REVISTA
BRASILEIRA
DE
ANESTESIOLOGIA
PublicaçãoOficialdaSociedadeBrasileiradeAnestesiologiawww.sba.com.br
SCIENTIFIC
ARTICLE
Sufentanil
in
combination
with
low-dose
hyperbaric
bupivacaine
in
spinal
anesthesia
for
cesarean
section:
a
randomized
clinical
trial
Alexandre
Dubeux
Dourado
a,
Ruy
Leite
de
Melo
Lins
Filho
a,
Raphaella
Amanda
Maria
Leite
Fernandes
a,∗,
Marcelo
Cavalcanti
de
Sá
Gondim
a,
Emmanuel
Victor
Magalhães
Nogueira
baUniversidadeFederaldePernambuco(UFPE),HospitaldasClínicas,Recife,PE,Brazil
bUniversidadeFederaldePernambuco(UFPE),HospitaldasClínicas,ProgramadeResidênciaMédicaemAnestesiologia,
Recife,PE,Brazil
Received4April2015;accepted12May2015 Availableonline13September2016
KEYWORDS
Sufentanil; Spinalanesthesia; Bupivacaine; Hyperbaric; Cesareansection
Abstract Adoubleblindrandomizedclinicaltrialofsufentanilasanadjunctinspinal anes-thesiaforcesareansectionand,thereby,beabletoreducethedoseofbupivacaine,alocal anesthetic, with the same result of ananesthetic block with higher doses but with fewer perioperativesideeffects,suchashypotension.
©2016SociedadeBrasileiradeAnestesiologia.PublishedbyElsevierEditoraLtda.Thisisan openaccessarticleundertheCCBY-NC-NDlicense( http://creativecommons.org/licenses/by-nc-nd/4.0/).
PALAVRAS-CHAVE
Sufentanil; Raquianestesia; Bupivacaína; Hiperbárica; Cesariana
Associac¸ãodesufentaniladosereduzidadebupivacaínahiperbárica emraquianestesiaparacesariana:ensaioclínicorandomizado
Resumo Ensaioclínico randomizadoduplamenteencobertosobreousodosufentanilcomo adjuvanteemraquianestesiaparacesarianae,possibilitandoareduc¸ãodadosedoanestésico local,abupivacaína,comomesmoresultadodebloqueioanestésicocomdosesmaiselevadas, mascommenosefeitoscolateraisnoperioperatório,comohipotensão.
©2016SociedadeBrasileiradeAnestesiologia.PublicadoporElsevierEditoraLtda.Este ´eum artigoOpen Accesssobumalicenc¸aCCBY-NC-ND( http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗Correspondingauthor.
E-mail:rafa.amanda120@gmail.com(R.A.Fernandes).
http://dx.doi.org/10.1016/j.bjane.2015.05.002
Introduction
Compared togeneral anesthesia, neuraxial anesthesiafor
cesarean section (C-section)presents advantages, suchas
reduced risk of intubation failure and aspiration of
gas-tric contents, reduced use of depressants (hypnotics and
opioids),andthemother’sconsciousnessthatallows
moth-ers undergo the birth experience.1,2 Spinal anesthesia is
currently the most widely used technique for C-section,
asit providesintensesensoryblockandrapidinstallation.
However,thistechniquecanbeaccompaniedbysignificant
hypotension,itsmostimportantsideeffect,withreported
incidenceof20---100%.3---5
Several strategies have been described to prevent the
occurrenceofhypotensioninC-section,suchasleftuterine
displacement, crystalloid or colloid administration, lower
limb wrapping, prophylacticuse of ephedrineor
phenyle-phrine,butnoneofthemeliminatedhypotension.6,7
Therelationshipbetweenthelocalanesthetic(LA)dose
used and the occurrence of maternal hypotension is well
established,withhigherdosesrelatedtoahigherincidence
ofhypotension.8However,thereductionofLAdoseleadsto
increasedincidenceofintraoperativepain.3,9
Ithasbeenshownthatthecombinationoflipophilic
opi-oids with local anesthetics in spinal anesthesiaallows LA
dosereductionandpromoteseffectiveanesthesiawithless
sideeffectsonmaternalhemodynamics.10---14
Sufentanil,highlysoluble,enhancesanalgesiaand
com-fortduringsurgery,withashortlatencyperiod(5---10min) anddurationofactionofupto7h.10,15---17The useof
mor-phine, soluble opioid, is recommended to ensure longer
postoperativeanalgesia.10,18
The aim of this study wasto compare the efficacy of
anesthesiaandtheincidenceof sideeffects betweentwo
hyperbaric bupivacaine doses in spinal anesthesia for
C-sectionwithcombinedsufentanilatthelowestdose.
Material
and
methods
After approval by the Ethics Committee, the
random-ized double-blind trial was started with 94 women,
aged 18---45 years, undergoing C-section under spinal
anesthesia. After obtaining written informed consent
and consulting the randomization table, patients were
allocated to one of two study groups: Group A
(bupiva-caine 12.5mg+morphine 80g)and Group S (bupivacaine
10mg+morphine80g+sufentanil5g).Syringes
prepara-tionandspinalanesthesiaadministrationwereperformedby
ananesthesiologistblindedtodatacollection.Allthe inves-tigatorsinvolvedinthestudywereblindedtotheassignment
ofeach group.Pregnantwomenunabletodecideontheir
participationinthe studyor unabletoprovidethe
neces-saryinformation,ASAIVorV,requiringemergencyobstetric
care,withahistoryofhypersensitivityorallergytoanyof
thestudydrugs,andthosewithanycontraindicationtothe
techniqueproposedwereexcluded.
Withthepatientinasittingposition,subarachnoid punc-turewasperformedintheL2---L3,L3---L4orL4---L5interspace,
with25Gor27GQuinckeneedles,andthedrugswere
admin-isteredaccordingtothegroupforwhichitwasrandomized.
Local anesthetics and opioids were delivered in separate
syringesforatotaltimeof15s.The patientswere
imme-diatelyplacedinthesupineposition,manuallyshiftingthe uterustotheleftatanangleof15◦.
Blood pressure and heart rate measurements were
recorded before spinal anesthesia and at every 3min in
thefirst15min, andat 30and 45min aftertheblockade.
Hypotension was defined as a decrease in systolic blood
pressure(SBP)upto20%frombaselineandcontrolledwith
intravenous ephedrine (5mg). Bradycardia, defined as a
heartratebelow80%ofbaselineorbelow50bpmof
base-linevalue,wastreatedwithintravenousatropine(0.5mg).
Thelevelofsensoryblockat T6dermatomewastestedat
5,10,and15minaftertheblockadebypinprickwitha22G
needle.
Symptomsoradverseevents,suchasnausea,vomiting,
drowsiness, pain above three on the Visual Analog Scale
(VAS>3)orabdominaldiscomfort,inadditiontotheneonate Apgarscoreinthefirstandfifthminute,needtouse
vaso-pressor for hypotension treatment, fetal extraction time,
anddurationofsurgerywererecorded.Abdominalpainor
discomfortduring surgerywastreated withfentanyl bolus
dosesof50g,repeatedattheassistant physician
discre-tion.
Postoperative nausea, vomiting, itching, and pain at
rest (VAS>3) at two, six, and 12h after anesthesiawere recorded.
Statistical analysis was performed using the Epi Info®
7.1.3.0 software. Quantitative variables were analyzed
usingmeanand standard deviation (SD)andsubmitted to
theStudentt-testandthefrequencyofqualitativevariables weresubmittedtothechi-squareandFisherexacttests(the
latterwhentheoccurrenceofvariableswaslessthanfive
andthistestcouldbeperformed).
Results
Intotal, 94patients wereselected andsubmittedto
ran-domization,46patientsinGroupAand48inGroupS.There
weretwolossesinGroup Aduetoinadequatecompletion
ofthequestionnaireandonelossin GroupSdue tospinal
anesthesiatotalfailureandneedforasecondpuncture.
Therewasnostatisticallysignificantdifferencebetween
thetwogroupsregardingageandbodymassindex(BMI)of
patientsandgestationalage(Table1).Themostcommonly
usedpuncturesitewasL3---L4,and27Gneedlewasthemost
usedinbothgroups(Table2).
Themeantimeforfetalextractionwas14.16±4.8min
forGroupAand14.51±4.7minforGroupS,andthemean
durationofsurgerywas56.06±11.75min forGroupAand
57.21±10.88minforGroupS(Table3).
Theuseofephedrinetotreathypotensionwassimilarin
bothgroups(30patientsinGroupAand36patientsinGroup S,68.18%and76.6%,respectively)(Table4).
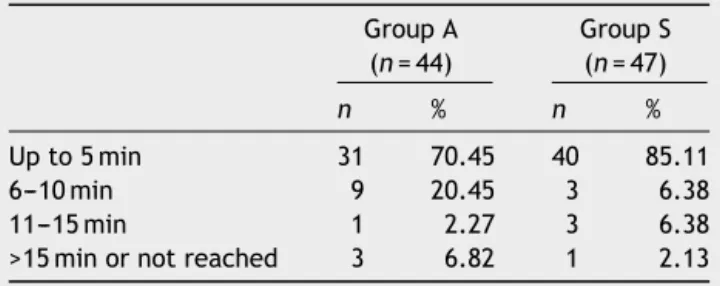
ThelatencytoreachT6dermatomewassimilarinboth
groups,althoughthenumberofpatientswhoreachedthis
levelin5minwashigherinGroupSthaninGroupA(85.11%
vs. 70.45%), but not statistically significant. Over 90% of
patients achieved this level up to 10min in both groups
Table1 Characteristicsofpatients.
GroupA(n=44) GroupS(n=47)
Mean SD Mean SD pa
Age(years) 26.0227 6.36 27.34 5.99 0.3114
BMI(kgm−2) 30.64 6.36 32.13 5.43 0.2322
Gestationalage(weeks) 37.9373 2.32 38.31 1.95 0.3976
SD,standarddeviation. aStudent’st-test.
Table2 Spinalpuncturedata.
GroupA GroupS
n=44 % n=47 % pa
Puncturelevel 0.5436
L2---L3 4 9.09% 2 4.26%
L3---L4 37 84.09% 43 91.49%
L4---L5 3 6.82% 2 4.26%
Needle 0.6615
25G 14 31.82% 17 36.17%
27G 30 68.18% 30 63.83%
aChi-squaretest.
Table3 Surgicaltimes.
GroupA(n=44) GroupS(n=47)
Mean SD Mean SD pa
Durationofbirth(min) 14.16 4.8 14.51 4.7 0.7252
Durationofsurgery(min) 56.06 11.75 57.21 10.88 0.6307
aStudent’st-test.
Intraoperativeincidenceofpruritusanddrowsinesswas
higherinGroupS(36.17%and23.4%)thaninGroupA(4.55%
and0%,respectively).
TherewasagreatertendencyofbradycardiainGroupS
(59.57%)thaninGroupA(43.18%),butthisdifferencewas
notstatisticallysignificant.
Therewasnosignificantdifferencebetweengroupsinthe
incidenceofhypotension,nausea,vomiting,decreased
oxy-gensaturationbyhemoglobin(SpO2),abdominaldiscomfort,
andintraoperativepain(Table6).
Table4 Intraoperativeneedofephedrine.a
GroupA(n=44) GroupS(n=47)
n % n %
Yes 30 68.18 36 76.6
No 14 31.82 11 23.4
ap=0.8074;chi-square.
TherewasnosignificantdifferencebetweenApgarscores
atoneand5min,95.45%ofnewbornsinGroupAand95.75%
ofnewbornsinGroupShadscoresbetween7and10at1min
andallinfantshadscorebetween7and10at5min(Table7). Postoperativeevaluation(Table8)showedahigher
inci-dence of pruritus 2h after intrathecal injection in Group
ScomparedtoGroupA(61.7%vs.30.23%,respectively),a
statisticallysignificantdifference.Therewasnosignificant
difference in the incidenceof pruritus duringrevaluation
Table5 LatencytimeofsensoryblocktoreachT6.a GroupA
(n=44)
GroupS (n=47)
n % n %
Upto5min 31 70.45 40 85.11
6---10min 9 20.45 3 6.38
11---15min 1 2.27 3 6.38
Table6 Intraoperativecomplications.
GroupA(n=44) GroupS(n=47)
n % n % p
Hypotension 36 81.82 37 78.72 0.7111a
Bradycardia 19 43.18 28 59.57 0.1178a
Nausea 17 38.64 16 34.04 0.6487a
Vomiting 9 20.45 6 12.77 0.3232a
Pruritus 2 4.55 17 36.17 c0.0001b
SpO2<95% 3 6.82 1 2.13 0.3504b
Drowsiness 0 0 11 23.4 c0.0005b
Abdominaldiscomfort 5 11.36 1 2.13 0.1032b
Pain 1 2.27 1 2.13 1.0b
a Chi-square. b Fisher’sexact. c p<0.05.
Table7 Apgarscores.
Valor Apgar,1mina Apgar,5minb
GroupA(n=44) GroupS(n=47) GroupA(n=44) GroupS(n=47)
n % n % n % n %
<7 2 4.55 2 4.26 --- --- ---
---7---10 48 95.45 45 95.74 44 100 47 100
a p=0.6663;Fisher’sexact. b p=1;Chi-square
timesatsixand12h,aswellastheincidenceofnauseaand vomitingin alltimes(2, 6,and12h). Ofthefivepatients
whoexperiencedepisodesof vomitingin thereevaluation
after2h,onlyonepersistedwithvomitinginthe2nd
reval-uation(6h).Theotherfouroccurrencesintherevaluation
at6hcorrespondedtonewcases.
Regardingtheoccurrenceofpainatrest(VAS>3),there
wasahigherincidenceinGroup AcomparedtoGroupSin
therevaluationat6haftertheprocedure(18.18%vs.6.38%, respectively),butstatisticallynotsignificant.Theincidence ofpain(VAS>3)12hafter spinalanesthesia tendedtobe similarbetweengroups(Table9).
Table8 Postoperativesideeffects.
GroupA(n=44) GroupS(n=47) p
n % n %
Pruritus
After2hc 13 30.23 29 61.7 0.0027a,c
After6h 13 30.23 18 38.3 0.4212a
After12h 6 13.95 8 17.02 0.6883a
Nausea
After2h 5 11.63 5 12.77 0.8692a
After6h 3 6.98 4 8.51 0.5502b
After12h 4 9.3 1 2.13 0.1538b
Vomiting
After2h 1 2.33 4 8.51 0.2094b
After6h 1 2.33 4 8.51 0.2094b
After12h 0 0 0 0
Table9 Postoperativepainatrest(VAS>3).
GroupA(n=44) GroupS(n=47) p
n % n %
After2h 3 6.82 4 8.51 0.5372b
After6h 8 18.18 3 6.38 0.0795b
After12h 13 29.55 13 27.66 0.0396a,c
aChi-square. b Fisher’sexact. c p<0.05.
Discussion
To obtain adequate anesthesia for C-section, an intense
blockadecovering fromthe sacral (S2---S4) tothe visceral
fibers(T4---T12)is needed.Ablockadewithsuchextension
resultsinhypotensionbyblockingthesympatheticfibers.10
Thelocalanestheticcommonlyusedis0.5%hyperbaric
bupi-vacaineatdosesrangingfrom7.5to15mg.Theuseof10mg
aloneor 8mg combinedwith opioidsis reported as‘‘low
dose’’bysomeauthors,10whileothersconsider‘‘lowdose’’
onlywhenthebupivacainemassdoesnotexceed8mg.3
Althoughtheliteratureshowatrendtowardbupivacaine
dosereduction uptodosesaslow as8mg,either withor
withouttheadditionoflipophilicopioids,reducingthisdose
tolevelsbelow 10mgwithout epidural catheterinsertion
maybeunsafeduetothepotentialriskoffailurein
obtain-inganadequate levelofblockadeor inadequateblockade
duration for surgical time, which increases the need for
intravenousanalgesicagents(fentanyl)orconversionto gen-eralanesthesia.3,8,9
Higherincidenceofintraoperativehypertensionhasbeen
was reported in patients receiving intrathecal sufentanil
at a dose of 5g.17 Other studies of sufentanil at doses
ranging from2.5 to 10g failedto establish a significant
relationshipbetweentheuseofintrathecalsufentaniland
hypotension,3,4,15,19 aresultsimilartothatobservedinour
study.However, it is possible that the addition of
sufen-tanilisresponsibleforthenon-occurrenceoftheexpected
decreasein the incidenceof hypotensionwith the
hyper-baricbupivacainedosereducedfrom12.5to10mg.
Althoughtherewasahigherincidenceofbradycardiain
patients whoreceived sufentanil, this differencewasnot
statisticallysignificant. Thisrelationship wasnotobserved previouslyinastudythatevaluatedtheincidenceof brady-cardiainpatientsreceivingsufentanil(10g)ormorphine
(200g)bythesamerouteofadministration.15
Severalstudieshaveshownthatintrathecalsufentanilis
relatedtosedation15,17,19andthatsufentanilismore
sedat-ingthanspinalmorphine(30%vs.5%,respectively).15These
resultscoincide with thoseobserved by us,asdrowsiness
occurredin23.4%ofpatientsreceivingsufentanilandwas
absentinthegroupthatreceivedonlymorphineasan
adju-vant.
Astudycomparingdifferentdosesofbupivacainealone
forC-sectionunderspinalanesthesiareported35%incidence
ofintraoperativepainwithadoseof8mg,20%withadose
of10mg,andabsent withadose of 12mg.9 We found no
differencesintheoccurrenceofthiseffectwhencomparing
hyperbaricbupivacaine(12.5mg)combinedwithonly
mor-phine withhyperbaric bupivacaine (10mg)combinedwith
morphine and sufentanil. It is probable that the addition
of sufentanil is responsible for maintaining the quality of
analgesiawitha dose reduction,which isin line withthe
resultsobservedinotherstudiesshownthattheadditionof
lipophilicopioiddrasticallyreducestheoccurrenceof
intra-operativepainwhenbupivacaineisusedatdosesbetween
8and10mg.3,9,20
Whentheincidenceofprurituswascomparedbetween
patients who received intrathecal bupivacaine alone and
patients who received sufentanil (10g) or morphine
(200g)combinedwithbupivacaine,it wasobserved that
theincidencewassignificantlyhigherinpatientsreceiving
sufentanil comparedtothosereceivingmorphine(30% vs.
10%,respectively).15Itwasalsoshownthatpruritusresulting
fromthe use of intrathecal sufentanil is dose-dependent,
rangingfrom34.3%withadoseof2.5gto68.6%withadose
of 5g.17 Pruritusinducedby intrathecalmorphine isalso
dose-dependent,especiallyindosesabove0.1mg.21Inour
study,asignificantlyhigherdifferenceinprurituswasseen inpatientsreceivingsufentanil,intheintraoperativeperiod
andatthefirstassessment2hafteranesthesia,sufentanil
actionperiod.
Thus,in thepresent study,thedose reduction of 0.5%
hyperbaric bupivacaine from 12.5 10mg, combined with
sufentanil(5g)at thelowestdose, maintainedthesame
qualityofanesthesia, butdidnotreduce theincidenceof
hypotension and increased the incidence of pruritus and
sedation.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.BirnbachDJ, BrowneIM.Anesthesia for obstetrics.In: Miller RD,FleisherLA,Wiener-KronishJP,YoungWL,editors.Miller’s anesthesia,vol.2,7thed.Philadelphia: Churchill-Livingstone-Elsevier;2010.p.2219---20.
4.RoofthooftE,VandeVeldeM.Low-dosespinalanaesthesiafor caesareansectiontopreventspinal-inducedhypotension.Curr OpinAnaesthesiol.2008;21:259---62.
5.Chinachoti T, Tritakarn T. Prospective study of hypotension and bradycardia during spinal anesthesia with bupivacaine: incidence and risk factors, part two. J Med Assoc Thai. 2007;90:492---501.
6.CynaAM, Andrew M,Emmett RS, et al. Techniquesfor pre-ventinghypotension duringspinal anaesthesiafor caesarean section.CochraneDatabaseSystRev.2006,http://dx.doi.org/ 10.1002/14651858.CD002251.pub3.ANCD002251.
7.VercauterenMP,Coppejans HC, HoffmannVH, et al. Preven-tionof hypotension bysingle5mg dose ofephedrine during smalldosespinalanesthesiainprehydratedcesareandelivery patients.AnesthAnalg.2000;90:324---7.
8.Fan SZ, Susetio L, Wang YP, et al. Low dose ofintrathecal hyperbaricbupivacainecombined withepidurallidocainefor cesareansection--- abalanceblocktechnique.AnesthAnalg. 1994;78:474---8.
9.ChoiDH,AhnHJ,KimMH.Bupivacaine-sparingeffectoffentanyl inspinalanesthesiaforcesareandelivery.RegAnesthPainMed. 2000;25:240---5.
10.DyerRA,JoubertIA.Low-dosespinalanaesthesiaforcaesarean section.CurrOpinAnaesthesiol.2004;17:301---8.
11.CrowhurstJA,BirnbachDJ.Small-doseneuraxialblock:heading towardthenewmillennium.AnesthAnalg.2000;90:241---2. 12.Ben DB, Miller G, Gavriel R, et al. Low dose
bupivacaine-fentanylspinalanesthesiaforcesareandelivery.RegAnesthPain Med.2000;25:235---9.
13.ChoiDH,AhnHJ,KimJA.Combinedlow-dosespinal-epidural anesthesia versus single shot spinal anesthesia for elective cesareandelivery.IntJObstetAnesth.2006;15:13---7.
14.GhaziA,RajaY.Combinedlow-dosespinal---epidural anaesthe-siaversussingle-shotspinalanaesthesiaforelectivecaesarean delivery.IntJObstetAnesth.2007;16:90---1.
15.VeenaA,AmitA,JagdishSP,etal.Comparisonofintrathecal sufentanil and morphine inaddition to bupivacaine for cae-sareansectionunderspinalanesthesia.AnaesthPainIntensive Care.2010;14:99---101.
16.DahlgrenG,HultstrandC,JakobssonJ,etal.Intrathecal sufen-tanil,fentanyl,orplaceboaddedtobupivacaineforcesarean section.AnesthAnalg.1997;85:1288---93.
17.Bang YS, Chung KH, Lee JH, et al. Comparison of clini-cal effects according to the dosage of sufentanil added to 0.5%hyperbaricbupivacainefor spinalanesthesia inpatients undergoing cesareansection.Korean JAnesthesiol. 2012;63: 321---6.
18.Braga AA, Frias JAF, Braga FS, et al. Raquianestesia em operac¸ão cesariana. Emprego da associac¸ão de bupivacaína hiperbárica(10mg)adiferentesadjuvantes.RevBras Aneste-siol.2012;62:775---87.
19.LeeJH,ChungKH,LeeJY,etal.Comparisonoffentanyland sufentanil added to 0.5% hyperbaric bupivacaine for spinal anesthesia inpatientsundergoingcesareansection.KoreanJ Anesthesiol.2011;60:103---8.
20.BograJ,AroraN,SrivastavaP.Synergisticeffectof intrathe-calfentanylandbupivacaineinspinalanesthesiaforcesarean section.BMCAnesthesiol.2005;5:5.