Abstracts of Papers of the 2nd Annual Russian-Korean Conference "Current Issues in Chemistry and Biotechnology of Natural Products". 11 Anticoagulant effect of a serine protease inhibitor preparation from the hepatopancreas of the king crab (Paralithodes camchaticus). 27 Contrastive analysis of composition and bactericidal activity of different fractions of Siberian pine essential oil.
30 Gene design for the optimal expression in yeast of the genetically modified constructs, including preS and S genes of A and D genotypes of the hepatitis B virus. Synthesis of biologically active [N-(2-ethoxyethyl)piperidyl-4]propargyl derivatives of natural alkaloids and their synthetic analogues. 103 The study of qualitative and quantitative characteristics of the Kazakhstan plant type, Polygonum Alpinum.
123 Cytokinin activity testing of mechanochemical preparation of rice husks using chickpea (Cicer arietinum L.) culture in vitro. 148 On the issue of standardization of the anti-cancer drug "Leykoefdin" based on the herb Ephedra Equisetina.
New Sesquiterpene Lactones of Plants
Structure of Molecules, Properties, Distribution in the Nature
Tree Raw Material from Siberia as a Source of Immune System, Growth and Development Stimulators for Use on Plants
Successes in Chemistry of Tricyclic Quinazoline Alkaloids and Their Analogues
LC-NMR-MS on Natural Products Chemistry Research – Possibility and Limits
The Bioconversion of Native Starches
Chemical Constituents of Hammada elegans (Bunge) Botsch and Their Antimicrobial Activity
Synthesis and Biological Activity of New Derivatives of Mono- and Sesquiterpenoides of Essential Oils
Phytochemical Study of Cyperus alternifolius L. as a
Flavonoids and Polysaccharides of Larch Wood, Isolation and Purification Technology and Experimental-Industrial Production
Creation
Anticoagulative Effect of the Preparation of Serine Proteases Inhibitor from Hepatopancreas
Skeletal and Oxidative Transformations of Some Lead Triterpenic Acids for the Structure-Anticancer Activity
Relationships Studies
Seasonal Fluctuations of Phycobiliproteins and Sulfated Polysaccharides in Ahnfeltiopsis flabelliformis (Harv.) Masuda
Rhodophyta, Phyllophoraceae)
St. John’s Wort Flowers Composition
Cellulose from Cereal Waste and Miscanthus Biomass
Biosynthesis of Astaxanthin in Heterologous Hosts by Marine Bacterial crtW and crtZ Genes
Optimization of Gelatin-Gum Arabic Complexed Microcapsules for Encapsulation of Allyl Isothiocyanate
Anticarcinogenic Activity of Aaptamine and Its Derivatives Isolated from Marine Sponge Aaptos sp
Sulphated Polysaccharides of Brown Seaweeds
History and Modern Day of the Investigation
New “Green” Way to Epoxydes of Terpenes
Plant Coumarins of Some Apeaceae Species Growing in Siberia and Mongolia
Study on Chemical Constitutes of the Volatiles from Dracocephalum nutans L
Plants Extracts
A Developed Technology of Fir Needles Complex Processing
Mechanochemistry of Plant Materials
Some Studying Results of Kazakhstan Wild-Growing Plants and the Ways of Their Research
Contrastive Analysis of Composition and Bactericidal Activity of Various Fraction of Siberian Pine Essential Oil
Doping agents and Oriental Herbs in Korea
Synthesis and cytotoxic activity of 7,10-dihydroxy-12H-benzo[b]xanthene-6,11-dione derivatives Analogues of Bikaverin. Benzo[b]xanthene-6,11-dione derivatives Analogues of Bikaverin. We found that benzopyranonafazarines 2 are substrates for the synthesis of potentially biologically active xanthones such as bikaverine and its analogues 1. The starting benzopyranonafazarines 2 were obtained by condensation of 2-hydroxynaphtazarines with 2-hydroxy- or 2-methoxy-benzaldehyde 2-bromo-3- hydroxynaphtazarines 3 and bromine derivatives 4 are the products of pyranonaftazarines 2-bromination.
Other xanthenediones 5,6 were obtained by reaction of bromo derivatives 4 with various nucleophilic reagents such as acetone (enol form) and succinimide.
Gene-Design for the Optimal Expression in Yeast of the
Genetically Modified Constructions Including preS and S-genes of A and D Genotypes of Hepatitis B Virus
Content of Alkaloids in the Different Parts of Sophora flavescens Soland
Synthetic Transformations of Alkaloids, Terpenoids and Coumarins of Siberian Flora for Search of New Pharmaceutics
A New Multifunctional Agents Synthesized on Natural Triterpenoid Platforms
The Study of Antioxidant Activity of Natural Organic Acids
Medications Standardization Problems
The advantage of such a method is a clear color transition from dark blue to golden yellow, which allows us to define the equivalence point in an easy and accurate way. Certain excessive percentages of the results can be ignored because phytopreparations have a very low content that can be affected by simultaneous oxidation according to the method described above. Based on experimental data obtained, a temperature of 20-30°C and a concentration of 0.25% should be optimal reaction conditions.
As ethylene alcohol is able to precipitate both polysaccharides and albumins, we have performed additional studies for albumin content, namely the ability of polysaccharides/inability of albumin to dissolve in 10% trichloroacetic acid. Based on such further investigation, we proposed to introduce correction coefficient to analytical formula of said method. The advantage of this method is high selectivity, and furthermore, the present method is of non-indicative type, which makes the analysis very simple.
The small amount of titrant for standardization forces the use of microburettes, which is the only drawback of the proposed method, as it increases the degree of human factor in accuracy and reproducibility. The experiment proved that a decrease in pH increases the amount of titrant, making the results definitely higher, while an increase in pH causes the reverse relationship.
New in Ecdysteroids Chemistry: the Synthesis of Minor Phytoecdysteroids and Ecdysteroids Analogues
Resources and Biotechnology of the Ecdysteroid-Containing Plants
Biological Activity of Sulfated Polysaccharides from Red Seaweed of Pacific Coast
Synthesis of Biological Active
N-(2-Ethoxyethyl)piperidyl-4]propargyl Derivatives of Natural Alkaloids and Their Synthetic Analogs
Isolation of Substances from Plants of Hipophae rhamnoides L
Species and Study on Their Phytochemical and Antioxidant Properties
Synthesis and Biological Activity of Sesquiterpene Lactone Modified by Natural Alkaloids
Chemical Constituents of Leonurus turkestanicus
Anticancerogenic Activity of Alkaloids from Haplophyllum perforatum
Quantification of Ecdysteroids in Insect by HPLC-MS
Chemical Composition of the Essential Oil of Thymus punctulosus
Improving Effects of Semen Flax Extract on Hemorheological Abnormalities in Ovariectomized Rats
Synthesis of C-20 Pyridine Analogs of Lupane-Type Triterpenoids
Synthesis of six 3-methyl-6-(1-methylethenyl)cyclohex-3-ene-1,2-diol stereoisomers with high optical purity. diol stereoisomers with high optical purity. It is known that the biological activity of the terpenoids is sufficiently dependent on their absolute configuration. In this work, we successfully synthesized six of all eight stereoisomers of compound 1 with high optical purity starting from the widely distributed monoterpenes (+)- and (-)-pinene in accordance with the scheme.
Gas-Chromatographic Analysis of the Volatile Compounds of Halostachys belangeriana
The Synthesis of Hybrid Structures Consisted of Sesquiterpene Methylene Lactone and Alkaloid
On Phytoadaptive Activity of Lonicera caerulea L
Enzymatic Hydrolysis of Miscanthus Conversion Products
Synthesis and Antioxidant Activity of Alkylthiomethyl Derivates of Quercetin and Dihydroquercetin
Obtaining Mannanooligosaccharide Additive by Means of the Mechanoenzymatic Hydrolysis of Yeast Biomass
Research of Siberian Fir Phenol Compounds Using Chromatographic Profiling Method
Steam-distillation and Simultaneous Hydrodistillation – Solvent Extraction in Essential Oil Preparation from Fresh Plant Material
Enantiomeric Composition of Monoterpene Hydrocarbons Isolated from Siberian Conifers
Chromatographic Isolation and Hightroughput Screening of Potencial Cytostatic on the Base of Extracts
Clerodane Diterpenoids from Pulicaria Gnaphalodes
Flavonoid from Scutellaria holosericea
Qualitative Analysis of Coumarins in Seeds Nigella sativa L., Growing in Uzbekistan
Flavonoid from Roots Scutellaria schachristanica
Optimization of Method of Isolation of Leucomisine from Artemisia leucodes Schrenk
Preparation of -Pinene Oxide from Turpentine
Chemical Research of Marsh Cinquefoil (Comarum palustre) Grows in the Krasnoyarsk Territory
Ultrasound Assisted Enzymatic Hydrolysis of Cellulose as a Method of Improvement of Biologically Active Substances
Extraction
Mannich-Type Three Component Condensation of α-Substituted Caran-4-one Oxime with Formaldehyde and Secondary Amine
Quantitative Determination of Betulin and Lupeol in Birch Bark Extracts by HPLC
Unusual Reactions of (+)-2- and (+)-3-Carenes with Aldehydes on K10 clay
Chemical Transformation of Cycloartane Triterpenoids
Perspectives of Ecdysten Use in the Treatment of Giardiasis as Mono- and Co-infection
Investigation of Capillary-Protective Composition on the Base of Dihydroquercetin and Lipoic Acid
Investigation of Biological Activity of the Stereoisomeric Halogenhydrines of Ludartine
Synthesis of Tetrahydroquinoline-Barbituric Acid-Terpene Hybrids
One-pot Synthesis of 3-Aminoacridine Derivatives
Obtain and Properties of Cryogel of Polyvinyl Alcohol
Isolation of Arglabin from CO 2 -Extraction of
Data on the distribution of the plant Ferula foetida (Bunge) Regel belonging to the family – Apiaceae (Umbelliferae) was given in the article. It has been noted that the dried juice of the roots of Ferula foetida is used in oriental fork medicine as anti-inflammatory, analgesic, antispasmodic and antiparasitic drugs for the treatment of various diseases. The toxicity and pharmacological activity (estrogen) of the alcoholic extract of the plant Ferula foetida, collected in the Bukhara district, were investigated on laboratory animals.
Synthetic Modification on the B Ring of Labdane-Type Diterpenoids
Studying Alkaloids of Haplophyllum griffithianum
Chemical Fixation of Betulonic Acid on Water Soluble Polyvinyl Alc ohol
Synthesis of New Esters of Peoniflorin, the Major Monoterpene Glycoside of Paeonia anomala L. Roots
Synthesis of New Chiral Ligand from (+)-2-Carene
The New Method of Salicylates Extraction
Triterpenoids of Picea and Pinus Species
Synthesis of Betulin Esters by Interaction of Organic Acids with Birch Bark
New Methods of Allobetulin Synthesis
Palladium-Catalyzed Amination in the Synthesis of Diamino Furocoumarin Derivatives
The Flavonoids of Some Species of Kazakhstan Rumex L
Solid State Mechanochemical Reaction of Hypericin in Plant Material
Phytochemical study of Artemisia pontica L
Extractive Compounds of Punica granatum L. Flowers
Dissolution Rate Control of Nitrogen Containing Forage Additives
Institute of Biology of Komi Scientific Center of the Ural Branch of the Russian Academy of Sciences. Today, more than 40 furostanol and spirostanol glycosides are extracted from this plant, with its carbohydrate chains including D-glucose, a D-xylose, D-galactose, L-rhamnose and L-pectin sugar. The analysis of the steroid glycoside content of nine species from the botanical garden collection of the Institute of Biology of the Komi Science Center (RAS) – A.
After separation of SSG into closely spaced fractions and individual materials by column and thin layer chromatography, reverse phase chromatography (RPC) and analysis of the obtained products using specific reagents, it was determined that the obtained SSG contained from six to ten individuals. substances of the nature spiristanol and furastanol. Two individual materials were separated from the sum of spirostanol glycosides using column chromatography on alumina and silica gel. Analysis by thin-layer chromatography, reversed-phase chromatography (RPC) and chromatographic mass spectrometry has allowed the identification of one of them as deltonin - the spirostanol glycoside originally obtained from the roots of Dioscorea deltoid Dioscorea deltoidea [2].
It is found that diosgenin is genin SG found both in an unbound state, and in products of acid hydrolysis of sum SG. From sum FG after gel chromatography on Sephadex G-25, separations in a chromatographic column, furostanol glycoside two were ensured – deltoside and protodioscin, identified by means of a thin layer chromatography, RPC and hormato mass spectrometry.
Sterols as a Part of Neutral Lipids in Some Allium L. (Alliaceae) Species
Substance from Plant Limonium Myrianthum as the Basis for Creation of New Medical Products
Preparation and Solid-State Characterization of Solvates of Betuline and Its Mechanocomposites with Polymers
Reaction of (-)-Cytisine with Thiourea
Naphthoquinone Substituted Labdanes via Diels-Alder Reaction
Alkaloids of Korolkowia Sewertzowi – Original
Antiarrhythmic Drugs of Different Mechanisms of Action
The Study of Qualitative and Quantitative Characteristics of the Kazakhstan Type of Plant, Polygonum Alpinum
Correction of Hypoxia of States at Animals Individual and Integral Materials from the Plant Convolvulus subhirsutus
Obtaining and Hepatoprotective Action of the Drug from Codonopsis clematideae
Influence of Steroidal Alkaloids on the Steroidogenesis at Rats
Synthesis of Bromo- and Iodo-Berberine
Synthesis and Antioxidant Activity of Alkylthiomethyl Derivates of Hydroquinone, Resorcinol and Pyrocatechol
A Convenient Synthesis of 3- and 3,10-Functionalized Indolizino[8,7-b]indole Derivatives from Harmine
These compounds can be produced from natural raw materials1 or synthesized.232-4 Literature data regarding the synthesis of dihydrofurocoumarins presented only a few references.3,4. We determined that -hydroxy-4-[hydroxy(phenyl)methyl]-3-methyl-3,4-3 dihydrofuro[3,2-c]coumarin 1 was formed upon interaction of 4-hydroxycoumarin with 2-acetyl-3 -phenyloxirane.
Alkaloids and Flavonoids from Roots of Sophora flavescens Soland
Isolation and Identification
Novel Methods of Mechano-Enzymatic Modification and Recovery of Agriculture Waste
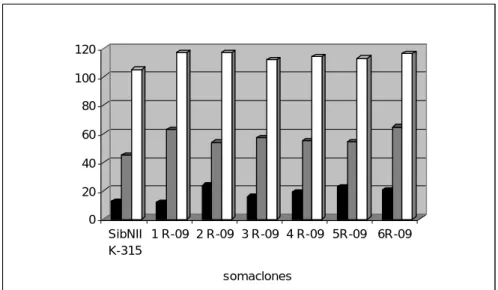
Unique Siberian Soybean Cultivar SibNIIK-315 and Tendency of Plant Breeding
Effects of Hybrid Macromolecular Phenolic Antioxidants on Whole Blood Viscosity
Ecdysteroids of Serratula quinquefolia Bieb. ex Willd
Working out Optimal Technology for the Substance Isolation from Cuscuta campestris Yuncker
Prediction of Its Biological Activity (PASS)
Chemical Study of Artemisia succulenta Ldb
Antimicrobic Activity in vitro of the Extracts Containing Phytoecdysteroids
Two Major Stilbene Glycosides of Pinus sibirica Bark
Isolation and Chemical Transformation
Distribution of Enantiomers of Monoterpene Hydrocarbons in Siberian Plants of the Apiaceae (Umbelliferae) Family
Synthesis of New Lappaconitine Derivatives Containing Indolizine Framework
Testing Cytokinin Activity
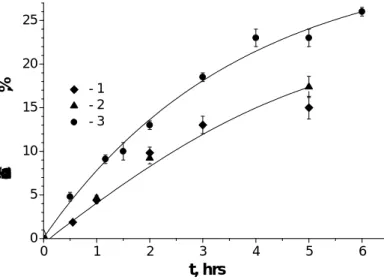
Cathepsin L–Like Protease
Synthesis and Spatial Structure of
Dependence of N-Desacetyllappaconitine Hydrobromide from the Method of Its Preparation
Analytical Control of Production
Chromatographic Method of Purifying Substance of Acsaritmin
Spectrophotometric Method of Analysis of Substance of Acsaritmin Preparation
BAS Accumulation and in vitro Propagation of Salix High Productive Genotypes
Distribution of Saponins in Fabaceae
Polyprenols of Cotton Leaves and Their Pharmacological Activity
Isolation of Betulin from Birch Bark by Supercritical Alcohols
Cryoconservation of Cultures of Cells of Endemic Species of Plants
Previously1, we found a new transformation resulting from the reaction of triterpenoid seven-membered cyclic anhydride 1 with benzylamine or p-methoxybenzylamine in THF followed by treatment with oxalyl chloride (“one-pot” conditions). Reaction of compound 1 with phenethylamine in CHCl3 leads to amine acids 2a and 2b, which were isolated by column chromatography over SiO2 in 57% and 42% respectively. In the presence of 6-7-fold excess of oxalyl chloride in THF, compounds 2a and 2b were completely converted to compounds 3 and 4, which were isolated by column chromatography over SiO2 in 76% and 83%, respectively.
Thus, desired compounds such as 3 can be obtained from 2-amino acids, while 3-amino acids and cyclic imides are not intermediates of present reaction.
Extractive Substances from Twigs of Picea obovata L
Influence of Oxime Pinostrobine on Lipidic Spectrum at Rats with Experimental Diabetes
The Neutral Compounds of Aerial Parts of Geranium saxatile Kar et Kir
Microcalorimetric Method for Study of Antioxidant Activity of Natural Lipid Fractions
Oxidation Usnic Acid by Organic Peracids
Chemical Contaminations of Camhorosma monspeliacum
The Separation of Biological Active Complexe from Genus Tamarix by Using Nanostructure Sorbents
The Basidiomycete Lentinula edodes Lectins Interaction with the Selected Organoselenium Diphenyl Substances
A Preparative Method for Isolation of Fucoxanthin from the East Sea Brown Algae Eisenia bicyclis by Centrifugal Partition
Chromatography
A preparative method for the isolation of fucoxanthin from the East Sea brown alga Eisenia bicyclis by centrifugal separation.
Preparative Separation of Chlorogenic Acid by Centrifugal Partition Chromatography from Highbush Blueberry (Vaccinium
Anthocyanins from Azalea (Rhododendron mucronulatum) Flowers
The rapid identification of anthocyanins from blueberry, Vaccinium corymbosum by LC–NMR/MS
To the Question of Standardization of Anticancer Drug
Leykoefdin» on the Basis of the Herb of Ephedra Equisetina
Investigation of Standardization of Rosa corymbifera Phytopreparation
The temperature limit for reaction with ammonium iron alum is set by imbalance of chelate complex, which is developed in high temperature environment. Low rate of oxidation-reduction reaction with ferricyanide indicates its low temperature limit, whereas ascorbic acid oxidation caused by increased temperature gives high temperature limit for reaction. Definition of concentration limits for reactions was performed based on contrast of transit of staining of working solutions.
Based on experimental data, we can say that the most sensitive, precise and reproducible method of authenticity control of phytopreparations is reactions with ammonium iron alum (hardening agents), concentrated ammonia (flavonoids), ferricyanide (vitamin C), α-naphthol (polysaccharides).
Perspectives of Production of Supplement with Antitumor Effect, Based on Polysaccharides from Brown Algae
Biocompozites on the Base of Natural Sorbents, Synthetic and Natural Polymers
Molecular Characteristics of Water-Soluble Sulphated Polysaccharides from Enteromorpha prolifera and Their
Anticancer and Immunomodulatory Activities
Coagulation Ability of Humic Acids from Mechanochemically Activated Peat
Bismuth Compounds for Medicine Based on Natural Products
Two billion people worldwide are infected; 360 million suffer from chronic HBV infection and over 520,000 die each year (50,000 from acute hepatitis B and 470,000 from cirrhosis or liver cancer) (Kane, M. HBV surface antigen (HBsAg) is the established serological marker routinely used for the diagnosis of acute or chronic HBV infection, the screening of blood or organ donors and the surveillance of persons at risk of acquiring or transmitting HBV. The aim of this study is to obtain hybridomas secreting monoclonal antibodies that are reactive on HBsAg.
For this purpose, mice were immunized with partially purified recombinant HBsAg obtained using baculovirus technology from transgenic B.
Retina Protection Properties of 4-Methyl-2,6-diisobornylphenol
New Flavonol Glycoside from Limonium gmelinii
Isolation and Identification of 26-Hydroxyecdysteroids