w w w. s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Characterization
of
intestinal
absorption
of
C
-glycoside
flavonoid
vicenin-2
from
Lychnophora
ericoides
leafs
in
rats
by
nonlinear
mixed
effects
modeling
Gabriela
A.
Buqui
a,
Sherwin
K.B.
Sy
b,
Matilde
Merino-Sanjuán
c,
Dayana
R.
Gouvea
a,
Suzana
L.
Nixdorf
d,
Elza
Kimura
e,
Hartmut
Derendorf
b,
Norberto
P.
Lopes
a,
Andrea
Diniz
a,e,∗aNPPNS(NúcleodePesquisaemProdutosNaturaiseSintéticos),DepartamentodeFísicaeQuímica,FaculdadedeCiênciasFarmacêuticasdeRibeirãoPreto,UniversidadedeSão
Paulo,RibeirãoPreto,SP,Brazil
bDepartmentofPharmaceutics,CollegeofPharmacy,UniversityofFlorida,Gainesville,USA cDepartamentodeFarmaciayTecnologia,UniversitatdeValencia,Valencia,Spain dDepartamentodeQuímica,UniversidadeEstadualdeLondrina,Londrina,PR,Brazil eDepartamentodeFarmácia,UniversidadeEstadualdeMaringá,Maringá,PR,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received15January2015 Accepted12April2015 Availableonline27April2015
Keywords: Vicenin-2 Flavonoid Intestinalabsorption Pharmacokinetic
a
b
s
t
r
a
c
t
Vicenin-2(apigenin-6,8-di-C--d-glucopyranoside)ispresentinhydroalcoholicextractsoftheBrazilian
speciesLychnophoraericoidesMart.,Asteraceae,leaves,andthebiologicaleffectsofthiscompoundhave beendemonstratedincludinganti-inflammatory,antioxidantandanti-tumoreffectsinratmodels.Given thepotentialofthiscompoundasapharmacologicalagent,theaimsofthisinvestigationwereto eval-uatetheextentofintestinalabsorptionofvicenin-2,andtodeterminetheintestinalpermeationprofile usinganinsitusingle-passintestinalperfusiontechnique.AvalidatedHPLC–UVmethodwasappliedto measuretheamountofunabsorbedvicenin-2inthegutafteranoraladministrationof180mgkg−1in
fiverats.Anonlinearmixedeffectsmodelwasusedtodeterminetheabsorptionpharmacokinetic param-etersassumingafirstorderabsorptionandactivesecretionprocessesforthiscompound,whereinthe activesecretionwascharacterizedbyazero-orderprocess.Thepopulationpharmacokineticparameters obtainedwere0.274min−1forthefirst-orderabsorptionrateconstant,16.3%min−1forthezero-order
rateconstant;thefinalpercentageoftheoriginaldosethatwasabsorbedinvivowas40.2±2.5%.These parametersindicatedthatvicenin-2wasrapidlyabsorbedinthesmallintestine.Incontrasttoliterature informationindicatingnoabsorptionofvicenin-2inCaco-2cells,ourresultssuggestedthatvicenin-2 canbeabsorbedinthesmallintestineofrats.Thefindingsupportsfurtherinvestigationofvicenin-2as aviableoralphytopharmaceuticalagentfordigestivediseases.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Thereisanenormousgrowthofworldwideinterestinherbal medicinesinboth thedevelopedand developingcountriesover thelastdecades.Theincreasingmarketforbotanicalproductshas attractedmuchinterestofsomepharmaceuticalcompanies,which hasinturnmotivatedpre-clinicalpharmacologicalstudiesaswell ascontrolledandrandomizedclinicaltrialstoprovethesafetyand efficacyofherbalproducts(Calixto,2000).Inadditiontoshowing pharmacologicalactivities,thepharmacokineticpropertiesofthese
∗Correspondingauthorat:DepartamentodeFarmácia,UniversidadeEstadualde Maringá,Maringá,PR,Brazil.
E-mail:adiniz@uem.br(A.Diniz).
agentsarekeyfactorsindeterminingwhetheracompoundcould beaviablemedicinalproduct(SyandDerendorf,2014;Syetal.,
2014).
Withgrowinginterestsinpolyphenoliccompoundsas pharma-cologicalagents, flavonoid,belongingtothisgroup,is themost studiedclassofcompounds;theirpharmacologicalactivitiesand pharmacokineticbehaviorshavebeenwellcharacterized. Polyphe-noliccompoundsoftenhavepoorbioavailability,giventhattheyare substratesofbothinfluxandeffluxtransportersandarealso sub-jectedtopre-systemicmetabolism(Barnes,2004;Geeetal.,2000). PhysiologicalpH,formationofconjugatedmetabolitesincluding glucuronidemetabolitesduringitspassagethroughtheenterocytes andevenbiotransformationbyintestinalmicrobiotaareknown toaffectthedispositionofvicenin-2presystemically(Gobbo-Neto
etal.,2005).
http://dx.doi.org/10.1016/j.bjp.2015.04.001
Mostof thestudiesof flavonoidabsorptionwereperformed ontheaglyconeorO-glycosylformswhichhaveunstable glyco-sidicbondsthatareeasilyhydrolyzed.Incontrast,vicenin-2(1), whosechemicalstructureisshownbelow,isaC-glycosylflavonoid thattendstobemorestableagainsthydrolysisthantheO-glycosyl flavonoids.
HO
HO
HO
HO
HO HO
O
O O
O
1
OH
OH
OH OH
OH
Thiscompoundisfoundinthehydroalcoholicextractsoftheleaves oftheBrazilianspeciesLychnophoraericoidesMart.,Asteraceae,and someofitsbiologicalactivitieshavealreadybeencharacterized. Inrecentstudy,investigatorshavedemonstratedthatL.ericoides extractswhicharerichinvicenin-2wereeffectiveas prophylac-ticagentagainstthediseaseprogressionofcoloncancerintherat model(Fernandesetal.,2011).Otherpharmacologicalactionof thiscompoundincludedanti-inflammatoryandantioxidant prop-erties(Gobbo-Netoetal.,2005).Thesepromisingpharmacological effectspromptedustoinvestigateandcharacterizetheintestinal absorptionofvicenin-2.
Theinvitroandinsituabsorptionmodels,suchasCaco-2cell monolayers,evertedgutsacsand perfusedanimalintestine,are commonlyusedtoinvestigatetransportmechanisms,toclassify permeability,andtopredictinvivoabsorptionofdrugsinhumans
(Lennernasetal.,1997).Theinsitusingle-passintestinalperfusion
techniquehasanadvantagesuchthatitiscarriedoutinlive exper-imentalanimalswithintactbloodsupplyandfunctionalnervous system.Thismethodologyisfoundtobesimpleandhighlyaccurate forpredictingintestinalabsorptioninhumans(Fagerholmetal., 1996).Theaimsofthisinvestigationaretoevaluatetheintestinal absorptionofvicenin-2,toobtaintheintestinalpermeationprofile forthisglycosyl-flavonoid,andtodevelopamathematicalmodel describingitsabsorption.
Materialsandmethods
Chemicals
AllsolventsforchromatographicanalysiswereHPLCgrade.All otherreagentswereP.A.grade.Thevicenin-2(1)wasisolatedfrom LychnophoraericoidesMart.,Asteraceae,accordingtothe method-ologypreviouslydescribed(Gobbo-Netoetal.,2005).
Single-passintestinalperfusionstudies
All animal experiments were conducted using protocols approvedbytheAnimalExperimentandEthicsCommitteeof Lon-drinaStateUniversity(protocol107/09).MalealbinoWistarrats, weighingfrom190to250gwereusedfortheperfusionstudies. Priortoeachexperiment,theratswerefastedovernight,12–18h
priortoexperimentation.Waterwasfreelyavailableforthese ani-malsduringthefastingperiod.
The in situ single-pass perfusion follows the procedure in publishedreports (Fagerholm et al., 1996).Briefly, theanimals wereanesthetizedwithanintra-peritonealinjectionof40mgkg−1 of thiopental solution and were placed on a heated surface maintainedat37◦C.For theperfusion, 10ml isosmoticsolution
(282–297mOsml−1)waspreparedcontaining5%ofTween80(v/v), bufferedatpH6.4withavicenin-2doseof180mgkg−1(n=5).
The remaining amountof drugin the intestinal lumen was collected in the volume of 200l per sample and measured every 5min, for a total time of 30min, by a validated high-performance liquid chromatography and ultraviolet detection (HPLC–UV)method.Thesamplescollectedwerefirstcentrifuged at140×gfor15minandthenfrozenat−40◦C. Nodrug
degra-dationwasdetectedafterfreeze-thawcycle.Waterreabsorption wasevaluatedforeachanimal.Thisprocessfollowsapparent zero-orderkinetics(Martin-Villodreetal.,1986;Ruiz-Balagueretal., 2002)andtheremainingvicenin-2concentrationswereproperly corrected.
Analyticalprocedures
Intestinalperfusedsampleswereassayedforvicenin-2 concen-trationusingHPLC–UV.Intestinalperfusedsampleswerediluted in100lofmethanol:water(1:1,v/v)solution,filteredacrossa 0.45Mmembrane(Millipore®)andanalyzedbychromatographic
system.ThechromatographicsystemconsistsofaShimadzu®
HPLC systemwhichincludedLC10ADpump,UVdetector,Class-VP sys-tem,Rheodyne®7125manualinjectorwitha20lloop.AWaters®
C18analyticalcolumnNova-Pak(3.9×150mm)andguardcolumn C-18(5mm,Hamilton)wasused.Themobilephasewasamixture (20:80,v/v)ofmethanolandultra-purifiedwater,bothcontaining 2%aceticacidatpH2.3;theflowratewas0.8mlmin−1.The wave-lengthusedwas330nm.Calibrationcurvescovering7.0–40.0mM L–1vicenin-2concentrationsintheluminalsampleswereprepared.
Pharmacokineticanalysis
Thevicenin-2concentrationsineachsamplerepresentedthe remainingconcentrationintheintestinallumen.Itwasassumed thatnodegradationoccurredduringtheexperimentsincenoother chromatographicpeakwasobservedat254nmand330nm,which werethewavelengthsthatproducethemaximumexcitationand emissionforflavonoidandphenolrings.
Thefinalpercentageabsorbed(%Abs)wasdeterminedusingEq.
(1):
%Abs= Ct30
Ct0
×100% (1)
where Ct30 is thevicenin-2 concentrationat thelast sampleat 30minandCt0istheinitialvicenin-2concentration.
Flavonoids are substrates for the efflux protein expressed in enterocyte membranes. Among the transporters, the P-glycoproteinwasthemoststudied(LiandPaxton,2013;Tianetal., 2009).Given thattheflavonoids are subjecttoactive secretion bytheenterocytes,boththeMichaelis–Mentenequationandthe zero-orderprocesstodescribedrugsecretionintothelumenwere evaluated,similartothemodelspreviouslydescribed(Munozetal.,
2005).
Model1isafirst-orderabsorptionandzero-ordersecretion pro-cess:
dA
Model 2 consists of a first-order absorption and Michaelis–Mentenfunctionrepresentingactivesecretion:
dA
dt =−ka·A+ VmsAE
Kms+AE (3)
Model3encompassesMichaelis–Mentenabsorptionandactive secretionprocesses:
dA dt =−
VmA Km+A+
VmsAE
Kms+AE (4)
Model4incorporatesacosinefunctiontotheactivesecretion processinModel2:
dA
dt =−ka·A+ VmsAE
Kms+AE
·(cos2t+1) (5)
Model5alsoincorporatesacosinefunctiontothezero-order secretionprocessinModel1:
dA
dt =−ka·A+k0·(cos
2t+1) (6)
wheredA/dtistheabsorptionrate,Aistheremainingvicenin-2 concentrationsinthegut,karepresentsthefirstorderabsorption rateconstant,Vmsreferstothemaximumsecretionrate,Kms is theconcentrationatwhichthesecretionishalfmaximal,Vmisthe maximumabsorption,Kmistheconcentrationwhichresultsinhalf maximumabsorption,k0isthezero-ordersecretionprocess,and AEissupposedlythevicenin-2concentrationintheenterocytethat isachievedinfirst5min.GiventhatAandAEareproportional,A, representingtheamountremaininginthegut,wasusedforAE.
Theremainingconcentrationofvicenin-2reportedasa percent-ageoftheinitialdoseineachsamplewasusedinthemodelfit. ThemodelslistedinEqs.(1)–(5)werefittedtothedatafromall animals,usingNonmem®versionVII.2(Buquietal.,2015;Munoz
etal.,2005;Sy etal.,2013).Thefirstorderconditional
estima-tionwithinteraction,usingsubroutineADVAN9andtoleranceof 5wasused.Between-animalvariabilityin themodelparameter wasassumedtobelog-normallydistributed.TheBayesianestimate ofindividualmodel-predictedvicenin-2remainingconcentration wasevaluatedwithandwithoutweightingfactorsbyusing addi-tiveorproportionalerrormodelsorthecombinationofboth.Given theexploratorynatureofthis study,modelselectionwasbased onmaximumlikelihoodstatistics,goodness-of-fitplots (consist-ingof populationandindividualpredictionsversusobservations
andconditionalweightedresidualsversustimeandindividual pre-dictions)andvisualpredictivechecks(VPC,with1000simulated profiles).Forhierarchicalmodels,thedifferenceinobjective func-tionvaluewas-squareddistributed.Ap-valueof0.01wasused asthecriteriaforselectingamorecomplexmodeloverareduced one,correspondingtothedifferenceinobjectivefunctionvalueof 6.63.Theevaluationofprecisionintheestimatedparametervalue wasbasedontherelativestandarderror.
Therobustnessofthefinalmodelandparameterimprecision wasevaluatedusinganon-parametricbootstrapprocedure.The algorithminvolvesrepeatedrandomsamplingofanimalsinthe study,withreplacementoftheoriginaldatasetineachsubsequent samplingtoproduceanotherdatasetofthesamesizeastheoriginal, butwithadifferentlistofanimals.There-samplingwasrepeated 500times.Thefinalpopulationpharmacokineticmodelwasfitted toeachofthebootstrapdatasetsandasetofmodelparameters weredeterminedforeach run.Themedianand 95%confidence intervalswerecomputedandcomparedtothevaluesfromthe orig-inalNonmem®analysis.PerlSpeaksNonmem®3.5.5runningactive
Perl® 5.10.1(Active StateSoftware Inc.,Vancouver,BC,Canada)
wereusedtomanagepost-NonmemanalysisandXpose®4running
onR®2.14.0forgraphicalevaluation.
Results
AchromatographicmethodusingHPLC–UVwasdevelopedand validatedforthequantificationofvicenin-2thatremainedinthe gutofratsovera30minperiod.Thestandardcurveforcalibration showedexcellentlinearplotsrelatingthepeakareatosolute con-centration(r2>0.9990);theinterceptofthelinearregressiondid notsignificantlydifferfromzero.Accuracywasevaluatedby cal-culatingtherelativeerror,whichwaslessthan15%.Precisionwas evaluatedbycalculatingthecoefficientofvariation,whichwasless than5%.Theseresultswereconsideredsatisfactory.
Theextentofvicenin-2absorptioncomputedfromEq.(1)was 40.2±2.5%.Theabsorptionprofilesofvicenin-2insixratswere evaluatedusingfivemodels.Theparameterestimatesforthefive modelsarelistedinTable1.Assomestudieshaveindicatedthat flavonoidsaresubstratesofeffluxtransportersoftheATPbinding cassettefamily(ABCB1,p-glycoprotein)(Barnes,2004;Fagerholm
etal.,1996;Geeetal.,2000),aMichaelis–Mentenkineticwas
incor-poratedtodescribetheactivesecretionprocessinModels2through
Table1
Populationpharmacokineticmodelsandmodelparameterestimatesforvicenin-2absorptioninrats.
Modeldescription
Absorption First-order First-order Michaelis-Menten First-order First-order
Secretion Zero-order Michaelis–Menten Michaelis–Menten Michaelis–Mentenwithcosinefunction Zero-orderwithcosinefunction
ModelNo. 1 2 3 4a 5a
Parameter
ka(min−1) 0.274(11%) 0.28(9.2%) 0.502 0.291
Vm(%min−1) 91.7(105%)
Km(%) 47,000(130%)
k0(%min−1) 16.3(9.4%) 8.78
Vms(%min−1) 17.2(7.3%) 6.99(84%) 9.55
Kms(%) 18.2(1.8%) 1.15(295%) 12.1
(min−1) 1.21 1.88
Interindividualvariability
%CVofka 8.4(75%) 8.2(77%) 60.5 0.0892
%CVofVm 16.6(90%)
%CVof 14.0 0.4
Residualvariability
Residualerror 0.00154(25%) 0.00154(25%) 0.00148(26%) 0.00142 0.00134
MOFV 118.43 118.4 117.143 151.05 117.78
Valuesreportedasmean(relativestandarderror,%).
100
90
80
70
60
50
40
Unabsorbed fla
v
onoid (%)
Time (min)
0 5 10 15 20 25 30 0
Rat No : 1 Rat No : 2 Rat No : 3 Rat No : 4 Rat No : 5 5 10 15 20 25 30 0 5 10 15 20 2530
0 5 10 15 20 2530 0 5 10 15 20 25 30 30
20
10
0
Fig.1.Plotofremainingunabsorbedvicenin-2asapercentageoftheinitialconcentrationintheintestinallumenversustimeusingtheratsingle-passperfusionmodel(n=5). ThesolidlinesrepresentindividualBayesianpredictedvaluesanddottedlinesarethepopulation-predictedvalues.Theactualobserveddataarerepresentedbytriangular symbols.
4.Theminimumobjectivefunctionvalues(MOFV)inTable1were comparable for allfive models,exceptfor Model 4, whichwas approximately33pointsgreaterthantheotherfourmodels.The saturable absorptionmodel (Model3)was consideredunstable giventhatthemagnitudeofthestandarderroroftheparameter estimateswasverylargerangingfrom84%to295%.
WeinitiallyevaluatedModel2giventhattheactivesecretionis asaturableprocess.Theparameterestimateswere0.28min−1for thefirstorderabsorptionrateconstantka,17.2%min–1and18.2% forVmsandKmsoftheactivesecretionprocess,respectively.The relativestandarderroroftheparameterestimatesrangedfrom1.8% to9.2%,whichweremarkedlysmallerthanthoseofModel3.We furtherevaluatedareducedmodelbyusingazero-ordersecretion processtoreplacetheMichaelis–Mentenprocess(Model1).The MOFVofModels1and2wereidentical,suggestingthatthemore complexMichaelis–Mentenactivesecretiondoesnotprovide sig-nificantadvantageoverthemoreparsimoniouszero-orderprocess. TheparametersofModel1were0.274min−1and16.3%min−1for kaandzero-ordersecretionk0,respectively.Theprecisionofthe pharmacokineticparametersandtheirvariabilitywereconsidered acceptableforbothModels1and2.
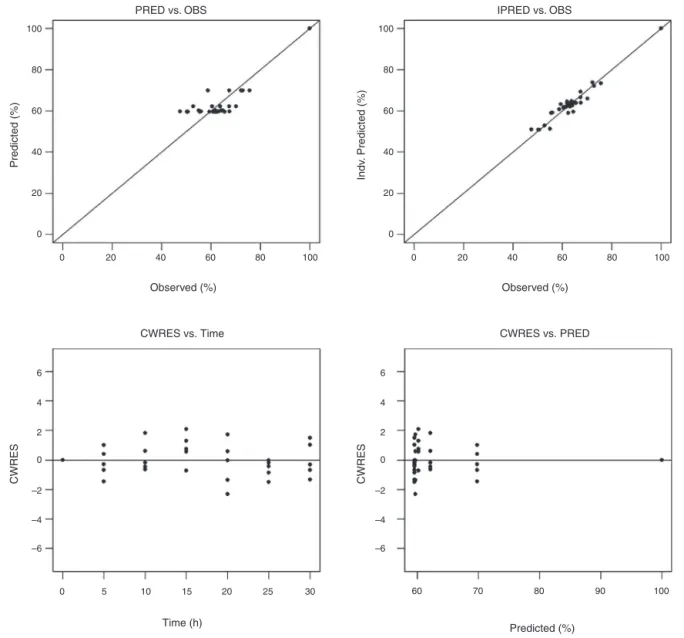
Theabsorptionprofilesofvicenin-2inthefiveratsareplotted inFig.1withtheremainingamountasapercentageofthe origi-naldoseversustimerepresentedbytrianglesymbols.Themodelfit ofEq.(1),whichhasfirst-orderabsorptionandzero-order secre-tionprocesses, totheobserved dataare representedasdashed linesandsolidlinesforthepopulationpredictedandindividual predictedcurvesinthesamefigure,respectively.Giventhe iden-ticalMOFVofbothModels1and2,theindividualplotofModel 2isidenticaltothatshowninFig.1.Theplotforeachanimalis presentedwiththetoppanelstripindicatedbyanimalnumber. Therewasagoodagreementbetweenmodelpredictionandthe observedpercentageunabsorbedvicenin-2.Theplotsofthemodel predicted(PRED)andindividualpredicted(IPRED)concentrations versusobserveddata(OBS)areshownonthetopgraphsofFig.3
forthefinalmodeldescribingvicenin-2intestinalabsorption.The conditionalweightedresiduals(CWRES)versustimeandCWRES
versusPREDplotsinthebottomgraphsofFig.2showthatmostof thedatalieswithin2unitsfromthezero-ordinate.
From theindividualplots and thediagnostic plotof CWRES versus TIME in Figs. 1 and 2 respectively, we noticed an alternating sinusoidal pattern with a period of approximately 25min.Asinusoidal functioncos2t+1wasincorporatedtothe Michaelis–Mentenandzero-ordersecretionprocessesinModels4 and5.Giventherangeofvaluesofacosinefunctionisbetween−1 and1,thecosinefunctionwassquaredandtranslatedby1unitto avoidnegativeandzerovalues.Bothmodelsachievedsuccessful convergencebutmatrixsingularitywasencountered. Incorporat-ing thesinusoidal functiondidnot give anadvantage over the reducedmodels1and2,astheMOFVswereeitherthesameor increased.
ThebootstrapanalysesforbothModels1and2arereportedin
Table2.Themedianvaluesweresimilartothepopulation
param-eterestimatesoftheoriginaldataandthe95%confidenceinterval (CI)containedtheparameterestimatesforModel1.The param-etersoftheMichaelis–MentenactivesecretionprocessinModel 2weresmallerthanthebootstrapmedianandmean,suggesting thatthere maybemultiplelocalminimaor possibleparameter non-identifiabilityforVmsandKms.Wheninterindividual variabil-itywasintroducedtoeitherVmsorKms,modelconvergencewas achievedwithboundaryproblems.
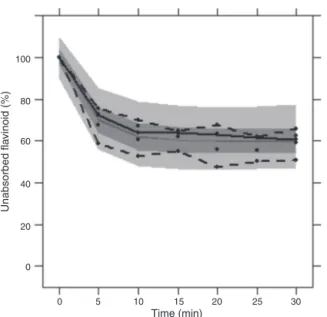
ThedegeneratevisualpredictivecheckinFig.3showedthatthe 2.5thand97.5thpercentilesofthesimulatedresultsfromModel1 containedtheindividualdataandtheobserveddata.These diag-nosticsindicatedthatthepopulationestimatesinthefinalmodel wereaccurateandstable.
Discussion
Predicted (%)
Observed (%) PRED vs. OBS
Time (h)
0 20 40 60 80 100 0
20 40 60 80 100
Indv
. Predicted (%)
Observed (%) IPRED vs. OBS
0 20 40 60 80 100 0
20 40 60 80 100
CWRES
CWRES vs. Time CWRES vs. PRED
0 5 10 15 20 25 30 –6
–4 –2 0 2 4 6
–6 –4 –2 0 2 4 6
CWRES
Predicted (%)
60 70 80 90 100
Fig.2. Goodness-of-fitplotforthefinalvicenin-2intestinalabsorptionpopulationmodel.IPRED,individualpredictedconcentration;OBS,observedconcentrations;PRED, modelpredictedconcentrations;CWRES,conditionalweightedresiduals.
Becausethecurrentstudyisperformedinliveanimals,theactive transportprocessisintact.Thedifferenceintheextentof absorp-tionbetweenthetwotechniquessupportedthenotionthatthe efflux pumps play an active role in limiting the absorption of vicenin-2,asvicenin-2canonlybeabsorbedthroughpassive trans-portacrossthemembraneintheBMCassay(Molero-Monfortetal.,
2001).TheBMCprediction methodwasbased onthechemical propertiesofvicenin-2andestimatedavalue70%largerthanwhat theinvivomodelhadfound.
Absorption studies of vicenin-2 in Caco-2 cells showed that vicenin-2 were not absorbed in the conditions that were tested (Gouvea et al., 2014). The possible explanationsfor the
Table2
StabilityofModels1and2usingnonparametricbootstrap.
ModelNo. 1 2
Mean(RSE%) Median(95%CI) Mean(RSE%) Median(95%CI)
Parameter
ka(min−1) 0.277(10.6) 0.279(0.217,0.327) 0.39(18.8) 0.388(0.285,0.563)
k0(%min−1) 16.4(9.0) 16.4(13.1,18.8)
Vms(%min−1) 35.5(32.1) 36.7(17.4,61.0)
Kms(%) 29.0(51.7) 31.2(0.98,52.3)
Interindividualvariability
%CVofka 7.7(26) 8.4(0.7,11.1) 4.0(0.45,9.3)
Residualvariability
Residualerror 0.00151(26) 0.00151(0.00074,0.00228) 0.00156(28.2) 0.00157(0.00074,0.00242)
MOFV 113.5(8.7) 114.9(83.0,126.4) 112.8(9.7) 114.2(83.0,127.8)
Time (min)
Unabsorbed fla
vinoid (%)
0 5 10 15 20 25 30 0
20 40 60 80 100
Fig.3. VPCplotforthefinalvicenin-2intestinalabsorptionpopulationmodel,where theobserveddataareincircles,themedianinsolidlineand2.5thand97.5th per-centilesofthepredictionindashedlines.Thedarkergrayshaderepresentsthe90% confidenceintervalofthemedianandthelightergrayshadesarethe90%confidence intervalsofthe2.5thand97.5thpercentiles.
discrepanciesbetweentheirstudyandthecurrentstudyarelow dosesandthesiteofabsorption.TheinvestigatorsusingCaco-2 cellstestedonlylowconcentrations at25 and50Mvicenin-2, whereasthepresentstudyusedadoseof180mgkg−1.Atthisdose, theconcentrationofdrugattheabsorptionsiteisapproximately 3.6mgml−1,whichisequivalentto6000M.Itislikelythatthe permeationprocessmayalsobedifferentinthecolonversusthat inthesmallintestine.Caco-2cellsarederivedfromcoloncancer cellswhereasmajorityofvicenin-2absorptionintheinvivomodel inthecurrentstudyislikelytohaveoccurredinthesmallintestine. Thesinglepassintestinalperfusiontechniqueillustratedthat therateofvicenin-2absorptionwasrapidbuttheextentof absorp-tionat 40%can beclassifiedas poorlyabsorbed. The collective informationpointstovicenin-2beingabsorbedbythesmall intes-tine butnot likely absorbedin thecolon. Thispharmacokinetic characteristicofvicenin-2suggeststhepotentialforthiscompound asa local anti-inflammatoryor anti-oxidativeagentfor intesti-nal diseases. This property may possibly explain how extracts fromL.ericoideswereeffectivebothasaprophylacticagentand treatmentforcoloncancerintheanimalmodel(Fernandesetal., 2011).Thedoseof90mgkg−1exhibitedefficacioussystemic anti-inflammatoryactivity(Gobbo-Netoetal.,2005)buthigherdose (180mgkg−1)wasusedin thisworkinorder toguarantee that theremainingconcentrationismeasurablebyHPLCassuminga rapidintestinalabsorptionofvicenin-2.Theresultsuggeststhat theextentofvicenin-2absorptioninthesmallintestinecouldbe sufficienttoelicitpharmacologicaleffectatthesiteofaction.The informationgeneratedfromthisstudycanbeusefulforguiding targetsandformulationsforthepotentialdevelopmentofnovel phytomedicinescontainingvicenin-2againstcoloncancer,Crohn’s diseaseandotherintestinalinflammatoryinjuries.However,more studiesarestillneededtoconfirmthishypothesis.
This study also evaluated the kinetics of vicenin-2 absorption using a first-order absorption and zero-order or Michaelis–Mententype secretionintotheintestinallumen.The Michaelis–Menten secretionprocess combined witheither first orderorMichaelis–Mentenorthecombinationofbothfor absorp-tionwaspreviouslyusedtodescribetheabsorptionbehaviorof ritonavirinrats,alsoassumingthatritonavirissubjecttoefflux transport(Munozetal.,2005).Theinvestigatorshaveshownthat
theapplicationoftwo Michaelis–Mentenfunctionsmayleadto instabilityandtheMichaelis–Mentenactivesecretionprocesscan becollapsedtoazero-order process.Theirresultscorroborated withourfindings.Ourstudyhasshownthatfurthermodel reduc-tiontoafirst-orderabsorptionandzero-ordersecretionprocesses canadequatelydescribetheabsorptionkineticsofvicenin-2.The differencesinthetwomodelingstrategywerethattheirstudywas conductedinfourdoseswhereasourstudyhadonlyonedoselevel, andtheirmodelswerefittedtothedrugconcentrationsreported inmicromolarunitwhereasthedatafromthepresentstudywere modeled onthepercent of thedosethat wasunabsorbed. The modelsutilizedinthisstudydescribedwelltheabsorptionprofiles ofvicenin-2intheinvivoratmodel.
Insummary,theabsorptionofvicenin-2iscomplexandlikely non-linear.Thisstudycharacterizedthegastro-intestinal absorp-tionkineticsofvicenin-2andhadshownthepotentialroleofactive secretionintothelumenofliveanimals.Furtherinvestigationof vicenin-2as anoral pharmacologicalagentissupportedby the findingsofthisstudy.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Authors’contributions
GABandMMSdevelopedtheanimalmodel.NPLwas responsi-bleforthecollectionofplantsampleaswellasidentificationand confirmationoftheherbalproduct.DRGisolatedthevicenin-2.SLN andEKdevelopedtheanalyticalmethodology.SKBSandHDwere responsiblefordataanalysisand thedevelopmentofthe math-ematicalmodels.ADandNPLdesignedthestudy,supervisedthe laboratoryworkandwrotethemanuscript.Alltheauthorsread thefinalmanuscriptandapprovedthesubmission.
Acknowledgements
This study was supported by a grant from the Fapesp and Fundac¸ãoAraucária(024/2007)andtheCAPES(BEX1525/06-9).
References
Barnes,S.,2004.Theimportanceofinvivometabolismofpolyphenolsandtheir biologicalactions.In:Meskin,M.,Bidlack,W.,Davies,A.,Lewis,D.,Randolph,R. (org.),Phytochemicals:MechanismsofAction.CRCombiPress,BocaRaton,pp. 51–59.
Buqui,G.A.,Gouvea,D.R.,Sy,S.K.,Voelkner,A.,Singh,R.S.,daSilva,D.B.,Kimura,E., Derendorf,H.,Lopes,N.P.,Diniz,A.,2015.Pharmacokineticevaluationof avicu-larinusingamodel-baseddevelopmentapproach.PlantaMed.81,373–381. Calixto,J.B.,2000.Efficacy,safety,qualitycontrol,marketingandregulatory
guide-linesforherbalmedicines(phytotherapeuticagents).Bras.J.Med.Biol.Res.33, 179–189.
Diniz,A.,Escuder-Gilabert,L.,Lopes,N.P.,Gobbo-Neto,L.,Villanueva-Camanas,R.M., Sagrado,S.,Medina-Hernandez,M.J.,2007.Permeabilityprofileestimationof flavonoidsandotherphenoliccompoundsbybiopartitioningmicellarcapillary chromatography.J.Agric.Food.Chem.55,8372–8379.
Fagerholm,U.,Johansson,M.,Lennernas,H.,1996.Comparisonbetween permeabil-itycoefficientsinratandhumanjejunum.Pharm.Res.13,1336–1342. Fernandes,C.R.,Turatti,A.,Gouvea,D.R.,Gobbo-Neto,L.,Diniz,A.,Ribeiro-Silva,
A.,Lopes,N.P.,Garcia,S.B.,2011.TheprotectiveroleofLychnophoraericoides Mart.(Brazilianarnica)in1,2-dimethylhydrazine-inducedexperimentalcolon carcinogenesis.Nutr.Cancer63,593–599.
Gee,J.M.,DuPont,M.S.,Day,A.J.,Plumb,G.W.,Williamson,G.,Johnson,I.T.,2000. Intestinaltransportofquercetinglycosidesinratsinvolvesbothdeglycosylation andinteractionwiththehexosetransportpathway.J.Nutr.130,2765–2771. Gobbo-Neto,L.,Santos,M.D.,Kanashiro,A.,Almeida,M.C.,Lucisano-Valim,Y.M.,
Lopes,J.L.,Souza,G.E.,Lopes,N.P.,2005.Evaluationoftheanti-inflammatoryand antioxidantactivitiesofdi-C-glucoflavonesfromLychnophoraericoides (Aster-aceae).PlantaMed.71,3–6.
Lennernas,H.,Nylander,S.,Ungell,A.L.,1997.Jejunalpermeability:acomparison betweentheusingchambertechniqueandthesingle-passperfusioninhumans. Pharm.Res.14,667–671.
Li,Y.,Paxton,J.W.,2013.TheeffectsofflavonoidsontheABCtransporters: conse-quencesforthepharmacokineticsofsubstratedrugs.Expert.Opin.DrugMetab. Toxicol.9,267–285.
Martin-Villodre,A.,Pla-Delfina,J.M.,Moreno,J.,Perez-Buendia,D.,Miralles,J., Col-lado,E.F.,Sanchez-Moyano,E.,delPozo,A.,1986.Studiesonthereliabilityofa bihyperbolicfunctionalabsorptionmodel.I.Ring-substitutedanilines.J. Phar-macokinet.Biopharm.14,615–633.
Molero-Monfort,M.,Escuder-Gilabert,L.,Villanueva-Camanas,R.M.,Sagrado,S., Medina-Hernandez,M.J.,2001.Biopartitioningmicellarchromatography:an invitrotechniqueforpredictinghumandrugabsorption.J.Chromatogr.B: Biomed.Sci.Appl.753,225–236.
Munoz,M.J.,Merino-Sanjuan,M.,Lledo-Garcia,R.,Casabo,V.G.,Manez-Castillejo, F.J.,Nacher,A.,2005.Useofnonlinearmixedeffectsmodelingfortheintestinal absorptiondata:applicationtoritonavirintherat.Eur.J.Pharm.Biopharm.61, 20–26.
Ruiz-Balaguer,N.,Nacher,A.,Casabo,V.G.,MerinoSanjuan,M.,2002.Intestinal transportofcefuroximeaxetilinrats:absorptionandhydrolysisprocesses.Int. J.Pharm.234,101–111.
Sy,S.K.,Derendorf,H.,2014.Pharmacometricsinbacterialinfections.In:Schmidt, S.,Derendorf,H.(org.),AppliedPharmacometrics,1sted.Springer,NewYork, pp.229–258.
Sy,S.K.,Singh,R.P.,Shilbayeh,S.,Zmeili,R.,Conrado,D.,Derendorf,H.,2013. Influ-enceofCYP3A56986A>GandABCB13435C>Tpolymorphismsonadverse events associated with tacrolimus in Jordanian pediatric renal transplant patients.Clin.Pharmacol.Drug.Dev.2,67–78.
Sy,S.K.,Wang, X., Derendorf,H., 2014.Introductionto pharmacometrics and quantitativepharmacologywithanemphasisonphysiologicallybased phar-macokinetics.In:Derendorf,H.,Schmidt,S.(org.),AppliedPharmacometrics. Springer,NewYork,pp.1–64.