w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Zeaxanthin
from
Porphyridium
purpureum
induces
apoptosis
in
human
melanoma
cells
expressing
the
oncogenic
BRAF
V600E
mutation
and
sensitizes
them
to
the
BRAF
inhibitor
vemurafenib
Camille
Juin
a,
Raimundo
Gonc¸
alves
de
Oliveira
Junior
a,c,
Audrey
Fleury
a,
Chloé
Oudinet
a,
Lior
Pytowski
a,
Jean-Baptiste
Bérard
b,
Elodie
Nicolau
b,
Valérie
Thiéry
a,
Isabelle
Lanneluc
a,
Laureen
Beaugeard
a,
Grégoire
Prunier
a,
Jackson
Roberto
Guedes
Da
Silva
Almeida
c,
Laurent
Picot
a,∗aLIttoralENvironnementEtSociétésUMR7266,CentreNationaldelaRechercheScientifique,UniversityofLaRochelle,LaRochelle,France
bLaboratoireBiotechnologiesetRessourcesMarines/LaboratoirePhysiologieetBiotechnologiedesAlgues,InstitutFranc¸aisdeRecherchepourl’ExploitationdelaMer,Nantes,France cCenterforStudiesandResearchonMedicinalPlants,FederalUniversityofSanFranciscoValley,Petrolina,PE,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received16January2018 Accepted30May2018 Availableonline15June2018
Keywords: Cancer Carotenoid Melanoma Microalgae Phytoplankton Zeaxanthin
a
b
s
t
r
a
c
t
Zeaxanthin,anabundantcarotenoidpresentinfruits,vegetablesandalgaewasreportedtoexert antipro-liferativeactivityandinduceapoptosisinhumanuvealmelanomacells.Italsoinhibiteduvealmelanoma tumorgrowthandcellmigrationinnudemicexenograftmodels.Herewereportthatzeaxanthin puri-fiedfromtherhodophytePorphyridiumpurpureum(Bory)K.M.Drew&R.Ross,Porphyridiaceae,promotes apoptosisintheA2058humanmelanomacelllineexpressingtheoncogenicBRAFV600Emutation. Zeax-anthin40M(IC50)inducedchromatincondensation,nuclearblebbing,hypodiploidy,accumulationof cellsinsub-G1phase,DNAinternucleosomalfragmentationandactivationofcaspase-3.Westernblot analysisrevealedthatzeaxanthininducedup-regulationofthepro-apoptoticfactorsBimandBidand inhibitionofNF-Btransactivation.Additionally,zeaxanthinsensitizedA2058melanomacellsinvitro tothecytotoxicactivityofvemurafenib,aBRAFinhibitorwidelyusedfortheclinicalmanagementof melanoma,suggestingitspotentialinterestasdietaryadjuvantincreasingmelanomacellssensitivityto chemotherapy.
©2018SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Melanomas accountfor lessthan 2%of skincancersbut are
responsible for 80% of theirmortality (Armstrong and Kricker,
2001;Grange,2005;LeiterandGarbe,2008;MacKieetal.,2009).
Melanomacellsarecharacterizedbymutationsthatconferthem
a strong resistance to anticancer drugs-induced apoptosis and
selectiveadvantagesforcellsurvival,proliferationandmetastasis
(Locatellietal.,2013).Particularly,mutationsintheBRAF
onco-genarefoundin70%ofmalignantmelanoma(Daviesetal.,2002;
Haluskaetal.,2006;Dankortetal.,2009;Dutton-Regesteretal.,
2012;JangandAtkins, 2014)and leadtoover-activationofthe
MAPkinasepathwaythatstimulatescellproliferation.Most
anti-cancerdrugsonlydelaytheearlygrowthofmelanomatumorsbut
failtoprovidealong-termcurebecauseoftherapidacquisition
ofdrugresistance(Locatelliet al.,2013;Spagnoloet al.,2014).
∗ Correspondingauthor.
E-mail:laurent.picot@univ-lr.fr(L.Picot).
Additionally,melanomacellsdisplaypronounced
neoangiogene-sis and a highability toescape immune cell that explain why
the5-yearsurvivalrateformetastaticmelanomarangesfrom5
to10%,withamediansurvivaloflessthan8months(Marneros,
2009;Mathieuetal.,2012).Brainmetastasisarepresentin75%
ofadvancedmelanomapatientsandconstituteamajorcauseof
mortalitybecauseofthelowpermeabilityoftheblood-brain
bar-riertochemotherapeuticdrugs(Halletal.,2000).Thesearchfor
cytostatic,antimetastaticandantiangiogenicmoleculesinplants
and algaehas establishedthat carotenoidshave a great
poten-tialasnaturalantimelanomacompounds(Hashimotoetal.,2011;
Tanakaetal.,2012;Firdousetal.,2010;Gagezetal.,2012;Baudelet
etal.,2013; Reboul,2013;Chungetal.,2013; Kimetal.,2013;
Xuetal.,2015;Luetal.,2015;Chenetal.,2017).Thesepigments
havenooraltoxicity,areresorbedbyenterocytes,transportedin
bloodafterperosconsumption(Burrietal.,2001;Hashimotoetal.,
2011;Reboul,2013)and canintegratecellmembranes(Reboul,
2013;Oliveira-Junioretal.,2016)andreachtumorcellswherethey
exertcytotoxic,cytostatic,antimetastatic,anti-inflammatoryand
antiangiogenicactivities(Sugawaraetal.,2006;Gagezetal.,2012).
https://doi.org/10.1016/j.bjp.2018.05.009
© Raymond Kaas. IFREMER.
4 µm
Fig.1. PorphyridiumpurpureumstrainCCAP1380.3.©RaymondKaas.IFREMER.
Fucoxanthin,acarotenoidpresentinbrownmicroalgaeand
sea-weedsinhibitsmelanomacellsandtumorgrowthinvitroandinvivo
(Chungetal.,2013;Kimetal.,2013).Italsolimitsmelanoma
metas-tasisinmurinemodels(Chungetal.,2013;Kumaretal.,2013),
suggestingthatitalsohasaclinicalefficacyinhumans.Werecently
demonstratedthatzeaxanthin(1),anabundantcarotenoidfoundin
variousdietarysources(corn,spinach,saffron,seaweeds,
microal-gae) inhibitsthe in vitrogrowth of the highly invasivehuman
melanomacelllineA2058(Baudeletetal.,2013).Zeaxanthinalso
inducedapoptosisintwohumanuvealmelanomacelllines(SP6.5
andC918)withoutimpairingthecellviabilityofnoncanceruveal
melanocytes(Bietal.,2013;Xuetal.,2015).Zeaxanthin-induced
apoptosiswasassociated toa decreaseintheexpressionofthe
antiapoptoticproteins Bcl-2 and Bcl-xL and an increase in the
expressionoftheproapoptoticproteinsBakandBax(Bietal.,2013).
Zeaxanthinalsoevokedthereleaseofmitochondrialcytochromec
inthecytosolandcaspase-9and-3activation(Bietal.,2013).In
thepresentreport,weperformedadditionalexperimentstofurther
elucidatethemolecularmechanismsofzeaxanthinpro-aptoptotic
activityin melanoma cells and assessed its ability to sensitize
melanomacellstoaBRAFinhibitorusedtotreatclinicalmelanoma.
WeselectedthehighlyinvasiveA2058humanmelanomacellline
asarelevantclinicalmodelexpressingtheV600EBRAFoncogenic
mutation(Dutton-Regesteretal.,2012).
Materialandmethods
Microalgaeculture,harvestandfreeze-drying
Porphyridium purpureum (Bory) K.M.Drew & R.Ross CCAP
1380.3 (bangiophyceae, rhodophyte) (Fig. 1) was grown at
120molm−2s−1irradiance.Cellsweregrowninfourunitsof
50-lcolumn photobioreactorswith35‰ salinityseawaterenriched
byWalnemedium(Walne,1966;Juinetal.,2015).Batchcultures
weremaintainedat20◦Cundercontinuouslightprovidedby
flu-orescentlamps(PhilipsTLD58W865)andbubbledwith0.22m
filteredaircontaining3%CO2(v/v).Microalgaewereharvestedafter
12–16daysofgrowthandseparatedfromtheculturemediumbya
two-stepprocess.Firststepusedaclarifierseparator(Clara20,Alfa
LavalCorporateAB,Sweden)at100lh−1,9000
×g,atroom
tem-perature.Steptwousedasoftcentrifugationat4000×g,20mn,at
4◦Ctoseparatetheslurry.Algalpastewasfreeze-driedat−55◦C
andP<1hPa,onafreeze-dryerequippedwithaHetoLyoPro3000
condenserandaHetocoolingtrap(Thermo,France).
PurificationandcharacterizationofPorphyridiumpurpureum zeaxanthin
Porphyridiumpurpureumpigmentswereextractedin ethanol
using a mixer mill extraction process developed in our group
(Seriveetal.,2012).Zeaxanthin(1)identificationwasconfirmed
afterseparationbyanalytical RP-HPLC(Zapataetal.,2000)and
cross-checkanalysisofitspolarity,absorptionspectrum,maximal
absorptionwavelengths,bandIII/IIratioandfragmentation
pro-fileinUPLC-MSE(Royetal.,2011;Baudeletetal.,2013;Juinetal.,
2015),in comparisonwithstandardzeaxanthin (Sigma–Aldrich,
France). Purezeaxanthin wasthencollectedbypreparative
RP-HPLC (Pasquet et al., 2011)in glass vials, dried under reduced
pressure in a Buchi R-210 rotatory evaporatorat 40◦C (Buchi,
France)andstoredat−80◦Cbeforeuseincellcultureexperiments.
Cellculture
A2058(ATCC® CRL-11147TM,LGC ATCCStandards,France) is
amelanomacelllineestablishedfrommetastaticcellsremoved
fromthelymphnodeofa43yearsoldmalecaucasianpatient.It
constitutesaclinicallyrelevantmodeltoassessthecytotoxicityof
newantimelanomadrugsasitcombineshighinvasive,metastatic
andchemoresistancepotentialswithagenemutationprofileoften
encounteredinhumanmelanomas(V600EmutationinBRAFand
mutationsinthePTENandP53genes)(Dankortetal.,2009).Cells
wereroutinelygrownasmonolayers,at37◦Cina5%CO2–95%air
humidifiedatmosphere,inDMEM(Fischerscientific,France)
sup-plementedwith10%heat-inactivated(56◦C,30min)FCS(Dutscher,
France)towhichwereaddedpenicillin100Uml−1 and
strepto-mycin100gml−1.
DeterminationofzeaxanthinIC50inA2058melanomacells
Purifiedzeaxanthin(1)wassolubilizedinDMSOat6mM(stock
solution)anddilutedincellculturemediumtoobtain5–60M
solutions.ThefinalDMSOconcentrationinthecellculturemedium
waslower than 1%,tested as a negative control and validated
asanoncytotoxicconcentration.Theantiproliferativeactivityof
zeaxanthinwasdeterminedusingtheMTTassay(Sigma–Aldrich,
France)aspreviouslydescribed(Puteyetal.,2007;Baudeletetal.,
2013;Hedidietal.,2016).IC50wasdeterminedusingthefreeGraph
padPrismsoftwareusingthe“sigmoidaldoseresponse”(variable
slope)function.
Nuclearmembranemodification,chromatincondensationand DNAfragmentation
Sub-confluent A2058 melanoma cells were trypsinized and
2×105cellswereseededin6-wellplates,inafinalvolumeof3mlof
controlmediumorculturemediumcontainingzeaxanthin40M
(IC50)orstaurosporine2M.Cellsweregrownfor72hat37◦Cand
washedinPBS0.1MpH7.4,beforebeingfixedwithformaldehyde
3%for30minat37◦C.CellswerethenrinsedinPBS,permeabilized
withTritonX-1001%inPBSandstainedwithDAPI2gml−1for
1hat37◦C.Cellswererinsed,mountedonglassmicroscopeslides
andobservedusingaLeicaepifluorescencemicroscopeequipped
withanepifluorescenceAfilterblock(excitation340–360nm)and
AnnexinV-Cy3and6-CFDAdetectionassay
ApoptosiswasevaluatedbyusingdoublestainingwithAnnexin
V-Cy3(red) and6-carboxyfluoresceindiacetate (6-CFDA,green)
(Sigma–Aldrich®,France).Cells(5×103cells/well)wereincubated
inconventionalcultureconditionsfor24h.Then,cellsweretreated
withzeaxanthin (IC50,40M) for72handstaurosporine
(posi-tivecontrol,1M)for24h.CellswerefurtherwashedwithPBS,
suspendedinbindingbufferandstainedwithAnnexinVand
6-CFDAsolutionfor10min.DAPIsolutionwasalsoaddedtothewells
forDNAlabelling.Finally,cellswereobservedunderfluorescence
microscope(ZEISSAxioObserver).
Flowcytometricdetectionofapoptoticcells
Melanoma cells were grown in control culture medium or
treatedwithzeaxanthin40Mfor72hbeforebeingstainedfor
30minat37◦CinPBScontainingpropidiumiodide(PI100gml−1)
andRnaseA(100gml−1)(MolecularProbes,France).Cellswere
washedandsuspendedin1mlPBSbeforebeinganalyzedusing
aFACSCantollfluxcytometer(BDBiosciences,France)equipped
withanaircooledblueLASER(=488nm,20mW).Lightdiffusion
parameters(forwardandlateralscatterlights)wereoptimizedto
definethesizethresholdexcludingcellulardebrisandcellclusters
forsingle-cellfluorescenceanalysis.PIfluorescencewasmeasured
usingaFL3filter(—— 670—— nm)andanalyzedusingtheBDFACSDiva
Software(BDBiosciences,France).Distributionofmelanomacells
inthedifferentcellcyclephasesandhypodiploidywasdetermined
accordingtotheirDNAcontentasmeasuredbythefluorescence
intensityofPI:Diploidcells(2n):G0/G1phase;Replicativecells
(2n<DNAcontent<4n):SPhase;Tetraploidcells(4n):G2/Mphase;
hypodiploidcells(DNAcontent<2n):apoptoticSub-G1Phase.
DNAinternucleosomalfragmentation
Aftertreatment withcontrol culture medium or zeaxanthin
40M,106 melanomacellswerewashedwithPBSandlysedin
400l lysis buffer (Tris–HCl 10mM pH 8 NaCl 150mM, EDTA
40mM,SDS1%,proteinaseK0.2mgml−1)for3hat56◦C.Thecell
lysatewascentrifuged(11,000×g,15min,4◦C)andtheDNA
con-tainedinthesupernatantwasextractedfor15minusingamixof
phenol/chloroform/isoamylalcohol(PCI)(25/24/1,v/v/v)atpH9.
Themixwascentrifuged(11,000×g,15min,4◦C),andtheaqueous
phasewascollectedtoprecipitateDNAusingsodiumacetate3M
in1mlabsoluteethanol(1night,−20◦C).TheprecipitatedDNA
wascentrifugated(11,000×g,30min,4◦C),thesupernatantwas
discardedandthepelletwasdried5minat60◦C.TheDNA
pel-letwassuspended in50lUltrapure watercontainingRNaseA
100gml−1 for 30minat37◦C. TwentymicrolitersoftheDNA
extractwereloadedandseparatedonanagarose/tris-borate-EDTA
1%gelfor30minat100V.Gelswerestainedwithethidium
bro-mide,andobservedusingaUVtransilluminator.
Caspase-3colorimetricassay
Caspase-3 activation was quantified using a commercial
assay based on the hydrolysis of Ac-DEVD-pNA (CASP3C kit,
Sigma–Aldrich,France).
Western-blot
A2058cells wereincubatedincontrolculturemedium orin
thepresenceofzeaxanthin(1)40Mfor72h.Thecellswere
col-lectedand lysedin a lysis buffer(HEPES 50mM pH7.4 CHAPS
5mMDTT5mM).Totalproteinswereseparatedby10%SDS-PAGE
and then transferred to a nitrocellulose membrane. The
mem-braneswereblockedwith5%(w/v)nonfatdrymilkinTBSfor1h
andchangedtoanappropriatedilutionofspecificprimary
anti-bodies against Bid,Bim,Bak,Bcl-xL, IB␣,NF-Bp65,p-NF-B
(Ser536),IKK␣,IKKand-actin(Ozyme,France)inmilkovernight
at4◦C.Thesecondaryantibodieswerehorseraddish
peroxidase-conjugatedanti-rabbitIgG(1:5000).Signalsweredetectedusing
theChemiDocTMimagingsystem(Biorad,France).
SensitizationofA2058melanomacellstotheBRAFinhibitor Vemurafenib
VemurafenibwasobtainedfromSelleckchem,France,diluted
toa10mMstocksolutioninPBS0.1MpH7.4,beforefurther
dilu-tionincellculturemedium.A2058cellswereincubatedfor72h
incontrolculturemediumorinthepresenceofzeaxanthin40M,
Vemurafenib(0.1,1and5M)orinamixofzeaxanthin40Mand
Vemurafenib(0.1,1and5M).Antiproliferativeactivitywas
cal-culatedusingtheMTTassayandpotentiationoftheVemurafenib
antiproliferativeactivitybyzeaxanthinwasexpressedasthe
per-centageofgrowthinhibitionincreaseascomparedtoVemurafenib
alone.
Statistics
Antiproliferativeactivityofzeaxanthinwasexpressedas
per-centagegrowthinhibition±SEMfromthreeindependentassays.
The normal distribution of absorbance data in control and
treated cells was demonstrated using the Hartley’s Fmax test
to confirm homogeneity of absorbances variances. The
statis-tical significance of proliferation differences between control
and treated cells was then investigated by an unpaired
Stu-dent’s t test, using a free online calculator developed by
Institut Pierre Louis d’Epidémiologie et de Santé publique
UMR S 1136 INSERM University Pierre et Marie Curie Paris
(http://marne.u707.jussieu.fr/biostatgv/?module=tests).
Results
PurificationofzeaxanthinfromPorphyridiumpurpureum
RP-HPLCoftheethanolicextractofP.purpureumgavea
chro-matogram containingeightmajor peaksat436nm(Fig.2).The
firstpigmentelutingasamajorpeakat24.582minwasidentified
aszeaxanthin(1)asitpresentedamedianpolaritywithmaximal
absorptionwavelengthsat452and481nm(Fig.3),aIII:IIbandratio
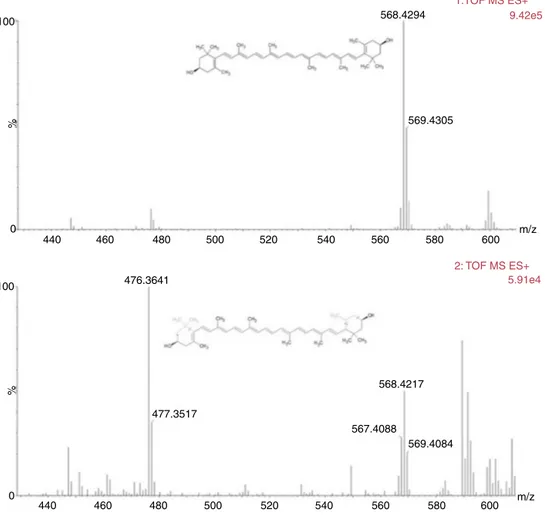
of29.03%(Fig.3),ahighresolutionmolecularweightof568.4294
(Fig.4)andaMSEfragmentationpatterncharacteristicof
zeaxan-thin(Fig.4)(Juinetal.,2015).
ExposuretozeaxanthininhibitstheproliferationofA2058 melanomacells
A 72h exposure to increasing concentrations of zeaxanthin
[0–60M]inducedadose-dependentreductioninthenumberof
A2058cellsascomparedtotheuntreatedcontrol,reaching60%
growthinhibition.TheIC50valueofzeaxanthinwasdeterminedas
40MusingthefreeGraphpadPrismsoftware“sigmoidaldose
response”(variableslope)function(Fig.5).
Exposuretozeaxanthinevokedcytotoxicityandnuclear fragmentationinA2058cells
A2058 cells incubated in control medium showed a regular
epithelialshape,exceptformitoticcellsexhibitingaroundshape
mAU
300 400
200
100
0
5 10 15 20 25 30 35
36.925
31.845
33.143
26.584
24.582
32.622
25.607
31.453
min Zeaxanthin Chloroph
yll
α
Chloroph
yll
α
allomers
Chloroph
yll
α
epimer
β,β
-carotene
Fig.2. RP-HPLCChromatogramofPorphyridiumpurpureumethanolextractat436nmobtainedusingtheVanHeukelemandThomasanalysis(VanHeukelemandThomas, 2001).ThepigmentprofilewasidenticaltothatpreviouslyreportedinSeriveetal.(2017).
Norm
80
60
40
20
0
300 400 452 481500 600
Band II
Band III
III:II band ratio=29.03%
700 nm
100
Fig.3.Identificationofthepigmentelutingat24.582minaszeaxanthin(1)basedonabsorptionspectrum,maximalabsorbancewavelengthsandIII:IIbandratio.
controlcellswasroundandshowednosignofDNAcondensation,
blebbingorshrinkage(Fig.6B).Thetreatmentwithstaurosporine
2M(nonselectivekinasesinhibitor,controlapoptosisinducer)
evokedahighcytotoxicityevidencedbycellshrinkage(Fig.6C)and
DNAcondensationinthenucleus(Fig.6D).A72htreatmentwith
zeaxanthin40Mhalfloweredthecelldensity,evokedrounding
ofthecells(Fig.6E)andnuclearfragmentation(Fig.6F),suggesting
ablockadeinthecellcycleandapoptosisinduction.
ZeaxanthininducesapoptosisinA2058cells
Toevaluatetheeffectofzeaxanthinoncelldeath,double
flu-orescencestainingwithAnnexinVand6-CFDAwasperformedto
differentiateliveandapoptoticcells.6-CFDAisusedtomeasure
viability.Inthissense,livecellswillonlystainwith6-CF(green),
cellsinearlyapoptosiswillstainbothwithAnnexinV(red)and
6-CF(green),andcellsinlateapoptosiswillonlystainwithAnnexin
VandDAPI.After72hoftreatment,zeaxanthin40Mincreased
thenumber ofAnnexinVand 6-CFDAdouble-stainedcells, and
AnnexinVandDAPIstainedcellscomparedtocontrol,indicating
enhancementofapoptosis(Fig.7).Asimilarresultwasobserved
forstaurosporine(1M),aknownpro-apoptoticagent.
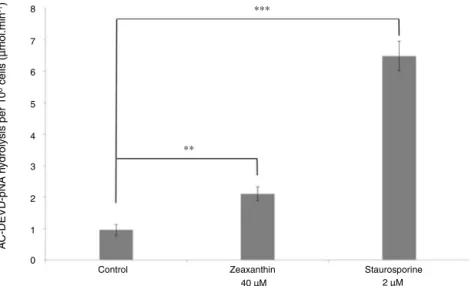
Exposuretozeaxanthinevokedcaspase-3activation,
internucleosomalfragmentationandhypopolyploidyinA2058 cells
Activationofcaspase-3isconsideredasacentraleventfor
inte-gratingpro-apoptoticstimuliandactivatingdownstreameffector
caspasesandDNAsesduringcancercellapoptosis.Thebasal
activ-ityofcaspase-3waslowinA2058cellsgrownfor72hincontrol
cellculturemedium (Fig.8).Treatmentwithzeaxanthin 40M
for72hinducedaverysignificantincreaseincaspase-3activity
(Studentttest,p<0.01),asdemonstratedbyhydrolysisofthe
spe-cificchromogenicsubstrateAc-DEVD-pNA(Fig.8).Treatmentwith
staurosporine2m,usedasapositivecontrolforcaspase-3
acti-vation,inducedahighlysignificantincreaseincaspase-3activity
(Studentttest,p<0.001).
Asone of the main consequences of caspase-3 activation is
thesubsequentDNAfragmentationbycaspase-activatedDNases,
100
100 476.3641
477.3517
568.4217
567.4088
569.4084 569.4305 568.4294
1:TOF MS ES+ 9.42e5
5.91e4 2: TOF MS ES+
0
0 440
440
480
480
500
500
520
520
540
540
560
560
580
580
600
600 m/z
m/z 460
460
%
%
Fig.4. MSEfragmentationpatternofzeaxanthin(1)isolatedfromPorphyridiumpurpureum.
60
40
20
0
0 20 40 60
Zeaxanthin concentration (µM)
(% control,2000 cells g
ro
wn f
or 72h)
Gro
wth inhibtion of A2058 melanoma cells
Fig.5. GrowthinhibitionofA2058cellinthepresenceofzeaxanthin(1).A2058 cellsweregrownfor72hinacellculturemediumcontainingincreasing concen-trationsofzeaxanthin.Theantiproliferativeactivityofzeaxanthinwasobservedfor concentrationssuperiorto5MandIC50wasdeterminedas40M.
fragmentationinA2058cellstreatedwithzeaxanthin.Evaluationof
cellcycleprogressionbyflowcytometryrevealedtheappearance
ofasub-G1cellpopulationafterexposuretozeaxanthin40M,
characteristicofdyingcells(Fig.9).Quantificationusingtheflux
cytometersoftwareindicatedthat2.9±0.4%ofcontrolcellswerein
sub-G1phaseincomparisonwith23.6±3.7%inzeaxanthin-treated
cells.
Agarose gel electrophoresis confirmed the internucleosomal
fragmentation ofDNA extracted from A2058cells treated with
zeaxanthin40Morstaurosporine2M(Fig.10),demonstrating
theactivationofcaspase-activatedDNasebyzeaxanthin.
Zeaxanthinstimulatestheexpressionofthepro-apoptoticfactors BimandBidandinhibitsthenucleartranslocationofNF-Bin A2058melanomacells
It was previously reported that zeaxanthin decreased the
expression of antiapoptotic proteins (Bcl-2 and Bcl-xL) and
increasedtheexpressionofproapoptoticproteins(BakandBax)
in zeaxanthin-treated uveal melanoma cells (Bi et al., 2013).
To complete the identification of signaling pathways involved
in zeaxanthin-induced apoptosis of melanoma cells (Bi et al.,
2013), theexpression of pro-apoptotic,anti-apoptotic and
pro-inflammatoryfactorswereinvestigatedbywestern-blotanalysis.
Zeaxanthin 40M respectively induced a high and moderate
expression increase of the pro-apoptotic factors Bim and Bid
(Fig.11)thatcouldbeinvolvedintheonsetofapoptosis.The
expres-sionofthepro-apoptoticfactorBakwasunchanged.Thelevelof
phosphorylatedNF-Bp65(Ser536)wasdecreasedwhileIB␣and
Ikk␣wereup-regulated,indicatingthatzeaxanthininhibitedthe
nucleartranslocationofNF-Bandsubsequentlydown-regulated
theexpressionofpro-inflammatorygenes.Ikkand
unphosphory-latedNF-kBexpressionswereunchanged(Fig.11).
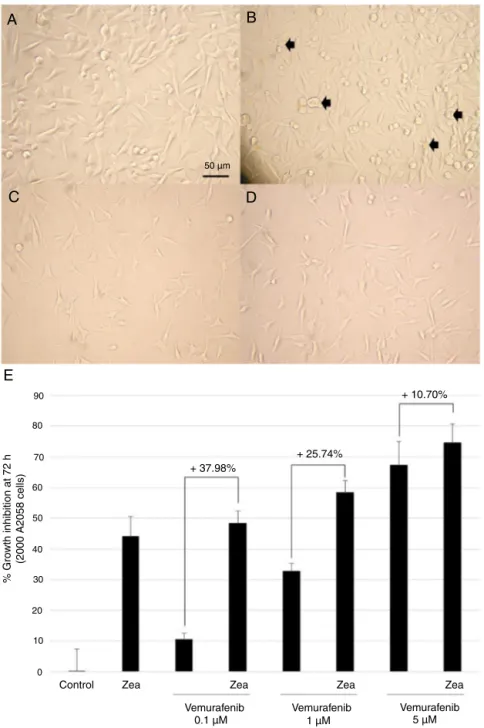
Zeaxanthinpotentiatestheantiproliferativeactivityof VemurafenibinA2058melanomacells
Toassessthecapacityofzeaxanthintopotentiatethegrowth
A
B
D
F
20 µm 20 µm 20 µm
E
C
100 µM
100 µM
100 µM
Fig.6.CytotoxicityandnuclearfragmentationinA2058cellsexposedtozeaxanthin40M.A2058cellsweregrownfor72hinacontrolcellculturemedium(AandB)orin amediumcontainingstaurosporine2M(CandD)orzeaxanthin40M(EandF).Treatmentwithstaurosporinevokedcellroundingandchromatincondensation(Cand D)whilezeaxanthinevokedcellrounding(E),chromatincondensationandnuclearfragmentation(F).
6-CFDA
Staurospor
ine
z
eaxanthin
Control (A2058)
Annexin V DAPI Merge
Fig.7.ZeaxanthininducesapoptosisofA2058melanomacells.AnnexinV(red)and 6-CFDA(green)doublestainingofapoptoticcellswasexaminedbyfluorescence microscopy.A2058cellsinearlyapoptosisshowedbothgreenandredstains;A2058 cellsinlateapoptosisshowedredandbluestains;andcontrol(untreated)cells stainedgreenonly.Cellstreatedwithzeaxanthin(40M,for72h)orStaurosporine (1M,for24h)wereconsideredinearlyorlateapoptosiscomparedtocontrol.Scale bar:50m.
clinicaltreatmentofmetastaticmelanoma,A2058melanomacells
weretreatedfor72hwithzeaxanthin40M,vemurafenib5Mor
thecombinationofbothmolecules.ControlA2058cellsexhibited
aregularepithelialmorphologyandbecamesub-confluentin72h
(Fig.12A),withahighproportionofmitoticcellsindicatingahigh
proliferationrate.Zeaxanthin40Minduceda reductionincell
density,cellshrinkage,DNAcondensationevidencedbythe
obser-vationofnucleargranulationsand appearanceofapoptoticcells
(blackarrows)(Fig.12B).Vemurafenib5Mhadadrasticeffect
onA2058cellproliferationandmorphology,evidencedbyalow
celldensity,cellbodyshrinkage,cellthinningandDNA
conden-sationwiththepresenceof nucleargranulations(Fig.12C).The
morphologyofcellstreatedwiththecombinationofzeaxanthin
and vemurafenibwas similarto thatobserved withthe
vemu-rafenibtreatmentalone (Fig.12D). As compared tothecontrol
cellculturemedium,zeaxanthin40Minduced44.1±6.4%growth
inhibition, vemurafenib 0.1M10.40±2.12, 1M 32.74±2.71,
and5M67.3±7.7%growthinhibitionand thecombination of
both48.38±3.98,58.48±3.80and74.5±6.2%growthinhibition,
respectively.Thuszeaxanthin40Minduceda37.98,25.75and
10.7%increaseoftheantiproliferativeactivityofvemurafenib0.1,
1and5M,respectively(Fig.12E).Thisvaluewasintherangeof
calculatedstandarddeviationsofantiproliferativeactivities.
Discussion
Advancedmelanomahaveabadprognosisasmostmolecules
usedincancerchemotherapyareineffectiveinkillingmetastatic
melanomacellswhichareconstitutivelyoradaptativelyresistant
to pro-apoptotic drugs. The developmentof targeted therapies
usingBRAFinhibitorshassignificantlyimprovedthetreatmentof
metastaticmelanomasastheV600EBRAFoncogenicmutationis
foundinmorethan70%ofclinicalcases.Howevermostpatients
eventuallydevelopresistancemechanismsthatultimatelyleadto
therapeuticimpasses. In this view,many researchprojectsaim
toidentifynaturalmoleculeswithcytostatic,antimetastaticand
8
7
5 6
4
3
2
1
∗∗
∗∗∗
0
Control Zeaxanthin Staurosporine
40 µM 2 µM
A
C-DEVD-pNA h
ydrolysis per 10
6 cells (
µ
mol.min
-1)
Fig.8. Zeaxanthininducescaspase-3activationinA2058melanomacells.A2058cellsweregrownfor72hinacontrolcellculturemediumorinamediumcontaining zeaxanthin40Morstaurosporine2M(positivecontrolforapoptosisinduction).
900
700
800
600
500
400
300
200
100
102 103 104 105
0
Control cell culture medium
Zeaxanthin 40 µM
IP fluorescence
900
700
800
600
500
400
300
200
100
102 103 104 105
0
Number of A2058 cells
Sub-G1
Sub-G1
G0
G0 G1 S
G1 S G2/M
G2/M
Fig.9.CellcycleanalysisofA2058cellsgrownfor72hincontrolcellculturemedium(A)orcellculturemediumcontainingzeaxanthin40M(B).Themajorityofcontrolor treatedcellswereinthequiescence(G0)orpre-replicative(G1)phases.Zeaxanthininducedtheappearanceofasub-G1peak,characteristicofhypodiploidiccellsundergoing celldeaths.
andcouldbeusedtopotentiatetheefficiencyofchemotherapy
andimmunotherapyandslowtheemergenceofresistance
mech-anisms(Craggetal., 1997;Caltagirone etal.,2000; Nilesetal.,
2003;Mesquitaetal.,2009;VanGoietsenovenetal.,2010;Pasquet
etal.,2011;Ahmadetal.,2013;Zhangetal.,2014;Alqathamaand
Prieto,2015;Mirzaeietal.,2016).Manycarotenoidsmeetallthese
activities,astheydisplayhighcytotoxicityintumorcellsfrom
var-ioushistologicalorigins,includingchemoresistantmelanomacell
lines,andexertsignificantantitumoralactivityinvivoby
10000 pb
MW - Zea stau
3000 pb
1000 pb
500 pb
Fig.10.AgarosegelelectrophoresisofDNAextractedfromA2058cellsincubatedfor 72hintheabsence(−)orpresenceofzeaxanthin40M(zea)orstaurosporine2M (stau).Observationofasmearinthezeaxanthinandstaurosporinelanesrevealed theactivationofcaspase-activatedDNasesandinternucleosomalfragmentationof DNAinapoptoticcells.
Kumaretal.,2013;Chenetal.,2017).Moreover,mostcarotenoids
havenooraltoxicityanddonotweakentheimmunesystem(Chew
and Park, 2004;Pechinskii and Kuregyan,2014; Ghodratizadeh
etal.,2014).Microalgaeconstituteanoptimalsourcetoproduce
carotenoidsforpharmaceuticalapplicationsastheycombinethe
advantagesofsynthesizingthewidechemodiversityofcarotenoids,
withhighproductionyields,withouttheneedoffreshwater,
agri-culturalsurfacesorpesticidestobegrown(Mimounietal.,2012).
Selection of hyper-producing strains combined tooptimization
oftheirgrowthconditions and purificationprocesses allowthe
recoveryofhighamountsofcarotenoidsdevoidofchemicalsor
endotoxins.Inthepresentreport,wedemonstratethat
zeaxan-thin,anabundantcarotenoid presentinmicroalgae,thatcanbe
easily obtained in high amounts from the rhodophyte P.
pur-pureum,inducesapoptosisinhumanmelanomacells expressing
theoncogenicBRAFV600Emutationandpotentiatesthe
antipro-liferativeactivityofvemurafenib,aBRAFinhibitorusedinpatients
withadvancedmetastaticmelanomas.Zeaxanthin(1)was
previ-ouslyreportedtohavenooraltoxicityanddecreasetheincidence
of variouscancers afteroral ingestion(Thurnhamand Howard,
2013;Xuetal.,2013).Itwasalsoreportedtoexertpro-apoptotic
activity in humanuveal melanoma cells (SP6.5 and C918) and
limit uveal melanoma invasivity without impairing the
viabil-ity of non canceruveal melanocytes (Xu et al.,2015; Bi et al.,
2013).InA2058melanomacells,zeaxanthinIC50wasdetermined
as40M,aconcentrationinducingcellrounding,chromatin
con-densation,nuclearfragmentation,hypodiploidy,cellapoptosisin
early and late stages, accumulation of cells in sub-G1 phase,
DNAinternucleosomalfragmentationand activationof
caspase-3.Zeaxanthin-inducedapoptosiswasaccompaniedbyinhibition
ofNF-Bandup-regulationofthepro-apoptoticfactorsBimand
Bid,demonstratingtheinvolvementofthemitochondrialsignaling
pathwayinapoptosistriggering,aspreviouslyreportedinuveal
melanomamodels(Bietal.,2013).Theobservationthat
zeaxan-thinwasabletomoderatelypotentiatetheinvitroantiproliferative
activityofvemurafenibisapromisingresultofourstudyasit
sug-gestsitspotentialinterestasanutritionaladjuvantincreasingthe
sensitivityoftumorcellstoBRAFinhibitors.Bypotentiatingthe
antiproliferativeeffectofBRAFinhibitors,zeaxanthinmayallowto
decreaseBRAFinhibitorseffectivedoses,limittheiradverseeffects
inpatientsanddelaytheemergenceofresistancemechanisms(Chu
etal.,2012;Zimmeretal.,2012;Anforthetal.,2015;Welshand
Corrie,2015).Themolecularmechanismsinvolvedinthisincrease
ofsensitivityaswellaspreclinicalandclinicalrelevanceof
com-bining carotenoidswithBRAFinhibitorswillhave tobefurther
Ctrl
ß-action
ß-action pNF-kB NF-kB kDa
kDa
kDa
37
37 87 85 37 39 70 70
30
25
25
20
(Ser 536)
IkBα
Ikkα
Ikkβ
β-action Bcl-XL
Bim
Bak
Bid
Ctrl
Ctrl
Zea Zea
Zea
A
B
C
D
E
9080
70
60
50
40
30
20
10
0
Control Zea
+ 37.98%
+ 25.74%
+ 10.70%
% Gro
wth inhibition at 72 h
(2000 A2058 cells)
Vemurafenib
Vemurafenib Vemurafenib
5 µM 1 µM
0.1 µM
Zea Zea Zea
50 µm
Fig.12. ZeaxanthinsensitizesA2058melanomacellstotheBRAFinhibitorvemurafenib.TwothousandsA2058melanomacellsweregrownfor72hincontrolcellculture medium(A),mediumcontainingzeaxanthin40M(B),vemurafenib5M(C),oramixofzeaxanthin40Mandvemurafenib5M(D).Zeaxanthininduceda37.98,25.75 and10.70%increaseoftheantiproliferativeactivityofvemurafenib0.1,1and5M,respectively(E).
explored,asthisstrategycouldextendthedurationof
chemother-apyefficiency.In summary,thisstudyconfirms thepotentialof
zeaxanthintolimitthegrowthofchemoresistantmelanomacells,
confirmsthemajorinterestofphytoplanktoncarotenoidsas
natu-ralanticancermoleculesdevoidoforaltoxicity,andsuggestsforthe
firsttimetheinterestofcombiningacarotenoidtoBRAFinhibitors
topotentiatetheirchemotherapeuticefficiencyinmelanomacells.
Authors’contributionandresponsibility
JBBandENproducedP.purpureumbiomass.CJ,RGOJ,AF,CO,LPy,
IL,GPandLPperformedthepigmentextraction,purification,cell
culture,western-blotandapoptosisexperiments.CJperformedthe
HRMSanalysis.LBandCJperformedthefluxcytometryanalysis.LP
designedtheexperiments,interpretedthedata,directedthestudy
andwrotethemanuscriptincollaborationwithVTandJRGDSA.LP
takesresponsibilityfortheintegrityofthework,frominceptionto
finishedarticle.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thatnoexperimentswereperformedonhumansoranimalsfor
thisstudy.
Confidentialityofdata. Theauthorsdeclarethatnopatientdata
appearinthisarticle.
Righttoprivacyandinformedconsent.Theauthorsdeclarethat
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
WearegratefultotheFrenchcancerleague(Comité17dela
LigueNationalecontreleCancer)forfinancialsupportandtothe
Poitou-CharentesregionforCJ’sPhDgrant.Wealsothankthe
“Can-céropôleGrandOuest,axeValorisationdesproduitsdelameren
cancérologie”forscientificsupport.LPisgratefultotheBrazilian
SocietyofPharmacognosyforitskindinvitationtotheXthBrazilian
SymposiumofPharmacognosyinPetrolinainSeptember2015.We
thankDrRaymondKaasfromIFREMERforhiskindpermissionto
usethemicrophotographyofPorphyridiumpurpureumandAntoine
Bonnet(PlatformfortheHighResolutionAnalysisofBiomolecules,
UniversityofLaRochelle,France)forexcellenttechnicalassistance
withHRMS.
References
Ahmad,I.,Muneer,K.M.,Tamimi,I.A.,Chang,M.E.,Ata,M.O.,Yusuf,N.,2013. Thy-moquinonesuppressesmetastasisofmelanomacellsbyinhibitionofNLRP3 inflammasome.Toxicol.Appl.Pharmacol.270,70–76.
Alqathama,A.,Prieto,J.M.,2015.Naturalproductswiththerapeuticpotentialin melanomametastasis.Nat.Prod.Rep.32,1170–1182.
Anforth,R.,Carlos,G.,Clements,A.,Kefford,R.,Fernandez-Pe ˜nas,P.,2015. Cuta-neousadverseeventsinpatientstreatedwithBRAFinhibitor-basedtherapies formetastaticmelanomaforlongerthan52weeks.Br.J.Dermatol.172,239–243. Armstrong,B.K.,Kricker,A.,2001.TheepidemiologyofUVinducedskincancer.J.
Photochem.Photobiol.B63,8–18.
Baudelet,P.H.,Gagez,A.L.,Bérard,J.B.,Juin,C.,Bridiau,N.,Kaas,R.,Thiéry,V.,Cadoret, J.P.,Picot,L.,2013.AntiproliferativeactivityofCyanophoraparadoxapigments inmelanoma,breastandlungcancercells.Mar.Drugs11,4390–4406. Bi,M.C.,Rosen,R.,Zha,R.Y.,McCormick,S.A.,Song,E.,Hu,D.N.,2013.Zeaxanthin
inducesapoptosisinhumanuvealmelanomacellsthroughBcl-2family pro-teinsandintrinsicapoptosispathway.Evid.Based.Complement.Alternat.Med., http://dx.doi.org/10.1155/2013/205082.
Burri,B.J.,Neidlinger,T.R.,Clifford,A.J.,2001.Serumcarotenoiddepletionfollows first-orderkineticsinhealthyadultwomenfednaturallylowcarotenoiddiets. J.Nutr.131,2096–2100.
Caltagirone,S.,Rossi,C.,Poggi,A.,Ranelletti,F.O.,Natali,P.G.,Brunetti,M.,Aiello,F.B., Piantelli,M.,2000.Flavonoidsapigeninandquercetininhibitmelanomagrowth andmetastaticpotential.Int.J.Cancer87,595–600.
Chen,Y.T.,Kao,C.J.,Huang,H.Y.,Huang,S.Y.,Chen,C.Y.,Lin,Y.S.,Wen,Z.H.,Wang, H.M.D.,2017.AstaxanthinreducesMMPexpressions,suppressescancercell migrations,andtriggersapoptoticcaspasesofinvitroandinvivomodelsin melanoma.J.Funct.Foods31,20–31.
Chew,B.P.,Park,J.S.,2004.Carotenoidactionontheimmuneresponse.J.Nutr.134, 257S–261S.
Chu,E.Y.,Wanat,K.A.,Miller,C.J.,Amaravadi,R.K.,Fecher,L.A.,Brose,M.S., McGet-tigan,S.,Giles,L.R.,Schuchter,L.M.,Seykora,J.T.,Rosenbach,M.,2012.Diverse cutaneoussideeffectsassociatedwithBRAFinhibitortherapy:a clinicopatho-logicstudy.J.Am.Acad.Dermatol.67,1265–1272.
Chung,T.W.,Choi,H.J.,Lee,J.Y.,Jeong,H.S.,Kim,C.H.,Joo,M.,Choi,J.Y.,Han,C.W.,Kim, S.Y.,Choi,J.S.,Ha,K.T.,2013.Marinealgalfucoxanthininhibitsthemetastatic potentialofcancercells.Biochem.Biophys.Res.Commun.439,580–585. Cragg,G.M.,Newman,D.J.,Snader,K.M.,1997.Naturalproductsindrugdiscovery
anddevelopment.J.Nat.Prod.60,52–60.
Dankort,D.,Curley,D.P.,Cartlidge,R.A.,Nelson,B.,Karnezis,A.N.,Damsky,W.E.,You, M.J.,DePinho,R.A.,McMahon,M.,Bosenberg,M.,2009.Braf(V600E)cooperates withPtenlosstoinducemetastaticmelanoma.Nat.Genet.41,544–552. Davies,H.,Bignell,G.R.,Cox,C.,Stephens,P.,Edkins,S.,Clegg,S.,Teague,J.,Woffendin,
H.,Garnett,M.J.,Bottomley,W.,Davis,N.,Dicks,E.,Ewing,R.,Floyd,Y.,Gray,K., Hall,S.,Hawes,R.,Hughes,J.,Kosmidou,V.,Menzies,A.,Mould,C.,Parker,A., Stevens,C.,Watt,S.,Hooper,S.,Wilson,R.,Jayatilake,H.,Gusterson,B.A.,Cooper, C.,Shipley,J.,Hargrave,D.,Pritchard-Jones,K.,Maitland,N.,Chenevix-Trench,G., Riggins,G.J.,Bigner,D.D.,Palmieri,G.,Cossu,A.,Flanagan,A.,Nicholson,A.,Ho, J.W.C.,Leung,S.Y.,Yuen,S.T.,Weber,B.L.,Seigler,H.F.,Darrow,T.L.,Paterson,H., Marais,R.,Marshall,C.J.,Wooster,R.,Stratton,M.R.,Futreal,P.A.,2002.Mutations oftheBRAFgeneinhumancancer.Nature417,949–954.
Dutton-Regester,K.,Irwin,D.,Hunt,P.,Aoude,L.G.,Tembe,V.,Pupo,G.M., Lana-gan,C.,Carter,C.D.,O’Connor,L.,O’Rourke,M.,Scolyer,R.A.,Mann,G.J.,Schmidt, C.W.,Herington,A.,Hayward,N.K.,2012.Ahigh-throughputpanelfor identi-fyingclinicallyrelevantmutationprofilesinmelanoma.Mol.CancerTher.11, 888–897.
Firdous,A.P.,Sindhu,E.R.,Ramnath,V.,Kuttan,R.,2010.Anti-mutagenicand anti-carcinogenicpotentialofthecarotenoidmeso-zeaxanthin.AsianPac.J.Cancer Prev.11,1795–1800.
Gagez,A.L.,Thiery,V.,Pasquet,V.,Cadoret,J.P.,Picot,L.,2012.Epoxycarotenoidsand cancer.Curr.Bioact.Compd.8,109–141.
Ghodratizadeh,S.,Kanbak,G.,Beyramzadeh,M.,Dikmen,Z.G.,Memarzadeh,S., Habibian,R.,2014.Effectofcarotenoid-cryptoxanthinoncellularandhumoral immuneresponseinrabbit.Vet.Res.Commun.38,59–62.
Grange,F.,2005.Epidemiologyofcutaneousmelanoma:descriptivedatainFrance andEurope.Ann.Dermatol.Venereol.132,975–982.
Hall,W.A.,Djalilian,H.R.,Nussbaum,E.S.,Cho,K.H.,2000.Long-termsurvivalwith metastaticcancertothebrain.Med.Oncol.17,279–286.
Haluska,F.G.,Tsao,H.,Wu,H.,Haluska,F.S.,Lazar,A.,Goel,V.,2006.Genetic alter-ationsinsignalingpathwaysinmelanoma.Clin.CancerRes.12,2301s–2307s. Hashimoto,T.,Ozaki,Y.,Mizuno,M.,Yoshida,M.,Nishitani,Y.,Azuma,T.,Komoto,
A.,Maoka,T.,Tanino,Y.,Kanazawa,K.,2011.Pharmacokineticsoffucoxanthinol inhumanplasmaaftertheoraladministrationofkombuextract.Br.J.Nutr.107, 1–4.
Hedidi,M.,Erb,W.,Bentabed-Ababsa,G.,Chevallier,F.,Picot,L.,Thiéry,V.,Bach,S., Ruchaud,S.,Roisnel,T.,Dorcet,V.,Mongin,F.,2016.SynthesisofN-pyridylazoles usingadeprotometalation-iodolysis-N-arylationsequenceandevaluationof theirantiproliferativeactivityinmelanomacells.Tetrahedron72,6467–6476. Jang,S.,Atkins,M.B.,2014.TreatmentofBRAF-mutantmelanoma:theroleof
vemu-rafenibandothertherapies.Clin.Pharmacol.Ther.95,24–31.
Juin,C.,Bonnet,A.,Nicolau,E.,Bérard,J.B.,Devillers,R.,Thiéry,V.,Cadoret,J.P.,Picot, L.,2015.UPLC-MSEprofilingofphytoplanktonmetabolites:applicationtothe identificationofpigmentsandstructuralanalysisofmetabolitesinPorphyridium purpureum.Mar.Drugs13,2541–2558.
Kim,K.N.,Ahn,G.,Heo,S.J.,Kang,S.M.,Kang,M.C.,Yang,H.M.,Kim,D.,Roh,S.W.,Kim, S.K.,Jeon,B.T.,Park,P.J.,Jung,W.K.,Jeon,Y.J.,2013.Inhibitionoftumorgrowth invitroandinvivobyfucoxanthinagainstmelanomaB16F10cells.Environ. Toxicol.Pharmacol.35,39–46.
Kumar,S.R.,Hosokawa,M.,Miyashita,K.,2013.Fucoxanthin:amarinecarotenoid exertinganti-cancereffectsbyaffectingmultiplemechanisms.Mar.Drugs11, 5130–5147.
Leiter,U.,Garbe,C.,2008.Epidemiologyofmelanomaandnonmelanomaskincancer –theroleofsunlight.In:Sunlight,Vitam.DSki.Cancer.Springer,NewYork,NY, pp.89–103.
Locatelli,C.,Filippin-Monteiro,F.B.,Creczynski-Pasa,T.B.,2013.Recentadvancesin thebiology,therapyandmanagementofmelanoma.InTech.
Lu,M.,Zhang,Y.,Zhao,C.,Zhou,P.,Yu,L.,2015.Analysisandidentificationof astax-anthinanditscarotenoidprecursorsfromXanthophyllomycesdendrorhousby high-performanceliquidchromatography.Z.Naturforsch.C65,489–494. MacKie,R.M.,Hauschild,A.,Eggermont,A.M.M.,2009.Epidemiologyofinvasive
cutaneousmelanoma.Ann.Oncol.20(Suppl.6vi),1–7.
Marneros,A.G.,2009.Tumorangiogenesisinmelanoma.Hematol.Oncol.Clin.North Am.23,431–446.
Mathieu,V.,deLassalle,E.M.,Toelen,J.,Mohr,T.,Bellahcène,A.,Van Goietsen-oven,G.,Verschuere,T.,Bouzin,C.,Debyser,Z.,DeVleeschouwer,S.,VanGool, S.,Poirier,F.,Castronovo,V.,Kiss,R.,Feron,O.,2012.Galectin-1inmelanoma biology and related neo-angiogenesis processes. J. Invest. Dermatol. 132, 2245–2254.
Mesquita,M.L.,Paula,J.E.,Pessoa,C.,Moraes,M.O.,Costa-Lotufo,L.V.,Grougnet, R.,Michel,S.,Tillequin,F.,Espindola,L.S.,2009.Cytotoxicactivityof Brazil-ianCerradoplantsusedintraditionalmedicineagainstcancercelllines.J. Ethnopharmacol.123,439–445.
Mimouni,V.,Ulmann,L.,Pasquet,V.,Mathieu,M.,Picot,L.,Bougaran,G.,Cadoret, J.P.,Morant-Manceau,A.,Schoefs,B.,2012.Thepotentialofmicroalgaefor theproductionofbioactivemoleculesofpharmaceuticalinterest.Curr.Pharm. Biotechnol.13,2733–2750.
Mirzaei,H.,Naseri,G.,Rezaee,R.,Mohammadi,M.,Banikazemi,Z.,Mirzaei,H.R., Salehi,H.,Peyvandi,M.,Pawelek,J.M.,Sahebkar,A.,2016.Curcumin:anew candidateformelanomatherapy?Int.J.Cancer139,1683–1695.
Niles,R.M.,McFarland,M.,Weimer,M.B.,Redkar,A.,Fu,Y.M.,Meadows,G.G.,2003. Resveratrolisapotentinducerofapoptosisinhumanmelanomacells.Cancer Lett.190,157–163.
Oliveira-Junior,R.G.,Thiéry,V.,Sergent,O.,Chevanne,M.,Picot,L.,2016.Could fucox-anthininteractionwithlipidraftsmediateitscytotoxicityincancercells?J. Oceanogr.Mar.Res.4,144.
Pasquet,V.,Morisset,P.,Ihammouine,S.,Chepied,A.,Aumailley,L.,Berard,J.B., Serive,B.,Kaas,R.,Lanneluc,I.,Thiery,V.,Lafferriere,M.,Piot,J.M.,Patrice,T., Cadoret,J.P.,Picot,L.,2011.Antiproliferativeactivityofviolaxanthinisolated frombioguidedfractionationofDunaliellatertiolectaextracts.Mar.Drugs9, 819–831.
Pechinskii,S.V.,Kuregyan,A.G.,2014.Theimpactofcarotenoidsonimmunity (Review).Pharm.Chem.J.47,509–513.
Putey,A.,Joucla,L.,Picot,L.,Besson,T.,Joseph,B.,2007.Synthesisoflatonduine derivativesviaintramolecularHeckreaction.Tetrahedron63,867–879. Reboul,E.,2013.AbsorptionofvitaminAandcarotenoidsbytheenterocyte:focus
ontransportproteins.Nutrients5,3563–3581.
Roy,S.,Llewellyn,C.,Egeland,E.,Johnsen,G.(Eds.),2011. Phytoplankton Pig-ments:Characterization,ChemotaxonomyandApplicationsinOceanography (Cambridge Environmental Chemistry Series).Cambridge University Press, Cambridge,pp.728–822,http://dx.doi.org/10.1017/CBO9780511732263.032. Serive,B.,Kaas,R.,Bérard,J.B.,Pasquet,V.,Picot,L.,Cadoret,J.P.,2012.Selectionand
optimisationofamethodforefficientmetabolitesextractionfrommicroalgae. Bioresour.Technol.124,311–320.
newcarotenoidsandporphyrinscharacteristicofdistinctstrainsandtaxonomic groups.PLOSONE12,e0171872.
Spagnolo,F.,Ghiorzo,P.,Queirolo,P.,2014.OvercomingresistancetoBRAFinhibition inBRAF-mutatedmetastaticmelanoma.Oncotarget5,10206–10221. Sugawara,T.,Matsubara,K.,Akagi,R.,Mori,M.,Hirata,T.,2006.Antiangiogenic
activ-ityofbrownalgaefucoxanthinanditsdeacetylatedproduct,fucoxanthinol.J. Agric.FoodChem.54,9805–9810.
Tanaka, T., Shnimizu, M., Moriwaki, H., 2012. Cancer chemoprevention by carotenoids.Molecules17,3202–3242.
Thurnham,D.I.,Howard,A.N.,2013.Studiesonmeso-zeaxanthinforpotential toxi-cityandmutagenicity.FoodChem.Toxicol.59,455–463.
VanGoietsenoven,G.,Hutton,J.,Becker,J.P.,Lallemand,B.,Robert,F.,Lefranc, F.,Pirker,C.,Vandenbussche,G.,VanAntwerpen,P.,Evidente,A.,Berger,W., Prévost,M.,Pelletier,J.,Kiss,R.,Kinzy,T.G.,Kornienko,A.,Mathieu,V.,2010. TargetingofeEF1AwithAmaryllidaceaeisocarbostyrilsasastrategytocombat melanomas.FASEBJ.24,4575–4584.
VanHeukelem,L.,Thomas,C.,2001.Computer-assistedhigh-performanceliquid chromatographymethoddevelopmentwithapplicationstotheisolationand analysisofphytoplanktonpigments.J.Chromatogr.A910,31–49.
Walne,P.,1966.Experimentsinthelarge-scalecultureofthelarvaeofOstrea edulis(L.).In:Fish.Investig.Ser.II.LondonHerMajesty’sStation.Off.,pp.iii. 53.
Welsh,S.J.,Corrie,P.G.,2015.ManagementofBRAFandMEKinhibitortoxicitiesin patientswithmetastaticmelanoma.Ther.Adv.Med.Oncol.7,122–136. Xu,X.,Zhang,L.,Shao,B.,Sun,X.,Ho,C.T.,Li,S.,2013.Safetyevaluationof
meso-zeaxanthin.FoodControl32,678–686.
Xu,X.L.,Hu,D.N.,Iacob,C.,Jordan,A.,Gandhi,S.,Gierhart,D.L.,Rosen,R.,2015.Effects ofzeaxanthinongrowthandinvasionofhumanuvealmelanomainnudemouse model.J.Ophthalmol.,http://dx.doi.org/10.1155/2015/392305.
Zapata,M.,Rodriguez,F.,Garrido,J.,2000.Separationofchlorophyllsandcarotenoids frommarinephytoplankton:anewHPLCmethodusingareversedphaseC8 columnandpyridine-containingmobilephases.Mar.Ecol.Prog.Ser.195,29–45. Zhang,L.,Wei,Y.,Zhang,J.,2014.Novelmechanismsofanticanceractivitiesofgreen teacomponentepigallocatechin-3-gallate.AnticancerAgentsMed.Chem.14, 779–786.