-J, IL-2, IL-4, IL-6, IL-7, IL-10, IL-12, and IL-15) and in periodontal disease (INF-J, TNF-D, IL-1 IL-4, IL-6, and IL-10)8,66,85. Furthermore
preliminary studies in human synovial tissue suggest that constitutive STAT activity is observed in rheumatoid arthritis35,39. Other recent study have
shown that STAT3 and STAT5 activations were noted on the ligature-induced model of experimental periodontitis20. To date, no studies with STAT
inhibitors are available in periodontal disease and in rheumatoid arthritis, despite the potential role of these proteins in expression of important genes
Among the four JAKs, JAK3 and Tyk2 have been the focus of most interest in terms of drug development whereas experimental deficiency of JAK1 or JAK2 is lethal. Therefore, targeting these kinases would not be expected to be good targets58,68. At moment, no Tyk2 inhibitor was
developed. Tyk2 was involved in signaling by type I IFNs84. Targeting Tyk2 would be a useful strategy
for the treatment of Th1 mediated disorders such as arthritis75. The JAK3 antagonist CP-690550
H)K ) rheumatoid arthritis88 (Figure 4).
It is now clear that JAK/STAT pathway have a & and progression. This pathway can affect the ' & * * & modulation of this pathway on cytokine signaling increases the possibility that these proteins may prove to be excellent targets for the discovery of drugs that can manipulate cytokine outcomes to resolve disease.
4- CONCLUSIONS
Periodontal diseases are one of the most ) & & clear that both the pathogenesis and the clinical manifestations of this disease are, at least in & & responses. Wherefore, the importance of the & be recognized by the fact it represents the opportunity to explore new treatment approaches. The adjunctive use of modulation of host response with traditional mechanical periodontal therapy has ) ) of periodontal disease.
Improved knowledge of signal transduction mechanisms and gene regulation involved in immune responses, notably in pathways involving *!" ( #$^%% create new therapeutic targets useful in treating ` & blockade may be more effective than targeting ) ( important in several other physiological processes and therefore their inhibition can also result in undesirable side effects.
The development of effective drugs targeting host response mechanisms may represent a new approach in adjunctive treatment of periodontal disease. Although these drugs offer great potential to modulate host response, a notable limitation of ( ) effects. Drugs that inhibit destruction of the connective tissue in one periodontal site also interfere with wound healing at another, or worse, can predispose the patient to opportunist and/or acquired infections in other organ systems when these drugs are administered systemically. Thus, & ) of periodontal disease may cause problems in other parts of body. Nevertheless, preliminary results indicate that the therapeutic potential of some of these drugs are promising for the management of & chronic diseases. In the future, it is possible that host modulating drugs will provide new adjunctive therapeutic strategies for periodontal treatment.
ACKNOWLEDGEMENTS
REFERENCES
1- Albandar JM. Global risk factors and risk indicators for periodontal diseases. Periodontol 2000. 2002;29:177-206. 2- Alexander MB, Damoulis PD. The role of cytokines in the pathogenesis of periodontal disease. Curr Opin Periodontol. 1994:39-53.
3- Ambili R, Santhi WS, Janam P, Nandakumar K, Pillai MR. Expression of activated transcription factor nuclear factor-kappaB in periodontally diseased tissues. J Periodontol. 2005;76:1148-53. 4- Badger AM, Griswold DE, Kapadia R, Blake S, Swift BA, Hoffman SJ, et al. Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthritis Rheum. 2000;43:175-83.
5- Baeuerle PA, Baichwal VR. NF-kappa B as a frequent target && * & Immunol. 1997;65:111-37.
6- Baker PJ. The role of immune responses in bone loss during periodontal disease. Microbes Infect. 2000;2:1181-92.
7- Baldassare JJ, Bi Y, Bellone CJ. The role of p38 mitogen-activated protein kinase in IL-1 beta transcription. J Immunol. 1999;162:5367-73.
8- Berglundh T, Donati M. Aspects of adaptive host response in periodontitis. J Clin Periodontol. 2005;32:87-107.
* " # ' " " _) therapeutic targets by using adenovirus: blocking NF-kappaB inhibits both inflammatory and destructive mechanisms in & & & * Proc Natl Acad Sci USA. 1999;96:5668-73.
10- Brown LJ, Löe H. Prevalence, extent, severity and progression of periodontal disease. Periodontol 2000. 1993;2:57-71. 11- Cho H, Lee KH, Colquhoun AN, Evans SA. Invasive oral aspergillosis in a patient with acute myeloid leukaemia. Aust Dent J. 2010;55:214-8.
12- Chopra P, Kulkarni O, Gupta S, Bajpai M, Kanoje V, Banerjee ) <*@@@ selective and orally active inhibitor of p38 MAP kinase. Int Immunopharmacol. 2010;10:467-73.
13- Ciesielski CJ, Andreakos E, Foxwell BM, Feldmann M. TNFalpha-induced macrophage chemokine secretion is more dependent on NF-kappaB expression than lipopolysaccharides-induced macrophage chemokine secretion. Eur J Immunol. 2002;32:2037-45.
14- Cohen P. Targeting protein kinases for the development of * & & ` " +@@* @* _\ & V^ ^* % ) pharmacodynamics, and safety of VX-702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: results of two randomized, double-blind, placebo-controlled clinical studies. Arthritis Rheum. 2009;60:1232-41.
16- De Repentigny L, Lewandowski D, Aumont F, Hanna Z, Jolicoeur P. Oral mucosal cell response to Candida albicans in transgenic mice expressing HIV-1. Methods Mol Biol. 2009;470:359-68. 17- Dhasmana DJ, Dheda K, Ravn P, Wilkinson RJ, Meintjes G. >& & >* patients receiving antiretroviral therapy: pathogenesis, clinical manifestations and management. Drugs. 2008;68:191-208. 18- Dinarello CA. Therapeutic strategies to reduce IL-1 activity in & ` 2004;4:378-85.
19- Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular & & & effect of periodontal treatment. J Periodontol. 2000;71:1535-45. 20- Garcia de Aquino S, Manzolli Leite FR, Stach-Machado DR, Francisco da Silva JA, Spolidorio LC, Rossa C Jr. Signaling pathways associated with the expression of inflammatory mediators activated during the course of two models of experimental periodontitis. Life Sci. 2009;84:745-54.
21- Garlet GP, Cardoso CR, Campanelli AP, Martins W Jr., Silva JS. Expression of suppressors of cytokine signaling in diseased periodontal tissues: a stop signal for disease progression? J Periodontal Res. 2006;41:580-4.
22- Garlet GP, Martins W Jr, Fonseca BA, Ferreira BR, Silva JS. Matrix metalloproteinases, their physiological inhibitors and & ( ) in human periodontal disease. J Clin Periodontol. 2004;31:671-9. 23- Gemmell E, Seymour GJ. Immunoregulatory control of Th1/ % ( ) 2004;35:21-41.
24- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225-60.
* _< ? % V > resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401-16.
26- Gilston V, Jones HW, Soo CC, Coumbe A, Blades S, Kaltschmidt C, et al. NF-kappa B activation in human knee-joint synovial tissue & \ " ^ % 1997;25:518S.
27- Goldstein DM, Gabriel T. Pathway to the clinic: inhibition of P38 MAP kinase. A review of ten chemotypes selected for development. Curr Top Med Chem. 2005;5:1017-29.
28- Golub LM, McNamara TF, Ryan ME, Kohut B, Blieden T, Payonk G, et al. Adjunctive treatment with subantimicrobial ' & and attachment loss in adult periodontitis. J Clin Periodontol. 2001;28:146-56.
29- Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79:1585-91.
30- Haddad JJ. Cytokines and related receptor-mediated signaling pathways. Biochem Biophys Res Commun. 2002;297:700-13. 31- Haddad JJ. VX-745. Vertex Pharmaceuticals. Curr Opin Investig Drugs. 2001;2:1070-6.
32- Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, et al. c-Jun N-terminal kinase is required for metalloproteinase ' \ & # Invest. 2001;108:73-81.
33- Hitti E, Iakovleva T, Brook M, Deppenmeier S, Gruber AD, Radzioch D, et al. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol Cell Biol. 2006;26:2399-407.
34- Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69-74.
35- Kasperkovitz PV, Verbeet NL, Smeets TJ, van Rietschoten JG, Kraan MC, van der Pouw Kraan TC, et al. Activation of the STAT1 pathway in rheumatoid arthritis. Ann Rheum Dis. 2004;63:233-9. 36- Kirkwood KL, Li F, Rogers JE, Otremba J, Coatney DD, Kreider JM, et al. A p38alpha selective mitogen-activated protein kinase inhibitor prevents periodontal bone loss. J Pharmacol Exp Ther. 2007;320:56-63.
37- Kirkwood KL, Rossa C Jr. The potential of p38 MAPK inhibitors to modulate periodontal infections. Curr Drug Metab. 2009;10:55-67.
38- Kortylewski M, Yu H. Stat3 as a potential target for cancer immunotherapy. J Immunother. 2007;30:131-9.
39- Krause A, Scaletta N, Ji JD, Ivashkiv LB. Rheumatoid arthritis synoviocyte survival is dependent on Stat3. J Immunol. 2002;169:6610-6.
42- Lane N, Armitage GC, Loomer P, Hsieh S, Majumdar S, Wang HY, et al. Bisphosphonate therapy improves the outcome of conventional periodontal treatment: results of a 12-month, randomized, placebo-controlled study. J Periodontol. 2005;76:1113-22.
43- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green _ ( & cytokine biosynthesis. Nature. 1994;372:739-46.
44- Leitao RF, Ribeiro RA, Chaves HV, Rocha FA, Lima V, Brito GA. Nitric oxide synthase inhibition prevents alveolar bone resorption in experimental periodontitis in rats. J Periodontol. 2005;76:956-63. 45- Li Q, Lu Q, Bottero V, Estepa G, Morrison L, Mercurio F, et al. Enhanced NF-kappaB activation and cellular function in macrophages lacking IkappaB kinase 1 (IKK1). Proc Natl Acad Sci USA. 2005;102:12425-30.
46- Liu D, Xu JK, Figliomeni L, Huang L, Pavlos NJ, Rogers M, et al. Expression of RANKL and OPG mRNA in periodontal disease: possible involvement in bone destruction. Int J Mol Med. 2003;11:17-21.
47- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145-51.
48- Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 2003;19:725-37. 49- Mbalaviele G, Anderson G, Jones A, De Ciechi P, Settle S, Mnich S, et al. Inhibition of p38 mitogen-activated protein kinase & # ' % 2006;317:1044-53.
50- McIntyre KW, Shuster DJ, Gillooly KM, Dambach DM, Pattoli MA, Lu P, et al. A highly selective inhibitor of I kappa B kinase, "^*@ ( \ & collagen-induced arthritis in mice. Arthritis Rheum. 2003;48:2652-9.
51- Medicherla S, Ma JY, Mangadu R, Jiang Y, Zhao JJ, Almirez R, et al. A selective p38 alpha mitogen-activated protein kinase inhibitor reverses cartilage and bone destruction in mice with collagen-induced arthritis. J Pharmacol Exp Ther. 2006;318:132-41. 52- Medzhitov R, Janeway CA Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295-8. 53- Miagkov AV, Kovalenko DV, Brown CE, Didsbury JR, Cogswell JP, Stimpson SA, et al. NF-kappaB activation provides the potential ( \ Proc Natl Acad Sci USA. 1998;95:13859-64.
54- Nishimoto N, Yoshizaki K, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1761-9. 55- O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109:S121-31. 56- Ohori M. ERK inhibitors as a potential new therapy for rheumatoid arthritis. Drug News Perspect. 2008;21:245-50. 57- Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991;26:230-42.
58- Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385-95.
59- Pargellis C, Regan J. Inhibitors of p38 mitogen-activated protein kinase for the treatment of rheumatoid arthritis. Curr Opin Investig Drugs. 2003;4:566-71.
60- Patil C, Rossa C Jr, Kirkwood KL. A c t i n o b a c i l l u s actinomycetemcomitans lipopolysaccharide induces interleukin-6 expression through multiple mitogen-activated protein kinase ) ` Immunol. 2006;21:392-8.
61- Patil C, Zhu X, Rossa C Jr, Kim YJ, Kirkwood KL. p38 MAPK regulates IL-1beta induced IL-6 expression through mRNA stability in osteoblasts. Immunol Invest. 2004;33:213-33.
62- Patil CS, Kirkwood KL. p38 MAPK signaling in oral-related diseases. J Dent Res. 2007;86:812-25.
63- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153-83.
64- Perdiguero E, Ruiz-Bonilla V, Serrano AL, Muñoz-Cánoves ) myoblast cell cycle exit: the p38alpha-JNK connection. Cell Cycle. 2007;6:1298-303.
65- Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation * * A systematic review. Ann Periodontol. 2003;8:12-37.
DD* V ( ^ ) ( mRNA expression in chronic adult periodontitis. J Dent Res. 1997;76:1833-9.
67- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180-6.
68- Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373-83.
69- Rogers JE, Li F, Coatney DD, Otremba J, Kriegl JM, Protter TA, et al. A p38 mitogen-activated protein kinase inhibitor arrests active alveolar bone loss in a rat periodontitis model. J Periodontol. 2007;78:1992-8.
70- Rossa C Jr, Liu M, Kirkwood KL. A dominant function of p38 mitogen-activated protein kinase signaling in receptor activator of nuclear factor-kappaB ligand expression and osteoclastogenesis induction by Aggregatibacter actinomycetemcomitans and
Escherichia coli lipopolysaccharide. J Periodontal Res. 2008;43:201-11.
71- Ryan ME. Clinical applications for host modulatory therapy. Compend Contin Educ Dent. 2002;23:1071-6,1079-80,1082. 72- Saklatvala J, Dean J, Clark A. Control of the expression of " ^ ^ *@D 73- Schindler CW. Series introduction. JAK-STAT signaling in human disease. J Clin Invest. 2002;109:1133-7.
74- Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921-8.
75- Shaw MH, Boyartchuk V, Wong S, Karaghiosoff M, Ragimbeau J, Pellegrini S, et al. A natural mutation in the Tyk2 pseudokinase domain underlies altered susceptibility of B10.Q/J mice to infection and autoimmunity. Proc Natl Acad Sci USA. 2003;100:11594-9. 76- Smith RJ. Therapies for rheumatoid arthritis: hope springs eternal. Drug Discov Today. 2005;10:1598-606.
77- Starr R, Hilton DJ. Negative regulation of the JAK/STAT pathway. Bioessays. 1999;21:47-52.
78- Sugita N, Kimura A, Matsuki Y, Yamamoto T, Yoshie H, Hara K. Activation of transcription factors and IL-8 expression in neutrophils stimulated with lipopolysaccharide from Porphyromonas gingivalis. > @+*D
79- Sweeney SE, Firestein GS. Mitogen activated protein kinase inhibitors: where are we now and where are we going? Ann Rheum Dis. 2006;65:iii83-8.
80- Sweeney SE, Firestein GS. Primer: signal transduction in rheumatic disease - a clinician's guide. Nat Clin Pract Rheumatol. 2007;3:651-60.
@* %( ^ *(" ( diseases. J Clin Invest. 2001;107:7-11.
82- Tan AE. Periodontal maintenance. Aust Dent J. 2009;54:S110-7.
83- Thalhamer T, McGrath MA, Harnett MM. MAPKs and their V& H`'K 2008;47:409-14.
85- Walker JG, Ahern MJ, Coleman M, Weedon H, Papangelis V, Beroukas D, et al. Expression of Jak3, STAT1, STAT4, and STAT6 &Z& #( ^%% ' dendritic cells in seropositive rheumatoid arthritis. Ann Rheum Dis. 2006;65:149-56.
86- Wang Z, Yang H, Tachado SD, Capó-Aponte JE, Bildin VN, Koziel H, et al. Phosphatase-mediated crosstalk control of ERK and p38 MAPK signaling in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:5267-75.
87- Weisman MH. What are the risks of biologic therapy in rheumatoid arthritis? An update on safety. J Rheumatol Suppl. 2002;65:33-8.
88- West K. CP-690550, a JAK3 inhibitor as an immunosuppressant for the treatment of rheumatoid arthritis, transplant rejection, psoriasis and other immune-mediated disorders. Curr Opin Investig Drugs. 2009;10:491-504.
89- Williams DH, Wilkinson SE, Purton T, Lamont A, Flotow H, Murray EJ. Ro 09-2210 exhibits potent anti-proliferative effects on activated T cells by selectively blocking MKK activity. Biochemistry. 1998;37:9579-85.
90- Williams RC, Beck JD, Offenbacher SN. The impact of new technologies to diagnose and treat periodontal disease. A look to the future. J Clin Periodontol. 1996;23:299-305.
91- Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, et al. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969-80.