ESCUELA TÉCNICA SUPERIOR DE INGENIEROS
INDUSTRIALES Y DE TELECOMUNICACIÓN. SANTANDER
INSIGHTS INTO THE ZSM-5 ZEOLITE MEMBRANE.
PERVAPORATION PERFORMANCE FOR ALCOHOL
DEHYDRATION
TRABAJO FIN DE MÁSTER (TFM)
MÁSTER UNIVERSITARIO EN INGENIERÍA QUÍMICA
POR LA UNIVERSIDAD DE CANTABRIA
Y LA UNIVERSIDAD DEL PAÍS VASCO/EUSKAL HERRIKO
UNIBERTSITATEA
Alumno: Paloma Ortiz Albo Fecha: Septiembre 2017 Firma
INDEX
ABSTRACT/RESUMEN 1
1. INTRODUCTION 3
1.1. ZEOLITE MEMBRANES 3
1.1.1. Zeolite membranes in pervaporation and dehydration of alcohols 3
1.1.2. Industrial applications of zeolite membranes 12
1.1.3. Potential applications 13
1.2. MORPHOLOGY AND STRUCTURE OF ZEOLITE MEMBRANES 15 1.3. TRANSPORT PHENOMENA THROUGH ZEOLITE MEMBRANES 17
1.4. OBJECTIVES 18
2. METHODOLOGY 19
2.1. ZSM-5 ZEOLITE MEMBRANES 19
2.2. PV EXPERIMENTS FOR ALCOHOLS DEHYDRATON 21
2.2.1. Pervaporation set-up and experimental plan 21
2.2.2. Analytical methods and calculations 22
2.3. MODELLING OF STRUCTURE AND adsorption IN ZEOLITES 24
3. RESULTS AND DISCUSSION 27
3.1. PERVAPORATIVE SEPARTION OF ALCOHOL/WATER MIXTURES
USING ZSM-5 ZEOLITE MEMBRANES 27
3.2. SIMULATIONS OF COMPETIVE ADSORPTION PHENOMENA 37 3.3. POST-TREATMENT AND RECOVERY OF PV PERFORMACE 41
4. CONCLUSIONS/CONCLUSIONES 43
5. NOMENCLATURE 45
FIGURES INDEX
TABLES INDEX
ABSTRACT/RESUMEN
Zeolite membranes are attracting attention as alternative separation technologies to conventional distillation especially for separation of close-boiling mixtures. In addition, zeolite membranes can also be integrated in membrane reactors. A few types of zeolite membranes, such as A-type and T-type, have been commercialized and applied in the industry for dehydration of solvents. However, applications of A-type and T-type membranes are limited due to their poor stability in acidic media and water-rich systems. To overcome these limitations, hydrophilic ZSM-5 zeolite membranes have been developed, although their long-term performance, the mass transport mechanism and the micro-structure of the membrane are still far from full understanding.
Therefore, the main objective of this master thesis was to study the performance of the synthesized ZSM-5 membranes in long-term pervaporation experiments, considering alcohol dehydration processes as application. The separation performance was studied considering different types of alcohol and composition of their aqueous mixtures. The post-treatment of the membrane was also investigated to recover the membrane performance.
Lastly, molecular simulations of competitive adsorption of water and alcohol molecules on ZSM-5 zeolitic pores using grand canonical ensemble molecular dynamics were studied. By comparing the information obtained by experiments to the results of the molecular simulations, the membrane micro-structure and the transport mechanism were discussed.
RESUMEN
Las membranas de zeolitas han surgido como interesante alternativa a tecnologías convencionales como la destilación en procesos como la separación de mezclas con compuestos con puntos de volatilidad cercanos. Además, las membranas basadas en zeolitas tienen el potencial de ser integradas en reactores. Unos pocos tipos de membranas, como las de tipo A y tipo T, han sido comercializadas y aplicadas en proceses industriales para la deshidratación de disolventes. Sin embargo, las aplicaciones de las membranas de zeolitas tipo A y tipo T son limitadas debido a su baja estabilidad en medio ácido y en sistemas con altos contenidos de agua. Para poder superar estas limitaciones, membranas hidrofílicas ZSM-5 han sido desarrolladas, aunque su operación a largo plazo, el mecanismo de transporte de materia y su microestructura se encuentran aún lejos de ser completamente comprendidos.
Por esta razón, el principal objetivo de este trabajo de fin de máster ha sido estudiar la operación de membranas sintéticas ZSM-5 en experimentos de larga duración, considerando la deshidratación de alcoholes como aplicación. El rendimiento de la separación ha sido estudiado considerando distintos tipos de alcoholes y distintas composiciones en la mezcla acuosa. El post-tratamiento ha sido también evaluado para poder recuperar el rendimiento de la membrana.
Por último, simulaciones moleculares de la adsorción competitiva de las moléculas de agua y alcohol en los poros zeolíticos de ZSM-5 usando el método “grand canonical ensemble molecular dynamics” han sido evaluadas. Mediante la comparación de la información obtenida mediante el trabajo experimental y los resultados de las simulaciones moleculares, la microestructura de la membrana y el mecanismo de transporte han sido evaluados.
1.
INTRODUCTION
1.1.
ZEOLITE MEMBRANESZeolites are crystalline microporous aluminosilicates with remarkable properties such as molecular sieving, preferential adsorption, and shape-selective catalysis. They are systems of regular channels of molecular dimensions with well-defined micropores. Their common structure is composed by silicon and aluminum atoms sharing oxygen atoms in a tetrahedral structure. However, other atoms can be found in a zeolite, such as B, Mg, P, Mn, Fe, among many others. Thus, the International Zeolite Association (Baerlocher, Ch.; McCusker 2017) gathers more than 200 different zeolites with different code and characteristics.
Zeolites can present hydrophobic or hydrophilic behavior, which can be related to their Si/Al ratio. The hydrophilicity of the zeolite comes attached to lower Si/Al ratios, which can be tuned by the synthesis procedure. However, the chemical resistance at acidic media is enhanced with higher Si/Al ratios, having compromising properties to consider (Bowen et al. 2004; Caro et al. 2005).
The interest of the synthesis and production of this type of inorganic material also relies on their attractive chemical, physical, and mechanical stability. Although zeolites have been applied by the petrochemical industry as catalyst in cracking and hydro-cracking processes (Csicsery 1984; Ward 1993), their separation properties have made them more than attractive to be supported in ceramic materials and use them as selective membranes. By the end of 1980s, the first attempts to develop zeolite membranes appeared and the interest in this topic has been increasing since then (Bowen et al. 2004; Gascon et al. 2012). The regular pore network of zeolite membranes gives them interesting sieving properties of molecular dimensions. Furthermore, zeolite membranes take advantage of adsorption and diffusion differences to separate mixtures.
Therefore, zeolite membranes create a wide and complex number of interesting possibilities for which a correct knowledge of the mass transport through the selective layer is required.
1.1.1. Zeolite membranes in pervaporation and dehydration of alcohols
Pervaporation (PV) process has been widely studied for chemical separation. The liquid feed mixture is in contact with the membranes and the product permeates through the membrane under a partial pressure gradient, that is usually created by applying vacuum or a sweep gas in the permeate side, (Baker 2007; Feng & Huang 1997) to reach the permeate side of the membrane as a vapor. PV has significant advantages in the separation of azeotropic and close boiling point mixtures since it can be applied to break this azeotrope by a mechanism other than liquid-vapor equilibria, without the high cost and energy consumption of the traditional distillation processes. If the feed mixture is vapor instead of liquid, the technology is then called vapor permeation (VP).
attracting applications are being studied for pervaporation processes such as separation of anhydrous organic mixtures (Bowen et al. 2004; Gascon et al. 2012; Kita et al. 1997; Urtiaga et al. 2006), application to esterification reactions (Li et al. 2009; Zhu et al. 2013). More extended information about potential applications under research is included in Section 2.1.3.
Among the commercialized applications of zeolite membranes, dehydration of alcohols has been one important operation in industrial processes. Multiple types of industries use effluents in which solvent dehydration is needed as purification and/or recovery step. Traditionally, distillation is the most applied technique for alcohol separation from aqueous solutions. However, since alcohols (like ethanol) form an azeotrope when high concentrations are desired, a more energy intensive technology is needed to reach the wanted purity, as azeotropic distillation (Abels et al. 2013; Bowen et al. 2004; Caro & Noack 2008; Chapman et al. 2008; Gascon et al. 2012; Wee et al. 2008).
Therefore, the combination of pervaporation and vapor permeation with distillation columns to obtain high concentrated ethanol gathered special interest (Urtiaga et al. 2006; Gorri et al. 2005). Furthermore, the interest of producing bioethanol for fuel usage has therefore increased the attention of using PV membranes. Their usage in biorefinery processes has been considered for the different steps included in bioethanol production. The fermentation product with a low concentration of ethanol (5-10%) needs to be concentrated up to >99% for fuel grade purity. From distillation, it is possible to obtain 80-90% of ethanol, and then combining pervaporation with zeolite membranes, the desired product can be obtained (Abels et al. 2013; Sato et al. 2008; Zhang et al. 2014). Cardona and Sanchez (2007) calculated the savings of integrating pervaporation into these common purification processes to be of 12%.
On the other hand, not only dehydration of ethanol has been attractive for zeolite membranes. The recovery of other alcohols from fermentation broths (Nomura et al. 2002) and the dehydration of solvents such as acetone and tetrahydrofuran (Urtiaga et al. 2004) have also been an interesting application for zeolite membranes and pervaporation systems.
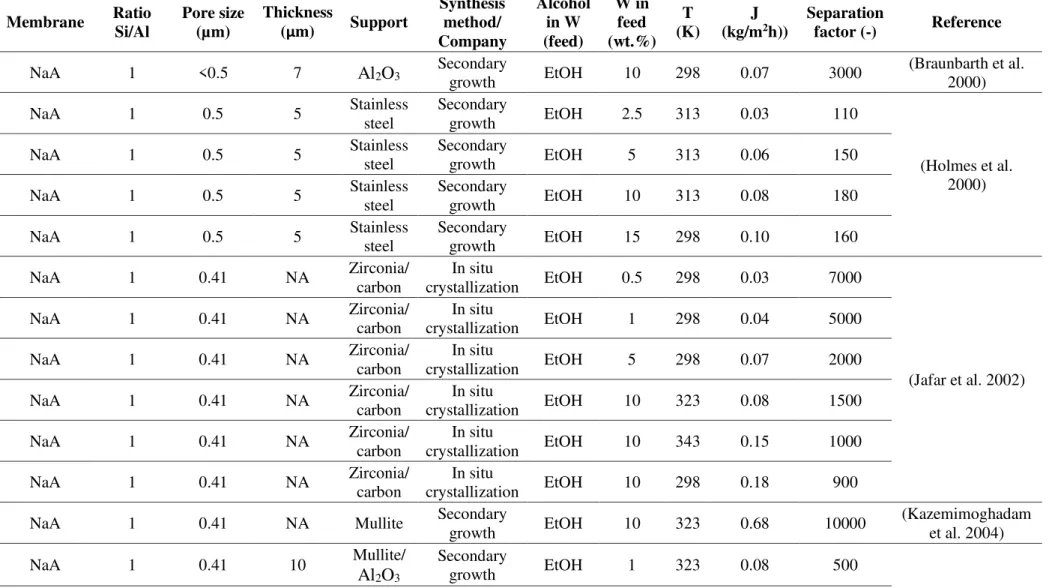
Table 1 summarizes a literature survey about zeolite membranes applied for alcohol and solvent dehydration.
The most widely studied supports were alumina, -alumina, and stainless steel. The alcohol solutions were analyzed in every case with water content under 10wt.%, which corresponds to the concentrations obtained under one simple distillation step. NaA zeolite membranes have been the most evaluated in ethanol dehydration. Okamoto and Kita (1994) developed a patent for NaA zeolite membrane for liquid separation through Mistui Chemical & Shipbuilding Co., Ltd. The water flux was as high as of 2.52 kg·m-2·h-1 and
a water/ethanol separation factor of 10000 was obtained by these membranes, synthesized as a zeolite NaA coating layer on a mullite support. A similar method was applied by Okamoto et al. (2000) using alumina supports. The authors analyzed the influence of ethanol concentration and temperature in the feed solution. The separation factors obtained were over 1200 in every case. Zhang et al. ( 2014) analyzed the stability over time for bioethanol and butanol dehydration process using a self-synthesized NaY membrane and studied the performance properties using different crystallization time of the zeolite. Ethanol dehydration gave similar flux results (2.1 kg·m-2·h-1) to NaA
differences of pore size between NaA zeolite (0.41 nm) and NaY zeolite (0.67 nm). Other zeolitic structures, such as FAU, MOR and ZSM-5, have been attracting attention specially in terms of synthesis method and their membrane performance for other alcohols dehydration. Since both types have pore sizes larger than NaA zeolite (0.65x0.70 nm for MOR, and 0.53x0.56 nm for ZSM-5), they are candidates for larger alcohols dehydration, e.g. propanol and butanol solutions. Attending to the new synthesis method, fluoride route and secondary growth methods without structure-directing agent (SDA) or organic template have been the latest evaluated for these new membranes (Zhang et al. 2014; Yao et al. 2017; Li et al. 2015; Zhu et al. 2012)
Besides the commented applications, as consequence of the poor stability of the commercialized NaA membranes in acidic media and water-rich solutions, other types of zeolite membranes have been evaluated to overcome this disadvantage. The influence of acid into the permeation properties has been studied by Hasegawa et al. (2010). Adding sulphuric acid into the feed solution destroyed the zeolite layer of NaA-type membranes, converting it into an amorphous silica-alumina layer. This phenomenon was appreciated when only 0.1 mL of the acid was added, although it was evaluated up to 10 mL. The dealumination of A-type zeolites studied by Rakoczy and Traa ( 2003) showed how NaA zeolite framework suffered a strong dealumination by trace content of acid and under high-concentration of water (> 20wt.%). Among the multiple types of zeolites, MFI and MOR zeolites were the mosts studied to overcome the chemical resistance to acid media (Zhu et al. 2013; Li et al. 2003a; Li et al. 2009; Gascon et al. 2012).
Table 1. Summary of zeolite membranes for alcohol dehydration processes.
Membrane Ratio Si/Al Pore size (µm) Thickness (
µm) Support
Synthesis method/ Company Alcohol in W (feed) W in feed (wt.%) T
(K) (kg/mJ 2h)) Separation factor (-) Reference
NaA 1 <0.5 7 Al2O3 Secondary growth EtOH 10 298 0.07 3000 (Braunbarth et al. 2000)
NaA 1 0.5 5 Stainless
steel
Secondary
growth EtOH 2.5 313 0.03 110
(Holmes et al. 2000)
NaA 1 0.5 5 Stainless
steel
Secondary
growth EtOH 5 313 0.06 150
NaA 1 0.5 5 Stainless
steel
Secondary
growth EtOH 10 313 0.08 180
NaA 1 0.5 5 Stainless
steel
Secondary
growth EtOH 15 298 0.10 160
NaA 1 0.41 NA Zirconia/ carbon
In situ
crystallization EtOH 0.5 298 0.03 7000
(Jafar et al. 2002) NaA 1 0.41 NA Zirconia/ carbon crystallization In situ EtOH 1 298 0.04 5000
NaA 1 0.41 NA Zirconia/ carbon
In situ
crystallization EtOH 5 298 0.07 2000
NaA 1 0.41 NA Zirconia/ carbon crystallization In situ EtOH 10 323 0.08 1500
NaA 1 0.41 NA Zirconia/ carbon crystallization In situ EtOH 10 343 0.15 1000
NaA 1 0.41 NA Zirconia/ carbon crystallization In situ EtOH 10 298 0.18 900
NaA 1 0.41 NA Mullite Secondary growth EtOH 10 323 0.68 10000 (Kazemimoghadam et al. 2004)
NaA 1 0.41 10 Mullite/ Al
2O3
Secondary
NaA 1 0.41 10 Mullite/ Al2O3
Secondary
growth EtOH 5 323 0.40 4800
(Kondo, M; Komori, M.; Kita, H.; Okamoto 1997)
NaA 1 0.41 10 Mullite/
Al2O3
Secondary
growth EtOH 10 348 0.77 46000
NaA 1 0.41 10 Mullite/ Al
2O3
Secondary
growth EtOH 10 393 2.16 42000
NaA 1 0.41 10 Mullite/
Al2O3
Secondary
growth EtOH 10 348 8.46 47000
NaA 1 0.41 NA Mullite
Mitsui Chemical & Shipbuilding
EtOH 10 348 2.52 10000 (Okamoto,K.; Kita 1994)
NaA 1 0.41 30 Al2O3 Secondary growth EtOH 0.5 348 0.01 5100
(Okamoto et al. 2000) NaA 1 0.41 30 Al2O3 Secondary growth EtOH 2 348 0.60 8000
NaA 1 0.41 30 Al2O3 Secondary growth EtOH 5 348 1.10 16000
NaA 1 0.41 30 Al2O3 Secondary growth EtOH 10 348 2.16 10000
NaA 1 0.41 30 Al2O3 Secondary growth EtOH 10 323 0.80 1700
NaA 1 0.41 30 Al2O3 Secondary growth EtOH 10 308 0.31 1200
NaA 1 0.4 3.5 TiO2 crystallization In situ EtOH 5 318 0.86 54000 (van den Berg et al. 2003)
NaX 1.3 0.74 20 NA crystallization In situ EtOH 10 348 0.99 410
X-type 1.3 0.74 NA NA In situ
crystallization EtOH 10 348 0.90 360
Y-type 1.9 0.74 NA NA In situ
crystallization EtOH 10 348 1.64 130
T-type 3.6 0.36x0.51 NA α-Al2O3 crystallization In situ EtOH 10 348 0.60 4400
MOR 7.5 0.67x0.7 30 α-Al2O3 crystallization In situ EtOH 10 293 0.14 3000 (Zhang et al. 2002)
NaA 1 0.42 5 - 10 Stainless steel
SMART Chemical Company Ltd.Co
tButOH 10 333 1.69 16000 (Gallego-Lizon et al. 2002)
ZSM-5 11.9 0.53x0.56 1.5 α-Al2O3 Secondary growth 2-PrOH 10 348 3.28 690
(Li et al. 2003b) MOR 5.1 0.65x0.70 3 α-Al2O3 Secondary growth 2-PrOH 10 348 0.26 330
Y-type 2.5 0.74 NA Al2O3 crystallization In situ THF 10 333 2.81 230
(Shiguang Li et al. 2001) MOR 6 0.65x0.70 NA Al2O3 crystallization In situ THF 6.7 333 1.26 11
ZSM5 25 0.53x0.56 NA Al2O3 crystallization In situ THF 6.7 333 0.90 3.5
A-type 1 0.42 NA Al2O3 crystallization In situ THF 6.7 333 0.67 49
MOR 10.2 0.65x0.70 35 α-Al2O3 crystallization In situ 1-PrOH 10 348 0.20 1800
(Lin et al. 2000) MOR 10.2 0.65x0.70 35 α-Al2O3 crystallization In situ 2-PrOH 10 348 0.21 190
NaA 1 0.41 30 Al2O3 Secondary growth MeOH 10 323 0.58 2100
(Okamoto et al. 2000) NaA 1 0.41 30 Al2O3 Secondary growth EtOH 10 348 2.16 10000
NaA 1 0.41 30 Al2O3 Secondary growth 1-PrOH 10 348 1.98 18000
NaA 1 0.41 30 Al2O3 Secondary growth 2-PrOH 10 348 1.77 10000
NaA 1 0.41 30 Al2O3 Secondary growth Acetone 10 323 0.90 5600
NaA 1 0.41 30 Al2O3 Secondary growth Dioxane 10 333 1.80 9000
NaA 1 0.41 30 Al2O3 Secondary growth DMF 10 333 0.90 9000
NaA 1 0.41 5 - 10 Ceramic
SMART Chemical Company Ltd.Co
THF 7
318-328 0.43-0.98 1420 (Urtiaga et al. 2003)
NaA 1 0.41 5 - 10 Ceramic
SMART Chemical Company Ltd.Co
Acetone 3 313-318
0.13-0.314 50 (Urtiaga et al. 2003)
MOR 7.5 0.65x0.70 30 α-Al2O3 crystallization In situ MeOH 10 298 0.46 800 (Zhang et al. 2002)
Silica >10000 0.3-0.5 0.1 Al2O3 Dip-coating EtOH 10 353 1.00 800
(Sekuli et al. 2005) Silica >10000 0.3-0.5 0.1 Al2O3 Dip-coating ButOH 2- 5 353 1.00 1000
Silica >10000 0.3-0.4 10-20 α-Al2O3 Pervatech BV IPA 15-25 343 2-3.2 NA (Urtiaga et al. 2004)
Mordenite NA 0.65 5 α-Al2O3 Secondary growth EtOH 15 423 0.06 60 (Navajas et al. 2007)
Ge-ZSM-5 Si/Ge=40 0.53 NA Steel In situ
crystallization EtOH 5 303 0.22 47 (Bowen et al. 2002)
FAU 1.1 0.74 2-3 α-Al2O3 Secondary growth EtOH 10 348 3.1 16
(Kumakiri et al. 2014) FAU 2.6 0.74 2-3 α-Al2O3 Secondary growth EtOH 10 348 3.8 50
NaY 1.8 0.67 6 Mullite
Fluoride mediated gel
with SDA-free
MeOH 10 333 0.6 15
(Zhang et al. 2014)
NaY 1.8 0.67 6 Mullite
Fluoride mediated gel
with SDA-free
EtOH 10 348 2.1 105
NaY 1.8 0.67 6 Mullite
Fluoride mediated gel
with SDA-free
IPA 10 348 2.5 360
NaY 1.8 0.67 6 Mullite
Fluoride mediated gel
with SDA-free
nButOH 10 348 2.6 1000
ZSM-5 NA NA NA α-Al2O3
Dip-coating seeded crystallization
IPA 10 348 1.68 8000 (Yao et al. 2017)
ZSM-5 NA NA NA α-Al2O3
Fluoride mediated gel
with SDA-free
ZSM-5 15 0.53x0.56 2 Mullite
Secondary growth with
SDA-free
IPA 10 348 0.85-1.85 200-5300 (Zhu et al. 2012)
ZSM-5 15 0.53x0.56 2 Mullite
Secondary growth with
SDA-free
EtOH 10 348 1.30 1700 (Zhu et al. 2012)
ZSM-5 15 0.53x0.56 2 Al2O3
Secondary growth with
SDA-free
IPA 10 348 0.80-3.25 150-6300 (Zhu et al. 2012)
ZSM-5 15 0.53x0.56 10 Mullite
Fluoride mediated gel
with SDA-free
EtOH 10 348 1.48 540 (Li et al. 2009)
MOR 7.7 0.65x0.70 <10 Mullite
Fluoride mediated gel
with SDA-free
EtOH 10 348 0.39 1000 (Li et al. 2009)
Abbreviations from Table 1: 1-PrOH: 1-propanol
2-PrOH: 2-propanol
2-ButOH: 2-butanol
DMF: dimethylformamide EtOH: ethanol
IPA: iso-propanol
nButOH: n-butanol
NA: not available
MeOH: methanol
tButOH: tert-butanol
1.1.2. Industrial applications of zeolite membranes
As main consequence of the constant technological improvement, membrane technology has offered many advantages over existing processes in terms of selectivity, energy consumption and flexibility by its modular design (Baker 2007). Furthermore, zeolite membranes have proved to be more effective in specific applications as in pervaporation processes, as previously commented.
The interest of using zeolite membranes for pervaporation or vapor permeation relies on their physical and chemical stability. Polymeric membranes have been also widely studied (Chapman et al. 2008), but they are limited by their insufficient stability under harsher operational conditions. Zeolite membranes can be applied under high temperatures with chemical and mechanical stability, with not swelling and higher chemical stability to a wider range of organic solvents (Bowen et al. 2004).
On the other hand, zeolite membranes have generally more complex synthesis processes having high importance the reproducibility of manufacturing and the production costs, which in polymeric membranes are generally lower. However, the important part of the cost of zeolite membranes is related to the ceramic support, which was estimated to be around 70% of total cost of the membrane (Caro et al. 2005). Therefore, different structures and sizes of the support are currently being studied to optimize the geometry and effective area of the membrane modules, while their robustness and stability make them interesting for industrial applications. O’Brien-Abraham and Lin ( 2010) summarized the most significant challenges for a zeolite membrane to be commercialized:
• High flux and selectivity • Reproducibility of synthesis
• Long-term stability without regeneration • High-temperature sealing
• Scaling-up of membrane synthesis and modules • Cost of membranes and modules
• Understanding of transport and separation mechanism through intramolecular and intermolecular paths
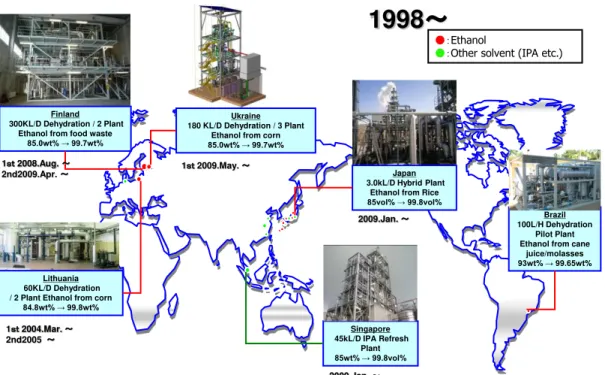
Figure 1. Current plants using zeolite membranes for alcohol and other solvents dehydration of Mitsui Zosen Machinery & Service Inc. (personal communication: Prof. Kumakiri, 2017).
Added to the Japanese company, others have commercialized zeolite membranes in the last decades: Busan Nano-Research Institute Inc. (subsidiary of Mitsui), SMART (U.K.), Inoceramic (Germany), fundamentally. Gascon et al. (2012) gathered information about other diverse interesting separations. They summarized the historical development of zeolite membranes as NaA and LTA-type for dehydration of alcohols, alone and in combination with distillation. Besides, several research works have widely tested the good performance of NaA commercial membranes for dehydration of several industrial effluents. Besides the studies related to ethanol dehydration (Hasegawa et al. 2010; Gascon et al. 2012; Kondo, M; Komori, M.; Kita, H.; Okamoto 1997), Urtiaga et al. (2003) tested the performance of Na-A type manufactured by SMART Chemical Company Ltd. (U.K.) for THF and acetone dehydration. Gallego-Lizon et. al (2002) studied the dehydration of tert-butanol by polymeric, microporous silica and zeolite membranes. Tert-butanol is a relatively large short chain alcohol which is applied as solvent in many industrial synthesis, and therefore, its recovery is a common demand. Commercial NaA zeolite membrane from SMART Chemical Company Ltd. (U.K.) showed the largest selectivity for different operational temperatures.
1.1.3. Potential applications
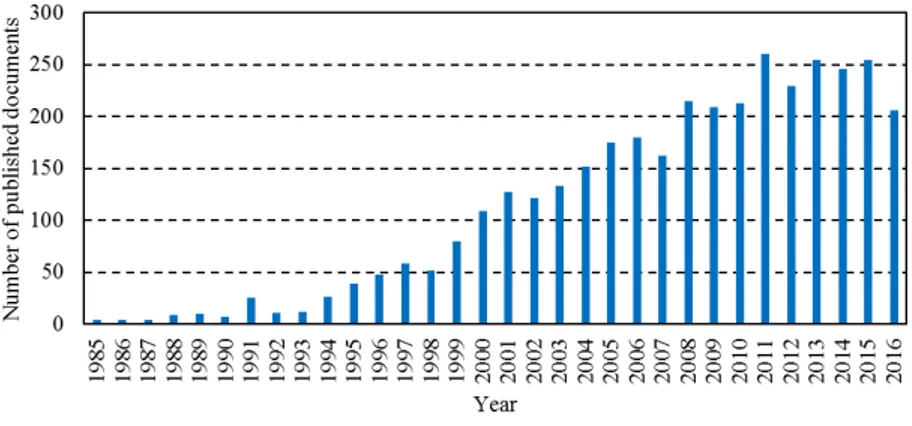
Alongside the successful commercialization of NaA zeolite membranes, this group of materials have been studied for several decades. Figure 2 shows the number of research studies published in the period 1985 to 2016 for “zeolite membrane” search (Scopus database). The growth of the interest for these membranes started around mid-90s, being currently stabilized although still with high interest, according to the number of publications per year.
Singapore 45kL/D IPA Refresh
Plant 85wt% → 99.8vol%
Japan 3.0kL/D Hybrid Plant
Ethanol from Rice 85vol% → 99.8vol%
Brazil 100L/H Dehydration
Pilot Plant Ethanol from cane
juice/molasses 93wt% → 99.65wt% Finland
300KL/D Dehydration / 2 Plant Ethanol from food waste
85.0wt% → 99.7wt%
Ukraine 180 KL/D Dehydration / 3 Plant
Ethanol from corn 85.0wt% → 99.7wt%
Lithuania 60KL/D Dehydration / 2 Plant Ethanol from corn
84.8wt% → 99.8wt%
●:Ethanol
●:Other solvent (IPA etc.)
1998
~
1st2008.Aug. ~
2nd2009.Apr. ~
1st2004.Mar. ~
2nd2005 ~
1st2009.May. ~
2009.Jan. ~
Figure 2. Evolution of publications of zeolite membranes (Source: Scopus.com).
Membrane separation can be also combined with reaction as a catalytic membrane reactor, which makes the process more efficient and compact in size. Zeolite Membrane Reactor (ZMR) have therefore showed a great potential industrial application in terms of optimized process but it is still far from real commercialization (Gascon et al. 2012). Diban et al. (2013,2014)20proposed the integration of hydrophilic and inert zeolite membrane in situ to remove water in dimethyl ether production using a packed bed catalytic reactor. The results showed the great importance of the zeolite membrane in the process, although further improvements in its selectivity and performance were still needed.
Other potential applications of zeolite membranes are natural gas sweetening, hydrogen generation, and air separation, among others (Caro & Noack 2008; Gascon et al. 2012). Kosinov et al. (2016) explained the latest developments of zeolite membranes for gas separation. They concluded that even if the amount of research works is important in the literature and with interesting performance, there are several drawbacks in their commercialization related to the high costs and reproducibility. Furthermore, the authors also pointed to the need of performing long-term experiments aimed at the stability analysis to identify the effective robustness of the synthesized zeolite membranes. Among other innovative liquid separation with great interesting, water desalination and separation of organic/organic mixtures are gathering importance. Although NaA membranes have reached industrial applications, MFI type membranes seem technically more suitable for seawater desalination by membrane distillation (MD) than polymeric membranes, due to their higher antifouling properties and the low resistance to aggressive media (alkaline or acid) of the polymer materials used in desalination (Gascon et al. 2012). Garofalo et al. (2016) studied the viability of MFI membranes for desalination of concentrated model saline solutions (0.2 -0.9 M NaCl) and with real samples. Applying vacuum membrane distillation (VMD), interesting fluxes up to 13.8 kg·m-2·h-1 and salt
rejection between 99.6-99.8% were obtained, which supports the effectivity of these membranes for these new applications.
addition, other organic/organic mixtures (e.g. ethanol/benzene, benzene/xylene, p-xylene/o-xylene) and membranes (A-type, Y-type, MFI, MOR) have been considered and summarized by Bowen et al. (2004). The separation performance compared to hydrophobic silicalite-1 was improved by the presence of aluminum in the zeolite structure and its resulting localized dipole (Bowen et al., 2004), and also, the molecular sieving had an important role in these separations due to the size of the molecules compared to the size of the zeolitic pore.
1.2.
MORPHOLOGY AND STRUCTURE OF ZEOLITE MEMBRANESThe inorganic structures of zeolites have been used as catalysts, adsorbents or deposited on porous support to obtain membranes. Their common structures O-T-O can vary in the tetrahedral framework by using different atoms, being Si, Al the most typical, B, Ge, Fe, and P.
The zeolite pores are determined by the rings made up by oxygen atmos. Bowen et al. (2004) classified different types of zeolites depending on the size of the zeolitic pores: small-pore, with eight-member oxygen rings; medium-pore consisting in ten-member rings; and large-pore zeolite, with twelve-member rings. Besides, these pores produced by the crystals of the zeolite can be found in one-, two- or three-dimensions and therefore, their orientation is another factor to consider. Appropriate synthesis conditions can lead to variations in the out-of-plane which affect the performance of the membrane (Xomeritakis et al. 1999; Lai et al. 2003).
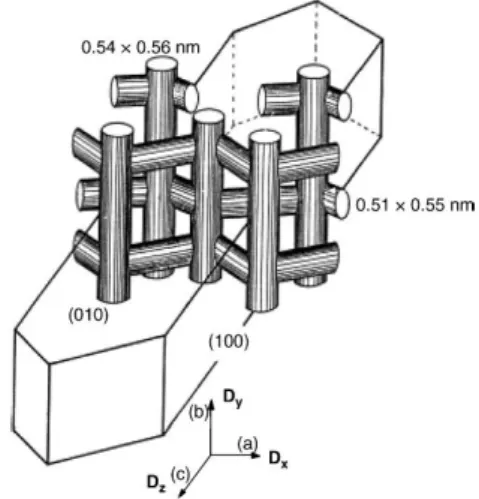
As an example of medium-pore zeolite, MFI structure has been commonly studied. MFI can be differentiated in two types: silicalite-1, composed by pure silica and hydrophobic; and ZSM-5, in which Al substitutes some Si atoms, providing hydrophilic properties. The ZSM-5 zeolite presents high acidic-media tolerance which makes it interesting to consider in several applications, as above commented.
ZSM-5 present a double dimension pore framework, schematically shown in Figure 3. Two pore types can be identified: straight with a size of 0.51 x 0.55 nm; and elliptical, 0.53 x 0.56 nm.
The substitution of silica by aluminum brings the introduction of negative charges into the zeolite. To compensate the charge, protons or other cations can be used. The result of using protons is the so-called H-ZSM-5, with catalytic activity at the acid sites (Bronsted acid site) produced by H+ and widely applied for hydrocarbons catalytic reactions. Other
cations have been widely studied, although the most typical is sodium.
The importance of the type and the location of these cations relies on their influence on the adsorption properties of the zeolite (Stave & Nicholas, 1995; García Sánchez, 2011). Particularly, the accurate location of the cations on ZSM-5 zeolite has been one of the main topics involving high resolution analytical techniques and theoretical models and simulations.
The ratio Si/Al can be experimentally measured and defines the amount of aluminum atoms per zeolite unit, but not their exact location in the zeolitic framework. To study the structure and the influence of the disposition of the atoms in the zeolite, simulation techniques have been applied. By dividing the zeolite in periodic unit cells, ZSM-5 consists of units with 96 T sites (Si or Al) and 192 atoms of O, and it can be divided in unique units with 12 potential sites for Al. Alvarado-Swaisgood et al. ( 1991) and Stave and Nicholas (1995) analyzed the bonds and the energy between the potential aluminum atom and its surroundings depending on the T-site and obtained as result the identification of the most preferential T-sites for Al: T6 < T12 < T9 < T3.
Besides, there are two rules for this complex location of Al over the T-sites: Löwenstein’s rule (no favorable Al-O-Al), widely accepted but with exceptions for other zeolites; and Dempsey’s rule (no favorable Al-O-Si-O-Al), not so widely accepted; and several studies about the favorable T-sites in terms of lowest energy state and bond energy between the atoms (Smit et al., 2008; Beerdsen et al., 2002).
Regarding the synthesis method of zeolite membranes, there are two main ways: in situ crystallization, in which the crystals grow directly on the support; or by secondary/seeded growth. This latter method allows a more uniform zeolite layer with better reproducibility. The synthesis mixture usually is composed of water, a silica and aluminum source, a mineralizing agent, and an organic structure-directing agent (SDA). Several studies have proved the importance of the selection and use of the SDA for the adequate orientation of the zeolite (Agrawal et al. 2015; Zhou et al. 2014). However, the synthesis of zeolite membranes without using an SDA has important advantages in terms of morphology and energy and cost savings of the membrane manufacturing. SDA are expensive chemicals and the post-calcination step for their removal from the membrane can lead to the formation of microcracks and modifications of the crystallinity due to the different thermal expansion properties between the ceramic support and the zeolite layer (Zhu et al. 2014; Zhu et al. 2012). Therefore, membrane preparation methods without using SDAs, as the one considered in this study, have the advantage of avoiding these effects (Zhu et al. 2012).
minimization of the effect of grain boundary effects; and avoiding stress-induced crack formation (Lai et al. 2003). The thickness and uniformity of the zeolite membranes can be characterized by surface and cross-section observation using scanning electron microscopy (SEM). To identify the crystallinity and zeolite framework structure, X-ray diffraction (XRD) is usually applied.
1.3.
TRANSPORT PHENOMENA THROUGH ZEOLITE MEMBRANESZeolite membranes, as nanopore material, carry out the separation using differences in the size of the molecules and/or in the adsorption and diffusion properties. Mass transport through zeolite membranes is driven by a chemical potential gradient between the feed and permeate sides of the membrane. Zeolites have pores with diameters in the range of a few angstroms and the separation can be described by the following steps: adsorption of molecules in the surface of the zeolite, transport from the external surface into the pores, the diffusion inside the pores, transport out of the pores to the external membrane surface, and posterior desorption out of the pore openings. Added to the several steps, the influence of co-existing molecules in each stage of the permeation is of great importance since multicomponent systems are considered.
Establishing the relation among transport mechanism at microscopic level with macroscopic measurable variables, such as density flux and selectivity of a membrane, is considered a complex task which has been studied for years.
Adsorption in zeolites has been widely studied (Bowen et al. 2004; Milestone & Bibby 2007b; Milestone & Bibby 2007a; Grenev & Gavrilov 2016) since zeolites can use the adsorption preference to separate mixtures. However, the influence of competitive adsorption is still an issue far from full understanding. The adsorption in zeolites is physical, and therefore, exothermic, and reversible. Two main variables are analyzed when the adsorption mechanism is studied: heat of adsorption and saturation coverage. Heat of adsorption of different alcohols and water in zeolites has been previously studied (Bowen et al. 2004), being the polarity and molecular size of the molecules the main factors influencing adsorption. On the other hand, saturation coverage is the maximum number of molecules adsorbed into the pores, which strongly depends on the structure, or total pore volume, of the zeolite.
Adsorption isotherms have been applied for modelling of pervaporation systems. Typically, the Langmuir approach (Eq. 1) is used.
𝑞
𝑖=
𝑞𝑖𝑚·𝐾 𝑖· 𝑝𝑖
1+𝐾𝑖· 𝑝𝑖 Eq. 1
Where qi, qmi, Ki, and pi are the loading (molecules/unit cell), maximum loading
(molecules/unit cell), adsorption constant (Pa-1), and partial pressure (Pa).
A way to represent mass transport in zeolites is the so called single-file diffusion. Although this theory was initially developed for colloidal particles, it was found useful for measuring diffusion coefficients through membranes with few defects.
the zeolite. In single-file diffusion, the Fickian diffusivity is assumed to be constant while the Maxwell-Stephan transport diffusivity varies with the coverage and therefore, the concentration dependence is upon to the interaction between the molecules (Nagumo et al. 2007).
The inhibition of the diffusion of faster molecules by slower permeating molecules inside the zeolite pores is expected to increase as coverage increases. The coverage increases with higher concentrations due to the adsorption of molecules in the pores, and therefore, the system is more constrained and the influence among molecules is greater. When pervaporation is applied, these higher coverages appear due to the liquid feed, reaching almost saturation (Nagumo et al. 2007).
1.4.
OBJECTIVESThe aim of this master thesis is to characterize the separation performance of ZSM-5 zeolite membranes used in pervaporation for alcohol dehydration. Experiments and simulations were designed in order to get insight into the mass transport mechanism and the influence of competitive sorption and diffusion phenomena. These unique, microporous inorganic membranes are studied for the dehydration of alcohol-water mixtures, a selected application usual in the chemical industry and therefore, of great interest.
The main objective can be summarized in three complementary specific objectives depending on the scope and method of characterization:
- Evaluating the long-term performance of the ZSM-5 membranes in pervaporation experiments with different feed compositions and operation conditions, with the aim of analyzing their influence over water permeation. Binary mixtures of water with ethanol and tert-butanol will be used as feed solutions, as the size of both alcohols are smaller and larger, respectively, than the size of the ZSM-5 pore size. - Defining a protocol for membrane regeneration.
2.
METHODOLOGY
2.1.
ZSM-5 ZEOLITE MEMBRANESHydrophilic ZSM-5 zeolite membranes were used in this study. The membrane samples were prepared as in previous works from the same laboratory, Green Chemical Processes from the division of Membrane Science and Engineering (Yamaguchi University), using the static secondary hydrothermal synthesis (Zhu et al. 2014; Zhu et al. 2013; Zhu et al. 2012),. In the present research, fixed preparation conditions were selected and the ceramic supports were -alumina tubes (o.d.: 12 mm; i.d.: 9 mm, porosity: 35.2%, pore size: 1.25 µm and length: 100 mm, Nikkato Corporation).
Three different ZSM-5 membranes were used in this study, named as Z1, Z2, and Z3. Prior to the experiments performed in this study, the membranes were characterized in terms of crystallinity by X-ray diffraction (XRD), scanning electron microscopy (SEM) and short PV experiments, giving as result similar morphology, crystallinity, thickness, and performance values for standard conditions.
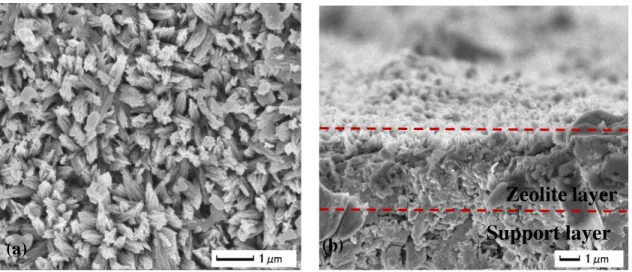
The crystallinity and morphology of the ZSM-5 membranes as reported by Zhu et al. (2012) are presented in Figure 4 and Figure 5a,b. The interest of these membranes relies on their attractive surface crystals, synthetized in the outer surface of the tubular supports. From Figure 5b, ZSM-5 zeolite layer can be identified with a thickness approximately of 5µm. The authors (Zhu et al., 2012) reported a nanorod surface when hydrothermal process without SDA was applied. The crystallinity otherwise corresponded to ZSM-5 zeolite, characterized by XRD equipment (Figure 4).
Figure 5. SEM images of surface (a) and cross section (b) of ZSM-5 zeolite membranes tested in this study.
When the synthetic membranes developed by Zhou et al. (2012) were compared to other ZSM-5 membranes, the structure of ZSM-5 crystals showed interesting differences. Figure 6 shows a surface SEM image of MFI membranes developed by Zhou et al. (2005). This membrane was prepared with tetrapropylammonium hydroxide (TPAH) as structure-directing agent. The crystals clearly show a different morphology compared to the ZSM-5 SDA-free membranes presented in Figure 5a. While MFI crystals from Figure 6 can be easily identified as the shape shown in Figure 3, the nanorod structure from Figure 5a was not obvious. The XRD peaks from Figure 4 from the SDA-free membranes were however of ZSM-5 crystals. Besides, the size of the crystals was significantly smaller (less than 1 µm) in Figure 5a, while Figure 6 shows ZSM-5 of several micros.
Figure 6. Surface SEM image of MFI zeolite membranes prepared with SDA (image taken from Zhou et al. (2005)).
The effect of the nanorod structure is still uncertain and thus, further investigations need to the performed in this matter in order to characterize the cause of the formation of these particular crystals and their role in the separation mechanism.
(a) (b)
2.2.
PV EXPERIMENTS FOR ALCOHOLS DEHYDRATON2.2.1. Pervaporation set-up and experimental plan
Synthesized ZSM-5 zeolite membranes were applied to dehydrate aqueous solutions of different alcohols. The pervaporation set-up available in the laboratory (Figure 7) was used to perform the dehydration experiments. The feed solution was heated up to 75°C and the membrane module was submerged in the feed mixture. The permeate vapor was condensed with liquid nitrogen in a trap tube. All the PV experiments were performed at atmospheric feed pressure and vacuum in the permeate side.
Figure 7. Pervaporation experimental set-up: 1-membrane, 2-membrane module, 3- thermal bath with stirrer, 4-feed sampler, 5- permeate traps tubes in liquid nitrogen, 6-vacuometer, 7-trap tube for vacuum pump, 8- oil-sealed rotary vacuum pump.
The membranes were initially characterized at ‘standard conditions’, defined as: 90wt.% of ethanol (EtOH)/ 10wt.% water (W) at 75°C for 4-5 hours. These operational conditions are commonly used in the literature for dehydration of highly concentrated alcohols. The experiments carried out in the laboratory can be divided in different topics, summarized in Table 2:
- Long-term stability: synthesized ZSM-5 membranes were used in long-term pervaporation experiments to analyze the robustness and stability of the membranes over operational time.
- Analysis of the influence of the composition of the feed: pervaporation experiments were carried out with different ethanol content in the feed solution. Besides, pure water and pure ethanol were considered.
1 2
3 4
5
6
7
8
FEED
PERMEATE
2 1
- Analysis of the size of the alcohol molecule: tert-butanol (tButOH) was selected to compare the PV performance when molecules with larger size (kinetic diameter = 6.2 Ȧ) than the ZSM-5 pore size (5.5 Ȧ) is considered.
- Stability under high water content conditions: pure water pervaporation and soaking periods in ultrapure water were performed to analyze their influence of the performance of the membrane.
Table 2. Experimental plan and conditions for ZSM-5 membranes for long-term PV experiments.
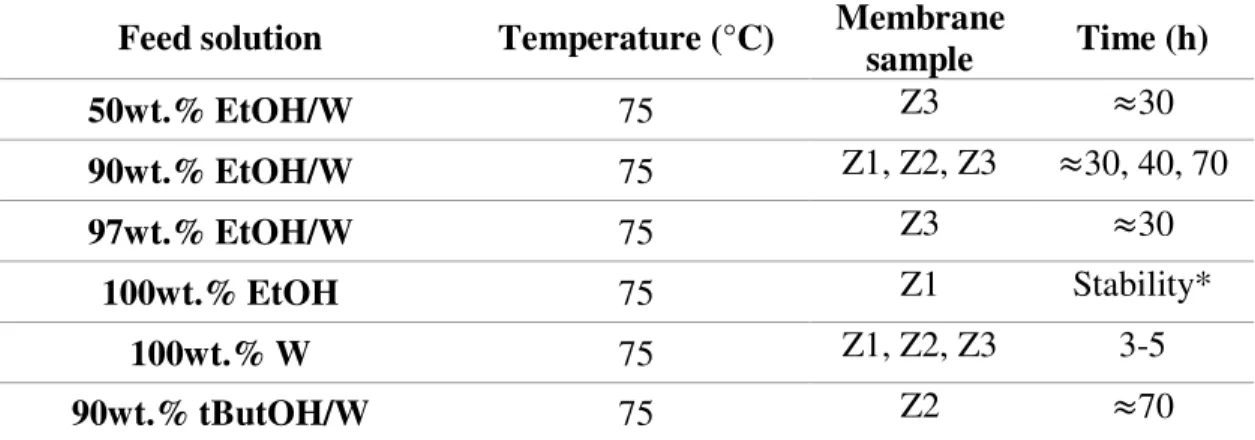
Feed solution Temperature (°C) Membrane sample Time (h)
50wt.% EtOH/W 75 Z3 ≈30
90wt.% EtOH/W 75 Z1, Z2, Z3 ≈30, 40, 70
97wt.% EtOH/W 75 Z3 ≈30
100wt.% EtOH 75 Z1 Stability*
100wt.% W 75 Z1, Z2, Z3 3-5
90wt.% tButOH/W 75 Z2 ≈70
*Pure ethanol pervaporation experiment was carried out until stability, that is, flux and permeate composition achieved steady state values.
Between PV experiments, the ZSM-5 membrane samples were kept drying at 80°C and the membrane regeneration was evaluated by soaking the membrane sample without sealing inside pure water at 75°C for 8, 24, and 48 hours, followed then by pure water PV and standard conditions PV at the same temperature.
2.2.2. Analytical methods and calculations
From pervaporation experiments, total flux and separation factor can be rapidly calculated by Eqs. 2 and 3.
𝐽 = 𝐴·𝑡𝑚 [=]𝑚𝑘𝑔2·𝑠 (Eq. 2)
𝛼𝑤/𝑖 = 𝑌𝑤⁄𝑌𝑖
𝑋𝑤
𝑋𝑖
⁄ [=] 𝑑𝑖𝑚𝑒𝑛𝑠𝑖𝑜𝑛𝑙𝑒𝑠𝑠 (Eq. 3)
Where m, A, t, denoted the mass of permeate condensed in the cooled trap for a known PV time (kg), effective area of membrane (m2) and test time (h).
Yw, Yi, Xw, and Xi are the
mass fractions of the components in the mixture: the mass fractions of components water (W) and alcohol (i) at the feed and permeate phases, respectively.
The compositions of the feed solution and the condensed permeate were measured by gas chromatography (Shimadzu, GC-17A).
Table 3. Equations considered for permeance calculation.
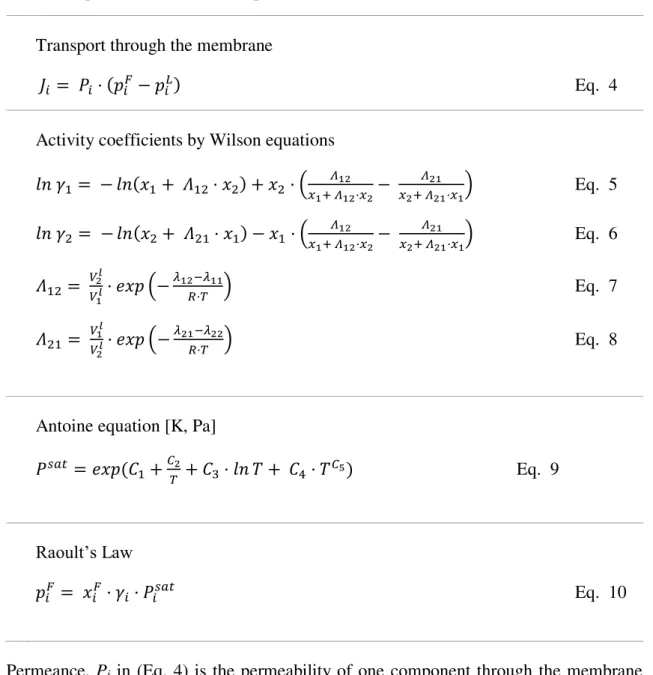
Transport through the membrane
𝐽𝑖 = 𝑃𝑖· (𝑝𝑖𝐹− 𝑝𝑖𝐿) Eq. 4
Activity coefficients by Wilson equations
𝑙𝑛 𝛾1 = − 𝑙𝑛(𝑥1+ 𝛬12· 𝑥2) + 𝑥2 · (𝑥 𝛬12
1+ 𝛬12·𝑥2−
𝛬21
𝑥2+ 𝛬21·𝑥1) Eq. 5 𝑙𝑛 𝛾2 = − 𝑙𝑛(𝑥2+ 𝛬21· 𝑥1) − 𝑥1· (𝑥 𝛬12
1+ 𝛬12·𝑥2−
𝛬21
𝑥2+ 𝛬21·𝑥1) Eq. 6 𝛬12 = 𝑉2
𝑙
𝑉1𝑙· 𝑒𝑥𝑝 (−𝜆12𝑅·𝑇−𝜆11) Eq. 7
𝛬21= 𝑉1
𝑙
𝑉2𝑙· 𝑒𝑥𝑝 (−
𝜆21−𝜆22
𝑅·𝑇 ) Eq. 8
Antoine equation [K, Pa]
𝑃𝑠𝑎𝑡 = 𝑒𝑥𝑝 (𝐶
1+𝐶𝑇2+ 𝐶3· 𝑙𝑛 𝑇 + 𝐶4 · 𝑇𝐶5) Eq. 9
Raoult’s Law
𝑝𝑖𝐹 = 𝑥𝑖𝐹· 𝛾𝑖· 𝑃𝑖𝑠𝑎𝑡 Eq. 10
Permeance, Pi in (Eq. 4) is the permeability of one component through the membrane
divided by the thickness of the zeolite layer, which is more useful for asymmetric and composite membranes (Feng & Huang 1997). The use of permeance instead of flux and selectivity allows the comparison of pervaporation experiments with different components in the feed solution and different concentrations.
Activity coefficients are calculated by Wilson parameters (Eq.5-8), and Antoine equation is used for saturation pressure at temperature in the feed solution (Eq. 9). Both equations are needed for partial pressure calculation at the feed side by Raoult’s law (Eq. 10).
Figure 8. Scheme of model considered for permeance calculations.
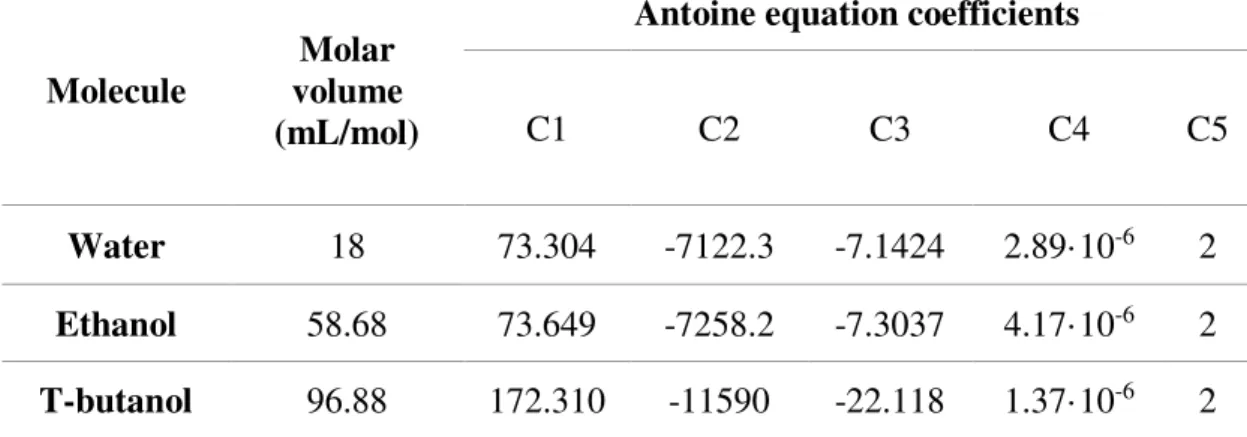
The parameters (Table 4 and Table 5) were taken from Perry’s Chemical Engineers Handbook (Perry & Green 2008).
Table 4. Parameters of water, ethanol and t-butanol needed for calculations.
Molecule volume Molar (mL/mol)
Antoine equation coefficients
C1 C2 C3 C4 C5
Water 18 73.304 -7122.3 -7.1424 2.89·10-6 2
Ethanol 58.68 73.649 -7258.2 -7.3037 4.17·10-6 2
T-butanol 96.88 172.310 -11590 -22.118 1.37·10-6 2
Table 5. Interaction energies for water, ethanol and t-butanol binary mixtures (Wilson model).
Mixtures 𝛌𝟏𝟐− 𝛌𝟏𝟏 (cal/mol) 𝛌𝟐𝟏− 𝛌𝟐𝟐 (cal/mol) Ethanol (i= 1)
Water (j = 2) 953.2792 325.0757
T-butanol (i=1)
Water (j = 2) 2304.092 828.7162
2.3.
MODELLING OF STRUCTURE AND ADSORPTION INZEOLITES
adding and correctly modelling the microporous ceramic membrane support, only the zeolite was considered in the simulations. The correct description of a zeolite with cations has been an important topic in the literature with several sensitive variations and considerations. Therefore, this work has followed the assumptions and simplifications recommended by Ari et al. (2009) and Wu et al. (2009) together with the recommendations of the software supplier (Accelrys Inc.).
ZSM-5 structure was developed taking as reference MFI structure from the Structure library folder from the software. The unit cell consisted in 96 Si and 192 O atoms, which corresponds to a Si/Al equal to cero. The next step therefore was to introduce Al into the structure. For this, Si atoms were exchanged by Al atoms, fulfilling the Si/Al ratio obtained experimentally. For the synthesized ZSM-5 membranes, the Si/Al ratio obtained from experimental measurements (Zhu et al. 2012) was approximately equal to 13, and that made a need of 7 Al atoms. To make the exchange possible, the symmetry property of the base structure was eliminated by making P1 symmetry.
Once the 7 Al atoms are located, it is necessary to adapt the charges of the unit cell to simulate the need of cations into the structure. The charge values were taken from the software recommendations: Si = 2.4 u, Al = 1.4 u, and O = -1.2 u. Accordingly, the structure required to balance and neutralize the excess of negative charges. Since the zeolite was synthesized with sodium as accompanying cation, 7 Na atoms were needed. The Sorption module was applied for location of the cations for the lowest energy position, the options selected for the simulation were listed in Table 6. The simulation was then run and the result was the base structure for the MS (Figure 9a,b,c).
Figure 9. ZSM-5 zeolite structure considered in this work (the scheme developed using Materials Studio software): a) transversal view XY plane, b) 3D view from XY plane
a)
with marked paths, c) 3D view from XZ plane with marked pores. Red lines = straight channels, and green lines = zig-zag channels
Atoms can be identified by their different color in Figure 9a. The connected atoms composed the aluminosilicate main structure: yellow ones are silica, red are oxygen and purple are aluminum. The no-connected light pink atoms are the sodium atoms, which were located at the lowest energy sites in the structure: near aluminum atoms, that is, where the unbalanced charge is.
Figure 9b,c represent the zeolite structure with the straight and zig-zag channels, coloured as grey volumes from different points of view so the channels can be easily determined. The transversal and inner view of the channels are shown in blue so the specific position of the molecules. Red lines in Figure 9b,c represent the straight channels of the zeolite, while green ones make reference to zig-zag channels.
Adsorption analysis were performed following the considerations taken by Yang et al. (2007) and adapted to this case of study and system.
The adsorption simulations were focused on the competitive adsorption analysis. The purpose by these MSs was to determine the number of adsorbed molecules for different bulk solutions and the visualization of the adsorbed species. The adsorption simulations were performed with the Sorption Module of the software tool. The parameters and options selected are summarized in Table 6.
Table 6. Sorption Module parameter for location of sodium cations and competitive adsorption analysis.
Parameter Location Competitive adsorption
Method Metropolis Metropolis
Forcefield cvff COMPASS
Charges Use current Forcefield assigned
Electrostatic forces Ewald Ewald
3.
RESULTS AND DISCUSSION
3.1.
PERVAPORATIVE SEPARTION OF ALCOHOL/WATERMIXTURES USING ZSM-5 ZEOLITE MEMBRANES
PV experiments with ZSM-5 zeolite membrane samples were performed at standard conditions (90wt.% EtOH/W at 75°C) and for a 90wt.%tButOH/W feed at 75°C. The experiments were carried out in PV experimental set-up from Figure 7, as described in Section 3.2.1.
Figure 10a-d and Figure 11a-d show the total flux obtained directly from the permeate trap tubes, water molar flux, ethanol/tert-butanol composition in permeate, and separation factor, respectively for both feed solutions considered.
(a)
Figure 10. Experimental results of total flux for PV using standard conditions (90wt.%EtOH/W at 75°C) for ZSM-5 zeolite membrane. Z1 and Z2 depict two different membrane samples. The second number after the hyphen depicts the number of the experiment performed with the same membrane sample.
Figure 10a-d gathers several shorts experiments that lasted between 10 and 20 hours, performed with the membrane sample Z1, and two long experiments performed with different membrane samples (Z1 and Z2) for 30 and 70 hours. Between short PV experiments, regeneration of the membrane was performed. Further information about the regeneration method and its limitations will be discussed in Section 4.3.
Total flux (Figure 10a) decreased over 60% after 70 hours, in good match with the evolution of water flux (Figure 10b) since water is the major component of the permeate phase. On the other hand, ethanol composition in the permeate side and separation factor varied up to 0.5wt.% EtOH and down to 2000 (Figure 10c,d), respectively.
(c)
All the experiments showed good reproducibility in terms of total flux and water flux, although ethanol composition and thus, separation factor, suffered high variations at early experimental values (until 10 hours). These variations can be related to the variations of maintenance method that was applied to the membrane between PV experiments. Low concentrations (case: 1-2,3,5) of ethanol at the beginning of the PV experiments corresponded to drying the membrane at 80°C before performing the experiments, while high concentrations (case: Z1-1,4) were obtained after soaking the sample in the feed solution prior to the experiments. Therefore, high values of ethanol can be related to a surface partial adsorption of ethanol, while clean and dried membrane surface avoids this phenomenon. However, water flux and steady-state values were not significantly altered by these different management of the membrane between experiments.
Regarding the PV results when tert-butanol/water feed was employed, total flux for Z2-1 (Figure 11a) showed an initial stability until 40 hours of experimental time. Afterwards, a strong decrease of 35% was appreciated, stabilizing in a value of 1.50 kg·m-2·h-1. Water
flux (Figure 11b) changed in a similar way to total flux, as it corresponded to the low tButOH content observed in the permeate phase (Figure 11c). TButOH composition in the permeate was maintained below 0.25 wt.% (Figure 11c) and the separation factor achieved extraordinary high values during the initial stages of the experiments, that were later stabilized around = 5000.
Figure 11. Experimental results of total flux for PV using 90wt.%tButOH at 75°C for ZSM-5 zeolite membrane.
TButOH is a large molecule (6.2 Ȧ )among the group of short chain alcohols. Its size is larger than the ZSM-5 zeolitic pore size (5.6x5.6 Ȧ). Therefore, it is expected to obtain
(a) (b)
high selectivity in the separation of tBuOH/water mixtures as a result of a molecular sieving mechanism. Attending to Figure 11c, the presence of tButOH on the permeate side can be justified by two possibilities: i) flexibility of the molecule (Milestone & Bibby 2007a) , and ii) the existence of some intercrystalline paths in the structure of the zeolitic layer. Unfortunately, there was not an available technique in the laboratory either to confirm or dismiss any of both hypothesis.
The duplication of tert-butanol experiment with another membrane was not possible due to the limitation of available membrane samples. In addition, membrane regeneration was applied after experiment Z2-1 without successful results. Total flux and selectivity obtained in experiment Z2-2 were approximately the same as at the end of the previous experiment. However, after Z2-2 experiments, one PV experiment using EtOH/water feed at standard conditions was performed. The values obtained were 0.61 kg·m-2·h-1 of total
flux and 3700 of separation factor. On the one hand, the latter values are still far from the values obtained with previous membrane samples when short PV experiments were considered. On the other hand, those values correspond, or even surpass, the steady state performance of the membrane in long term PV experiments.
Experimental results obtained in the present research were compared to the available literature data, previously summarized in Table 1, at standard conditions for several types of zeolite membranes. The comparison is shown in Figure 12 and Table 7. The literature search did not provide enough tBuOH PV data with zeolite membranes.
Figure 12. Comparison of total flux and separation factor for several types of zeolite membranes for EtOH/water pervaporation at standard conditions.
ethanol (4.5 Ȧ) are considered. However, although alternative zeolites did not present as good performance as the commercial NaA membranes, the need of alternative zeolite membranes was originated by the low chemical stability of NaA membranes. Regarding other types of zeolite membranes, the high fluxes reported for NaY membranes , can be explained by their large pore size and low Si/Al ratio, and therefore, hydrophilic character. However, the low separation factor of NaY membranes can be also related to their large pore size (0.74 nm), making them more suitable for dehydration of alcohols with larger molecular size than ethanol. Other membranes with similar characteristics such us T-type, NaX, MOR and other ZSM-5 membranes presented similar flux and selectivity results (Figure 12) . Regarding the preparation method, membranes synthesized by secondary growth method gave in general better performance. However, other variables as the thickness and the intercrystalline area of the zeolite layer have an important influence with high complexity to identify accurately into the pervaporation results.
It is important to point that the ZSM-5 structure presents the highest Si/Al ratio among the membranes considered in Figure 12, and therefore lower hydrophilicity than other zeolites used in membrane synthesis.
Table 7. Qualitative comparison of the membranes summarized in Figure 12 in terms of structure characteristics.
Zeolite type
Si/Al
ratio Hydrophilicity
Pore size
(nm) Comments
NaA 1 ++++ 0.41
Highest hydrophilicity, small pore → good separation performance. Weak acid
resistance
NaY 1.1-2.6 +++ 0.74 High hydrophilicity, large pore → high flux, low selectivity. Strong acid resistance
MOR 7.7 ++ 0.65x0.70
Large pore, low hydrophilicity → low flux and selectivity,
fluoride route. Strong acid resistance, low in water
X-type 1.3 +++ 0.74
High hydrophilicity, large pore size → Medium performance, in situ crystallization method.
Strong acid resistance
T-type 3.6 ++ 0.36x0.51
Medium performance, in situ crystallization method. High
acid resistance and hydrophilicity
ZSM-5 13-15 ++ 0.53x0.56
Medium pore size and performance, high selectivity and hydrophilicity. High acid
Therefore, after comparing the results of the ZSM-5 zeolite membrane, with other zeolite membranes under development and with the commercial NaA membranes, it can be concluded that the performance of the ZSM-5 membrane synthesized without SDA showed high potential for pervaporative dehydration of alcohols. In addition, the stability of ZSM-5 in acid and water-rich media makes them attractive to consider them as potential alternative for the NaA zeolite membranes that are currently in used.
Figure 13a,b shows the values of water and alcohol (ethanol and tert-butanol) permeances, calculated according to the mass transfer model described in Section 3.2.2. The data presented in Figure 13a,b correspond to Z1-5 and Z2-1 long-term experiments (40 hours of continuous PV).
Tert-butanol, as molecule with high molecular weight compared to ethanol, has lower amount of alcohol in the aqueous solution when the same mass composition is considered. 90wt.%EtOH/W corresponded to a molar fraction of water in liquid phase equal to 0.21, while 90wt.%tButOH/W gave as result 0.30 water mol fraction. Assuming that the permeate composition did not affect the driving force since total vacuum was considered, the different partial pressures calculated in the feed side by the model considered in Section 2.2.2. had an important role for performance values. The molar fraction of water in the case of tButOH/W was 50% higher than the content of water in 90wt.%EtOH/W, the water partial pressure calculated by Raoult’s law provided the same relation as themolar composition. However, the water flux was approximately 3 times higher in the case of tert-butanol dehydration (Figure 10b and Figure 11b).. The membrane permeance values allowed the comparison of water flux through the membrane for both alcohol mixtures considered, ethanol and tert-butanol, without the influence of the different water concentration gradients.
Both cases were compared to pure water permeance and pure ethanol permeance values obtained by pure water and pure ethanol PV experiments at 75°C.
Figure 13. Water permeance (a), and alcohol permeance (b) along experimental time for pure water and pure ethanol and 90wt.% alcohol mixtures of ethanol and tert-butanol. Pure water permeance was 1.75 and 1.4 times higher than water permeances in ethanol and tert-butanol mixtures (Figure 13a). In the case of EtOH/water mixtures, the results show a strong decrease of water permeance in the first 20 hours, and then, the slope is softened and tending to a steady state value, which corresponds to a total decrease of 60% respect the initial results obtained with the standard conditions. The initial decrease of water permeance observed in Figure 13a) can be related to the effect of the competitive alcohol adsorption within the zeolitic pores of the membrane. It is hypothesized that the adsorption of ethanol, a molecule with larger size than water, would be obstructing the passage of water molecules through the zeolitic pores. Similarly, ethanol permeance of pure ethanol was significantly higher than for 90 wt.% EtOH/W mixtures. In the case of feed EtOH/water mixtures, ethanol permeance increases over 25% during the long-term PV experiment. This behaviour can be also relayed to the adsoprtion of ethanol into the zeolitic pores, that would have enhanced the alcohol flux. No pure tert-butanol PV experiments were performed, so no possible comparison and analysis of water influence can be carried out for the heavier alcohol. tButOH permeance showed an initial and short decrease, which can be related to the period for membrane stabilization. The stability of water permeance is appreciated for the rest of the experimental time. However, tButOH permeance also show an increasing tendency.
These behaviours can be related to the molecular size the zeolitic pore size. EtOH, as small molecule, can enter inside the pores despite the hydrophilicity of the membrane. Once it is inside the zeolitic pore, EtOH adsorption and diffusion compete with water molecules, with the results of decreasing the water permeance, and therefore, an increase of ethanol permeance. On the other hand, tButOH is a big molecule and therefore, the separation is mainly controlled by size exclusion. T-BuOH cannot enter into the ZSM-5 pores and therefore, their effect on water flux is less notorious than in the case of ethanol. Figure 14a-b shows the water permeance values when different EtOH concentrations in the feed solution were considered: 50wt.%, 90wt.% and 97wt.%EtOH/W. The membrane sample selected for the three PV experiments was Z3, and after each experiment, membrane regeneration was applied.
The water permeance was affected by the content of water in the feed solution. Higher content of water in the feed corresponded to higher values of the water permeance (Figure
14a), even at the initial stages of the PV experimentsOne possible explanation is that the higher the alcohol content in the feed mixture, the higher the adsorption of ethanol in the ZSM-5 pores, giving as a consequence, the hindrance of the mobility of water molecules. However, the calculated values of ethanol permeance after 15-20 hours of continuous experiment stabilized with values under pure EtOH permeance, which can be related to the preference of the hydrophilic zeolite for water molecules. On the other hand, when high concentration of EtOH (97% EtOH) is considered, the permeation of EtOH is significantly higher.
Figure 14. Water permeance (a), and alcohol permeance (b) along experimental time for different ethanol compositions in the feed solution.
With the aim of further understanding of the effect of water and alcohol concentration on PV results, Figure 15 gathers the available literature data for alcohol dehydration processes applying ZSM-5 zeolite membranes. The data corresponds to the ones previously summarized in Table 1. Experimental results obtained in the present study were compared considering different alcohols and PV conditions.
(a)
As it was commented previously in Figure 12, ethanol dehydration experiments performed in the present research provided similar values of total flux and better separation factor values compared to the ones in the literature. Other alcohol dehydration case of study included 2-propanol and i-propanol aqueous solutions. No tert-butanol dehydration data were found in the literature for ZSM-5 membranes. In general terms, higher values of flux and separation factor were obtained when larger molecules were considered, which is in good agreement with the relation appreciated in the tert-butanol PV experiments performed in this study. Therefore, the size of the molecule has an important role in the performance of the membrane. Tert-butanol, as molecule bigger than propanol, provided even higher values of flux and separation factor.
Figure 15. Comparison of experimental alcohol/water PV results with the data available in the literature for ZSM-5 membranes.
As it was depicted in Figure 12, NaA zeolite membranes have attractive flux and selectivity performance for solvent dehydration process. However, as it is well known, their chemical stability is limited to determined media. In this study, the stability in acid solutions of NaA membranes synthesized in Yamaguchi University laboratory is evaluated, in order to further assess the limitations of the NaA membrane. A-type membranes were soaked into 1 M HCl and 50wt.% acetic acid/water over 12 hours at specific temperatures. The membranes were characterized by XRD and SEM before and after the soaking in the acid solutions.
Figure 16. XRD results for NaA membranes before acid treatment (a) and after soaking in HCl (b) and in acetic acid (c). The support peaks are marked with ( ) and the A-type zeolite peaks with ( ).
By SEM images (Figure 17a-f), it is possible to see the effects of the different acids over the NaA zeolitic crystals. Surface and cross section for each case in included.
(a) (b)
(c) (d)