RE
VISÃO RE
VIE
W
1 Hospital Gaffrée Guinle, Universidade Federal do Estado do Rio de Janeiro (Unirio). R. Mariz e Barros 775, Tijuca. 20270-004 Rio de Janeiro RJ Brasil. adsvieira@yahoo.com.br 2 Escola de Nutrição, Unirio. Rio de Janeiro RJ Brasil.
Effectiveness of n-3 fatty acids in the treatment
of hypertriglyceridemia in HIV/AIDS patients: a meta-analysis
Efetividade de ácidos graxos n-3 no tratamento
da hipertrigliceridemia em pacientes com HIV/AIDS:
uma meta-análise
Resumo A hipertrigliceridemia é comum em pacientes tratados com terapia antirretroviral e os ácidos graxos ômega 3 estão sendo usados como uma intervenção na redução triglicérides séricos (TG) nesses pacientes. O objetivo deste estudo é avaliar a eficácia do uso do ômega 3 no tratamen-to da hipertrigliceridemia em pacientes com HIV/ Aids em terapia antirretroviral. Este estudo é uma revisão sistemática com metanálise de ensaios clí-nicos randomizados. As bases de dados eletrônicas – PubMed, Cochrane e Lilacs foram pesquisadas. Cinquenta e um artigos foram encontrados. Nove incluídos na metanálise. A redução do nível de tri-glicerídios foi -77.55 mg (IC de -121,85 a - 33,25) no grupo com Omega 3. A análise considerando ensaios com mais de 1000 mg de EPA/DHA in-cluiu sete estudos e a heterogeneidade caiu para 0%. A redução das médias combinada foi -101.56 mg (IC de -145,76 a -57,37). A análise conside-rando ensaios com doentes que tinham mais do que 200 mg/dL, de triglicéridos iniciais incluiu também sete ensaios e a heterogeneidade caiu para 0%. A redução das médias combinada foi -114.15 mg (IC de -162,34 a -65,97). A suplementação de EPA/DHA reduz níveis de triglicérides séricos em pacientes com hipertrigliceridemia associada ao HIV/AIDS em uso de terapia antirretroviral
es-tável.
Palavras-chave HIV, HAART, Triglicerídeos, Ômega 3 e óleo de peixe
Abstract Hypertriglyceridemia is common in an-tiretroviral therapy-treated patients and Omega 3 fatty acids are being used as a intervention in re-ducing serum triglycerides (TG) in these patients. The objective of this study is to evaluate the effec-tiveness of the use of Omega 3 in the treatment of hypertriglyceridemia in HIV/AIDS patients on antiretroviral therapy. This study is a systematic review with meta-analysis of randomized clinical trials. Electronic databases – PubMed, Cochrane and Lilacs were researched. Fifty one articles were encountered. Nine were added to the meta-analy-sis. The reduction of triglycerides level was -77.55 mg (IC of -121.85 to -33.25) in Omega 3 groups. The analysis considering trials with more than 1000 mg of EPA/DHA included seven studies and the heterogeneity dropped to 0%.The reduc-tion of combined averages was -101.56mg (IC of -145.76 to -57.37). The analysis considering trials with patients that had more than 200 mg/dL of initial triglycerides included also seven trials and the heterogeneity dropped to 0%. The reduction of combined averages was -114.15 mg (IC of -162.34 to -65.97). EPA/DHA supplementation reduces serum triglycerides levels in patients with HIV/ AIDS-associated hypertriglyceridemia in stable
use of antiretroviral therapy.
Key words HIV, HAART, Triglycerides, Omega 3 and fish oil
Aline Doria Sobral Vieira 1
V
ie
ir
a
ADS,
S
il
ve
Introduction
Highly activity antiretroviral therapy (HAART) is reducing the morbidity and mortality of HIV-infected patients. With improved prognosis, the disease’s chronic aspect started to be han-dled with1,2. However, treatment is associated to
adverse reactions and HIV associated lipodys-trophy syndrome characterized by dyslipidemia, glycemic alterations and morphologic alterations as lipoatrophy and lipohypertrophy3.
In case of dyslipidemia, when associated to HIV-infection, it occurs low HDL levels, total cholesterol and LDL increase (TC) and rise of triglycerides, conditions that favor the risk of car-diovascular disease4,5. A few observational
stud-ies revealed that the incidence of cardiovascular event in HIV-infected patients on ART is higher than in the overall population6,7.
Hypertriglyceridemia is one of the most fre-quent metabolic alterations in ART patients8. In
a transversal study with 788 patients’ cohort, the authors identified the prevalence of 56% of hy-pertriglyceridemia9.
It has been discussed the effect of Omega 3 fatty acids in metabolic complications in ART patients. Almeida et al., in a review, concluded that Omega 3 supplementation resulted in sub-stantial reduction of serum triglycerides levels10.
In 2012, a new review showed that the reduction of triglycerides with Omega 3 fatty acids supple-mentation was the nutritional intervention that most gathered substantial scientific evidences11.
In a meta-analysis published by Oliveira et al.12 in 2011 with four controlled trials evaluating
the use of Omega 3 fatty acids in the reduction of serum triglycerides in ART HIV-patients, it was observed a reduction of -80.34 with interval con-fidence of (IC of -129.08 to -31.60) in Omega-3 treated groups. In another meta-analysis where the effects of dietetic intervention in HIV dyslip-idemia were analyzed it was observed a reduction of 99.11 (IC of -138.93 to -59.29) in triglycerides of the Omega 3-treated group13.
In another study of Jacobson14, the author
discuss that the degree of TG reduction would be associated on the initial levels of triglycerides and the dose of EPA/DHA used.
There was no uniformity across clinical trials in the dosage of the supplement used, with a vari-ation of 900 to 4000 mg/day of EPA/DHA. The same thing happens with the initial triglicerides dosage15,16.
New clinical trials were published16-18, and
better answers are necessary to the questions mentioned above.
The aim of this study is to evaluate the effec-tiveness of the use of omega 3 fatty acids in the treatment of hypertriglyceridemia in HIV/AIDS patients on HAART.
Method
This study consists in a systematic review with meta-analysis of randomized clinical trials to as-sess the use of Omega 3 fatty acids for the treat-ment of hypertriglyceridemia in HIV/AIDS pa-tients.
Studies with HIV/AIDS male and female, any ethnicity adult individuals on stable use of ARV’s with Omega 3 fatty acids-based interventions, at any dosage, compared with control group pla-cebo and serum triglycerides as outcome were included. Studies comparing equivalence or su-periority of n-3 supplement the drugs were ex-cluded.
The research was made in electronic data-bases – PubMed, Cochrane Central of Clinical Trials, and Lilacs, using the terms: HIV, HAART, triglycerides, hypertriglyceridemia, Omega3 and fish oil. The searches were made between May 2013 and August 2014, and the terms were used in the follow combination, aiming to find the greatest number of studies: HIV, Hypertriglycer-idemia, omega 3; HIV, HypertriglycerHypertriglycer-idemia, fish oil; HIV, triglycerides, omega 3; HIV, triglycerides, fish oil; HAART, Hypertriglyceridemia, omega 3; HAART, Hypertriglyceridemia,fish oil; HAART, triglycerides, omega 3; HAART, triglycerides, fish oil. Articles were obtained from specific bib-liographic references in manual search too. The quotes identified by the researchers were selected by two independent reviewers.
Jadad Scale19 was adopted to evaluate the
quality of the articles comprehending the ran-domization (1 point to randomized plus 1 for adequate randomization), blinding (1 point to blinding plus 1 for adequate blinding) and losses (only 1 point). So the total sum of points is five and five is the better classification. However, such classification was applied only to discuss differ-ent results of each study and not to discard them. Portions of the data were extracted inde-pendently by two reviewers.
Differences of weighted averages by the inver-sion of the variance of the study20 to evaluate the
esti-aúd
e C
ole
tiv
a,
22(8):2659-2669,
2017
mated using the fixed effects method20. In case
of heterogeneity, Dersimonian & Laird21 random
effects model was used. The presence of hetero-geneity was evaluated per the methods suggested by Deeks et al.20.
Initially, a graphic exploratory analysis was performed based in the visual inspection of the charts (forest-plot). Later, the chi-square test (χ2)
of homogeneity was calculated.
Because of the limitation of the test χ2, the
heterogeneity was investigated as well through statistic I2 proposed by Higgins & Thompson22.
Values below 30% mean mild heterogeneity, in-termediate values of 30% to 50%, moderate and above 50%, an elevated level of heterogeneity.
Multivariate models of meta-regression23
were adjusted to investigate possible sources of heterogeneity across the studies’ results.
It was adopted the sensibility analysis to ex-plore the robustness of the results. This analysis consists in the repetition of the procedures, ex-cluding, for example, non-published studies and of low methodological quality.
Stata 10.0 SE24 software was used for
statisti-cal analysis.
Results
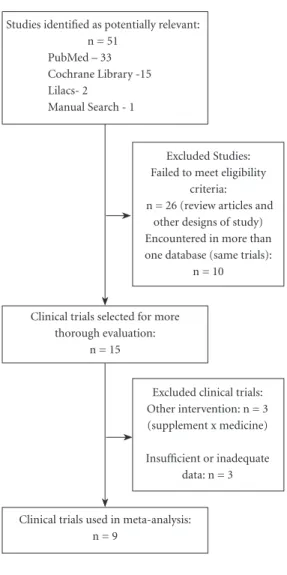
A total of 51 articles were encountered. At Pubmed 33, Cochrane 15, Lilacs 2 and 1 article as reference of references. Of a total of 51 arti-cles, only 9 met the eligibility criteria previously defined and were included in the meta-analysis (Figure 1).
Of the nine (9) clinical trials included in this meta-analysis with total of 448 patients, five (5) consider diet together with intervention17,25-28
and one of them adopted diet and physical work out28. The other four (4) did not address
co-in-terventions. The result of the quality analysis ac-cording to Jadad Scale was three trials (3) with five points, three trials (3) with four points and three trials (3) with Three points.
Six studies accepted the stable use of hypoli-pemiants for a minimum period (prior to the be-ginning of the study) of 8 weeks18; 3 months15,29;
6 months25; one of them accepted the use of
statin, but excluded who were on fibrates for 6 weeks before the beginning of the study28; and
on another, the use of fibrate or niacin was al-lowed, but the use of niacin for less or equal to 3 months prior to the beginning of the study was exclusion criteria26. Various TARV regimens
were described, using protease inhibitors (PIs), NRTI (nucleoside analog reverse-transcriptase
inhibitors) and NNRTI (non-nucleoside analog reverse-transcriptase inhibitors) in various com-binations; and the stable use for a period of ≥ 2 months27; ≥ 3 months16,18,26,28,29; ≥ 6 months15,17,25
was inclusion criteria.
Supplements used and dosages of EPA/DHA (eicosapentaenoic acid and docosahexaenoic acid) per day were:
Omacor® – 3360 mg26,29;
Maxepa® – 2700 mg25,1800 mg27;
Lovaza® – 3360 mg18;
Coromega® – 2900 mg28;
Salmon oil – 900 mg15;
Fish oil – 4000 mg17, 900 mg16
The studies included in this revision are shown in Chart 1.
Excluded Studies: Failed to meet eligibility
criteria: n = 26 (review articles and
other designs of study) Encountered in more than one database (same trials):
n = 10
Excluded clinical trials: Other intervention: n = 3 (supplement x medicine) Insufficient or inadequate
data: n = 3 Studies identified as potentially relevant:
n = 51 PubMed – 33 Cochrane Library -15 Lilacs- 2
Manual Search - 1
Figure 1. Flow chart with stages of obtaining of results. Clinical trials selected for more
thorough evaluation: n = 15
V ie ir a ADS, S il ve
Chart 1. Data summary of clinical trials included in the meta-analysis. Author/ Year/ Country Method/ timing (author/used) Intervention (n) Control
(n) Jadad Results (tg)
Baril JG et al.15, 2007.
Canada Clinical Trial, randomized, controlled, crossover. 24 weeks (author). 12 weeks (used). Salmon oil. EPA/DHA - 540/360mg. n = 26
Did not use placebo, did not receive oil (salmon) in the 12 initial weeks, but from 12th -24th
weeks. n = 31
3 I C mg/dL(SD) mmol/ L(SD) mg/dL(SD) mmol/ L(SD) 380,5 (203,5), reduction TG. P = 0.04
4.3 (2.3) 433.6 (212.3) 4.9 (2.4)
Peters BS et al.26, 2012.
England Clinical trial, randomized Double-blind, controlled. 12 weeks. Hypocholesterinic diet in use of fibrate or niacin. Omacor®. EPA/DHA - 1840/1520mg. n = 23
Hypocholesterinic diet in use of fibrate or niacin + placebo.
n = 25
5 I C mg/dL (SD) mmol/L (SD) mg/dL (SD) mmol/ L(SD) 342.4 (168.1), reduction TG. p = 0.02
3.78 (1.90) 425.6 (272.5) 4.81 (3.08) Carter et al.25, 2006.
Australia Clinical trial, randomized Double-blind, controlled. 14 weeks.
Maxepa®.
EPA/DHA - 1620/1080mg. n = 5
Dietary guidance for 6 weeks (NHFG) placebo (olive oil). n = 6
4 I C mg/dL (SD) mmol/L (SD) mg/dL (SD) mmol/ L(SD) 203.5 (163.7), reduction TG. p = 0.04
2.3 (1.85)
361.1(163.7) 4.08 (1.85)
De Truchis et al.27, 2007.
France Clinical trial, randomized Double-blind, controlled. crossover. 16 weeks (author). 8 weeks (used).
Maxepa®
EPA/DHA - 1080mg/720mg. n = 58
4 weeks diet (AHA) placebo. n = 62
5 I C mg/dL (SD) mmol/L (SD) mg/dL (SD) mmol/L (SD) 340 (180), reduction TG. p = 0.003
3.8 (2.30)
480 (310) 5.4 (3.5)
Wohl et al.28,
2005. USA Clinical Trial, randomized, controlled. 16 weeks (author). 4 weeks (used).
Coromega®.
EPA/DHA - 1750/1150mg. n = 24.
Diet and physical activity (AHA), no placebo.
n = 20
3 I C mg/dL (SD) mmol/L (SD) mg/dL (SD) mmol/L (SD) 306 (162), redução TG. p = 0,007
3.4 (1.8) 503 (421) 5.6 (4.7)
Thusgaardet al..29, 2009.
Denmark Clinical trial, randomized Double-blind, controlled. 12 weeks. Omacor® EPA/DHA - 1840/1520mg. n = 25
Placebo, 2 pills, 2g corn oil 2x/day. n = 23
4 I C mg/dL (SD) mmol/L (SD) mg/dL (SD) mmol/L (SD) 134.5 (85.8), reduction TG. p = 0.03
1.52
(0.97) 178.7 (170.7) 2.02 (1.93)
aúd e C ole tiv a, 22(8):2659-2669, 2017
Effect of the Omega 3 fatty acids on triglycerides in individuals with HIV/AIDS associated hypertriglyceridemia
Of the studies encountered and considering those with sufficient data for calculation purpos-es, nine clinical trials evaluated the outcome of
interest. Heterogeneity measured by I2 of
Hig-gins & Thompson22 was 43.7%. The results of
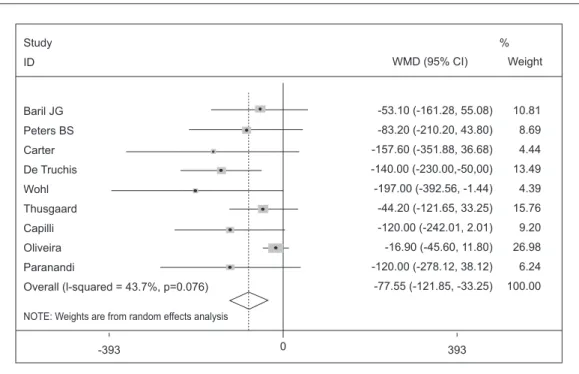
each study, their respective intervals of confi-dence (IC) of 95% and the summary measure are shown in Graphic 1. The reduction of combined averages through random effects was -77.55 mg/ dL (IC of -121.85 to -33.25; p = 0.001), that can be interpreted as presence of significant reduc-tion of triglycerides of the Omega 3group.
Year variables, classification of Jadad et al.19,
EPA and DHA dosage and initial triglycerides were analyzed to evaluate heterogeneity. Howev-er, none of the variables used explained the het-erogeneity.
As the AHA cardioprotective recommended dosage is approximately 1g/d and the recom-mended dosage to reduce triglycerides levels is 2 to 4g/d30 it was performed the analysis of
sub-groups considering studies with more than 1000 mg of EPA/DHA. Of the nine studies included, seven used more than 1000 mg of EPA/DHA and, when these seven were analyzed, the heteroge-neity measured by I2 of Higgins & Thompson22
dropped to 0%. The results of each study, their respective intervals of confidence (IC) of 95%
Chart 1. continuation
Author/ Year/ Country Method/ timing (author/used) Intervention (n) Control
(n) Jadad Results (tg)
Capili & Anastasi17,
2013. USA Clinical trial, randomized Double-blind, controlled. 10 weeks (author). 8 weeks (used).
Diet (NCEP-TLC). Fish oil.
EPA/DHA - 2400mg/1600mg n = 8
Diet (NCEP-TLC) placebo
n = 10
4 I C mg/dL (SD) mmol/L (SD) mg/dL (SD) mmol/L (SD) 169 (133), reduction TG. p = 0.013
1.90
(1.50) 289 (129)
3.26 (1.45)
Oliveira et al.16, 2014.
Brazil Clinical Trial, randomized, controlled. 24 weeks. Fish oil. EPA/DHA - 540mg/365mg. n = 31
Placebo, 3g soy oil/day n = 35
3 I C mg/dL (SD) mmol/L (SD) mg/dL (SD) mmol/L (SD) 141.7 (59.7), Non-significant reduction TG When compared with GC. p = 0.613
1.60
(0.67) 158.6 (59.0) 1.79 (0.66)
Paranandi A et al.14, 2014.
USA Clinical Trial, randomized, controlled. Crossover. 28 weeks (author) Washout (4 weeks) 12 weeks (used).
Lovaza®.
EPA/DHA - 1860/1500mg (12 weeks)
n = 17
Placebo (12 weeks.), washout (4 weeks) and 4 pills of Lovaza®
(12 weeks). n = 19
5 I C mg/dL (SD) mmol/L (SD) mg/dL (SD) mmol/L (SD) 215.6 (149.4), reduction TG. p = 0.001
2.43
(1.68) 335.6 (314.2) 3.79 (3.55)
V
ie
ir
a
ADS,
S
il
ve
and the summary measure are shown in Graphic 2. The reduction of combined averages through the random effects was
-101.56 mg/dL (IC of -145.76 to -57.37; p= 0.00), which can be interpreted as presence of significant reduction of triglycerides of the group that used more than 1000 mg of EPA/DHA.
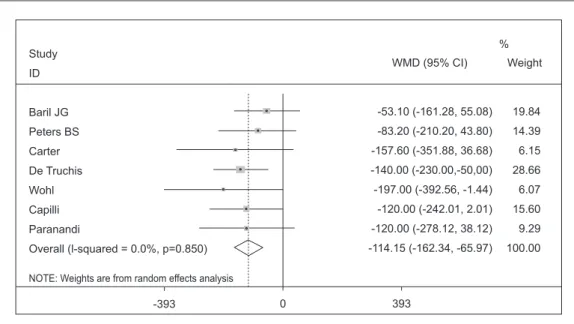
Was performed the analysis of subgroups in studies with patients that had more than 200 mg/dL serum triglycerides in the beginning of the study, a value defined as elevated level of triglyceride31. Of the nine studies used in this
meta-analysis, seven included patients with ini-tial average triglycerides over 200 mg/dL and
Graphic 2. Effectiveness of more than 1000mg of EPA/DHA to treat HIV/AIDS associated hypertriglyceridemia.
Study
ID
Peters BS
Carter
De Truchis
Wohl
Thusgaard
Capilli
Paranandi
Overall (l-squared = 0.0%, p=0.641)
NOTE: Weights are from random effects analysis
WMD (95% CI)
-83.20 (-210.20, 43.80)
-157.60 (-351.88, 36.68)
-140.00 (-230.00, -50.00)
-197.00 (-392.56,-1.44)
-44.20 (-121.65, 33.25)
-120.00 (-242.01, 2.01)
-120.00 (-278.12, 38.12)
-101.56 (-145.76, -57.37) Weight
12.11
5.17
24.11
5.11
32.56
13.12
7.81
100.00 %
393
-393 0
Graphic 1. Effectiveness of Omega 3 fatty acids to treat HIV/AIDS associated hypertriglyceridemia.
Study
ID
Baril JG
Peters BS
Carter
De Truchis
Wohl
Thusgaard
Capilli
Oliveira
Paranandi
Overall (l-squared = 43.7%, p=0.076)
NOTE: Weights are from random effects analysis
WMD (95% CI)
-53.10 (-161.28, 55.08)
-83.20 (-210.20, 43.80)
-157.60 (-351.88, 36.68)
-140.00 (-230.00,-50,00)
-197.00 (-392.56, -1.44)
-44.20 (-121.65, 33.25)
-120.00 (-242.01, 2.01)
-16.90 (-45.60, 11.80)
-120.00 (-278.12, 38.12)
-77.55 (-121.85, -33.25) Weight
10.81
8.69
4.44
13.49
4.39
15.76
9.20
26.98
6.24
100.00 %
393
aúd
e C
ole
tiv
a,
22(8):2659-2669,
2017
when these seven were analyzed, the heteroge-neity measured by I2 of Higgins & Thompson22
dropped to 0%. The results of each study, their respective intervals of confidence (IC) of 95% and the summary measure are shown in Graphic 3. The reduction of combined averages through random effects was -114.15 mg/dL
(IC from -162.34 to -65.97; p = 0.00), which can be interpreted as presence of significant re-duction of triglycerides of the group with initial serum triglyceride above 200 mg/dL.
Discussion
This meta-analysis with nine studies indicated a statistically significant reduction of serum tri-glycerides in the Omega 3 (EPA/DHA) group when compared to control group. Similar re-sults to the encountered in the meta-analysis of Oliveira e Stradling12,13. The poor result of the
triglycerides reduction in the present study can be explained by the inclusion of new clinical trials16-18 with specific characteristics discussed
next. The heterogeneity encountered was consid-ered moderate according to Higgins
&Thomp-son22. Two analysis of the subgroup were
con-ducted, the first excluding the studies that used
under 1000 mg EPA/DHA day15,16 dosages and
the second, excluding the studies with initial
tri-glycerides under 200 mg/dL16,29; in both studies,
the heterogeneity was 0%.
Studies of the overall population show that there is a relation between levels of triglycerides and the percentage of reduction. Individuals with higher triglycerides have major percent of reduc-tion of the EPA/DHA dosage used32. Balk et al.33
in a systematic review with meta-analysis verified that each raise of 1 g/d of fish oil was associated to the reduction of triglycerides levels of approx-imately 8 mg/dL. Additionally, each raise of 10 mg/dL in the initial levels of triglycerides is as-sociated to a decline of 1,6 mg/dL of triglycerides after consumption of fish oil. The degree of TG reduction would be contingent, therefore, on the initial levels of triglycerides and the dose of EPA/ DHA used14.
There was no uniformity across clinical trials in the dosage of the supplement used, with a vari-ation of 900 to 4000 mg/day of EPA/DHA. Only two clinical trials used values below 1000mg of EPA/DHA15,16 and presented different results.
Ol-iveira et al.16 did not find significant reduction
when compared to the control group while in the study of Baril et al.15 the reduction was
substan-tial. The difference among these studies is most likely in the initial levels of triglycerides: Olivei-ra, under 200m/dL, and Baril, above 400mg/dL, which is the justification of Baril’s study best re-sults.
Graphic 3. Effectiveness of Omega 3 to treat HIV/AIDS associated hypertriglyceridemia in patients with triglycerides over 200 mg/dL d.
Study
ID
Baril JG
Peters BS
Carter
De Truchis
Wohl
Capilli
Paranandi
Overall (l-squared = 0.0%, p=0.850)
NOTE: Weights are from random effects analysis
WMD (95% CI)
-53.10 (-161.28, 55.08)
-83.20 (-210.20, 43.80)
-157.60 (-351.88, 36.68)
-140.00 (-230.00,-50,00)
-197.00 (-392.56, -1.44)
-120.00 (-242.01, 2.01)
-120.00 (-278.12, 38.12)
-114.15 (-162.34, -65.97) Weight
19.84
14.39
6.15
28.66
6.07
15.60
9.29
100.00 %
393
V
ie
ir
a
ADS,
S
il
ve
In the present study the results were clear-ly better when the EPA/DHA was more than 1000mg and triglycerides was over than 200 mg/ dL. This becomes clear when look at the summa-ry measured: -77,55 (-121,85 to -33,25) all group; -101,56 (-145,76 to -57,37) >1000 mg EPA/DHA; -144,15 (-162,34 to -65,97) >200 mg/dL of tri-glycerides.
Other studies that also adopted the same dos-age of EPA/DHA18,26,29 and that had different
ini-tial levels of TG, also presented different results. The reduction was commensurate to the initial levels of triglycerides. The higher TG, major re-duction is encountered (Peters et al.26 > 400 mg/
dL, Paranandi et al.18 >200 mg/dL and Thusgaard
et al.29 < 200 mg/dL)
Our study has limitations, due to the reduced number of studies included, different dosages of Omega 3, difference of interventions associated with concomitant use of diets, use of hypolimi-ants, further to initial TG levels and differences of TARV regimen. Among them, it is possible to single out the absence of non-published studies, the impossibility of evaluating the existence of bias of publication due to the small number of studies included and heterogeneity.
The recommendations for the treatment of dyslipidemia, including HIV-patients hypertri-glyceridemia, are the same for the overall popula-tion. The guidelines of IDSA (Infectious Disease
Society of America)34 and ACTG (Adult AIDS
Clinical Trial Group)35 follow the
recommenda-tions of NCEP ATPIII31. EACS (European AIDS
Clinical Society)36 guidelines recommend healthy
diet, work out and weight control to reduce dys-lipidemia and among the drugs recommended for dyslipidemia management, it appears Omega 3 with dosage indication, Maxepa® 5g 2x/d(3000 mg EPA/DHA) and Omacor® 1g to 2g 2x/d (1680 mg to 3360 mg EPA/DHA) to reduce TG.
Publications about dyslipidemia manage-ment for HIV manage-mention fish oil (supplemanage-ment of EPA/DHA) as a safe and alternative treatment to reduce serum TG because it is well tolerated and does not present drug interactions with
antiret-roviral therapy37-39.Recommended dosage vary
from 2 to 9g/d and the supplements are indicated some times as fish oil, others as Omega 3 fatty acids and others as EPA/DHA37,38,40-42.
Fish oil is a source of Omega 3 fatty acid con-taining EPA/DHA. And 1g of fish oil (or Omega 3) is not necessarily equal to 1 g of EPA/DHA, it will depend on the prescribed formulation. For this reason, the selection of supplement with bigger concentration of EPA/DHA is essential
to reduce the quantity of required pills to get an effective result, thus improving the adherence to the treatment.
To achieve the dosage recommended by the AHA cardioprotective approximately 1g/day of EPA/DHA need to ingest ≈ 71g of salmon, 71-340 g of tuna, 57g of herring, 354 of cod daily43. The
recommendation for the reduction of TG is 2 to 4 g/of which prevents the use of fish consumption for this purpose.
In two studies44,45 comparing the use of
Ome-ga 3 and fibrates to reduce TG in HIV patients, the authors conclude that the reduction with fibrates is bigger, but it occurs also with Omega3 (n-3 polynsaturated fatty acids-PUFA, polyunsaturat-ed ethyl esters of n-3 fatty acids- PEE) showing improved tolerability, and this may potentially mean an effective and safe alternative to fibrates.
In another study46 evaluating the associated
use of fish oil supplement with fenofibrate, which initially separated the patients received treatment for 8 weeks and those who did not respond to treatment, received combination therapy for 10 weeks. In the group receiving fish oil only TG was reduced by 46%, as received fenofibrate was 58% and that received combination therapy reduction was 65.5%, concluding that the fish oil alone or combined the fenofibrate is safe and significantly reduces TG levels in HIV patients with hypertri-glyceridemia and that the combination therapy is effective for those who do not respond to therapy alone.
EPA/DHA dosage to reduce TG needs more trials. As there exists a relation between the initial TG and EPA/DHA dosage in the effective reduc-tion, the following questions need to be respond-ed: Will initial high TG respond better to a lower EPA/DHA dosage? And even why not ask wheth-er, depending on the case, would it be interesting to attempt initially only with EPA and DHA prior to prescribing fibrate in specific cases, as we are dealing with patients using multiple medicines daily?
Conclusions
The findings of this study led to the conclusion that supplementation with EPA/DHA in 900 to 4,000 mg/day dosages reduce the serum levels of triglycerides in HIV/AIDS associated hypertri-glyceridemia patients in stable use of antiretro-viral therapy.
tri-aúd
e C
ole
tiv
a,
22(8):2659-2669,
2017
glyceride and when offered in higher doses than 1000 mg/day.
Other clinical trials to attempt to determine EP/DHA dosages in different scenarios to pro-mote the reduction of TG are necessary, consid-ering the relation of initial TG and EPA/DHA dosage.
Collaborations
V
ie
ir
a
ADS,
S
il
ve
Referências
1. Brazil. Ministry of Health (MoH). Health Vigilance Secretariat. Program of STD/AIDS. Recommenda-tions on Antiretroviral Therapy in HIV Infected Adults. Brasília: MoH; 2008.
2. Oh J, Hegele RA. HIV-associated dyslipidaemia: pathogenesis and treatment. Lancet Infect Dis 2007; 7(12):787-796.
3. Valente AM, Reis AF, Machado DM, Succi RC, Chacra AR. Metabolic alterations in HIV-associated lipodys-trophy syndrome. Arq Bras Endocrinol Metabol 2005; 49(6):871-881.
4. Montessori V, Press N, Harris M, Akagi L, Montaner JS. Adverse effects of antiretroviral therapy for HIV infec-tion. CMAJ 2004; 170(2):229-238.
5. Friis-Moller N, Sabin CA, Weber R, d’Arminio Mon-forte A, El-Sadr WM, Reiss P, Thiebaut R, Morfeldt L, De Wit S, Pradier C, Calvo G, Law MG, Kirk O, Phillips AN, Lundgren JD. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med
2003; 349(21):1993-2003.
6. Sabin CA, d’Arminio Monforte A, Friis-Moller N, We-ber R, El-Sadr WM, Reiss P, Kirk O, Mercie P, Law MG, De Wit S, Pradier C, Phillips AN, Lundgren JD. Chang-es over time in risk factors for cardiovascular disease and use of lipid-lowering drugs in HIV-infected indi-viduals and impact on myocardial infarction. Clin In-fect Dis 2008; 46(7):1101-1110.
7. Saves M, Chene G, Ducimetiere P, Leport C, Le Moal G, Amouyel P, Arveiler D, Ruidavets JB, Reynes J, Bing-ham A, Raffi F. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis 2003; 37(2):292-298.
8. Campanela RC FB, Lagos SL, Gavriolovics BA, Vasquez TP. Hypertriglyceridemia and antiretroviral therapy in HIV patients - clinical cases. Bol Hosp San Juan de Dios
2003; 50(1):47-51.
9. Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadi-ponectinemia. Diabetes Care 2007; 30(1):113-119. 10. Almeida LB, Giudici KV, Jaime PC. Dietary intake and
dyslipidemia arising from combination antiretroviral therapy for HIV infection: a systematic review. Arq Bras Endocrinol Metabol 2009; 53(5):519-527.
11. Falco M, Castro Ade C, Silveira EA. Nutritional therapy in metabolic changes in individuals with HIV/AIDS.
Rev Saude Publica 2012; 46(4):737-746.
12. Oliveira JM, Rondo PH. Omega-3 fatty acids and hy-pertriglyceridemia in HIV-infected subjects on antiret-roviral therapy: systematic review and meta-analysis.
HIV Clin Trials 2011; 12(5):268-274.
13. Stradling C, Chen YF, Russell T, Connock M, Thom-as GN, Taheri S. The effects of dietary intervention on HIV dyslipidaemia: a systematic review and meta-anal-ysis. PLoS One 2012; 7(6):e38121.
14. Jacobson TA. Role of n-3 fatty acids in the treatment of hypertriglyceridemia and cardiovascular disease. Am J Clin Nutr 2008; 87(6):1981S-1990S.
15. Baril JG, Kovacs CM, Trottier S, Roederer G, Martel AY, Ackad N, Koulis T, Sampalis JS. Effectiveness and tol-erability of oral administration of low-dose salmon oil to HIV patients with HAART-associated dyslipidemia.
HIV Clin Trials 2007; 8(6):400-411.
16. Oliveira JM, Rondo PH, Yudkin JS, Souza JM, Pereira TN, Catalani AW, Picone CM, Segurado AA. Effects of fish oil on lipid profile and other metabolic outcomes in HIV-infected patients on antiretroviral therapy: a randomized placebo-controlled trial. Int J STD AIDS
2014; 25(2):96-104.
17. Capili B, Anastasi JK. Exploratory study: evaluating the effects of fish oil and controlled diet to reduce tri-glyceride levels in HIV. J Assoc Nurses AIDS Care 2013; 24(3):276-282.
18. Paranandi A, Asztalos BF, Mangili A, Kuvin J, Gerrior J, Sheehan H, Skinner SC, Tang AM, Wanke CA. Short communication: effects of omega-3 fatty acids on tri-glycerides and high-density lipoprotein subprofiles in HIV-infected persons with hypertriglyceridemia. AIDS Res Hum Retroviruses 2014; 30(8):800-805.
19. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding neces-sary? Control Clin Trials 1996; 17(1):1-12.
20. Deeks JJ, Altman DG, Bradburn MJ. Statistical meth-ods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Dav-ey Smith G, Altman DG, editors. Systematic Reviews in Health Care: meta-analysis in context. 2nd ed. London: BMJ Books; 2001. p. 285-312.
21. DerSimonian R, Laird N. Meta-analysis in clinical tri-als. Control Clin Trials 1986; 7(3):177-188.
22. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21(11):1539-1558. 23. Stern JEM. Investigating and dealing with publication
and other bases. In: Smith GD. Systematic reviews in Health care. London: Book B; 2001. p. 189-208. 24. STATACORP. Stata Statistical Software/SE. release 10.
College Station: Stata-Corp LP; 2007.
25. Carter VM, Woolley I, Jolley D, Nyulasi I, Mijch A, Dart A. A randomised controlled trial of omega-3 fatty acid supplementation for the treatment of hypertriglyceri-demia in HIV-infected males on highly active antiret-roviral therapy. Sex Health 2006; 3(4):287-290. 26. Peters BS, Wierzbicki AS, Moyle G, Nair D, Brockmeyer
N. The effect of a 12-week course of omega-3 polyun-saturated fatty acids on lipid parameters in hypertri-glyceridemic adult HIV-infected patients undergoing HAART: a randomized, placebo-controlled pilot trial.
Clin Ther 2012; 34(1):67-76.
aúd
e C
ole
tiv
a,
22(8):2659-2669,
2017
28. Wohl DA, Tien HC, Busby M, Cunningham C, Macin-tosh B, Napravnik S, Danan E, Donovan K, Hosseni-pour M, Simpson Junior RJ. Randomized study of the safety and efficacy of fish oil (omega-3 fatty acid) sup-plementation with dietary and exercise counseling for the treatment of antiretroviral therapy-associated hy-pertriglyceridemia. Clin Infect Dis 2005; 41(10):1498-1504.
29. Thusgaard M, Christensen JH, Morn B, Andersen TS, Vige R, Arildsen H, Schmidt EB, Nielsen H. Effect of fish oil (n-3 polyunsaturated fatty acids) on plasma lip-ids, lipoproteins and inflammatory markers in HIV-in-fected patients treated with antiretroviral therapy: a randomized, double-blind, placebo-controlled study.
Scand J Infect Dis 2009; 41(10):760-766.
30. Kris-Etherton PM, Harris WS, Appel LJ. Omega-3 fatty acids and cardiovascular disease: new recommenda-tions from the American Heart Association. Arterioscler Thromb Vasc Biol 2003; 23(2):151-152.
31. Executive Summary of The Third Report of The Na-tional Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285(19):2486-2497.
32. Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG. Dose-response effects of omega-3 fatty acids on triglycerides, inflamma-tion, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr 2011; 93(2):243-252.
33. Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic re-view. Atherosclerosis 2006; 189(1):19-30.
34. Dube MP, Stein JH, Aberg JA, Fichtenbaum CJ, Ger-ber JG, Tashima KT, Henry WK, Currier JS, Sprecher D, Glesby MJ. Guidelines for the evaluation and man-agement of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Associ-ation of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis
2003; 37(5):613-627.
35. Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zing-man BS, Horberg MA, Infectious Diseases Society of A. Primary care guidelines for the management of per-sons infected with HIV: 2013 update by the HIV med-icine association of the Infectious Diseases Society of America. Clin Infect Dis 2013;58(1):e1-34.
36. Lundgren JD, Battegay M, Behrens G, De Wit S, Guar-aldi G, Katlama C, Martinez E, Nair D, Powderly WG, Reiss P, Sutinen J, Vigano A. European AIDS Clinical Society (EACS) guidelines on the prevention and man-agement of metabolic diseases in HIV. HIV Med 2008; 9(2):72-81.
37. Aberg JA. Lipid management in patients who have HIV and are receiving HIV therapy. Endocrinol Metab Clin North Am 2009; 38(1):207-222.
38. Stein JH. Dyslipidemia in the era of HIV protease in-hibitors. Prog Cardiovasc Dis 2003; 45(4):293-304.
39. Blanco F, San Roman J, Vispo E, Lopez M, Salto A, Abad V, Soriano V. Management of metabolic complications and cardiovascular risk in HIV-infected patients. AIDS Rev 2010; 12(4):231-241.
40. Wohl DA, McComsey G, Tebas P, Brown TT, Glesby MJ, Reeds D, Shikuma C, Mulligan K, Dube M, Wininger D, Huang J, Revuelta M, Currier J, Swindells S, Ficht-enbaum C, Basar M, Tungsiripat M, Meyer W, Weihe J, Wanke C. Current concepts in the diagnosis and man-agement of metabolic complications of HIV infection and its therapy. Clin Infect Dis 2006; 43(5):645-653. 41. Silva EFR, Barbaro G. New options in the treatment of
lipid disorders in HIV-infected patients. Open AIDS J.
2009; 3:31-37.
42. Feeney ER, Mallon PW. HIV and HAART-Associated Dyslipidemia. Open Cardiovasc Med J 2011; 5:49-63. 43. Kris-Etherton PM, Harris WS, Appel LJ. Fish
consump-tion, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002; 106(21):2747-2757. 44. Manfredi R. Management of
hypertriglyceridae-mia caused by combination antiretroviral therapy in HIV-infected patients: role of omega-3 polyunsatu-rated fatty acids at different dosages, compared with fibrates. Int J STD AIDS 2010; 21(1):73-74.
45. Manfredi R, Calza L, Chiodo F. Polyunsaturated ethyl esters of n-3 fatty acids in HIV-infected patients with moderate hypertriglyceridemia: comparison with di-etary and lifestyle changes, and fibrate therapy. J Acquir Immune Defic Syndr 2004; 36(3):878-880.
46. Gerber JG, Kitch DW, Fichtenbaum CJ, Zackin RA, Charles S, Hogg E, Acosta EP, Connick E, Wohl D, Kojic EM, Benson CA, Aberg JA. Fish oil and fenofibrate for the treatment of hypertriglyceridemia in HIV-infect-ed subjects on antiretroviral therapy: results of ACTG A5186. J Acquir Immune Defic Syndr 2008; 47(4):459-466.
Artigo apresentado em 25/06/2015 Aprovado em 23/01/2016