Article
0103 - 5053 $6.00+0.00*e-mail: rgvaghei@yahoo.com
Poly(
N,N’
-dichloro-
N
-ethyl-benzene-1, 3-disulfonamide) and
N,N,N’,N’
-Tetrachlorobenzene-1,3-disulfonamide as Novel Catalytic Reagents for Synthesis
of Bis-indolyl, Tris-indolyl, Di(bis-indolyl), Tri(bis-indolyl) and Tetra(bis-indolyl)
Methanes under Solid-State, Solvent and Water Conditions
Ramin Ghorbani-Vaghei*,a and Hojat Veisia,b
aDepartment of Organic Chemistry, Faculty of Chemistry, Bu-Ali Sina University, 65174, Hamadan, Iran bDepartment of Chemistry, Payame Noor University, Songhor, Kermanshah, Iran
Descreve-se a preparação fácil e rápida de bis-indolil, tris-indolil, di(bis-indolil), tri(bis-indolil) e tetra(bis-indolil) metanos a partir de indole com diferentes aldeídos e cetonas, sob diferentes condições reacionais, usando poly(N,N´-dicloro-N-etil-benzeno-1,3-disulfonamida), [PCBS], e
N,N,N´,N´-tetraclorobenzeno-1,3-disulfonamida, [TCBDA], como novos catalizadores.
Easy and rapid preparation of bis-indolyl, tris-indolyl, di(bis-indolyl), tri(bis-indolyl) and tetra(bis-indolyl) methanesfrom indole with various aldehydes and ketones using poly (N,N’ -dichloro-N-ethyl-benzene-1,3-disulfonamide) [PCBS] and N,N,N’,N’ -tetrachlorobenzene-1,3-disulfonamide [TCBDA] as novel catalysts under various conditions are here described.
Keywords: TCBDA, PCBS, bis(indolyl)methanes, catalytic reagents
Introduction
Indole derivatives are widely distributed in nature and are known to possess broad spectrum of biological and pharmaceutical activities.1 Bis(indolyl)methanes are found in cruciferous plants and are known to promote beneicial estrogen metabolism2 and induce apoptosis in human cancer cell. Thus, the development of facile and environmental friendly synthetic methods for the preparation of these compounds constitutes an active area of investigation in pharmaceutical and organic synthesis.3-5
Synthetically the reaction of 1H-indole with aldehydes or ketones produces azafulvenium salts that react further with a second 1H-indole molecule to form bis(indol-3-yl)methanes.6 In recent years, synthesis of this class of molecules under mild conditions have been reported, with promoters such as montmorillonite clay K-10,7 trichloro-1,3,5-triazine,8 AlPW
12O40,
9sodium dodecyl sulfate
(SDS),10 ZrCl 4,
11 H
2NSO3H, 12 I
2,
13 zeolites,14 bentonite,15
In(OTf)3/ionic liquid,16 CuBr 2,
17Dy(OTf)
3/ ionic liquid, 18
HClO4-SiO2,19 InCl 3,
20 MW/ Lewis acids (FeCl
3, BiCl3, InCl3, ZnCl2, CoCl2),21 NaHSO
4 and Amberlyst-15, 22
sulfated zirconia,23 ZrOCl 2/SiO2,
24silica sulfuric acid
(SSA),25 TiO 2,
26 (NH
4)2HPO4,
27 acidic ionic liquid,28
NaBF4,29 metal hydrogen sulfates,30 tetrabutylammonium tribromide,31 superacid SO
4 2−/TiO
2,
32 NaHSO 4/ionic liquid,33 NBS,34 Ph
3CCl, 35 H
3PW12O40, 36 LiClO
4,
37 Zr(DS) 4,
38
and Bi(NO3)3·5H2O.39
Result and Discussion
In continuation of our interest in the synthesis of
N-halo sulfonamide and application of these reagents in organic synthesis,40-43 we report the synthesis of poly (N,N’ -dichloro-N-ethyl-benzene-1,3-disulfonamide) [PCBS] and N,N,N’,N’-tetrachlorobenzene-1,3-disulfonamide [TCBDA] as novel catalytic reagents in organic reactions (Scheme 1).
Many of the methods described above involve the use of expensive reagents, high catalyst loading, long reaction times and environmentally hazardous reagents. Therefore, we studied the use of poly (N,N’
-dichloro-N-ethyl-benzene-1,3-disulfonamide) and N,N,N’,N’
Since TCBDA and PCBS contain chlorine atoms which are attached to nitrogen atoms, it is also possible that they release Cl+in situ which can act as Lewis acid to activate the carbonyl oxygen to form the bis-indole derivatives.
Initially, we carried out the reaction of indole with benzaldehyde in the presence catalytic amount of N,N,N’,N’-tetrachlorobenzene-1,3-disulfonamide (TCBDA) in EtOH at room temperature. After 5 min the reaction completed with good yield (96%).
The applicability of the present methodology is further extended by performing the reaction in an aqueous media. In this reaction, we also found that, water in the presence of 5 mol% CTAB (N-cetyl-N,N,N-trimethylammonium bromide) as surfactant is essential for this reaction using TCBDA at room temperature. However, this process is green, environmentally friendly, clean, and could be carried out easily at room temperature without undesirable side reactions.
In recent years, there has been an increasing interest in reactions that proceed in the absence of solvents due to
their reduced pollution, low cost, simplicity in process and handling. Therefore, we decided to test this reaction under solvent-free conditions (grinding) with these reagents. We found that the reaction could be rapid with excellent yield, (98%, entry 1) using N,N,N’,N’ -tetrachlorobenzene-1,3-disulfonamide [TCBDA] (1 min) and using poly (N,N’ -dichloro-N-ethyl-benzene-1,3-disulfonamide) [PCBS] (1.5 min). In comparison to the reported methods, TCBDA and PCBS under solvent-free conditions were found to be eficient catalysts in terms of handling, temperature, yield and reaction times.
These results promoted us to investigate the scope and the generality of these new protocols for various aldehydes and ketones under optimized conditions. As shown in Table 1, a series of aromatic, aliphatic and heterocyclic aldehydes underwent electrophilic substitution reaction with indole smoothly to afford a wide range of substituted bis(indolyl)methanes in good to excellent yields. The electron deiciency and nature of the substituents on the aromatic ring effect the conversion Scheme 1.
Scheme 2. N
H CHO
N H
HN
N
H NH
+ 2
Condition A: TCBDA or PCBS, EtOH, 40 °C, 30 min, 95% Condition B: TCBDA or PCBS, solid-state, grinding, r.t., 10 min, 96% Condition C: TCBDA,CTAB, H2O, 100 °C, 120 min, 90%
Condition A
Condition B
Condition C
N
H NH
N H + 2
method A: 70 °C, 30 min, 90%45 method B: r.t. 10 min, 96%
method C: 100 °C, 200 min, 70%
O O
N
H O
HN
Table 1. Synthesis of bis(indolyl)methanes by the reaction of indole with aldehydes and ketones in various conditions
Entry Carbonyl compound TCBDA (method A) PCBS (method A) TCBDA (method B) PCBS (method B) TCBDA (method C)
time/min yield (%)a time/min yield (%)a time/min yield (%)a time/min yield (%)a time/h yield (%)a
1 CHO 5 96 10 90 1 98 1.5 98 2 9011
2 10 92 15 90 1.5 96 2 96 2.5 8011
3 5 98 10 95 1 99 2 98 3 8911
4 8 96 12 90 1 98 1.5 96 4 7811
5 Br CHO 15 92 15 87 2 92 2 92 3.5 7536
6 Cl CHO 15 89 16 85 1.5 95 2.5 90 3.5 8035
7 10 96 20 90 1 96 2 96 2 8235
8 O CHO 25 90 30 80 2.5 92 2.5 90 4 7025a
9 15 92 20 90 1 96 2 96 2 7911
10 20 90 30 85 1 98 2 96 5 7035
11 25 90 30 85 2 97 2-3 97 4 7035
12 30 78 40 75 2.5 96 3 96 5 7513
13 25 95 35 90 2 95 4 94 4.5 7211
14
S CHO 2 92 5 90 1 98 2 96 2 82
36
15 70 85 85 80 4 90 6 90 6 7013
16 120 80 120 75 4 90 6 90 9 6536
17 100 85 100 85 4.5 85 5 80 7 7511
18
O
110 78 120 70 5 80 6 80 6 7011
a Products were characterized from their physical properties, comparison with authentic samples and by spectroscopic methods.
rate; aromatic aldehydes having electron-withdrawing groups on the aromatic ring (Table 1, entries 10,11) react slower than electron-donating groups (Table 1, entries 4, 8, 9, 12). Furthermore, unsaturated aldehydes, such as cinnamaldehyde, give the corresponding bis(indolyl) methanes without polymerization or halogenation under the above reaction conditions. Ketones required longer reaction times, which is most probably due to the electron-donating and steric effects of the methyl group.
This reaction was further explored for the synthesis of tri-indolylmethane by the condensation of indol-3-carbaldehyde or isatin with two equivalents of indole under similar conditions with our methods in good to high yields (Scheme 2 and 3).
CH2
H2C
CH2 O O O HN HN NH NH H N H N CH2 CH2
CH2 O
O O OHC CHO CHO N H 6
DMSO/ 100°C / 10 min PCBS/ 95% TCBDA/ 98% d e Scheme 4. N H 2 N H 4 CHO CHO N
H NH
H N N
H NH
CHO
H N
b
c method A, B and C
method A, B and C 90-92%
95-98%
Scheme 3.
equivalents of indole to terephthaldialdehyde, gives (b)in good yield under various conditions (Scheme 3). Treatment of 4 equivalents of indole with terephthaldialdehyde gives the corresponding di(bis-indolyl methanes), (c) in excellent yield at room temperature under various conditions.38
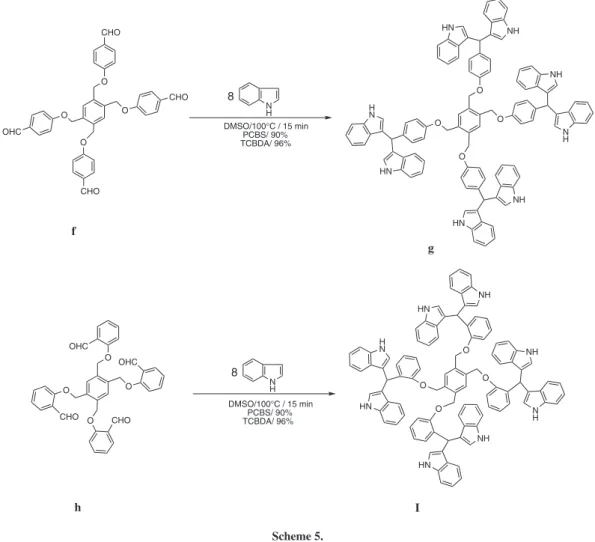
This reaction was further explored for the synthesis of tri(bis-indolyl)methane (e) and tetra(bis-indolyl)methanes (g, h) as new triarylmethanes, by the condensation of aldehyde (d)with 6 equivalents indole and aldehydes (f, h)with 8 equivalents indole under similar condition (relux in DMSO) in high yields (Scheme 4 and 5).
3-Substituted indoles were examined for this reaction under the above reaction conditions with aldehydes (Scheme 6). Since the more active site (C-3) in indole was blocked in this case electrophilic substitution took place at C-2 in indole giving the corresponding bis(indolyl)methane in high yield under various conditions. The results were summarized in Table 2.
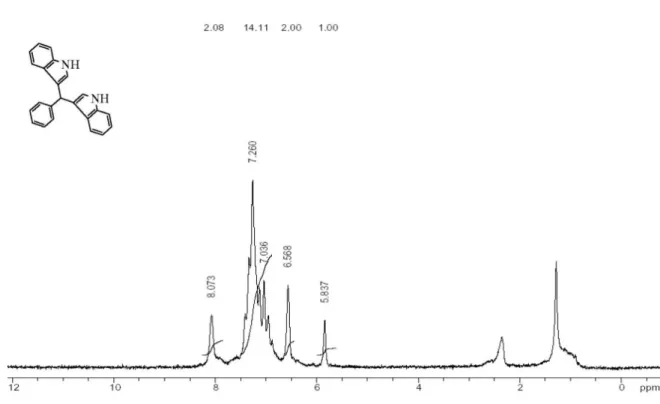
Our experiments also indicated that TCBDA and PCBS were reusable catalysts and after four runs, the catalytic activities of the reagents were almost the same as those of fresh catalysts. Thus, after the successful synthesis of
2-methoxyphenyl-3,3-bis(indolyl)methane in irst run (in solvent condition), which gave the corresponding product in 98% isolated yield (Table 1, entry 3), the N,N’-dichloro benzene-1,3-disulfonamide (TCBDA) catalyst was subjected to a second run reaction from which it gave the product in 85% yield; the average chemical yield for four consecutive runs was 60%. The reusability of catalysts are shown in Figure 1.
The chemo selectivity of the present methods is also demonstrated by competitive reactions of indol with arylaldehydes in the presence of aliphatic ones and ketones. For example, when a 1:1 mixture of benzaldehyde and propionaldehyde was allowed to react with two equivalents of indole in the presence of TCBDA and PCBS under various conditions, it was found that the arylaldehydes was chemo selectively converted to the corresponding bis(indolyl) methane but the aliphatic ones was converted slightly. Also, in an equimolar mixture arylaldehyde and ketone, only arylaldehyde was converted to the corresponding bis(indolyl)methane, whereas ketone remained (Scheme 7). The reaction was clean and the products were obtained in high yields without the formation of any side products such as N-alkylated products.
Table 2. The reaction of aromatic aldehydes with 3-substituted indoles under various conditions
Entry Carbonyl compound Indole Product Condition A Condition B Condition C
time/min yield (%)a time/min yield (%)a time/min yield (%)a
1 N H HOOC 3 N
H NH
COOH HOOC
n
n
n=3 OMe
30 95 10 96 120 9013
2 N
H COOH
N
H NH
COOH HOOC
Me
30 92 10 90 150 8013
3 CHO
N H
N
H NH 25 96 5 90 100 8525
4 N
H
N
H NH
NH
60 75 10 70 180 5044
a Products were characrerized from their physical properties, comparison with authentic samples and by spectroscopic methods f g O NH HN O N H NH O HN NH O H N HN O CHO O CHO O CHO O OHC N H 8
DMSO/100°C / 15 min PCBS/ 90% TCBDA/ 96% h I O NH HN O N H NH O HN NH O H N HN O O O O N H 8
Experimental
Procedure for the preparation of poly (N,N’-dichloro-N-ethyl-benzene-1,3-disulfonamide) [PCBS] and N,N,N’,N’-tetrachlorobenzene-1,3-disulfonamide [TCBDA]
A sample of white inely powdered poly (N -ethyl-disulfonamide) (1 g) or benzene-1,3-disulfonamide (1 g) was dissolved in a solution of NaOCl
Scheme 6.
N
H NH
R R/R/
N H
+
R/
RCHO
2
Condition A: TCBDA or PCBS, EtOH
Condition B: TCBDA or PCBS, solid-state, grinding, r.t. Condition C: TCBDA,CTAB, H2O, 100 °C
Condition A Condition B Condition C
Figure 1. Reusability of catalysts. Conditions: TCBDA (0.1 mmol) or PCBS (0.1 g), indole (4 mmol), 2-methoxybenzaldehyde (2 mmol), EtOH (5 mL) and time (10 min).
Scheme 7.
+ N
H NH NH
+ O CHO
O
95% 100%
method A:
method B:
method C:
98%
90% 100%
100%
CHO
N H NH
3
+
N
H NH NH
+ CHO
80% 15%
method A:
method B:
method C:
90%
65% 15%
10%
(50 mL, 14%), at 25 °C for 30 min. The color of the solution did not change. After this time, acetic acid (20 mL, 50%) was added to the solution. The insoluble chlorinated reagent was removed by iltration and washed with water (5 mL).
Analytical data for N,N,N’,N’-tetrachlorobenzene-1,3-disulfonamide
Synthesis of tri(bis-indolyl)methanes in DMSO catalyzed by TCBDA or PCBS
A mixture of indole (6.0 mmol), aldehyde, (d), (1.0 mmol) and catalyst PCBS (0.05 g) or TCBDA (0.05 mmol, 0.04 g) in DMSO (5 mL) was heated in an oil bath until 100 °C. The reaction mixture was iltered after 10 min. The residue was washed with EtOH (5 mL). Then, water (30mL) was added to the iltrate, and the product was precipated and iltered. The product was puriied by washing with cold EtOH (2 ×5 mL).
Synthesis of tetra(bis-indolyl)methanes in DMSO catalyzed by TCBDA or PCBS
A mixture of indole (8.0 mmol), aldehyde, (f or h), (1.0 mmol), and catalyst PCBS (0.05 g) or TCBDA (0.05 mmol, 0.04 g) in DMSO (5 mL) was heated in an oil bath until 100 °C. The reaction mixture was iltered after 15 min. The residue was washed with EtOH (5 mL). Then, water (30 ml) was added to the iltrate, and the product was precipated and iltered. The product was puriied by washing with cold EtOH (2 ×5 mL).
Analytical data for compound e
Light red solid, mp 208-210 oC. IR (KBr) ν max/cm
-1:
3421, 2950, 2900, 1635, 1506, 1457, 1377, 1216, 1173,
1082, 742. 1H NMR (300 MHz, DMSO-d
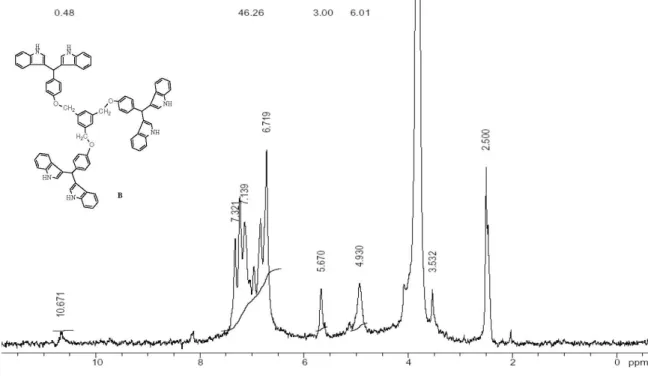
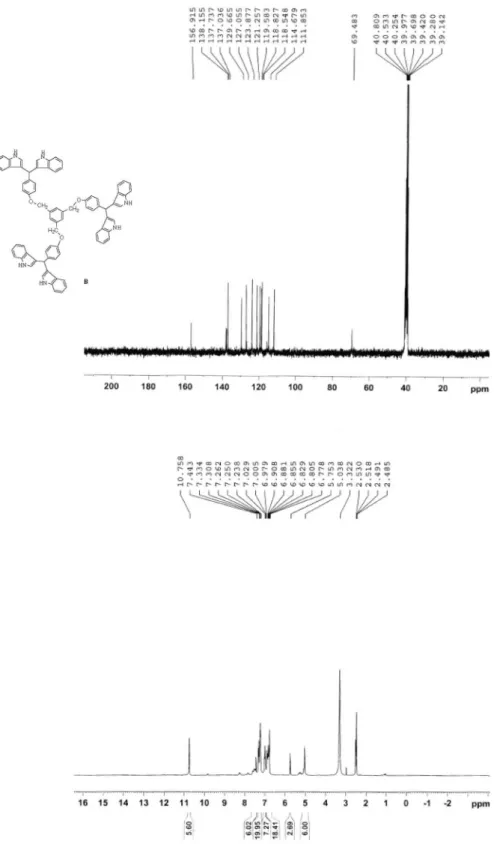
6) dH (ppm) 5.01 (s, CH2 benzylic, 6H), 5.72 (s, CH, 3H), 6.74-7.50 (m, CH aromatic, 46H), 10.71 (s, NH, 6H). 13C NMR (300 MHz, DMSO-d6) dc (ppm) 40.08, 69.48, 111.85, 114.67, 115.24, 118.54, 118.82, 119.58, 121.25, 123.87, 127.05, 129.66, 137.03, 137.73, 138.15, 156.91. Anal. Calc. for C78H60N6O3: C, 82.95; H, 5.35; N, 7.44. Found: C, 82.52; H, 5.21; N, 7.36.
Analytical data for compound g
Red solid, mp 250-251 °C d.IR (KBr) ν max/cm
-1: 3417,
2926, 2854, 1609, 1506, 1456, 1413, 1338, 1218, 1172, 1127, 1012, 743. 1H NMR (300 MHz, DMSO-d
6) dH (ppm) 5.08 (s, CH2 benzylic, 8H), 5.76 (s, CH, 4H), 6.82-7.65 (m, CH aromatic, 60H), 10.70 (s, NH, 8H). 13C NMR (300 MHz, DMSO-d6) dc (ppm) 41.05, 70.40, 111.45, 114.52, 115.12, 117.85, 118.85, 119.26, 120.85, 122.82, 126.65, 129.26, 136.76, 137.43, 138.63, 157.43. Anal. Calc. for C102H78N8O4: C, 82.79; H, 5.31; N, 7.57. Found: C, 82.12; H, 5.20; N, 7.35.
Analytical data for compound I
Red solid, mp 255-257 °C.IR (KBr) ν max/cm
-1: 3420,
2912, 2860, 1612, 1510, 1435, 1403, 1327, 1218, 1170,
1118, 735. 1H NMR (300 MHz, DMSO-d
6) dH (ppm) 5.10 (s, CH2 benzylic, 8H), 5.71 (s, CH, 4H), 6.75-7.85 (m, CH
807, 776, 675. 1H NMR (250 MHz, CDCl
3) d 7.95-8.09 (m, CH aromatic, 1H), 8.11-8.58 (m, CH aromatic, 2H), 8.79 (s, CH aromatic, 1H). m/e: 374 ([M+H]+), 339, 337, 321, 319, 305, 303, 272, 269, 267, 156, 154, 139, 125, 120, 104, 91, 77, 63. Anal. Calc. for C6H4N2S2O4Cl4: C, 19.25; H, 1.06; N, 7.48; S, 17.11. Found: C, 19.05; H, 1.16; N, 6.94; S, 16.28.
Analytical data for poly (N,N’-dichloro-N-ethyl-benzene-1,3-disulfonamide)
White solid, mp: 175-178 °C,IR (KBr) νmax/cm-1: 3050, 2950, 2900, 1578, 1462, 1418, 1377, 1303, 1168, 1081,
809, 779, 674, 603, 570. 1H NMR (250 MHz, CDCl
3) d 7.56-8.48 (b, CH aromatic).
Synthesis of bis(indolyl)methanes in ethanol catalyzed by TCBDA or PCBS (method A)
A mixture of indole (2.0 mmol), aldehyde or ketone (1.0 mmol) and catalyst [PCBS (0.05 g) or TCBDA (0.05 mmol, 0.04 g)] in EtOH (3 mL) was stirred at room temperature for the appropriate time (Table 1). The progress of the reaction was monitored by TLC (4:1, n-hexan/ acetone). After completion of reaction, the organic solvent was concentrated in vacuo and then CH2Cl2 (10 mL) was added, and the catalyst was removed by iltration. Evaporation of the solvent under reduced pressure gave the products. Further puriication was achieved by recrystalization from ethanol-water to afford pure products.
Synthesis of bis(indolyl)methanes in solid-state catalyzed by TCBDA or PCBS (method B)
A mixture of indole (2.0 mmol), aldehyde or ketone (1.0 mmol) and catalyst [PCBS (0.05 g) or TCBDA (0.05 mmol, 0.04 g)] were added to a mortar and the mixture was pulverized with a pestle. A spontaneouse reaction took place (1-6 min, Table 1, montored by TlC, 4:1, hexane/ acetone). After completion of the reaction, CH2Cl2 (10 mL) was added, and insoluble reagents were removed by iltration. The iltrate was evaporated under reduced pressure and the resulting crude material was puriied by recrystalization from ethanol-water to afford pure products.
Synthesis of bis(indolyl)methanes in H2O catalyzed by TCBDA and TBAB (method C)
aromatic, 60H), 10.65 (s, NH, 8H). 13C NMR (300 MHz, DMSO-d6) dc (ppm) 42.10, 70.41, 111.43, 114.60, 115.10, 117.88, 118.86, 119.36, 120.01, 120.85, 122.09, 122.90, 126.65, 129.25, 136.82, 137.45, 138.70, 157.45. Anal. Calc. for C102H78N8O4: C, 82.79; H, 5.31; N, 7.57. Found: C, 82.35; H, 5.18; N, 7.42.
Supplementary Information
Supplementary characterization data and 1H NMR spectra are available, free of charge at http://jbcs.sbq.org.br, as a PDF ile
Acknowledgments
We are thankful to Bu-Ali Sina University, Center of Excellence of Chemical Methods (CEDCM) for inancial support, and the University of Shefield for NMR, mass spectra and CHN. Also the author acknowledges Prof. I. Coldham from the University of Shefield for this useful comments and his kindness for hosting the author as a research visitor.
References
1. Sundberg, R. J.; The Chemistry of Indoles, Academic Press: New York, USA, 1996.
2. Zeligs, M. A.; J. Med. Food1998, 1, 67-68.
3. Chakrabarty, M.; Ghosh, N.; Basak, R.; Harigaya, Y.;
Tetrahedron Lett. 2002, 43, 4075.
4. Bandgar, B. P.; Shaikh, K. A.; Tetrahedron Lett. 2003, 44, 1959. 5. Reddy, A. V.; Ravinder, K.; Reddy, V. L. N.; Venkateshwer
Goud, T.; Ravikanth, V.; Venkateswarlu, Y.; Synth. Commun.
2003, 33, 3687.
6. Remers, W. A. In Heterocyclic Compounds; Houlihan, W. J., ed.; Interscience Publishers: New York, USA, 1972, p. 1. 7. Maiti, A. K.; Bhattacharyya, P.; J. Chem. Res. 1997, 424. 8. Sharma, G. V. M.; Reddy, J. J.; Lakshmi, P. S.; Krishna, P. R.;
Tetrahedron Lett. 2004, 45, 7729.
9. Firouzabadi, H.; Iranpoor, N.; Jafari, A. A. ; J. Mol. Catal. A: Chem. 2005, 244, 168.
10. Deb, M. L.; Bhuyan, P. J.; Tetrahedron Lett. 2006, 47, 1441. 11. Zhang, Z.-H.; Yin, L.; Wang, Y.-M.; Synthesis 2005, 1949. 12. Li, J.-T.; Dai, H.-G.; Xu, W.-Z.; Li, T.-S.; Ultrasonics Sonochem.
2006, 13, 24.
13. Bandgar, B. P.; Shaikh, K. A.; Tetrahedron Lett. 2003, 44, 1959.
14. Karthik, M.; Tripathi, A. K.; Gupta, N. M.; Palanichamy, M.; Murugesan, V.; Catal. Commun. 2004, 5, 371.
15. Penieres-Carrillo,G.; García-Estrada, J. G.; Gutiérrez-Ramírez, J. L.; Alvarez-Toledano, C.; Green Chem. 2003, 5, 337.
16. Ji, S.-J.; Zhou, M.-F.; Gu, D.-G.; Wang, S.-Y.; Loh, T.-P.; Synlett 2003,2077.
17. Mo, L.-P.; Ma, Z.-C.; Zhang, Z.-H.; Synth. Commun. 2005, 35, 1997.
18. Mi, X.; Luo, S.; He, J.; Cheng, J.-P.; Tetrahedron Lett. 2004,
45, 4567.
19. Kamble, V. T.; Kadam, K. R.; Joshi, N. S.; Muley, D. B.; Catal. Commun. 2007, 8, 498.
20. Pradhan, P. K.; Dey, S.; Giri, V. S.; Jaisankar, P.; Synthesis 2005, 1779.
21. Xia, M.; Wang, S. H.; Yuan, W. B.; Synth. Commun. 2004, 34, 3175.
22. Ramesh, C.; Banerjee, J.; Pal, R.; Das, B.; Adv. Synth. Catal.
2003, 345, 557.
23. Reddy, B. M.; Sreekanth, P. M.; Lakshmanan, P.; J. Mol. Catal. A: Chem. 2005, 237, 93.
24. Firouzabadi, H.; Iranpoor, N.; Jafarpour, M.; Ghaderi, A.;
J. Mol. Catal. A: Chem. 2006, 253, 249.
25. Pore, D. M.; Desai, U. V.; Thopate, T. S.; Wadgaonkar, P. P.;
ARKIVOC2006, 12, 75; Zoligol, M. A.; Salehi, P.; Shiri, M.; Sayadi, A.; Abdoli, A.; Keypour, H.; Rezaeivala, M.; Niknam, K.; Kolvari, E.; Mol. Divers. 2008,12, 203.
26. Hosseini-Sarvari, M.; Acta Chim. Slovenica 2007, 54, 354.
27. Dabiri, M.; Salehi, P.; Baghbanzadeh, M.; Vakilzadeh, Y.; Kiani, S.; Monatshefte for Chemie 2007, 138, 595.
28. Hagiwara, H.; Sekifuji, M.; Hoshi, T.; Qiao, K.; Yokoyama, C.;
Synlett 2007,1320.
29. Kamble, V. T.; Bandgar, B. P.; Bavikar, S. N.; Chin. J. Chem.
2007, 25, 13.
30. Niknam, K.; Zoligol, M. A.; Sadabadi, T.; Nejati, A.; J. Iran. Chem. Soc. 2006, 3, 318.
31. Lin, X. F.; Cui, S. L.; Wang, Y. G.; Synth. Commun. 2006, 36, 3153.
32. Zeng, X. F.; Ji, S. J.; Lett. Org. Chem. 2006, 3, 374.
33. Zhang, L. P.; Li, Y. Q.; Zhou, M. Y.; Chin. Chem. Lett. 2006,
17, 723.
34. Koshima, H.; Matsuaka, W.; J. Heterocycl. Chem. 2002, 1089.
35. Khalai-Nezhad, A.; Parhami, A.; Zare, A.; Moosavi Zare, A. R.; Hasaninejad, A.; Panahi, F.; Synthesis 2008, 617. 36. Azizi, N.; Torkian, L.; Saidi, M. R.; J. Mol. Catal. A: Chem.
2007, 275, 109.
37. Mehrazma, S.; Azizi, N.; Saidi, M. R.; Lett. Org. Chem. 2006, 3, 161.
38. Zoligol, M. A.; Salehi, P.; Shiri, M.; Tanbakouchian, Z.; Catal. Commun. 2007, 8, 173.
39. Khodaei, M. M.; Mohammadpoor-Baltork, I.; Memarian, H. R.; Khosropour, A. R.; Nikofar, K.; Ghanbary, P.; J. Heterocyclic Chem. 2008, 45, 1.
41. Ghorbani-Vaghei, R.; Zoligol, M, A.; Chegeny, M.; Veisi, H.;
Tetrahedron Lett. 2006, 47, 4505.
42. Zolfigol, M, A.; Ghorbani-Vaghei, R.; Mallakpour, S; Chehardoli, G.; Ghorbani Choghamani, A.; Yazdi Hosain, A.;
Synthesis2006, 1631.
43. Ghorbani-Vaghei, R.; Shahbazee, E.; Veisi, H.; Mendeleev Commun. 2005, 207.
44. Chakrabarty, M.; Sarkar, S.; Tetrahedron Lett. 2002, 43, 1351. 45. Wang, S.-Y.; Ji, S.-J.; Tetrahedron2006, 62, 1527.
Received: March 2, 2009
Supplementary Information
0103 - 5053 $6.00+0.00
*e-mail: rgvaghei@yahoo.com
P
oly (
N,N’
-dichloro-
N
-ethyl-benzene-1, 3-disulfonamide) and
N,N,N’,N’
-Tetrachlorobenzene-1,3-disulfonamide as Novel Catalytic Reagents for Synthesis
of Bis-indolyl, Tris-indolyl, Di(bis-indolyl), Tri(bis-indolyl) and Tetra(bis-indolyl)
methanes under Solid-State, Solvent and Water Conditions
Ramin Ghorbani-Vaghei*,a and Hojat Veisia,b
aDepartment of Organic Chemistry, Faculty of Chemistry, Bu-Ali Sina University, 65174, Hamadan, Iran bDepartment of Chemistry, Payame Noor University, Songhor, Kermanshah, Iran
3,3’-Bisindolyl-phenylmethane (Table 1, entry 1) Solid, mp 124-125 °C.IR (KBr) ν
max/cm
-1: 3402, 3050,
2986, 1615, 1600, 1455, 1112. 1H NMR (90 MHz, CDCl
3)dH
(ppm) 5.88 (s, 1H), 6.68 (s, 2H), 7.13-7.45 (m, ArH, 13H), 7.95 (br s, NH, 2H). 13C NMR (75 MHz, CDCl
3) dc (ppm) 31.6, 110.9, 111.9, 118.4, 119.5, 1121.2, 124.0, 126.3, 127.1, 128.5, 128.6, 137.0, 145.2. MS: (MS-EI) m/z 322.
3,3’-Bisindolyl-4-methylphenylmethane (Table 1, entry 2) Solid, mp 97-98 °C.IR (KBr) ν
max/cm
-1: 3452, 3112,
3045, 2950, 1604, 1523, 1210, 1052, 765. 1H NMR (90 MHz, CDCl3) dH (ppm) 2.38 (s, 3H), 5.85 (s, 1H), 6.70-7.55 (m, ArH, 14H), 7.85 (br s, NH, 2H).MS: (MS-EI) m/z
336. Anal. Calc. for C24H20N2: C, 85.68; H, 5.99; N, 8.33. Found: C, 85.45; H, 5.88; N, 8.14.
3,3’-Bisindolyl-2-methoxylphenylmethane (Table 1, entry 3) Red solid, mp 134-136 °C.IR (KBr) ν
max/cm
-1: 3408,
3056, 2932, 1597, 1486, 1450, 1335, 1102, 745. 1H NMR (90 MHz, CDCl3) dH (ppm) 3.82 (s, 3H), 6.32 (s, 1H), 6.61 (s, 2H), 6.81-7.40 (m, ArH, 12H), 7.80 (br s, NH, 2H). 13C NMR (75 MHz, CDCl
3) dc (ppm) 32.3, 56.0, 110.0, 110.8, 111.1, 119.2, 119.8, 120.2, 120.6, 121.9, 123.7, 127.2, 129.9, 132.5, 136.9, 157.1. Anal. Calc. for C24H20N2O: C, 81.79; H, 5.72; N, 7.95. Found: C, 81.24; H, 5.45; N, 7.86.
3,3’-Bisindolyl-4-methoxyphenylmethane (Table 1, entry 4) 1H NMR (90 MHz, CDCl
3) dH (ppm) 3.84 (s, 3H), 5.90 (s, 1H), 6.64-7.65 (m, ArH, 14H), 7.80 (br s, NH, 2H). 13C NMR (75 MHz, CDCl
3) d (ppm) 39.7, 55.6, 111.5, 114.0, 119.6, 120.4, 120.4, 122.3, 123.9, 127.5,130.0, 136.7, 137.1, 158.3.
3,3’-Bisindolyl-4-bromophenylmethane (Table 1, entry 5) Pink solid, mp 110-112 °C.IR (KBr) ν
max/cm
-1: 3410,
3054, 1487, 1455, 1089. 1H NMR (90 MHz, CDCl
3) dH
(ppm) 6.66 (s, 2H), 7.02-7.45 (m, ArH, 12H), 7.85 (br s, NH, 2H).MS: (MS-EI) m/z 361.
3,3’-Bisindolyl-4-chlorophenylmethane (Table 1, entry 6) mp 75-76 oC, FT-IR (KBr) ν
max/cm
-1: 3409, 2958, 1456,
1488. 1H NMR (300 MHz, acetone-d
6) d (ppm) 5.90 (s, 1H), 6.82 (s, 2H), 6.89-7.39 (m, 12H), 9.99 (s, 2H, N-H). 13C NMR (75 MHz, acetone-d6) d (ppm) 40.8, 112.1, 119.2, 120.1, 120.3, 121.9, 124.5, 128.1, 129.2, 138.0, 143.4.
3,3’-Bisindolyl-2-chlorophenylmethane (Table 1, entry 7) 1H NMR (90 MHz, CDCl
3) d (ppm) 7.89 (br s, 2H),
7.07-7.51 (m, 12H), 6.53 (s, 2H), 6.36 (s, 1H); 13C NMR (75 MHz,
CDCl3) d (ppm) 36.9, 110.1, 111.3, 119.3, 119.8, 122.0, 124.0, 126.7, 127.0, 128.3, 30.4, 130.7, 135.3, 136.7, 141.5.
3,3’-Bisindolyl-2-furylmethane (Table 1, entry 8) 1H NMR (90 MHz, CDCl
3)d (ppm) 7.74 (br s, 2H),
7.32-7.63 (m, 9H), 7.09 (s, 2H), 6.21-6.35 (m, 2H), 5.82 (s, 1H);
13C NMR (75 MHz, CDCl
3)d (ppm) 41.2, 102.1, 110.3, 111.8,
112.6, 119.4, 120.3, 121.5, 122.6, 131.0, 136.4, 141.2, 152.0.
3,3’-Bisindolyl-4-hydroxyphenylmethane (Table 1, entry 9) mp 212-213 oC, FT-IR (KBr) ν
max/cm
-1: 3420, 3239,
1455, 1511, 1616. 1H NMR (90 MHz, DMSO)d (ppm) 7.58
(br s, 2H), 6.69-7.24 (m, 13H), 6.67 (s, 2H), 5.85 (s, 1H).
13C NMR (300 MHz, DMSO-d
6) d (ppm) 31.12, 111.89, 115.28, 118.58, 119.19, 119.71, 121.30, 123.89, 127.17, 129.66, 135.71, 136.93, 137.10, 155.79.
3,3’-Bisindolyl-4-nitrophenylmethane (Table 1, entry 10) 1H NMR (90 MHz, CDCl
3)d (ppm) 8.19 (d, 2H, J 7.81),
1H); 13C NMR (75 MHz, CDCl
3) d (ppm) 44.5, 110.0,
112.1, 119.6, 119.8, 120.9, 121.3, 121.9, 130.2, 133.8, 136.2, 143.1, 145.2
3,3’-Bisindolyl-3-nitrophenylmethane (Table 1, entry 11) 1H NMR (90 MHz, CDCl
3)d (ppm) 8.46 (br s, 2H),
7.02-7.87 (m, 12H), 6.61 (s, 2H), 5.34 (s, 1H); 13C NMR
(75 MHz, CDCl3)d (ppm) 34.9, 111.5, 111.6, 119.5, 120.7, 121.9, 122.2, 124.3, 126.8, 129.6, 131.2, 132.6, 134.2, 136.8, 149.7.
3,3’-Bisindolyl-4-(N,N-dimethyl)phenylmethane (Table 1,
entry 12)
1H NMR (90 MHz, CDCl
3) dH (ppm) 3.28 (s, 6H), 5.75 (s, 1H), 6.76-7.84 (m, ArH, 14H), 7.82 (br s, NH, 2H).MS: (MS-EI) m/z 365.
3,3’-Bisindolyl-1-(2-phenylethylene)methane (Table 1,
entry 13)
1H NMR (90 MHz, CDCl
3) d (ppm) 5.92-6.07 (m, 2H), 5.76 (m, 1H), 6.71 (s, 2H), 6.89-7.68 (m, 15H), 8.02 (br s, 2H, NH). MS: (MS-EI) m/z 348.
3,3’-Bisindolyl-[2]thienylmethane (Table 1, entry 14) Brown solid, mp 185-186 °
C.IR (KBr) ν
max/cm
-1: 3412,
1715, 1452, 1260, 1105 cm-1. 1H NMR (90 MHz, CDCl 3)
dH (ppm) 6.18 (s, 1H), 6.87 (s, 2H), 6.92-7.48 (m, ArH, 11H), 7.98 (br s, NH, 2H). Anal. Calc. for C21H16N2S: C, 76.80; H, 4.91; N, 8.53. Found: C, 76.52; H, 5.10; N, 8.35.
3,3’-Bisindolyl-1-butylmethane (Table 1, entry 15) 1H NMR (90 MHz, CDCl
3) d (ppm) 0.80 (t, J 6.7 Hz, 3H), 1.25-1.29 (m, 6H), 4.62 (t, J 6.7 Hz, 1H), 6.80 (d, J
2.5 Hz, 2H), 6.89-7.68 (m, 8H), 8.12 (br s, 2H, NH). MS: (MS-EI) m/z 302.
3,3’-Bisindolyl-1-methyl-1-phenylmethane (Table 1, entry
16)
mp 165-167 oC. 1H NMR (90 MHz, CDCl
3) d (ppm) 2.40 (s, 3H), 6.67 (s, 2H), 6.95-7.43 (m, 13H), 7.91 (br s, 2H, NH). MS: m/z 336.
3,3’-Bisindolyl-1-methyl-1-(4-nitro)phenylmethane (Table
1, entry 17)
1H NMR (90 MHz, CDCl
3) d (ppm) 2.46 (s, 3H), 6.78 (s, 2H), 6.79-7.68 (m, 12H), 7.98 (br s, 2H, NH). MS: (MS-EI) m/z 381.
3,3’-Bisindolyl-[1,1]cyclohexane (Table 1, entry 18) Red solid, mp 115-116 °
C.IR (KBr) ν
max/cm
-1: 3478,
3020, 2935, 1603, 1522, 1421, 1335, 1216, 1099, 1017,
758, 699 cm-1. 1H NMR (90 MHz, CDCl
3) dH (ppm) 1.56 (m, 6H), 2.48 (m, 4H), 6.81 (s, 2H), 7.03-7.65 (m, 8H), 7.84 (br s, NH, 2H).
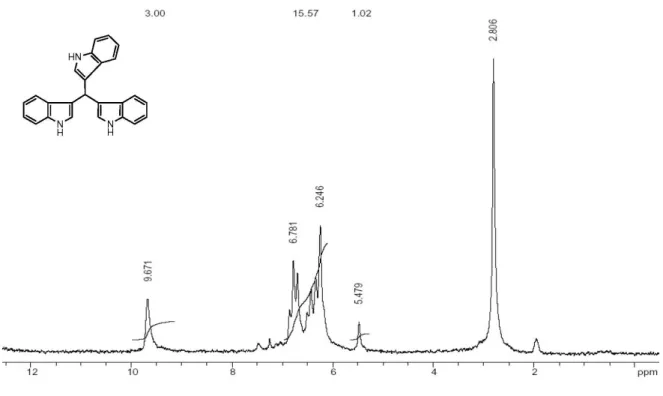
Analitical data for compound Tri-indolylmethane
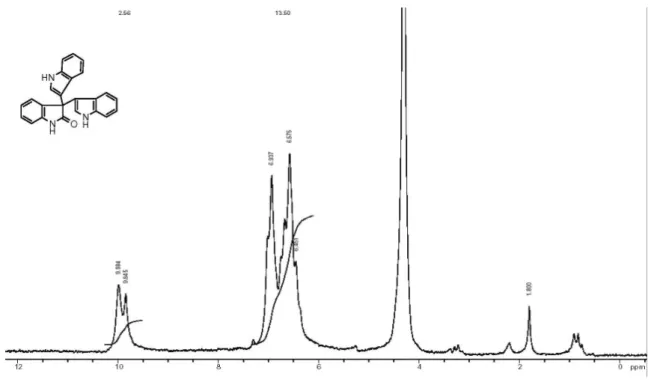
Yellow solid, mp 243-245 oC. 1H NMR (90 MHz, DMSO-d6) dH (ppm) 9.67 (br s, 2H), 6.24 (s, 3H), 6.27-6.87 (m, 12H), 5.47 (s, 1H). IR (KBr) ν
max/cm
-1: 3403, 3043,
2918 cm-1. MS: (MS-EI) m/z 361.
Analitical data for compound (a)
Yellow solid, mp 243-245 oC. 1H NMR (90 MHz, DMSO-d6) dH (ppm) 9.98 (br s, 2H), 9.81 (br s, 1H), 6.45 (m, 2H), 6.52-6.93 (m, 12H). 13C NMR (90 MHz, DMSO-d6) dc (ppm) 200.8, 160.5, 137.4, 136.8, 125.5, 124.4, 123.9, 121.0, 120.5, 118.3, 117.7, 117.0, 113.9, 111.7, 111.5. MS: (MS-EI) m/z 364.
Analitical data for compound (c)
Pink solid, mp 194-195 oC. FT-IR (KBr) ν
max/cm
-1: 3405,
3049, 1622, 1455, 1216 cm-1. 1H NMR (90 MHz, DMSO-d 6)
dH (ppm) 5.75 (s, CH, 1H), 6.29 (s, 4H), 7.05-7.40 (m, CH aromatic, 20H), 7.31 (br s, NH, 4H). 13C NMR (90 MHz, DMSO-d6) dc (ppm) 142.5, 136.7, 128.1, 126.8, 123.6, 120.9, 119.2, 118.4, 118.3, 111.5, 29.1. MS: (MS-EI) m/z 566.200.
Analitical data for compound (Table2, entry 1)
1H NMR (90 MHz, DMSO-d
6) dH (ppm) 10.68 (br s, 2H), 8.53 (br s, 2H), 6.75-7.55 (m, 12H), 6.35 (s, 1H), 4.12 (s, 3H), 2.55 (t, 2H), 2.23 (t, 2H), 1.78 (m, 2H).. Anal. Calc. for C32H32N2O5: C, 73.26; H, 6.15; N, 5.34. Found: C, 72.86; H, 6.18; N, 5.23. MS: (MS-EI) m/z 524.
Analitical data for compound (Table2, entry 2)
1H NMR (90 MHz, DMSO-d
6) dH (ppm) 10.59 (br s, 2H), 8.86 (br s, 2H), 6.92-7.62 (m, 12H), 6.22 (s, 1H), 5.22 (s, 4H), 2.30 (s, 3H). Anal. Calc. for C28H24N2O4: C, 74.32; H, 5.35; N, 6.19. Found: C, 73.92; H, 5.12; N, 5.79. MS: (MS-EI) m/z 452.
2,2’-Bisindolyl-phenylmethane (Table2, entry 3) 1H NMR (90 MHz, DMSO-d
6) dH (ppm) 7.94 (br s, 2H), 7.22-7.66 (m, 13H), 6.04 (s, 1H), 2.23 (s, 6H). Anal. Calc. for C25H22N2: C, 85.68; H, 6.33; N, 7.99. Found: C, 85.43; H, 5.95; N, 7.68. MS: (MS-EI) m/z 350.
2,2’-Bisindolyl-4-methoxyphenylmethane (Table2, entry 4) 1H NMR (90 MHz, DMSO-d
Figure S1. 1H NMR (CDCl
3) spectrum of 3,3
’-bisindolyl-phenylmethane (Table 1, entry1).
Figure S2. 1H NMR (CDCl
3) spectrum of 3,3
Figure S3. 1H NMR (CDCl
3) spectrum of 3,3
’-bisindolyl-4-(N,N-dimethyl)phenylmethane (Table 1, entry 12).
Figure S4. 1H NMR (CDCl
Figure S4. 1H NMR (CDCl
3) spectrum of compound (a). Same compound? Different spectrum?Again S4 ?
Figure S5. 1H NMR (DMSO-d
Figure S6. 1H NMR (DMSO-d
6) spectrum of compound tri-indolylmethane.
Figure S7. 1H NMR (DMSO-d
Figure S8. 1H NMR (DMSO-d
Figure S9. 1H NMR and 13C NMR (DMSO-d
Figure S10. 1H NMR (DMSO-d
6) spectrum of compound (g).
Figure S11. 1H NMR (CDCl
3) spectrum of compound 3,3
Figure S12. 1H NMR (CDCl
3) spectrum of compound 2,2
’-bisindolyl-phenylmethane (Table2, entry 3).
Figure S13. 1H NMR (CDCl
3) spectrum of compound 3,3