w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Anti-inflammatory
effects
of
methanolic
extract
of
green
algae
Caulerpa
mexicana
in
a
murine
model
of
ulcerative
colitis
Mariana
A.O.
Bitencourt
a,
Hylarina
M.D.
Silva
a,
Gisely
M.F.
Abílio
b,
George
E.C.
Miranda
c,
Adolpho
M.A.
Moura
d,
João
X.
de
Araújo-Júnior
e,
Ericka
J.D.
Silveira
a,
Barbara
V.O.
Santos
b,
Janeusa
T.
Souto
a,∗aDepartamentodeMicrobiologiaeParasitologiaeDepartamentodeOdontologia,UniversidadeFederaldoRioGrandedoNorte,Natal,RN,Brasil bProgramadePós-graduac¸ãoemProdutosNaturaiseBioativosSintéticosUniversidadeFederaldaParaíba,JoãoPessoa,PB,Brasil
cLaboratoriodeAlgasMarinhas,DepartamentodeEcologiaSistemática,UniversidadeFederaldaParaíba,JoãoPessoa,PB,Brasil dCentrodePesquisaAgeuMagalhaes,Fundac¸ãoOswaldoCruz,CidadeUniversitária,Recife,PE,Brasil
eLaboratoriodeQuímicaMedicinal,ProgramadePós-graduac¸ãoemCiênciasFarmacêuticas,UniversidadeFederaldeAlagoas,Maceió,AL,Brasil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received28April2015 Accepted1October2015 Availableonline26October2015
Keywords: Caulerpamexicana
Greenalgae Inflammation Ulcerativecolitis Cytokines Tissuedamage
a
b
s
t
r
a
c
t
Inflammatoryboweldiseases,whichincludeCrohn’sdiseaseandulcerativecolitis,arecharacterizedby chronicandrelapsedgutinflammation.Caulerpamexicanaisatypeofgreenmarinealgaethatcanbe foundintropicalareas,suchastheBrazilianCoastland.Thesemacrophytesexhibitinvitroandinvivo anti-inflammatorypropertiessuchastheabilitytoreducebothcellmigrationtodifferentsitesandedema formationinducedbychemicalirritants.TheaimofthisstudywastoexaminetheeffectoftheC.mexicana methanolicextractonthetreatmentofcolitisinducedbydextransodiumsulfate.Acuteexperimental colitiswasinducedinBALB/cmicebytreatmentwith3%dextransodiumsulfateorallyfor14days.During this14-dayperiod,C.mexicanamethanolicextract(2mg/kg/day)wasgivenintravenouslyonalternate days.Treatmentwiththemethanolicextractsignificantlyattenuatedbodyweightlossandsevereclinical symptoms.Thiswasassociatedwitharemarkableameliorationofcolonicarchitecturedisruptionand asignificantreductioninpro-inflammatorycytokineproduction.Theseresultssuggestthatthe anti-inflammatoryactionofC.mexicanamethanolicextractoncolorectalsitesmaybeausefultherapeutic approachforinflammatoryboweldiseases.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Inflammatorybowel diseases(IBD)includingCrohn’sdisease (CD)andulcerativecolitis(UC),arecharacterizedbychronicand relapsedgutinflammation.UCisassociatedwithintestinal inflam-mationandoftenresultsinweightloss,diarrheaaccompaniedby blood and mucus,fever, gastric dysmotilityand colon shorten-ing(Hendricksonetal.,2002;ByrneandViney,2006;Cho,2008). Traditionaltherapeuticagents,suchas5-aminosalicylates(5-ASA) andcorticosteroids,arestillusedtotreatIBD.Some immunomod-ulators,such asazathioprine and 6-mercaptopurine,as wellas antibioticsarebecomingimportantinsteroidresistantand steroid-dependent patients (Cho et al., 2011). Considering the serious sideeffects associatedwiththeconventionaltreatment, natural
∗ Correspondingauthor.
E-mail:jtsouto@cb.ufrn.br(J.T.Souto).
products,includingthosefrommarineorigin,havebeenstudiedto aidintheimprovementofIBDclinicalsymptoms(D’Orazioetal., 2012).
Caulerpamexicana isa green marinealgae, fromthe Bryop-sidalesOrderandtheCauleparceaeFamily, whichcanbefound especiallyintropicalareassuchastheBrazilianCoastland(Neto etal.,2008).Thesemacrophyteshavemanybioactivecompounds, suchaspolysaccharides,terpenesandflavonoids,whichhave dif-ferent pharmacological activitieswith antitumor,antiprotozoal, antiviral,antioxidant,antinoceptive,anti-inflammatoryand anti-coagulanteffects(Rochaetal.,2007;Souzaetal.,2009a,b;Matta etal.,2011;Torresetal.,2014).Recently,weshowedthataqueous andmethanolicextractsofC.mexicanahaveinvitroandinvivo
anti-inflammatory propertiesthat reduce thepro-inflammatory cytokine levels in macrophage culture supernatants stimulated withLPSand decreasethecell migration todifferentsitesthat occurs following stimulation with zymosan. The extracts also reduceedemaformationinducedbychemicalirritants(Bitencourt etal.,2011).
http://dx.doi.org/10.1016/j.bjp.2015.10.001
Theoral administrationofdextransulfate sodiumsalt (DSS) iswidelyusedasamodelforUC.Thismodelischaracterizedby mucosalinfiltration ofinflammatory cells, epithelialinjury and ulceration(Kimetal.,2010).Anotherstudyreportedthatmicewith DSS-inducedcolitisexhibitedphenotypiccharacteristicssimilarto humanacuteandchronicUC(Okayasuetal.,1990).Wepreviously showedthatC.mexicanaextractsexhibitanti-inflammatory activ-ity(Bitencourtetal.,2011).Therefore,theaimofthisstudywasto examinethetreatmenteffectsoftheC.mexicanamethanolicextract onDSS-inducedcolitis.
Materialsandmethods
ExtractionandisolationofthemethanolicextractofC.mexicana
ThegreenalgaeCaulerpamexicanawascollectedinthecoastal regionofBessabeach(7◦03′52′′S/34◦49′51′′W),JoaoPessoa,inthe ParaibaStateofBrazilinApril2008.Thespecimenwasidentified byDr.GeorgeEmmanuelCavalcantideMiranda.Voucher speci-mensofC.mexicana(JPB13985)havebeendepositedintheLauro PiresXavierHerbariumattheFederalUniversityofParaiba,Brazil. Freshalgaesampleswerewashed,dried,lyophilizedand exhaus-tivelyextractedwithmethanolinaSoxhletapparatus,toobtainthe methanolicextract.
Animals
MaleBALB/cmice(6–8weeksold)wereusedin the experi-ment.Allmicewerehousedfivepercageataroomtemperature of22±2◦Candundera12h:12hlight/darkcycle.Theyhadfree accesstofood andwater. Groups of fiveanimals wereused in eachtestgroupandcontrolanimalsreceivedsalineonly.Allinvivo
experimentswereapprovedby“EthicsCommitteeonAnimalUse, CEUA/UFRN,” under protocol number 044/2009, which was in accordancewiththeguidelinesoftheBrazilianCommitteefor Ani-malExperimentation(COBEA).
AssessmentofDSS-inducedcolitis
Previously we evaluate the dose-response of C. mexicana
methanolicextractinperitonitis,earswellingandairpouch mod-els,andthedosethatshowedbetteranti-inflammatoryeffectwas 2mg/kg,andthisischosentobeusedincolitismodel(Bitencourt et al., 2011). Our colitis model was developed following the methodologydescribedbyKimetal.(2010)andMelgaretal.(2006) withtheaimofevaluatecolitisduringitsacutephase.Therefore, experimentalcolitiswasinducedbygivingmicedrinkingwaterad libitumcontainingDSS3%(w/v)for14days.Miceweremonitored carefullyeverydaytoconfirmthattheyconsumedapproximately equalvolumesofDSS-containingwater.Fortheexperiment,the miceweredividedintothreeexperimentalgroups.Thefirstgroup waskeptasvehicle-treatedcontrolandtreatedwithsalineinthe samerouteasthemethanolicextract,andthesecondgroupwas givendrinkingwater withDSSonlythroughoutthe experimen-talperiod.The othergroupconsisted ofmice receiving3%DSS andadministeredmethanolicextract(2mg/kg/day)byintravenous routeonalternatedaysfor14days.Themicetreatmentwiththe methanolicextractbeganonthesamedaythatDSSadministration indrinkingwater.
Evaluationofdiseaseactivityindex(DAI)
Themicewerecheckeddailyforcolitisdevelopmentby mon-itoringbodyweight,grossrectalbleedingandstoolconsistency. Theoveralldiseaseseveritywasassessedbyaclinicalscoring sys-temonascaleof0–4. Briefly,thescoringwasasfollows:0,no
weightloss,nooccultbloodinthestoolsandnormalstool con-sistency;1,weightlossof1–5%,nooccultbloodandnormalstool consistency;2,5–10%weightloss,positiveforfecaloccultblood andloosestools;3,10–20%weightloss,positiveforfecaloccult bloodandloosestools;and4,greaterthan20%weightloss,gross rectalbleedinganddiarrhea.
Colontissueculture
Intestinetissuesamplescorrespondingtothetransversecolon wereexhaustivelywashedwithRPMI-1640mediumcontaining2% fetalbovineserumandgentamicinbeforebeingcutintosmaller pieces.Then,approximately0.5cmoftissuewasplacedin1mlof 10%FBScontainingRPMI-1460mediumin24-welltissueculture platesandincubatedfor24hat37◦Cin5%CO2.Thereafter,the supernatantswerecollectedfordeterminationofIL-6,IL-12,TNF-␣, IFN-␥andIL-17byELISA.
ELISAforcytokines
TheIFN-␥,IL-17,IL-12,TNF-␣andIL-6concentrationsinthe cell-freeculturesupernatantsofthecolontissuesweremeasured intriplicate,utilizingasetenzyme-linkedimmunosorbentassay kit(eBioscience,SanDiego,CA,USA)accordingtomanufacturer’s instructions.
Histopathology
Aftertheperiodof inductionofcolitisbyDSS,animalswere euthanized;fragmentsof0.5cmofascendantcolonwereobtained, fixedinasolutionof4%formalinandusedforhistologicalanalysis byconventionaltissuepreparationmethods.Tissuesampleswere viewedunderthelightmicroscope(40×)afterhematoxylinand eosin(H&E)staining.
Results
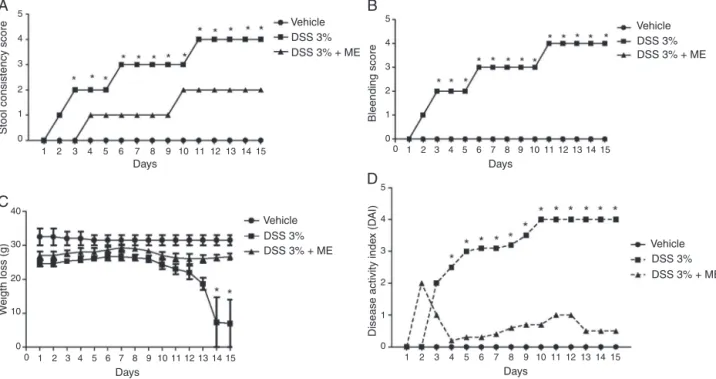
TreatmentofmicewithC.mexicanamethanolicextract decreasedtheDAIaftercolitisinduction
Theparameterusedtoevaluatethedevelopmentofthedisease wastheDAI,whichisassociatedwithobservedclinicalsymptoms suchasweightloss,stoolconsistency,fecalbloodanddiarrhea.In ourmodeltheDAIwasevaluatedindividuallyduringthe14daysof thestudy.ThehighestDAIscorewasobservedinDSSgroup(Fig.1D, closedsquares),whichexhibitedintenseweightlossfromthe11th day,asshowninFig.1C(closedsquares),aswellaspersistentand severediarrhea(Fig.1A)andfecalblood(Fig.1B).Ontheotherhand, thegrouptreatedwiththemethanolicextracthadweight devel-opmentsimilartothegroupthatreceivednormalwater(Fig.1C, closedcycles),lowdiseasedevelopmentrate(Fig.1D,closed tri-angles),withnobloodinthestool(Fig.1B,closedtriangles)and littlediarrhea(Fig.1A,closedtriangles)comparedwiththegroup receivingDSS.Overall,theDAIscoreoftheC.mexicana’s methano-licextract-treatedgroupwassignificantlylowerthanthatofthe DSScontrolgroupfromdayfourfollowingadministrationofDSS.
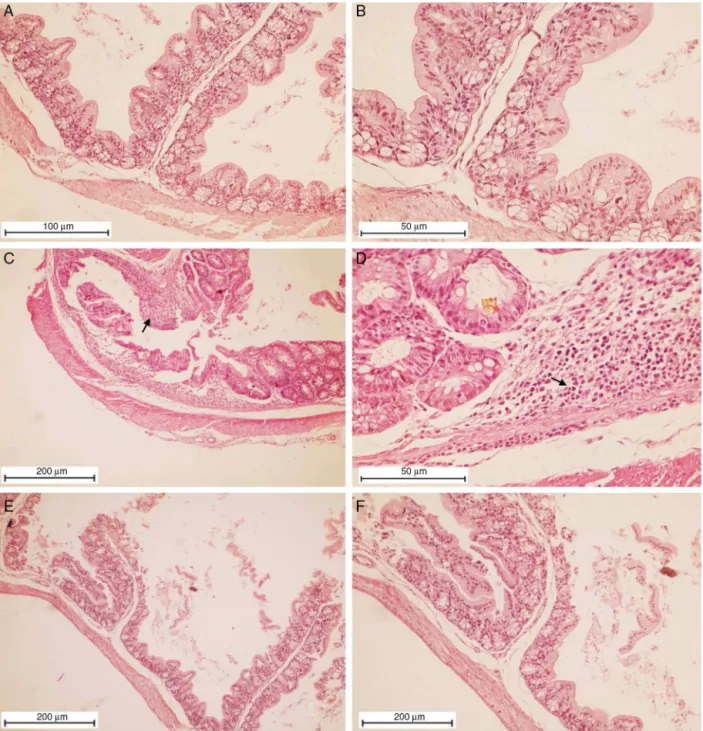
ThetreatmentofmicewithC.mexicanamethanolicextract amelioratedthetissuedamageinthecolonaftercolitisinduction
5
4
3
2
1
0
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
Vehicle DSS 3% DSS 3% + ME
Vehicle DSS 3% DSS 3% + ME
Stool consistency score
Days
A
W
eigth loss (g)
C
5
4
3
2
1
0
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
Vehicle DSS 3% DSS 3% + ME
Bleending score
Days
0 0 10 20 30 40
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
Days
B
5
4
3
2
1
0
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
Vehicle DSS 3% DSS 3% + ME
Disease activity inde
x (D
AI)
Days
D
Fig.1.Thediseaseactivityindex(DAI)inmicetreatedwithCaulerpamexicanamethanolicextractinthecolitismodel.(A)Stoolconsistencyscore,(B)bleedingscore,(C) weightlossscoreand(D)totalDAIscore.Themicewereadministered3%DSSintheirdrinkingwater(adlibitum)for14dayswithorwithoutmethanolicextract(ME) treatment(2mg/kg/dayi.v.upto14days);salinewasusedasanegativecontrol.ChangesinDAIlevelwereevaluateddailythroughoutthe15-dayexperimentalperiod. *p<0.05inthevehicle-treatedcontrolgroupvs.theDSS-inducedcolitisgroup;significancesbetweentreatedgroupsweredeterminedbyANOVA.
waterwithoutDSSpresentednormalcolonstructureswithcrypts and other morphological structures preserved (Fig. 2A and B). On theotherhand, colon samplesfromthe micethat received DSSshowedtypicalinflammatorychangesinthecolon architec-tureand a diffuseinflammatory infiltrate,composedmainlyby mononuclearcells.Atcertainpointstheinflammationwasmore intense,promotingsmallfociofglandulardestructionand mod-erateulceration,cryptdilationandgobletcelldepletionasshown inFig.2CandD.Althoughtheyhadaslightinflammatory reac-tiondecrease,thegrouptreatedwiththemethanolicextracthad an acute inflammatory infiltrate observed on theentire length ofthemucosathat wasintenseinsomeareas,leading to glan-dulardestructionandulcerationpoints.Insomeareas,abscesses withexudatewerepresent,althoughthesewerelessintenseand morefocalwhencomparedwiththegroupthatreceivedonlyDSS (Fig.2EandF).
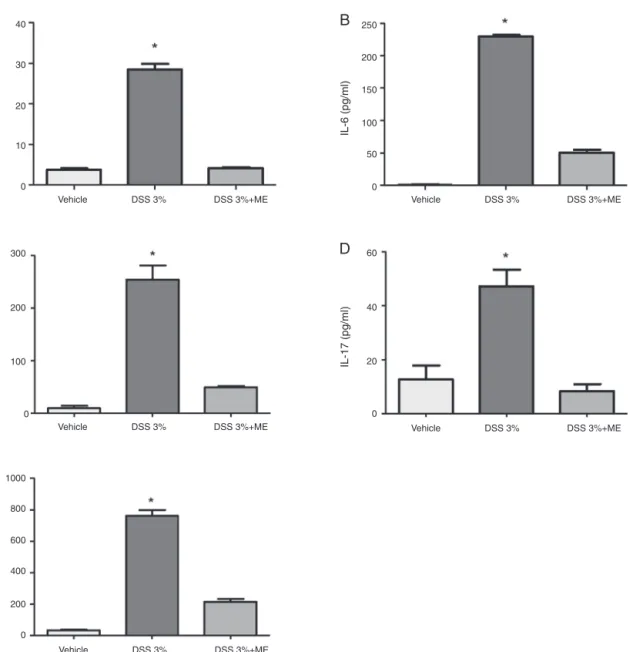
ThetreatmentofmicewithmethanolicextractofC.mexicana
decreasedTh1andTh17cytokineproductioninthecolonculture supernatant
Becausecytokinesareimportantmediatorsofinflammationin colitismodels,thenextstepwastoevaluatetheTh1andTh17 cytokinelevelsintheinvitrocolontissueculturesupernatant.The resultspresented inFig.3 showanintenseproduction ofIFN-␥ (Fig.3A),IL-6(Fig.3B),IL-12(Fig.3C),IL-17A(Fig.3D)andTNF-␣ (Fig.3E)inthecolonculturesupernatantsofmicethatreceived DSSintheirdrinkingwater.However,themicetreatedwiththe methanolicextractofC.mexicanaexhibitedasignificantdecreasein thelevelsofthesecytokinescomparedwiththegroupthatreceived DSSintheirdrinkingwater.
Discussion
UC,whichismajorformofIBD,isanonspecificinflammatory diseaseofthelargeintestine. Inaddition,it is alifelong illness with profound emotional and social impacts, it causes serious
intestinaltractdamageanditsprogressiontochronicUCcanleadto coloncancer(XavierandPodolsky,2007;ShanahanandBernstein, 2009).TheUCinducedinmicebydrinkingwatercontainingDSS leadstoclinicalsymptomssuchasweightloss,diarrhea,fecalblood, mucosalulcerationandcolonicshortening(Kimetal.,2010).Inthis study,weevaluatedthedevelopmentofthediseasefor14days (Melgaretal.,2006).ThehighestDAIscorewasobservedintheDSS groupthatdevelopedintenseweightloss,fecalbleedingand per-sistentandseverediarrhea.Ontheotherhand,theanimalstreated withthemethanolicextractdidnotshowtheclinicalsymptomsof colitisorweightloss.
BecauseUCischaracterizedbyanintenseinflammatory pro-cess, the ability of themethanolic extractto reduce thecolitis clinicalsymptomsdemonstratestheextract’santi-inflammatory activity.Inaddition,thecolonhistologicalanalysisshowsthatthe animalsthatreceivedwaterwithDSSpresentedtypical inflamma-torychangesintheircolonarchitecturethatwasslightlyattenuated whentheanimalsweretreatedwiththemethanolicextract.These effectsonclinicalsymptomsandonhistologicalparameterscould be due to the presence of antioxidant compounds, suchas  -caroteneand␣-tocopherolthathavebeenisolatedfromC.mexicana
(Sousaetal.,2008).Theinhibitionoffreeradicalsbythese com-poundscouldbedecisiveindecreasingtheoxidativestressthat causesdamagetotheintestinalepithelialbarrier(ZhuandLi,2012; Zhengetal.,2012).Furthermore,wereportedpreviouslythatthe
C.mexicanamethanolicextractinhibitscellmigrationinamodel ofperitonitis(Bitencourtetal.,2011)andwebelievethatthis inhi-bitioncouldalsobehappeninginthecolitismodelpresentedhere, contributingtotheattenuationoftheclinicalsymptomsaswell asleadingtotheslightimprovementonthehistologicalanalysis observedbytheinflammatoryinfiltratereductioninthecolonsof
C.mexicanatreatedanimals.
Fig.2. TissuedamageinthecolonofmicewithDSS-inducedcolitistreatedwithmethanolicextractofCaulerpamexicana.Salinewasusedasanegativecontrol(AandB). Representativesectionsofcolontissuefrommicethatreceivedintheirdrinkingwater3%DSSwhichweretreatedwithmethanolicextractofC.mexicana(2mg/kg,i.v.,up to14days),showinganimprovementininflammatoryprocessesandrestorationofmucosaltissue(EandF)andcolonsectionsfromanimalsthatwerenottreatedwiththe extractunderstudy(CandD),showingthedestructionofthemucosaltissuewhichhasbeenreplacedbyinflammatorygranulationtissue(arrow).Histologicalchangeswere determinedbyH&Estaining.OriginalmagnificationinAandFwas100×,inBandDwas200×andinCandEwas40×.
modelisreliableforstudyingthepathogenesisofUCandfor test-ingdrugs orphytochemicals for treatment(Cunha etal., 2013; Luceroetal.,1996).Theliteratureshowsthatthereisanimbalance betweenpro-inflammatoryandanti-inflammatorycytokinesinUC (Papadakis,2004).Pro-inflammatorycytokinesareresponsiblefor determiningthenatureof theimmuneresponse inUC andcan stimulatetherapidsynthesisandsecretionofinflammatory medi-ators,suchasreactiveoxygenandnitrogenspecies,leukotrienes, plateletactivatingfactorandprostaglandins(Neuman,2007).The hyperactivationofimmunecellsisanotherimportantfactorinIBD progressionandisassociatedwithhighlevelsofpro-inflammatory cytokinesfromTh1andTh17patternofimmuneresponse,such as TNF-␣, IL-6 and IFN-␥, which are known to be involved in colondamage(Gálvez,2014).Cytokinesareimportantmediators
ofinflammationandelevatedlevelsofpro-inflammatorycytokines areobservednotonlyintheinflamedgutofIBDpatientsbutalsoin animalswithDSS-inducedcolitis(Neuman,2007).Becauseofthis itisinterestingtoevaluateifthepatternofimmuneresponsein colitismodelinducedbyDSScouldbechangedbytreatmentwith themethanolicextractofC.mexicanaandthispatterncanbe eval-uatedbythetypeofTh1andTh17cytokinesproducedatthesite ofinjury.
0 10 20 30 40
IFN-γ
(pg/ml)
Vehicle DSS 3% DSS 3%+ME
Vehicle DSS 3% DSS 3%+ME
Vehicle DSS 3% DSS 3%+ME
A
0 200 400 600 800 1000
IFN-α
(pg/ml)
Vehicle DSS 3% DSS 3%+ME
E
0 50 100 150 200 250
IL-6 (pg/ml)
B
Vehicle DSS 3% DSS 3%+ME
0 20 40 60
IL-17 (pg/ml)
D
0 100 200 300
IL-12 (pg/ml)
C
Fig.3. LevelsofTh1andTh17cytokinesobservedinculturedcolonictissueofmicewithDSS-inducedcolitistreatedwithCaulerpamexicanamethanolicextract.Micewere administered3%DSSintheirdrinkingwater(adlibitum)for14dayswithorwithoutC.mexicanamethanolicextract(2mg/kg/dayi.v.upto14days);salinewasusedasa negativecontrol.Theproductionof(A)IFN-␥,(B)IL-6,(C)IL-12,(D)IL-17Aand(E)TNF-␣weredeterminedasdescribedinMaterialandMethodssection.Valuesrepresent mean±SD(n=5)*p<0.05inthevehicle-treatedcontrolgroupvs.theDSS-inducedcolitisgroup;significancebetweenthetreatedgroupswasdeterminedbyANOVA.
methanolicextractexertsananti-inflammatoryeffectinUCby neg-ativelyregulatingthepro-inflammatorycytokinelevels.Reduction ofthelevelsoftheseinflammatorymediatorsmayhaveoccurredby twopossiblemechanisms.Thefirstisassociatedwiththecapacity oftheC.mexicanamethanolicextracttodirectlyinhibitcytokine secretionfrominnateimmunecells,aspreviouslydemonstrated (Bitencourtetal.,2011),andthelatterisrelatedtothereductionof cellmigrationtothecolonicmucosaasanindirectmechanismto decreasecytokinelevels.
Currently some studies have shown the efficacy of nat-ural products in the treatment of experimental IBD. These studies demonstrated that curcumin–piperine mixtures in self-microemulsifying(CUR-PIP-SMEDDS),ellargicacid,mangiferinand olipeinameliorate theDSS-induced colitis in mice throughthe reductioninDAIandhistopathologicallesion,and downregulat-inginflammatorymediatorssuchasMPOactivity,IFN-␥,TNF-␣ andIL-6levels,COX-2andiNOSexpressionandinterferingwith the signaling pathways of p38 MAPK, NF-B, and STAT3 (Dou et al., 2014; Li et al., 2015; Giner et al., 2011; Marín et al., 2013).
The green seaweed C. mexicana is composed of numerous compoundsendowedwithpotentialbiologicalactivities( Carrillo-Dominguezet al.,2002).Someof them couldcontribute tothe negativeeffectsmediatedbythemethanolicextractoncytokine secretionatthecolonicmucosaandoninflammatorycell migra-tion to the colon. In fact, compounds like caulerpin and other purifiedindolicalkaloidsfromCaulerpaspeciessuchasCaulerpa racemosa, have proven anti-inflammatoryactivity reducing cell migration in peritonitis models (Bamias and Cominelli, 2007) and sulphated polysaccharides isolated from C. mexicana has anti-inflammatory and antinociception activity (Carneiro et al., 2014).
Therefore,newstudiesareneededtoisolatethecompounds fromtheextract andevaluatetheirmechanismsof action. Fur-thermore,consideringthegreatreductionintheclinicalsymptoms andcytokinesecretion,butonlyslightameliorationof histologi-caldamage,itispossiblethattheassociationoftheC.mexicana
Conclusions
Insummary,ourdatashowthatthetreatmentof micewith methanolicextractofC.mexicanasignificantlyamelioratethe clin-icalsignsobservedinUCanddecreasethelevelsofcytokinefrom Th1(IFN-g,IL-12andTNF-a)andTh17(IL-6andIL-17)immune responsepatternandthisdecreasecouldbeassociatedwith reduc-tionoftissue damageobservedinthecolonoftheanimalsthat receivedDSS.Thereforethisextractappearspromisingforresearch onmetabolitesthatmaybeeffectiveinthetreatmentofUC.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Authors’contributions
MAOB and HMDS (MSc students) contributed to biological studies, runningthe laboratory work, analysisof the data and draftedthepaper.GMFA(MScstudent)contributetoC.mexicana
extractproduction.GECMcontributedincollectingplantsample and identificationand herbarium confection. AMAMcontribute withthemiceproduction.JXAJcontributedtocriticalreadingof themanuscript.EJDScontributetohistopathologyanalysis.JTSand BVOSdesignedthestudy,supervisedthelaboratoryworkand con-tributedtocriticalreadingofthemanuscript.Alltheauthorshave readthefinalmanuscriptandapprovedthesubmission.
Acknowledgments
The authors are grateful to the BNB (482012), CNPq (478655/2007-0)forthejointfundingofthisresearchprojectand byfellowshipsfromCAPES.
References
Bamias,G.,Cominelli,F.,2007.Immunopathogenesisofinflammatorybowel dis-ease:currentconcepts.Curr.Opin.Gastroenterol.23,365–369.
Bitencourt,M.A.O.,Dantas,G.R.,Lira,D.P.,Barbosa-Filho,J.M.,Miranda,G.E.C.,Santos, B.V.O.,Souto,J.T.,2011.AqueousandmethanolicextractsofCaulerpamexicana suppresscellmigrationandearedemainducedbyinflammatoryagents.Mar. Drugs9,1332–1345.
Byrne,F.R.,Viney,J.L.,2006.Mousemodelsofinflammatoryboweldisease.Curr. Opin.DrugDiscov.Dev.9,207–217.
Carneiro,J.G.,Rodrigues,J.A.,deSousaOliveiraVanderlei,E.,Souza,R.B.,Quinderé, A.L.,Coura,C.O.,deAraújo,I.W.,Chaves,H.V.,Bezerra,M.M.,Benevides,N.M., 2014.Peripheralantinociceptionandanti-inflammatoryeffectsofsulphated polysaccharidesfromthealgaCaulerpamexicana.BasicClin.Pharmacol.Toxicol. 115,335–342.
Carrillo-Dominguez,S.,Valdez,M.C.,Ramos,F.R.,Perez-Gil,F.,Rodriguez,I.S.,2002.
MarinealgaeofBajaCaliforniaSur,Mexico:nutritionalvalue.Arch.Latinoam. Nutr.52,400–405.
Cho,E.S.,Shina,J.S.,Noha,Y.S.,Cho,Y.W.,Hong,S.J.,Park,J.H.,Lee,J.Y.,Leea,K.T., 2011.Anti-inflammatoryeffectsofmethanolextractofPatriniascabiosaefoliain micewithulcerativecolitis.J.Ethnopharmacol.136,428–435.
Cho,J.H.,2008.Thegeneticsandimmunopathogenesisofinflammatorybowel dis-ease.Nat.Rev.Immunol.8,458–466.
Cunha,C.R.M.,Neto,S.A.M.,Silva,C.C.,Cortez,A.P.,Gomes,M.N.,Martins,F.I.,Alonso, A.,Rezende,K.R.,Menegatti,R.,Magalhães,M.T.Q.,Valadares,M.C.,2013. 4-Nerolidylcathecolandsyntheticanalogues:antioxidantactivityandtoxicity evaluation.Eur.J.Med.Chem.62,371–378.
D’Orazio,N.,Gammone,M.A.,Gemello,E.,Girolamo,M.,Cusenza,S.,Riccioni,G., 2012.Marinebioactives:pharmacologicalpropertiesandpotentialapplications againstinflammatorydiseases.Mar.Drugs10,812–833.
Dou,W.,Zhang,J.,Ren,G.,Ding,L.,Sun,A.,Deng,C.,Wu,X.,Wei,X.,Mani,S.,Wang,Z., 2014.Mangiferinattenuatesthesymptomsofdextransulfatesodium-induced colitisinmiceviaNF-BandMAPKsignalinginactivation.Int. Immunopharma-col.23,170–178.
Giner, E., Andújar, I., Recio, M.C., Ríos, J.L., Cerdá-Nicolás, J.M., Giner, R.M., 2011.Oleuropeinamelioratesacutecolitisinmice.J.Agric.FoodChem.59, 12882–12892.
Gálvez,J.,2014.RoleofTh17cellsinthepathogenesisofhumanIBD.Inflammation 25,1–14.
Hendrickson,B.A.,Gokhale,R.,Cho,J.H.,2002.Clinicalaspectsandpathophysiology ofinflammatoryboweldisease.Clin.Microbiol.Rev.15,79–94.
Kim,S.J.,Kim,M.C.,Um,J.Y.,Hong,S.H.,2010.Thebeneficialeffectofvanillicacidon ulcerativecolitis.Molecules15,7208–7217.
Li,Q.,Zhai,W.,Jiang,Q.,Huang,R.,Liu,L.,Dai,J.,Gong,W.,Du,S.,Wu,Q.,2015.
Curcumin–piperinemixturesinself-microemulsifyingdrugdeliverysystemfor ulcerativecolitistherapy.Int.J.Pharm.490,22–31.
Lucero,M.J.,Vigo,J.,León,M.J.,Martin,F.,Sánchez,J.A.,1996.Therapeuticefficacy ofhydrophilicgelsof␣-tocopherolandtretioininskinulcersinducedby adri-amycinhydrochloride.Int.J.Pharm.127,73–83.
Marín,M.,Giner,R.M.,Ríos,J.L.,Recio,M.C.,2013.Intestinalanti-inflammatory activ-ityofellagicacidintheacuteandchronicdextranesulfatesodiummodelsof micecolitis.J.Ethnopharmacol.150,925–934.
Matta,C.B.B.,Souza,E.T.,Queiroz,A.C.,Lira,D.P.,Araújo,M.V.,Cavalcante-Silva, L.H.A.,Miranda,G.E.C.,Araújo-Júnior,J.X.,Barbosa-Filho,J.M.,Santos,B.V.O., Alexandre-Moreira,M.S.,2011.Antinociceptiveandanti-inflammatoryactivity fromalgaeofthegenusCaulerpa.Mar.Drugs9,307–318.
Melgar,S.,Karlsson,A.,Michaelsson,E.,2006.Acutecolitisinducedbydextran sulfatesodiumprogressestochronicityinC57BL/6butnotinBALB/cmice: corre-lationbetweensymptomsandinflammation.Am.J.Physiol.288,G1328–G1338.
Neto,J.T.B.B.,Rodrigues,J.A.G.,Pontes,G.C.,Farias,W.R.L.,2008.Sulfated polysac-charidesofthealgaCaulerpasertularioides(GMEL.)Howe:analysisofmethods ofprecipitation.Rev.Bras.Eng.Pesca3,50–62.
Neuman,M.G.,2007.Immunedysfunctionininflammatoryboweldisease.Transl. Res.149,173–186.
Okayasu,I.,Hatakeyama,S.,Yamada,M.,Ohkusa,T.,Inagaki,Y.,Nakaya,R.,1990.
Anovelmethodintheinductionofreliableexperimentalacuteandchronic ulcerativecolitisinmice.Gastroenterology98,694–702.
Papadakis,K.A.,2004.Chemokinesininflammatoryboweldisease.Curr.Allergy AsthmaRep.4,83–89.
Rocha,F.D.,Pereira,R.C.,Kaplan,M.A.C.,Teixeira,V.L.,2007.Naturalproductsfrom marineseaweedsandtheirantioxidantpotential.Braz.J. Pharmacogn.17, 631–639.
Shanahan,F.,Bernstein,C.N.,2009.Theevolvingepidemiologyofinflammatory boweldisease.Curr.Opin.Gastroenterol.25,301–305.
Sousa,M.B.,Pires,K.M.S.,Alencar,D.B.,Sampaio,A.H.,Saker-Sampaio,S.,2008. ␣-and-carotene,and␣-tocopherolinfreshseaweeds.Ciênc.Tecnol.Aliment.28, 953–958.
Souza, E.T., Lira, D.P., Queiroz, A.C., Silva, D.J.C., Aquino, A.B., Mella, E.A.C., Lorenzo,V.P.,Miranda,G.E.C.,Araújo-Júnior,J.X.,Chaves,C.O.M.,Barbosa-Filho, J.M.,Athayde-Filho,P.F.,Santos,B.V.O.,Alexandre-Moreira,M.S.,2009a.The antinoceptiveandanti-inflammatoryactivitiesofcaulerpin,abisindolealkaloid, isolatedfromseaweedsofthegenusCaulerpa.Mar.Drugs7,689–704.
Souza,E.T.,Queiroz,A.C.,Miranda,G.E.C.,Lorenzo,V.P.,Silva,E.F.,Freire-Dias,T.L.M., Cupertino-Silva,Y.K.,Melo,G.M.A.,Santos,B.V.O.,Chaves,M.C.O., Alexandre-Moreira,M.S.,2009b.Antinociceptiveactivitiesofcrudemethanolicextractand phases,n-butanolic,chloroformicandethylacetatefromCaulerparacemosa (Caulerpaceae).Braz.J.Pharmacogn.19,115–120.
Torres,F.A.E.,Passalacqua,T.G.,Velasquez,A.M.A.,Souza,R.A.,Colepicolo,P., Gram-inha,M.A.S.,2014.Newdrugswithantiprotozoalactivityfrommarinealgae:a review.Braz.J.Pharmacogn.24,265–276.
Xavier,R.J.,Podolsky,D.K.,2007.Unravellingthepathogenesisofinflammatory boweldisease.Nature448,427–434.
Zheng,J.,Chen,Y.,Yao,F.,Chen,W.,Shi,G.,2012.Chemicalcompositionand antiox-idant/antimicrobialactivitiesinsupercriticalcarbondioxidefluidextractof Gloiopeltistenax.Mar.Drugs10,2634–2647.