w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Possible
mechanisms
of
antinociception
of
methanol
extract
of
Melastoma
malabathricum
leaves
Erman
Shah
Jaios
a,
Suzana
Abdul
Rahman
b,
Siew
Mooi
Ching
c,
Arifah
Abdul
Kadir
d,
Mohd.
Nasir
Mohd.
Desa
a,e,
Zainul
Amirudin
Zakaria
a,e,f,∗aDepartmentofBiomedicalSciences,FacultyofMedicineandHealthSciences,UniversitiPutraMalaysia,Selangor,Malaysia bDepartmentofBiomedicalSciences,KulliyyahofAlliedHealthSciences,InternationalIslamicUniversityMalaysia,Pahang,Malaysia cDepartmentofFamilyMedicine,FacultyofMedicineandHealthSciences,UniversitiPutraMalaysia,Selangor,Malaysia
dDepartmentofVeterinaryPre-ClinicalSciences,FacultyofVeterinaryMedicine,UniversitiPutraMalaysia,Selangor,Malaysia eHalalProductResearchInstitute,UniversitiPutraMalaysia,Selangor,Malaysia
fIntegrativePharmacogenomicsInstitute,UniversitiTeknologiMARA,Selangor,Malaysia
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received21August2015 Accepted4January2016 Availableonline14June2016
Keywords:
Melastomaceae Herbal Pain-killing Naturalproducts Drugdiscovery
a
b
s
t
r
a
c
t
MelastomamalabathricumL.,Melastomaceae,hasbeentraditionallyusedtorelievediversepain-related ailments.Theobjectivesofthepresentstudyweretodeterminetheantinociceptiveactivityofmethanol extractofM.malabathricumleavesandtoelucidatethepossiblemechanismsofantinociceptioninvolved usingvariousrats’models.Theextract(100,250,and500mg/kg)wasadministeredorally60minprior tosubjectiontotherespectivetest.Theinvivoaceticacid-inducedabdominalconstriction, formalin-inducedpawlicking,andhotplatetestswereusedasthemodelsofnociceptiontoevaluatetheextract antinociceptiveactivity.Furtherstudieswerecarriedouttodeterminetheroleofopioidand vanil-loidreceptors,glutamatesystemandnitricoxide/cyclicguanosinephosphate(NO/cGMP)pathwayin modulatingtheextractantinociceptiveactivity.Fromtheresultsobtained,M.malabathricumexhibited significant(p<0.05)antinociceptiveactivityinallthechemical-andthermal-inducednociception mod-els.Naloxone(5mg/kg),anon-selectiveopioidantagonist,failedtosignificantlyaffecttheantinociceptive activityofMEMMwhenassessedusingtheabdominalconstriction-,hotplate-andformalin-inducedpaw licking-test.M.malabathricumalsosignificantly(p<0.05)reversedthenociceptiveresponsein capsaicin-andglutamate-inducedpawlickingtest.Furthermore,onlyl-arginine(anitricoxideprecursor)alone,but
not,NG-nitro-l-argininemethylesters(l-NAME;aninhibitorofNOsynthase),methyleneblue(MB;an
inhibitorofcGMP),ortheircombinationthereof,significantly(p<0.05)blocktheantinociceptiveactivity ofM.malabathricum.Inconclusion,M.malabathricumexertedanon-opioidantinociceptiveactivityat thecentralandperipherallevelspartlyviatheinhibitionofvanilloidreceptorsandglutamatergicsystem, andactivationoftheNO-mediated/cGMP-independentpathway.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Someofthemostcommonailmentsthataffectedmillionsof peopleworldwidearepainandinflammation(Raghavetal.,2006; Rangetal.,2011).Therefore,researchesinthefieldsofpainand inflammation,particularlyonfindingappropriatedrugs totreat them, have tremendously increased these past few years. This claimissuggestedbasedontherapiddevelopmentsinthefield ofsyntheticandmedicinalchemistrythatsawincreaseinreports onnewly synthesizedrugs includingthoseforthetreatmentof pain and inflammation. However, many of synthetic drugs are
∗ Correspondingauthor.
E-mail:zaz@upm.edu.my(Z.A.Zakaria).
withdrawnlatelyaftertheirintroductionintothemarketbecause of the adverse side effects associated with their prolongused. For example, chronic usedof morphine, a currently prescribed analgesic drug, has been associated with the development of toleranceanddependence.Therefore,alternativeagentswithless or,possibly,nounwantedsideeffectsarerequiredandplant-based natural products are and have been the important sources of thosealternativeagents(Verpoorte,1998).Naturalproductsfrom plants have long been recognized as the important sources of therapeuticallyeffectivemedicines(Craggetal.,2003).
MelastomamalabathricumL.(familyMelastomaceae)isa flow-eringplantnativetotheSoutheastAsianregionincludingMalaysia. ItisthesolespeciesinthegenusMelastomaandhasbeenclassified asaweed, andcanbefoundtogrowextensivelyinthe waste-landareas.Commonlycalledthe“StraitsRhododendron”andlocally
http://dx.doi.org/10.1016/j.bjp.2016.01.011
knowntotheMalayas“Senduduk”,M.malabathricum hasbeen widelyusedintheMalayaswellasotherstraditionalmedicines (Rajenderan, 2010).Accordingto severalreports, the leaves, in particular, have been applied either pounded, grounded or as decoction,byseveraltribesandpopulationtotreatailmentssuch asstomachulcers,dysenteryanddiarrhoea,thoseassociatedwith pain(i.e.,toothacheandstomachache),toacceleratewound heal-ing,for post-natalcareand preventionofscarsfromsmall pox infection,and,postpartumremedy(Grosvenoret al.,1995;Ong andNordiana,1990;Sharmaetal.,2001;Sulaimanetal.,2004; Roositaetal.,2008).Scientifically,theleavesofM.malabathricum havebeenreportedtoexertnoacutetoxicityand(Sunilsonetal., 2009),antibacterial (Grosvenoret al.,1995; Wiartet al.,2004), antiviral(Grosvenoretal.,1995),antioxidant(Nazlinaetal.,2008), cytotoxic (Nazlina et al., 2008), anticoagulant (Manicam et al., 2010),antiulcer(Hussainetal.,2008),antidiarrheal(Sunilsonetal., 2009),anti-inflammatory(Susantietal.,2008;Zakariaetal.,2008), antinociceptive(Sulaimanet al.,2004;Zakaria etal.,2008)and antipyretic(Zakariaetal.,2008)activities.Phytochemicalscreening of the M. malabathricum leaves demonstrated the presence of flavonoids,triterpenes,tannins,saponins,steroids(Zakariaetal., 2008;Simanjuntak,2008;Faravani,2009),alkaloids(Zakariaetal., 2008), glycosides and phenolics (Simanjuntak, 2008; Faravani, 2009).However,thephytochemicalanalysisofmethanolextract ofM.malabathricumleaves(MEMM)revealedonlythepresenceof flavonoids,glycosides,phenolics,triterpenes,tannins,saponinsand steroidsbutnoalkaloids.ThepreviousantinociceptiveactivityofM. malabathricumleaves,inparticular,hasbeeninvestigatedusingthe aqueousandethanolextractoftheleaveswithattemptonlymade todeterminetheroleofopioidreceptorsreported.Inthepresent study,themethanolextractofM.malabathricumleaves(MEMM) wasusedtofurtherstudytheantinociceptiveactivityofM. mala-bathricumandthemechanismsofactioninvolved.Themechanisms ofactiontobestudiedincludetheroleofopioidandvanilloid recep-tors,glutamatesystemandnitricoxide/cyclicguanosinephosphate (NO/cGMP)pathway.
Materialsandmethodology
PlantcollectionandpreparationofMEMM
The leaves of Melastoma malabathricum L. was collected betweenJuneandJuly2012aroundtheUniversitiPutraMalaysia (UPM), Malaysia and certified by a botanist, Dr. Shamsul Khamis, fromthe Institute of Bioscience (IBS), Universiti Putra Malaysia(UPM),Serdang,Selangor,Malaysia.Avoucherspecimen (ACP0017)hasbeenearlierdepositedattheHerbariumofthe Lab-oratoryofNaturalProducts,IBS,UPM,Malaysia.Theprocedurefor preparationofMEMMwascarriedoutasdescribedin detailby Zakariaetal.(2008).
Drugsandchemicals
Thefollowingreagentsanddrugswereused:methanol(Fischer Scientific, UK), DMSO, formalin, acetic acid, morphine, acetyl-salicylic acid (ASA), naloxone, capsaicin, glutamate, l-arginine, NG-nitro-l-argininemethylesters(l-NAME)andmethyleneblue (MB)(Sigma,USA).Thedrugswerepreparedbydissolvingthemin distilledwater.TheMEMMwasdissolvedinvehicle(10%DMSO) immediatelybeforeused.Allsolutionswereadministeredinthe volumeof10ml/kg.
Animals
MaleSpragueDawley(SD)rats(180–200g;8–10weeksold)and maleICRmice(25–30g;5–7weeksold),purchasedfromCheNur
Supplier,Selangor,Malaysia,weretransferredtotheAnimal Hold-ingUnit,InternationalIslamicUniversityMalaysia(IIUM),Pahang, Malaysiaand allowed toacclimatizefor one weekprior to the experimentation.Theanimalswerecaredandhandledaccording totheproceduresdescribedindetailbyMohd.Sanietal.(2012). Thestudyprotocolofthepresentstudywasapprovedbythe Ani-malEthicsCommitteeofInternationalIslamicUniversityMalaysia [IIUM/IACUCApproval/2016/(9)(58)]andwereperformedin accordancewiththeIntegratedCentreforResearchofAnimalCare andUse(ICRACU)guidelines.
AcutetoxicitystudyofMEMM
Acutetoxicitystudieswerecarriedoutaccordingtothe “Guide-line for Testing of Chemicals – Acute Oral Toxicity – Fixed DoseProcedure(OECDNo.423)”(OECD,2002).Ratswerefasted overnightpriortotheadministrationoftestsolutions.Thetreated groupobtainedasingledoseof5000mg/kgMEMMwhileonegroup eachobtainedeithervehicleordistilledwater(10ml/kg)bygavage. Then,theanimalsweremonitoredseparatelyatleastonceduring thefirst30minafterdosing,occasionallyduringthefirst24hand dailythereafterfor14days.Foodandwaterwereprovidedad libi-tum.Themortality,bodyweightandbehaviouralscreeningwere recordeddailyfor14daysaftertreatment.Theratsthatsurvived wereeuthanizedandmacroscopicanalysisandtheweightofvital organswererecorded.Theseorganswerefixedin10%formalinfor histologicalassessment.
Antinociceptiveactivity
Aceticacid-inducedabdominalconstrictiontest
Theacetic-acid-inducedabdominalconstrictiontestwas car-riedoutaccordingtothemethoddescribedindetailbyCollieretal. (1968)butwithslightmodifications. Themice(n=6)were pre-treatedorally(p.o.)with10%DMSO(negativecontrol),100mg/kg ASA(positivecontrol),orMEMM(100,250,and500mg/kg)prior toassessmentusingtheabdominalconstrictiontest.Sixtyminutes aftertherespectivetest solutionadministration,themicewere injectedviaintraperitoneal(i.p.)routewithphlogisticagent(0.6% aceticacid).Theanimalswereimmediatelyplacedindividuallyinto glasscageand5minwereallowedtoelapse.Theabdominal con-strictionresultingfromtheinjectionofacetic acidconsistsofa contractionoftheabdominaltogetherwithastretchingofatleast onehindlimb.Thenumberofabdominalconstrictionsproducedin theseanimalswascountedcumulativelyfor25min. Antinocicep-tiveactivity,indicatedbythereductioninthemeanofthenumber ofabdominalconstrictionsinthetestgroupscomparedtothe con-trolgroup,wascalculatedasthepercentageinhibitionofabdominal constrictions(percentageofinhibitorylevel)usingthefollowing formula:[meanof(control−testgroup)/controlgroup×100%].
Hotplatetest
The hotplate test wascarried out accordingtothe method describedbyHunskaaretal.(1986)butwithslightmodifications. The mice(n=6) werepre-treated (p.o.)with10% DMSO (nega-tivecontrol),5mg/kgmorphine(positivecontrol),orMEMM(100, 250,and500mg/kg)priortoassessmentusingthehotplatetest. Thetemperatureofthemetalsurface(UgoBasile7280)wassetat 50±0.2◦C.Sixtyminutesaftertherespectivetestsolution
Formalin-inducedpawlickingtest
Theformalininducedpawlickingtest(alsoknownas forma-lintest)wascarriedoutasdescribedbyHunskaarandHole(1987) butwithslightmodifications.Rats(n=6)wereadministeredp.o. with10%DMSO(negativecontrol),5mg/kgmorphineor100mg/kg ASA(bothactasthepositivecontrols),orMEMM(100,250,and 500mg/kg)priortoassessmentusingtheformalintest.Painwas inducedbyinjecting50lof5%formalininthesub-plantarregion oftherighthindpaw60minafterthetestsolutions administra-tion.Immediatelyafterthephlogisticagentadministration,therats wereindividuallyplacedinatransparentglasscageobservation chamber.Theamountoftime thattheanimalspentlickingthe injectedpaw,consideredasanindicatorofpain,wasrecordedfor thedurationof30minintwophases,knownastheearly(0–5min) andlate(15–30min)phases.
MechanismsofantinociceptiveactivityofMEMM
Capsaicin-inducedpawlickingtest
Thecapsaicin-inducedpawlickingtestwasusedtoinvestigate theroleofvanilloidreceptorsinthemodulationofMEMM antinoci-ceptiveactionandtheprocedureadoptedhasbeendescribedin detailbySakuradaetal.(1992)butwithslightmodifications.Rats werepre-treatedorallywith10%DMSOorMEMM(100,250,and 500mg/kg)60minpriortoassessmentusingtherespectivetest. Sixtyminaftertheadministrationoftestsolutionscapsaicinwas injected(1.6g/paw,20l)intotheintraplantar(i.pl.)regionofthe rat’srighthindpaw.Immediatelyafterthephlogisticagent admin-istration,theratswereindividuallyplacedinatransparentglass cageobservationchamberandobservedindividuallyfor5minafter thecapsaicininjection.Theamountoftimetheanimalsspent lick-ingtheinjectedpawwasrecordedwithachronometerandwas consideredasanindicationofnociception.
Glutamate-inducedpawlickingtest
The glutamate-induced paw licking test was used to inves-tigate the role of glutamatergic system in the modulation of MEMMantinociceptiveactionandtheprocedureadoptedhasbeen described by Luiz et al. (2007) with slight modifications. Rats werepre-treatedorallywith10%DMSOorMEMM(100,250,and 500mg/kg)60minpriortoassessmentusingtherespectivetest. Avolumeof20lofglutamate(10mol/paw,innormalsaline) wasinjected viai.pl.routeintherighthindpawofrats60min followingthetestsolutionsadministration.Immediatelyafterthe phlogisticagentadministration,theratswereindividuallyplaced inatransparentglasscageobservationchamberandobserved indi-viduallyfrom0to15minaftertheglutamateinjection.Theamount oftimetheanimalsspentlickingorbitingtheinjectedpawwas recordedwithachronometerandwasconsideredasanindication ofnociception.
Involvementofopioidreceptor
Todeterminetheroleofopioidreceptorsinthemodulationof MEMMantinociceptiveactivity,sixgroupsofanimals(n=6)were pre-treated(i.p.)withanon-selectiveopioidantagonist,naloxone (5mg/kg;i.p.)for15minfollowed bytheoral administrationof themosteffectiveMEMMdose(500mg/kg)or10%DMSO.Sixty minuteslater, theanimalswereassessedusing theacetic acid-inducedabdominalconstrictiontest,hotplatetestorformalintest, respectively(Mohd.Sanietal.,2012).
Involvementofnitricoxide/cyclic-guanosinemonophosphate (NO/cGMP)pathway
To determine the role of nitric oxide/cyclic-guanosine monophosphate (NO/cGMP) pathway in the modulation of MEMMantinociceptive activity,themethod described indetail
by Mohd. Sani et al. (2012) was adopted with slight modifi-cations. In this study, the mice (n=6) were pre-treated with 20mg/kgl-arginine,l-NAME,MB,ortheirrespectivecombination (l-argininewithl-NAMEorl-argininewithMB)followed5min later bypre-treatment with10% DMSOor MEMM(500mg/kg), respectively.Sixtyminuteslater,theanimalswereassessedusing theabdominalconstrictiontest.
HPLCandGCMSanalysisofMEMM
TheHPLCanalysisofMEMMhasbeencarriedoutpreviously andthedetailedmethodwaspublishedbyKamisanetal.(2014). Fromtheanalysis,flavonoidsweregenerallydetectedbased on theUV–vis spectrawavelengthwhilequercitrinwasspecifically detectedbasedonthecomparisonofchromatogramobtainedfor MEMMagainstthoseofseveralpureflavonoids.Inadditiontothe HPLCfinding,GCMSanalysiswasalsoperformedonMEMM.
GC–MSanalysisofMEMMwasperformedusingAgilent7890A (Agilent Technologies) coupled with MSD quadrupole detector 5975C(AgilentTechnologies).Separationofanalytesbygas chro-matographywascarriedoutusingtheHewlettPackardHP-5MS silica capillary column (30m×0.25mm×0.25mm).For GC–MS detection,anelectronionizationsystemwithionizingenergyof 70eVwasused.Heliumgas(99.999%)wasusedasthecarriergas atconstant flow rate1ml/min and an injectionvolumeof 1l wasemployed(splitratioof1:10);injectortemperature250◦C;
ion-sourcetemperature280◦C. Theoventemperature was
pro-grammedfrom100◦C(isothermalfor2min),withanincreaseof
10◦C/minto200◦C,then12◦C/minto280◦C,endingwitha17min
isothermalat280◦C.Massspectraweretakenat70eV;ascan
inter-valof 0.5sandfragments from45 to450Da. TotalGC running timewas35.50min.Therelative%amountofeachcomponentwas calculatedbycomparingitsaveragepeakareatothetotalareas. Thesoftwareadoptedtohandlemassspectraandchromatograms wasaTurbomass.Fortheidentificationofcompounds, interpreta-tiononmassspectrumGC–MSwasconductedusingthedatabase ofNationalInstituteStandardandtechnology(NIST)havingmore than62,000patterns.Thespectrumoftheunknowncomponent wascomparedwiththespectrumoftheknowncomponentsstored intheNISTlibrary.
Results
AcutetoxicityeffectofMEMM
Allanimalsinthetreatmentandcontrolgroupsdemonstrated anincreaseinbodyweightatweeks1and2incomparisontoday 0.Neitheralterationinthebehaviouralpatternnormortalitywas observedthroughoutthedurationofexperimentation.Inaddition, nochangesinfoodandwaterintake,andbehaviourweredetected amongtheanimals.Thevitalorgansshowednosignificantchanges intheirrespectiverelativeweightwhiletherespectivemicroscopic analysisdemonstratednosignsoftoxicity(datanotshown). More-over,thesingleoraladministrationof5000mg/kgMEMMdidnot generateanysignoftoxicityinthetreatedanimalsafter14days. Basedontheseobservations,theextractwassuggestedtopossess anLD50thatisgreaterthan2000mg/kgbodyweight.
Aceticacid-inducedabdominalconstrictiontest
100
Number of constr
iction 80 60 40 ∗∗∗ ∗∗∗ ∗∗∗ ∗∗∗ # # 20
10% DMSO –
100 250 500 5+500 100 + – – – – – – + – – – – – – + – – – – – – + – – – – – – + – – – – – – + MEMM NLX+MEMM ASA 0
Fig.1.Antinociceptiveactivity ofMEMM assessedbythe aceticacid-induced abdominalconstrictiontestinmice.Aceticacidadministratedbyintraperitoneally 60minbeforepre-treatedwithDMSOasvehicle(control),acetylsalicylicacid(ASA), orMEMM(100,250and500mg/kg).Alltreatmentsadministratedviaoralroute. ***Datadifferedsignificantly(p<0.001)whencomparedto 10%DMSO-treated group.#Datawasnotsignificantwhencomparedtogether.
effectivenesswhencomparedtothepositivecontrol(100mg/kg ASA).
Hotplatetest
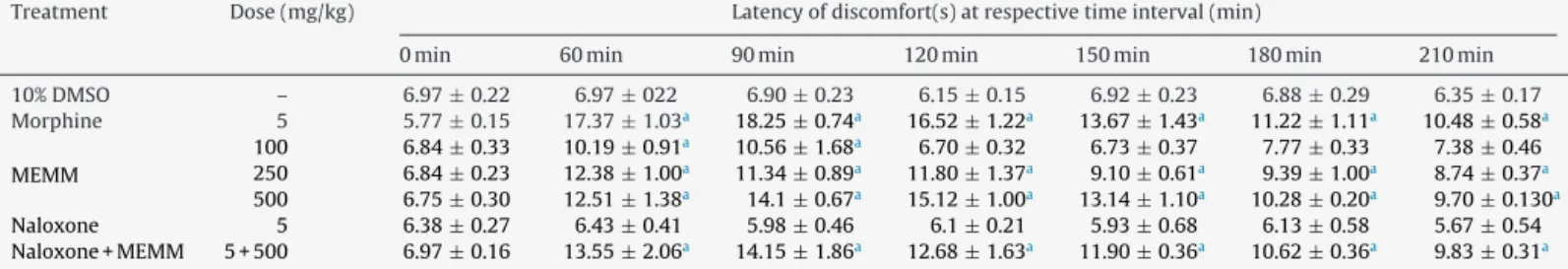
Table1showstheantinociceptiveprofileoforally-administered MEMMasassessedusingthehotplatetest.TheMEMM,atthedose of250mg/kg,exhibitedsignificant(p<0.001)activityatthe inter-valtimeof60,90and120minwhilethe500mg/kgMEMMexerted significant(p<0.001)antinociceptionuntiltheendofexperiment. Co-administrationwithnaloxone(5mg/kg;i.p.)failedtoinhibit the500mg/kgMEMMantinociceptiveactivity.Overall,the5mg/kg morphinedemonstratedthemosteffectiveeffectwhencompared totheMEMMatalldosesused.
Formalin-inducedpawlickingtest
MEMMdemonstratedasignificant(p<0.001)antinociceptive activityinadose-dependentmannerinbothphasesofthe formalin-inducedpawlickingtestasshowninFig.2AandB,respectively. IncomparisontoMEMM,5mg/kgmorphinealsoattenuatedboth phaseswhile100mg/kgASAonlyreducedthenociceptioninthe late phase. Overall, morphine was effective than the ASA and MEMMinbothphasesoftheformalintest.
Capsaicin-inducedpawlickingtest
TheantinociceptiveprofileofMEMMassessedusing capsaicin-induced paw licking test is shown in Fig. 3. All doses of MEMMdemonstratedasignificant(p<0.05)anddose-dependent
150
A
B
100 50 10% DMSO MEMM 100 250 500 500+5 100 5 – + – – – – – + – – – – – – – – + – – – – – – – + – – – – – – – + – – – – – – – + – – – – – – – + MEMM+Naloxone ASA Morphine 10% DMSO MEMM 100 250 500 500+5 100 5 – + – – – – – + – – – – – – – – + – – – – – – – + – – – – – – – + – – – – – – – + – – – – – – – + MEMM+Naloxone ASA Morphine P a w licking time (s)
P
a
w lic
king time (s)
(50.08%) (59.48%) (67.02%) (68.26%) (9.71%) (84.58%) ∗∗∗ ∗∗∗ ∗∗∗ ∗∗∗ ∗∗∗ ∗∗∗ 250 200 150 100 (70.6%) ∗∗∗ (78.2%)
∗∗∗ (82.5%)∗∗∗ (85.6%)∗∗∗
(86.3%) ∗∗∗ (96.9%) ∗∗∗ 50 0 0
Fig.2. AntinociceptiveactivityofMEMMassessedusingtheformalin-inducedpaw lickingtest.(A)EffectofMEMMrecordedattheearlyphase(0–5min),and;(B) effectofMEMMrecordedatthelatephase(15–30min).Eachcolumnrepresentsthe mean±SEMof6rats.Theratswerepre-treatedwithvehicle(10%DMSO)ascontrol, MEMM(100,250,and500mg/kg,p.o.),acetylsalicylicacid(ASA,p.o.),ormorphine (5mg/kg,p.o.),60minbeforei.pl.injectionofformalin.Theasterisksdenotethe significancelevelsascomparedtocontrol,***p<0.001byone-wayANOVAfollowed byDunnett’sposthoctest.***Datadifferedsignificantly(p<0.05)whencompared tothe10%DMSO-treatedgroup.
antinociception against capsaicin-induced nociception with the percentageofanalgesiarangingbetween29%and64%.
Glutamate-inducedpawlickingtest
Fig.4showstheantinociceptiveprofileofMEMMassessedusing theglutamate-inducedpawlickingtest.AlldosesofMEMMalso exerted a significant (p<0.05) and dose-dependent antinociep-tionagainstglutamate-inducednociceptionwiththepercentage ofanalgesiarangingfrom17%to72%.
Involvementofopioidreceptors
The effects of non-selective opioid antagonist (naloxone, 5mg/kg)onMEMMantinociceptiveactivitywasevaluatedusing
Table1
AntinocicpetiveactivityofMEMMassessedbythehotplatetestinmice.
Treatment Dose(mg/kg) Latencyofdiscomfort(s)atrespectivetimeinterval(min)
0min 60min 90min 120min 150min 180min 210min
10%DMSO – 6.97±0.22 6.97±022 6.90±0.23 6.15±0.15 6.92±0.23 6.88±0.29 6.35±0.17 Morphine 5 5.77±0.15 17.37±1.03a 18.25±0.74a 16.52±1.22a 13.67±1.43a 11.22±1.11a 10.48±0.58a
MEMM
100 6.84±0.33 10.19±0.91a 10.56±1.68a 6.70±0.32 6.73±0.37 7.77±0.33 7.38±0.46
250 6.84±0.23 12.38±1.00a 11.34
±0.89a 11.80
±1.37a 9.10
±0.61a 9.39
±1.00a 8.74 ±0.37a
500 6.75±0.30 12.51±1.38a 14.1
±0.67a 15.12
±1.00a 13.14
±1.10a 10.28
±0.20a 9.70 ±0.130a
50 40 30 20 10 0 Control (10% DMSO) Lic
king time (s)
100 (29.3) ∗∗∗ (41.7) ∗∗∗ (63.9) ∗∗∗ 250 MEMM (mg/kg) 500
Fig.3. AntinociceptiveactivityofMEMMassessedusingthecapsaicin-inducedpaw lickingtestinrats.Eachcolumnrepresentsthemean±SEMof6rats.Theratswere pre-treatedwithvehicle(10%DMSO)ascontrolorMEMM(100,250and500mg/kg,
p.o.)60minbeforeofcapsaicin(1.6g/paw,20l,i.pl.).Theasterisksdenotethe significancelevelsascomparedtocontrol,***p<0.001byone-wayANOVAfollowed byDunnett’sposthoctest.
50 40 30 20 10 0 Control (10% DMSO) Lic
king time (s)
100 (17.0) ∗∗∗ (41.8) ∗∗∗ (71.8) ∗∗∗ 250 MEMM (mg/kg) 500
Fig.4.AntinociceptiveactivityofMEMMassessedusingtheglutamate-induced pawlickingtestinrats.Eachcolumnrepresentsthemean±SEMof6rats.The ratswerepre-treatedwithvehicle(10%DMSO)ascontrolorMEMM(100,250and 500mg/kg,p.o.)60minbeforeofglutamate(10mol/paw,20l,i.pl.).The aster-isksdenotethesignificancelevelsascomparedtocontrol,***p<0.001byone-way ANOVAfollowedbyDunnett’sposthoctest.
theabdominalconstrictiontest,hotplatetest,andformalintestand areshowninFig.1,Table1,andFig.2AandB,respectively.Naloxone didnotsignificantly(p<0.05)reversedtheantinociceptiveactivity of500mg/kgMEMMwhenassessedusingtheabdominal constric-tiontest,hotplatetestandformalintest.
InvolvementofNO/cGMPpathway
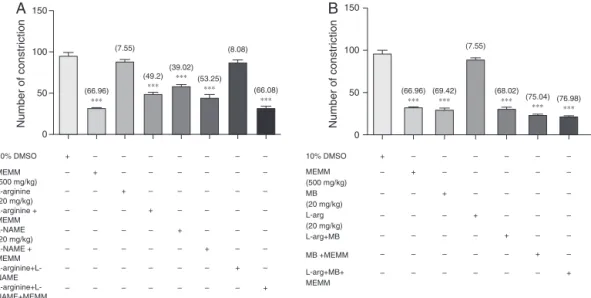
Fig.5Ashowstheeffectofl-arginine,l-NAME,ortheir com-binationonantinociceptiveactivityof500mg/kgMEMMassessed usingtheabdominalconstrictiontest.Pre-treatmentofratswith l-arginine alone didnotaffecttheacetic acid-induced nocicep-tion but significantly (p<0.05) reversed the antinociception of 500mg/kgMEMM.Ontheotherhand,pre-treatmentofratswith l-NAMEaloneexertedsignificant(p<0.05)antinociceptiveactivity butfailedtoenhancetheextract’santinociceptiveactivity. More-over,pretreatmentwithbothl-arginineandl-NAMEalsofailedto significantlychangedtheextract’santinocicpetion.Therefore,only thepresenceofNOdonor(l-arginine)significantlyaffectsMEMM antinociceptiveactivity.
Fig.5Bshowstheeffectofl-arginine,MBortheircombination onantinociceptiveactivityof500mg/kgMEMMassessedusingthe abdominalconstrictiontest.Pre-treatmentofratswithMBalone produceasignificant(p<0.05)antinociceptiveeffect,whichisnot affected by earlypre-treatment with l-arginine. Moreover, MB aloneorincombinationwithl-argininedidnothelptoincreasethe antinociceptiveactivityofMEMM.Thus,inhibitorofcGMPpathway didnotaffecttheextract’santinociception.
GCMSanalysisofMEMM
A total of 30 peaks were identified from MEMM with the major compounds constituted of 9-octadecenamide (48%), 3-methyl quinoline (7.8%), propanoic acid (5.8%), methyl- -d-galactopyranoside (6.8%), methyl-␣-d-glucopyranoside (3.9%), hexadecanoicacid,methylester(3.4%),hexadecanoicacid(2.9%), hexadecanamide(2.9%),furfural(2.3%), pyrogallol(2.3%), myris-tamide(1.8%),9,12,15-octadecatrienoicacid,methylester(2.1%), 2-methyl-l-mannomethylpyranoside (1.7%), ␥-conicein (1.6%),
150
A
B
(66.96)
∗∗∗
10% DMSO +
– – – – – – – – + – – – – – – – – + – – – – – – – – + – – – – – – – – + – – – – – – – – + – – – – – – – – + – – – – – – – – + MEMM (500 mg/kg) L-arginine (20 mg/kg) L-arginine + MEMM L-NAME (20 mg/kg) L-NAME + MEMM L-arginine+L-NAME L-arginine+L-NAME+MEMM
10% DMSO +
– – – – – – – + – – – – – – – + – – – – – – – + – – – – – – – + – – – – – – – + – – – – – – – + MEMM (500 mg/kg) MB (20 mg/kg) L-arg (20 mg/kg) L-arg+MB MB +MEMM L-arg+MB+ MEMM (49.2) ∗∗∗ (39.02) ∗∗∗ (53.25) ∗∗∗ (66.08) ∗∗∗ (66.96) ∗∗∗ (69.42) ∗∗∗ (68.02) ∗∗∗ (75.04) ∗∗∗ (76.98) ∗∗∗ (7.55) (8.08) (7.55) 100
Number of constr
iction
50
0
150
100
Number of constr
iction
50
0
Fig.5.InvolvementofNO/cGMPpathwayinthemodulationofantinociceptiveactivityofMEMM.(A)Effectofl-arginine,l-NAMEandtheircombinationonantinociceptive
activityofMEMMassessedusingtheabdominalconstrictiontest.(B)Effectofl-argininemethyleneblue(MB)andtheircombinationonantinociceptiveactivityofMEMM
dodecanedioicacid,dimethylester(1.6%)and9-octadecenoicacid (1.3%).
Discussion
Themanagementofpainusingcurrentlyavailableanalgesics havebeenover shadowedbyvariousadverseeffects.Morphine, whichhasbeenthedrugofchoiceforthetreatmentofpain,has beenknowntocausedependenceandtoleranceuponitsprolong used(Rangetal.,2011).Inanattempttocontributetowards find-ingnewanalgesicdrugwithlowor,possibly,noadverseeffects, thepresentstudywasconductedtodeterminetheantinociceptive potentialofMEMMusingvariouschemical-andthermal-induced modelsofnociceptioninlaboratoryanimals.Althoughtherehave been three reports on the antinociceptive activity of M. mala-bathricumleaves, thethree papers reportedtheuseof ethanol, aqueousand chloroformextractsastheirsourceof antinocicep-tivestudyandthatthoseextractswereadministeredsystemically, which areeither bytheintraperitoneal or subcutaneousroutes (Sulaimanetal.,2004;Zakariaetal.,2006;2008).Incontrastto thosereports,thepresentstudyusedmethanolextractthatwas administered orally into therats. Although the use of ethanol extractalmostresemblesthecurrentusedofmethanolextract,the formeradministrationviatheintraperitonealroutedidnot repre-sentthetraditionalwayofconsumingtheextract.Moreover,the aqueousandchloroformextractsofM.malabathricumhavebeen proventoexertantinociceptiveactivityand,therefore,justifythe useofintermediatesolventlikemethanoltoextractoutboththe antinociceptive-bearingpolaradnon-polarbioactivecompounds fromM.malabathricum.
Inthepresentstudy,MEMMwasfoundtoexert antinocicep-tiveactivity at both theperipheral and central levelswiththe non-opioid-mediatedactivityseenatbothlevels.Thenon-opioid mechanismissuggestedbasedonourfindingsthatnaloxonefailed toinhibittheextract’sactivitywhenassessedusingall nocicep-tiveassays.Interestingly,theantinociceptiveactivityofMEMMis alsosuggestedtoinvolveinhibitionofthevanilloidreceptorsand glutamatergicsystemasMEMM attenuatedboth the capsaicin-andglutamate-inducedmodelsofnociception.Moreover,MEMMis alsosuggestedtoexertantinociceptiveactivityviathemechanisms ofactionthatdidnotinvolveactivationoftheNO/cGMPpathwayas pre-challengingtheextractwithl-NAMEorMBalsofailedtoinhibit MEMMantinociceptiveactivity.Thefindingthatonlyl-arginine, burnotl-NAMEorMB,significantlyreversedtheantinociceptive activityofMEMMsuggestedthatthepresenceofNOaffectedthe extractviaapathwaythatwasindependentofcGMPaction(Cui etal.,2005).
Severalothermechanismsofactioncouldbeproposedbased ontheresultsobtained.Painsensationcanbeproducedbyvarious typesofstimuli(i.e.,mechanical,thermalandchemical),hencethe existenceofmechanosensitive,thermosensitiveand chemosensi-tivepainreceptorsaresuggested(Tandonetal.,2003).Theextract abilitytoinhibitatleasttwoofthestimuli,whichisrepresented bytherespectiveaceticacid-inducedabdominalconstrictionand formalin-inducedpawlicking tests,andthehot-platetest,isan indicativeofitsabilitytoinhibittheperipheralandcentral nocicep-tivemechanisms.Moreover,MEMMalsoattenuatedbothphasesof theformalintestand,takingthesethingstogether,itisplausible tosuggestthatMEMMpossessesthecharacteristicofcentrally act-inganalgesics.However,sinceMEMMdidnotworkontheopioid receptorsattheperipheralandcentrallevels,itmightbearight candidateforthedevelopmentofanalgesictoreplacemorphine.
Severalmechanismsofantinociceptioncouldalsobesuggested based onthe nociceptivemodels applied in thepresent study. The acetic acid-induced abdominal constriction test is a typi-cal model for assessment of peripheralantinociceptive activity
of neworpotentialanalgesicagents. Otherthan beinga sensi-tivemodel(Collieretal.,1968;Bentleyetal.,1981;Mohd.Sani etal.,2012), thisassayrepresentsthestimulation ofperipheral nociceptivemechanismviatheaceticacid-inducedreleaseof sev-eralpro-inflammatoryornociceptive-endogenousmediators(i.e., bradykinin, serotonin,histamine, substanceP or prostaglandins (PGE2 and PGF2␣)) (Deraedt et al., 1980; Ribeiro et al., 2000; Tandonetal.,2003),whichcausedsubsequentactivationof periph-eralnociceptiveneuronswithintheperitonealcavity.Inaddition, thenociceptiveresponseinducedbyaceticaciddependsonthe production ofnitric oxide(NO)(Larsonet al.,2000).Moreover, theacetic acid-inducednociceptionobservedusing the abdom-inal constriction test wasattenuated byboth the peripherally-andcentrally-mediatedanalgesics.Therefore,ourpresentresults implythat MEMMmayexertedinpartaperipherally-mediated antinociceptiveactivity,which maytoacertainextentresulted fromtheinhibitionofthesynthesisoractionofsomeofthe pro-inflammatorymediatorsmentionedabove.Inaddition,theextract mightalsocausedecreaseintheproductionofNOand/orcytokines, therebyinterferingwiththemechanismsofsignaltransductionin theprimaryafferentnociceptors.
Nevertheless,theabdominalconstrictiontestisnotaspecific testascertaintypeofnon-analgesicslikemusclerelaxantscanalso givefalsepositiveresults,whichcouldleadtomisinterpretationof theresults(LeBarsetal.,2001;Mohd.Sanietal.,2012).Therefore, additionalassessmentofMEMM’santinociceptivepotentialusing othermodelsofnociceptionneedtobeconducted.Toconfirmthe possiblemechanismsofantinociceptioninvolved,theMEMMwas furthersubjectedtothehot-plateandformalin-inducedpaw lick-ingtests.Thesetestsareconsideredasthemorespecifictestsfor determiningtheinvolvementofthecentraland/orperipherallevels ofantinociception(Mohd.Sanietal.,2012).
The hot plate test, which measured thermal-induced noci-cpetionatthesupra-spinalandspinallevels,isanociceptivemodel suitableforassessingthepotentialofanycompounds/extractsto exertantinociceptive activityat centrallevel. It hasthe advan-tageofbeingselectiveandsensitiveonlytothecentrally-,butnot peripherally-actinganalgesics(HosseinzadehandYounesi,2002; Giglioetal.,2006).Theability ofMEMMtoprolongthelatency to feeling discomfort indicates the extract potential to inhibit the thermal-induced nociception and, therefore, suggested the involvement of centrally-mediated antinociception. Taking into accounttheabilityofMEMMtoattenuatenociceptivestimuliwhen assessedusingtheabdominalconstrictionandhotplatetests,it isplausibletosuggestthatMEMMexertsperipheralandcentral antinociceptiveactivityand,hence,demonstratedthe characteris-ticofstronganalgesics.
locatedatthesensoryC-fibresthatreflectcentrally-mediatedpain (McNamaraetal.,2007).Thelatephase,ontheotherhand, cor-respondstotheinflammatory-mediatednociceptiveresponse,and representstheperipherally-actingnociception.This inflammatory-inducednociceptionisduetotheactionofvariousinflammation mediatorsreleasedduetodamagetothecellsresultingfrom forma-lininjection(Paradaetal.,2001).ItwasreportedthatsubstanceP andbradykininactasmediatorsinthefirstphaseresponse,while histamine,serotonin,prostaglandinandbradykinin areinvolved inthenociceptiveresponseofthesecondphase.Itiswell estab-lishedthat,centrally actingdrugs(i.e.,opioids)caninhibit both phases;however,peripherallyactingdrugs(i.e.,NSAID)onlyinhibit thesecondphase(Tjolsenetal.,1992;Mohd.Sanietal.,2012).In thepresentstudy,MEMMattenuatedbothphasesofnociception furtherconfirmingtheextract’scentrally-actingeffect.
Intheearlypartofthediscussion,MEMMissuggestedto prob-ablypossessacharacteristicofstrongopioidanalgesicsbasedon theextractabilitytoinhibitthechemically-andthermally-induced nocicpetivestimuliandtoattenuatethenociceptiveresponseinthe twophasesofformalintest.However,pre-treatmentwith nalox-onedidnotaffecttheantinociceptiveactivityofMEMMagainstall modelsofnociception.Thisfindingcontradictedpreviousreport madebySulaimanetal.(2004),whodemonstratedthe involve-mentofopioidsysteminthemodulationofantinociceptiveactivity ofethanolextractofM.malabathricum(EEMM).Thisdiscrepancy couldbedue,particularly,tothedifferentinrouteof administra-tionoftherespectiveextract.Theeffectofrouteofadministration canbeseeninreportbyMatsumotoetal.(2004),whereinmorphine wasreportedtoexertweakantinociceptiveefficacywhengivenvia theoralrouteincomparisontothesubcutaneousroute.Inthiscase, EEMMwasgivenintraperitoneallyincomparisontoMEMM,which wasgivenorally.ThebioactivecompoundsinMEMMmighthave lostordecreasedinitsopioidactionduetometabolismprocesses intheliverfollowingtheextract’soraladministrationin compar-isontoEEMM,whichwasnotmetabolizedbyliverfollowingits intraperitonealadministrationanddirectlytransfertothesiteof action.Theprocessesmighthavedestroyedsomeofthe antinoci-ceptivecompounds,possiblythoseactingattheopioidreceptors. Tofurthersupportthecontradictionmentioned above,areport byRebolledoetal.(2012)couldbeusedtoexplainthe discrep-ancyinopioidreceptorsroleontheantinociceptionofMEMMand EEMM.AccordingtoRebolledoetal.(2012),thepolarcompounds wereeasilydigestedthanthenon-polarcompoundsandthiscould beusedtoexplaintheinabilityofnaloxonetoblocktheextract antinociceptionwhenadministeredorally,butnotsystemically.
Furthertestswerealsoconductedtoexaminetheinvolvement ofMEMMinthemodulationofnociceptivetransmissionviathe vanilloidreceptors,glutamatergicsystemandNO/cGMPpathway. Todeterminetheroleofvanilloidreceptorsinthemodulationof antinociceptiveactivityofMEMM,thecapsaicin-inducedpaw lick-ingtestwerecarriedoutinrats.Capsaicin,thepungentsubstance fromchilli peppers, hasbeen repeatedly usedin pain research foritsabilitytoinducebothhyperalgesiaandanalgesia, depend-ingontheconcentrationandrouteofapplication(Numazakiand Tominaga,2004).CapsaicinhastheabilitytoactivateC-orA
∂
-fibres inafferentneuronsthroughstimulationofTRPV1receptorsthus allowingtheinfluxofCa2+ and Na+ leadingtoneurogenicpain.Inaddition,TRPV1receptorsareconsideredtobeintegratorsof noxiouschemical and physicalstimuli that canbeactivatedby capsaicin,heatandlowpH(NumazakiandTominaga,2004). Pre-viousstudieshavealsoshownthatcapsaicininducestherelease ofneurokinins,neuropeptides,excitatoryaminoacids(glutamate andaspartate), nitricoxide(NO) andpro-inflammatory periph-eralmediators,besidespromotingthetransmissionofnociceptive informationtothespinalcordandtheactivationofvanilloid recep-tors(CaterinaandJulius,2001).Thevanilloidreceptorsalsocan
besensitizedoractivatedbyseveralinflammatorymediators(i.e., bradykinin,nitricoxideandprostaglandin)andstudieshaveshown thatactivationofthesereceptorscausesasharpincreasein inflam-matorymediatorlevels(Cortrightand Szallasi,2004;Numazaki andTominaga,2004).Inthepresentstudy,MEMMreversedthe capsaicin-inducednociceptioninadose-dependentmanner indi-catingthattheextractwasalsoeffectiveinattenuatingnociceptive transmissionmodulatedviathevanilloidreceptorsaswellas inter-feringwiththerelease/action ofthose inflammatorymediators. Thelatterabilitymightexplaintheextractpotentialtoinhibitthe abdominalconstrictiontestandthesecondphaseoftheformalin test,whicharerelatedtotheinflammatory-mediatednociception. InanattempttoassesstheabilityofMEMMtointerferewith theglutamate-mediatednociceptivetransmission,the glutamate-inducedpawlickingtest wasperformed.Glutamate,oneofthe important excitatory amino acids, is most wide spread in the central nervous system (CNS) (Neugebauer, 2002). It acts as a major excitatory neurotransmitter where they participate in a greatnumberofphysiologicalandpathologicalstates.Glutamate induces nociceptive transmission at the peripheral, spinal and supra-spinalsitesviadifferenttypesofglutamatereceptors(i.e., AMPA,KainateandNMDAreceptors)(Neugebauer,2001a,b). Addi-tionally,thenociceptiveresponseinducedbyglutamateisgreatly mediated by both activation of N-methyl-d-aspartate (NMDA) and ␣-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate (non-NMDA)receptors,aswellasreleasingofNOorsomeNO-derived substances (Beirithet al., 2002). Therelease of NO will trigger thesynthesisof pro-inflammatorymediators suchas cytokines, whichhelptoenhancetheinflammatory reaction(Beirithetal., 1998,2002).Moreover,therewerereportsthattheactivationof glutamatereceptorscontributetothemaintenanceofperipheral nociceptiveprocessassociatedwithinflammation(asseeninthe latephaseofformalintest)(Neugebauer,2002),whilethepresence ofglutamatereceptorsantagonistinhibitedtheinflammatory(late phase),butnotneurogenicphaseoftheformalintest(Bhaveetal., 2001).Inthepresentstudy,oraladministrationofMEMMexerted a dose-dependentinhibition of theglutamate-induced nocicep-tiveresponse.Therefore,itisstronglysuggestedthattheMEMM antinociceptiveactivityagainsttheglutamate-inducednociception occursthroughtheinteractionofrespectiveextractwithany gluta-matereceptorsintheglutamatergicsystemorbyinterferingwith theNOproduction.
ToinvestigatetheroleofNO/cGMPpathwayinthemodulation ofMEMMantinociceptiveactivity,theextractwerepre-challenged againstl-arginine(actsasaNOdonor),l-NAME(actsasaninhibitor ofNOsynthase)andMB(actsasaninhibitorofcGMPpathway).NO, abiologicalmoleculefoundinsideandbetweencells,isamajor playerin physiological functionssuchas theimpulse transmis-sioninthecentraland peripheralnervoussystems(Garthwaite and Boulton,1995).It hasbeenreported that NOis involve in themechanismofnociceptionatthesupraspinalandperipheral sitesbyacting asa pro-nociceptiveor anantinociceptiveagent dependingonthedosespresence(Ferreiraetal.,1991;Machelska etal.,1997).Fromtheresultsobtained,onlyl-argininesignificantly reversed MEMM’s antinociception suggesting that thepresence of NOreduced butdid not inhibitthe extract’santinociceptive potential.Hence,itisplausibletosuggestthatMEMMworksvia theNO-mediated/cGMP-independentpathway. Ithasbeen well acknowledgedthatNOexertsvariousbiologicalrolesthatare medi-atedinacGMP-independentmanner.Forexample,NOhasbeen showntointeractdirectlyandindirectlywithvariousinhibitory neurotransmitterssuchasGABA,glycine,opioid,andmuscarinic receptormechanisms(Ichinoseetal.,1998;Cuietal.,2005).
of quercitrin in the extract (Kamisan et al., 2014).The ability of quercitrin to inhibit the pro-inflammatory mediators, espe-ciallycytokines,engagedin painmodulationhasbeenreported (Comaladaetal.,2005;Gadottietal.,2005),thus,suggestedthe compoundtopartlyresponsiblefortheobservedantinociceptive activityofMEMM.Inthepresentstudy,GCMSanalysiswas per-formed on MEMM and approximately 53% of the constituents detectedwerefattyacidamide,namely9-octadecenamide, hex-adecanamideand myristamide.Thepresent of fattyacidamide might contribute to the observed antinociceptive activity of MEMMbasedonpreviousreportsthatseveralfattyacidamides demonstratedantinociceptiveactivity(DrayandDickenson,1991; Déciga-Campos et al., 2007; Barrière et al., 2013). Derivatives of 9-octadecenamide (Dray and Dickenson, 1991) and hexade-canamide(Déciga-Camposetal.,2007),inparticular,havebeen proventoexertantinociceptiveactivityand,hence,arebelieved tocontributeto MEMM’santinociceptive activity.Nevertheless, further studies are needed to refine and validate these early findings.
Conclusion
In conclusion, MEMM exerted a non-opioid antinocicepitve activity at the peripheral and central levels via mechanisms involvingmodulationofthevanilloidreceptors,glutamatergic sys-tem, and NO-mediated/cGMP-independent pathway. Moreover, the antinociceptive activity of MEMM might be attributed to thepresenceofflavonoid-basedbioactivecompounds,including quercitrin.Theabilitytoexertanon-opioidantinociceptive activ-ityattheperipheralandcentrallevelsuggeststhattheextractcould beagoodcandidateforthedevelopmentofnewanalgesicdrugthat islackofdependence/toleranceeffectsseenwithmorphine.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethattheyhave fol-lowed theprotocolsof theirworkcenter onthe publicationof patientdata.
Right to privacy and informed consent. The authors have obtainedthewritteninformedconsentofthepatientsorsubjects mentionedinthearticle.Thecorrespondingauthorisinpossession ofthisdocument.
Authors’contribution
ESJcarried outtheexperimentsand draftedthemanuscript. SARandAKAparticipatedintheexperimentaldesign,andhelped todraftthemanuscript.SMCandMNDinvolvedinthestatistical analysisandmanuscriptpreparation.ZAZconceivedofthestudy, participatedinitsdesignandhelpedtodraftthemanuscript.All authorsreadandapprovedthefinalmanuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
References
Barrière, D.A., Mallet, C., Blomgren, A., Simonsen, C., Daulhac, L., Libert, F., Chapuy, E., Etienne, M., Högestätt, E.D., Zygmunt, P.M., Eschalier, A., 2013. Fattyacidamidehydrolase-dependentgeneration ofantinociceptive drug metabolites acting on TRPV1 in the brain. PLOS ONE 8, e70690,
http://dx.doi.org/10.1371/journal.pone.0070690.
Beirith,A.,Santos,A.R.S.,Calixto,J.B.,2002.Mechanismsunderlyingthenociception andpawoedemacausedbyinjectionofglutamateintothemousepaw.Brain Res.924,219–228.
Beirith,A.,Santos,A.R.,Rodrigues,A.L.,Creczynski-Pasa,T.B.,Calixto,J.B.,1998.
Spinalandsupraspinalantinociceptiveactionofdipyroneinformalin,capsaicin andglutamatetests.Studyofthemechanismofaction.Eur.J.Pharmacol.345, 233–245.
Bentley,G.A.,Newton,S.H.,Starr,J.,1981.Evidenceforanactionofmorphineandthe enkephalinsonsensorynerveendingsinthemouseperitoneum.Br.J. Pharma-col.73,325–332.
Bhave, G.,Karim, F., Carlton,S.M.,Gereau, I.V.R.W., 2001. Peripheral groupI metabotropicglutamatereceptorsmodulatenociceptioninmice.Nat.Neurosci. 4,417–423.
Caterina,M.J.,Julius,D.,2001.Thevanilloidreceptor:amoleculargatewaytothe painpathway.Annu.Rev.Neurosci.24,487–517.
Collier,H.O.,Dinneen,L.C.,Johnson,C.A.,Schneider,C.,1968.Theabdominal con-strictionresponseanditssuppressionbyanalgesicdrugsinthemouse.Br.J. Pharmacol.32,295–310.
Comalada,M.,Camuesco,D.,Sierra,S.,Ballester,I.,Xaus,J.,Gálvez,J.,Zarzuelo,A., 2005.Invivoquercitrinanti-inflammatoryeffectinvolvesreleaseofquercetin, whichinhibitsinflammationthroughdown-regulationoftheNF-kappaB path-way.Eur.J.Immunol.35,584–592.
Cortright,D.N.,Szallasi,A.,2004.Biochemicalpharmacologyofthevanilloidreceptor TRPV1.Anupdate.Eur.J.Biochem.271,1814–1819.
Cragg,G.M.,Newman,D.J.,Snader,K.M.,2003.Naturalproductindrugdiscovery anddevelopment.J.Nat.Prod.66,1022–1037.
Cui, X., Zhang, J., Ma, P., Myers, D.E., Goldberg, I.G., Sittler, K.J., Barb, J.J., Munson,P.J.,CintronAdel,P.,McCoy,J.P.,Wang,S.,Danner,R.L.,2005. cGMP-independent nitric oxide signaling and regulation of the cell cycle.BMC Genomics,http://dx.doi.org/10.1186/1471-2164-6-151.
Déciga-Campos,M.,Montiel-Ruiz,R.M.,Navarrete-Vázquez,G.,López-Mu ˜noz,F.J., 2007.Palmiticacidanaloguesexhibitingantinociceptiveactivityinmice.Proc. WestPharmacol.Soc.50,75–77.
Deraedt,R.,Jougney,S.,Delevalcese,F.,Falhout,M.,1980.Releaseofprostaglandins EandFinanalgogenicreactionanditinhibition.Eur.J.Pharmacol.51,17–24.
Dray,A.,Dickenson,A.,1991.Systemiccapsaicinandolvanilreducetheacute algogenicandthelateinflammatoryphasefollowingformalininjectioninto rodentpaw.Pain47,79–83.
Faravani,M.,(PhDthesis)2009.ThepopulationbiologyofStraitsRhododendron (MelastomamalabathricumL.).UniversityofMalaya,KualaLumpur,Malaysia.
Ferreira,S.H.,Duarte,I.D.,Lorenzetti,B.B.,1991.Themolecularmechanismofaction ofperipheralmorphineanalgesia:stimulationofthecGMPsystemvianitric oxiderelease.Eur.J.Pharmacol.201,121–122.
Gadotti, V.M., Schmeling, L.O., Machado, C., Liz, F.H., Meyre-Silva, C., San-tos, A.R., 2005. Antinociceptive action of the extract and the flavonoid quercitrinisolatedfromBauhiniamicrostachyaleaves.J.Pharm.Pharmacol.57, 1345–1351.
Garthwaite,J.,Boulton,C.L.,1995.Nitricoxidesignalinginthecentralnervous sys-tem.Annu.Rev.Physiol.57,683–706.
Giglio,C.A.,Defino,H.L.A.,Da-Silva,C.A.,De-Souza,A.S.,DelBel,E.A.,2006. Behav-ioralandphysiologicalmethodsforearlyquantitativeassessmentofspinalcord injuryandprognosisinrats.Braz.J.Med.Biol.Res.39,1613–1623.
Grosvenor,P.W.,Gothard,P.K.,McWilliam,N.C.,Supriono,A.,Gray,D.O.,1995.
Medicinal plants from Riau Province, Sumatra,Indonesia. Part1: Uses.J. Ethnopharmacol.45,75–95.
Hosseinzadeh,H., Younesi,H.M.,2002. Antinociceptiveand anti-inflammatory effectsofCrocussativusL.stigmaandpetalextractsinmice.BMCPharmacol.,
http://dx.doi.org/10.1186/1471-2210-2-7.
Hunskaar,S.,Berge,O.G.,Hole,K.,1986.Amodifiedhot-platetestsensitivetomild analgesics.Behav.BrainRes.21,101–108.
Hunskaar,S.,Hole,K.,1987.Theformalintestinmice:dissociationbetween inflam-matoryandnon-inflammatorypain.Pain30,103–114.
Hussain,F.,Abdulla,M.A.,Noor,S.M.,Ismail,S.,Ali,H.M.,2008.Gastroprotective effects ofMelastoma malabathricum aqueous leafextract against ethanol-inducedgastriculcerinrats.Am.J.Biochem.Biotechnol.4,438–441.
Ichinose,F.,Mi,W.D.,Miyazaki,M.,Onouchi,T.,Goto,T.,Morita,S.,1998.Lackof correlationbetweenthereductionofsevofluraneMACandthecerebellarcyclic GMPconcentrationsinmicetreatedwith7-nitroindazole.Anesthesiology89, 143–148.
Kamisan,F.H.,Yahya,F.A.,Mamat,S.S.,Kamarolzaman,M.F.F.,Suhaili,Z., Mohtar-rudin,N.,Ching,S.M.,Teh,L.K.,Salleh,M.Z.,Abdullah,M.N.H.,Zakaria,Z.A., 2014. Effect of methanol extract of Dicranopteris linearis against carbon tetrachloride-induced acute liverinjuryin rats.BMC Complim. Alt.Med.,
http://dx.doi.org/10.1186/1472-6882-14-123.
Larson,A.A.,Kovacs,K.J.,Cooper,J.C.,Kitto,K.F.,2000.Transientchangesinthe syn-thesisofnitricoxideresultinlong-termaswellasshort-termchangesinacetic acid-inducedwrithinginmice.Pain86,103–111.
Luiz,A.P.,Moura,J.D.,Meotti,F.C.,Guginski,G.,Guimaraes,C.L.,Azevedo,M.S., Rodrigues,A.L.,Santos,A.R.,2007.Antinociceptiveactionofethanolicextract obtainedfromrootsofHumiriantheraamplaMiers.J.Ethnopharmacol.114, 355–363.
Manicam,C.,Abdullah,J.O.,MohdTohit,E.R.,Seman,Z.,Sieo,C.C.,Hamid,M.,2010.
InvitroanticoagulantactivitiesofMelastomamalabathricumL.aqueousleaf extract:apreliminarynovelfinding.J.Med.PlantsRes.4,1464–1472.
Machelska,H.,Labuz,D.,Przewlocki,R.,Przewlocka,B.,1997.Inhibitionofnitric oxidesynthaseenhancesantinociceptionmediatedbyMu,DeltaandKappa opi-oidreceptorsinacuteandprolongedpainintheratspinalcord.J.Pharm.Exp. Ther.282,977–984.
Matsumoto,K.,Horie,S.,Ishikawa,H.,Takayama,H.,Aimi,N.,Ponglux,D., Watan-abe,K.,2004.Antinociceptiveeffectof7-hydroxymitragynineinmice:discovery ofanorallyactiveopioidanalgesicfromtheThaimedicinalherbMitragyna speciosa.LifeSci.74,2143–2155.
McNamara,C.R.,Mandel-Brehm,J.,Bautista,D.M.,Siemens,J.,Deranian,K.L.,Zhao, M.,2007.TRPA1mediatesformalin-inducedpain.Proc.Natl.Acad.Sci.104, 13525–13530.
Mohd.Sani,M.H.,Zakaria,Z.A.,Balan,T.,Teh,L.K.,Salleh,M.Z.,2012. Antinoci-ceptive activity of methanol extract of Muntingia calabura leaves and the mechanisms of action involved. Evid. Based Complim. Altern. Med.,
http://dx.doi.org/10.1155/2012/890361.
Nazlina,I.,Norha,S.,NoorZarina,A.W.,Ahmad,I.B.,2008.Cytotoxicityandantiviral activityofMelastomamalabathricumextracts.Malays.J.Appl.Biol.37,53–55.
Neugebauer,V.,2001a.Metabotropicglutamatereceptors:noveltargetsforpain relief.ExpertRev.Neurother.1,207–224.
Neugebauer,V.,2001b.Peripheralmetabotropicglutamatereceptors:fightthepain whereithurts.TrendsNeurosci.24,550–552.
Neugebauer,V.,2002.Metabotropicglutamatereceptors–importantmodulatorsof nociceptionandpainbehavior.Pain98,1–8.
Numazaki,M.,Tominaga,M.,2004.NociceptionandTRPchannels.Curr.DrugTargets CNSNeurol.Disord.3,479–485.
Ong,H.C.,Nordiana,M.,1990.Malayethno-medicobotanyinMachang,Kelantan, Malaysia.Fitoterapia70,502–513.
Parada,C.A.,Tambeli,C.H.,Cunha, F.Q.,Ferreira,S.H.,2001.Themajorroleof peripheralreleaseofhistamineand5-hydroxytryptamineinformalin-induced nociception.Neuroscience102,937–944.
Raghav,S.K.,Gupta,B.,Agrawal,C.,Goswami,K.,Das,H.R.,2006.Anti-inflammatory effectofRutagraveolensL.inmurinemacrophagecells.J.Ethnopharmacol.104, 234–239.
Rajenderan,M.T.,2010.Ethnomedicinalusesandantimicrobialpropertiesof Melas-tomamalabathricum.SEGiRev.3,34–44.
Rang,H.P.,Dale,M.M.,Ritter,J.M.,Flower,R.J.,Henderson,G.,2011.Pharmacology, 7thed.ElsevierChurchillLivingstone,Edinburgh,UK.
Rebolledo,R.,Abarzúa,J.,Zavala,A.,Quiroz,A.,Alvear,M.,Aguilera,A.,2012.The effectsoftheessentialoilandhydrolateofcanelo(Drimyswinteri)onadultsof Aegorhinussuperciliosusinthelaboratory.Cien.Inv.Agr.39,481–488.
Ribeiro,R.A.,Vale,M.L.,Thomazzi,S.M.,Paschoalato,A.B.,Poole,S.,Ferreira,S.H., 2000.Involvementofresidentmacrophagesandmastcellsinthewrithing noci-ceptiveresponseinducedbyzymosanandaceticacidinmice.Eur.J.Pharmacol. 387,111–118.
Roosita,K.,Kusharto,C.M.,Sekiyama,M.,Fachrurozi,M.,Ohtsuka,R.,2008.Medicinal plantsusedbythevillagersofaSundanesecommunityinWestJava,Indonesia. J.Ethnopharmacol.115,72–81.
Sakurada,T.,Katsumata,K.,Tan-No,K.,Sakurada,S.,Kisara,K.,1992.Thecapsaicin testinmiceforevaluatingtachykininantagonistsinthespinalcord. Neuro-pharmacology31,1279–1285.
Sharma,H.K.,Chhangte,L.,Dolui,A.K.,2001.Traditionalmedicinalplantsin Mizo-ram,India.Fitoterapia72,146–161.
Shibata,M.,Ohkubo,M.,Takahashi,T.,Inoki,H.,1989.Modifiedformalintest; char-acteristicbiphasicpainresponse.Pain38,347–352.
Simanjuntak,M.,(M.S.thesis)2008.Ekstraksi,fraksinasi:komponenekstrakdaun tumbuhansenduduk(Melsatomamalabathricum)sertapengujianefeksediaan krimterhadappenyembuhanlukabakar.UniversitasSumateraUtara,Sumatera, Indonesia.
Sulaiman,M.R.,Somchit,M.N.,Israf,D.A.,Ahmad,Z.,Moin,S.,2004. Antinocicep-tiveeffectofMelastomamalabathricumethanolicextractinmice.Fitoterapia75, 667–672.
Sunilson,J.A.J.,Anandarajagopal,K.,Kumari,A.V.A.G.,Mohan,S.,2009. Antidiar-rhoealactivityofleavesofMelastomamalabathricumL.IndianJ.Pharm.Sci.71, 691–695.
Susanti,D.,Sirat,H.M.,Ahmad,F.,MatAli,R.,2008.Bioactiveconstituentsfromthe leavesofMelastomamalabathricumL.J.IlmiahFarmasi.5,1–8.
Tandon,O.P.,Malhotra,T.V.,Tandon,S.,D’silva,I.,2003.Neurophysiologyofpain: insighttoorofacialpain.IndianJ.Physiol.Pharmacol.47,247–269.
Tjolsen,A.,Berge,O.G.,Hunskaar,S.,Rosland,J.H.,Hole,K.,1992.Theformalintest: anevaluationofthemethod.Pain51,5–17.
Verpoorte,R.,1998.Explorationofnature’schemodiversity–theroleofsecondary metabolitesasleadsindrugdevelopment.DrugDiscov.Today3,232–238.
Wiart,C.,Mogana,S.,Khalifah, S.,Mahan,M.,Ismail,S.,Buckle,M.,Narayana, A.K.,Sulaiman,M.,2004.Antimicrobialscreeningofplants usedfor tradi-tional medicineinthe stateofPerak, PeninsularMalaysia.Fitoterapia75, 68–73.
Zakaria,Z.A., Raden Mohd Nor,R.N.S., Abdul Ghani,Z.D.F.,Hanan Kumar, G., Sulaiman,M.R.,Fatimah,C.A.,2006.Antinociceptiveandanti-inflammatory propertiesofMelastomamalabathricumleaveschloroformextractin experimen-talanimals.J.Pharmacol.Toxicol.1,337–345.