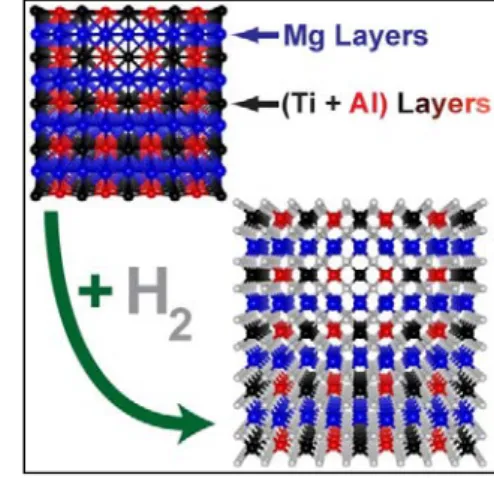
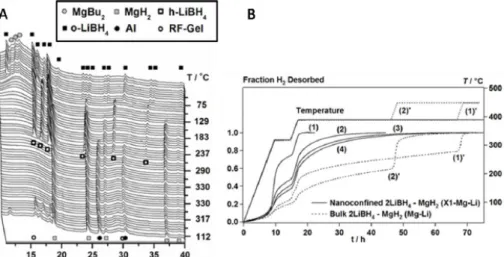
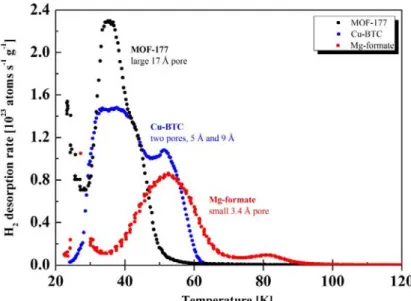
The presentation will explain the status of various hydrogen storage systems and discuss current challenges and future goals. Capacitance fading due to the large electrode volume change (83%) during charge/discharge is also a major issue.
Surface-Modified Advanced Hydrogen Storage Alloys for Hydrogen Separation and Purification
Effect of Mg on Structure and Thermodynamics of La-Mg-Ni Hydrides Synthesised by Hydrogen Metallurgy and Studied by in
Thermodynamic Optimization of the System Pd-Rh-H-D-T
Hydrogen in Nanostructured Materials
Structure and Bonding in Metal Borohydrides
Hydrogen Diffusion and Partial Reversibility of Transition Metal- Doped Magnesium Hydrides
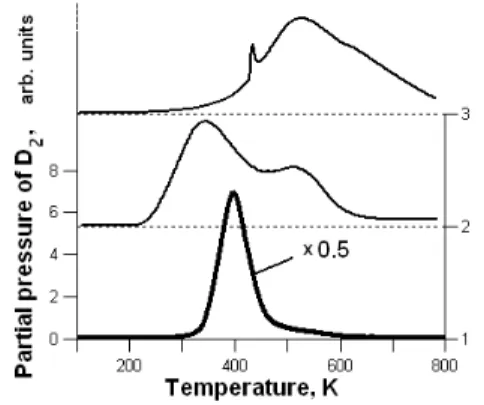
A systematic study of the structural phase transition occurring around 220 K in NH3BH3 and in its deuterated analogues was also performed by combining anelastic spectroscopy and differential scanning calorimetry. These results provided evidence that nanoconfinement drastically affects the thermodynamic properties of the starting material.
Classical Diffusion of Interstitial Hydrogen Revisited
Hydrogen-Related Properties of Metal and Alloy Nanoparticles
Inhibition of Hydrogen Chemisorption on Uranium Surfaces by Traces of Water Vapor
Principles of So-Called d-Metal Catalyst Interfaces for Very Fast Hydrogenation Kinetics of Magnesium
The Thermodynamics and Hydrogen Sorption Mechanism of Borohydrides
Effect of Interstitial Elements and/or Pressure on the Magnetic Properties of Some Iron Rich Intermetallic Compounds
Nuclear Magnetic Resonance Studies of Atomic Motion in Borohydrides
Developing Novel Materials and Methods for Hydrogen Storage
Synthesis of Mg-Ti Alloys with HCP, FCC, BCC Structures and Their Hydrides with FCC Structure
The ionic conductivity of Li(BH4) jumps by three orders of magnitude at about 390 K due to its structural transition from a low-temperature (LT) phase to a high-temperature (HT) phase. The HT phase of Li(BH4) can be stabilized by the addition of lithium halides, resulting in increased ionic conductivity at room temperature (RT) [5-8].
Nanoconfining Metal Hydrides: Impact on Thermodynamics, Kinetics and Reversibility of Hydrogen Sorption
Nanophase Aspects of Hydrogen Storage Materials - a Theoretical Study
Thermodynamics of Nano-Cluster Complex Hydrides Using First- Principles Density Functional Theory
Reaction Mechanism and Kinetics of MgH 2 /Borohydrides Based Reactive Hydride Composites
Metal hydrides have been investigated with a focus on hydrogen-metal bonding and hydrogen-hydrogen interaction for their densified states realized under high pressure. My talk will cover recent results on SR studies of the structural, electronic and magnetic properties of metal hydrides under high pressure.
Oral Presentations
Hydrogen Storage for Stationary and Portable Electricity Supply
A MgH 2 Tank – PEM-FC Coupling
Hydride Fuel Cells: from Solid to Liquid Fuel
Optical Hydrogen Sensor Based on the Elastic Clamping Effect
W Alloy Membranes for Hydrogen Purification
Following this concept, Nb-based alloys with high hydrogen permeability and strong resistance to hydrogen embrittlement were designed and developed. For this purpose, the alloy has effects on the hydrogen solubility and the resistance to hydrogen.
Hydrogen Permeation through the Pd-V/Nb-Pd Composite Membranes
Surface Effects and Thermal Degradation
Hydrogen-Palladium Temporary Gradient Material
Novel Behaviour in Aluminium Hydride at High Pressures
The Elastic Fields Generated byal Hydride Particle on a Free Surface of a Metal and their Effect on its Growth
Raman and X-ray High Pressure Investigations on Terbium and Dysprosium Trihydrides
The Effect of Film Thickness on the Thermodynamics of the Hydrogen- Vanadium Thin Films System
Effect of film thickness on the thermodynamics of the hydrogen-vanadium thin film system.
The Effect of Hydrogen on the Electrical Resistivity of Iron-Vanadium Alloy Thin Films
Di-Vacancies and the Hydrogenation of Mg-Ti Films with Short-Range Chemical Order
Structure and Hydrogenation Study of Nickel Substituted NdCo 4 B
Positron Lifetime Measurements for Monitoring Vacancies and Vacancy Clusters in Hydride Forming Materials
Simulation Study on Structure Variation of Metallic Nanoparticles due to Hydrogenation
Vibrational Properties of CeNiSn-Hydrides
Structural Studies of Hydrogen Storage Materials using Total Scattering and the PDF Method
Phase Stability in the LiNH 2 :MgH 2 System
In-Situ Neutron Scattering Studies of BCC Ti-Cr-V-Mo Alloy Hydrogen Storage Materials
Structure Modification of Nanocrystalline Mg-Nb Films
In this study, we investigated the dependence of composition and rare earth elements on the crystal structure of Mg2-xRExNi4 hydrides using in-situ and ex-situ X-ray diffraction (XRD) and ab-initio calculations. These results show that the difference in the structure of the hydrides depends on the ratio of Mg and RE, i.e.
Neutron Diffraction Study of BCC Alloys Containing Ferrovanadium
The Use of 3-D Laser Confocal Microscopy to Image Metal - Hydrogen Interactions
Diffusion of Deuterium in Zr-2.5Nb Alloy under Neutron Irradiation
Stabilization of Lattice Defects in HPT-deformed Palladium Hydride
Hydrogen Dynamics in Icosahedral and Amorphous Zr-based Alloys by Diffusion and Fast Field Cycling Nuclear Magnetic Relaxation Methods
Atomic probe analysis of hydrogen in V-6at%Fe film and Fe/V-6at%Fe multilayer film.
Atom Probe Analysis of Hydrogen in V-6at%Fe Film and Fe/V-6at%Fe Multi-Layered Film
Hydrogen Sorption Mechanisms at Various Hydrides Surfaces and Towards Synthetic Fuels
Irradiation Treatment Effects on Hydrogen Storage Alloy
Cooperative Effects at Formation and Decomposition of Magnesium Hydride in Powders
Real-time Observation of Hydrogen Absorption Dynamics for Pd Nanoparticles
Thermodynamic and Kinetic Characterization of H-D Exchange on β- Metals
Hydrogenation Kinetics of Aluminum Under High Pressure and High Temperatures
Kinetics of Dehydrogenation of MgH 2 and AlH 3
Halide Stabilized LiBH
4 : a Room Temperature Lithium Fast-Ion Conductor
Low Energy Regeneration of Aluminum Hydride
Diborane -the Key to Reversible Hydrogen Storage in Borohydrides
Theoretical Study on Improved Hydrogen Storage Materials
Neutron Spectroscopy of Magnesium Dihydride
Improved Dehydrogenation of Ca(BH 4 ) 2 Comibend with LiNH 2
Silicon-Based Hydrides for Hydrogen Storage: Relationship Between Hydrogenation Properties and Crystallographic Structures
The effects of alkali metal borohydrides on the crystal structure and hydrogen storage properties of potassium borohydride.
The Effects of Alkali Metal Borohydrides on the Crystal Structure and Hydrogen Storage Properties of Calcium Borohydride
To complete the study of RE-TM-Mg ternary diagrams (RE = rare earth and TM = transition metal), the high domain content of magnesium has been investigated. Different RE (Gd, La, Nd and Ce) as well as different transition elements (Ni and Cu) were investigated and two new compounds were discovered: La11Cu9Mg81 and Gd13Ni9Mg78. For Gd13Ni9Mg78 the determination of structural parameters is more complex due to the difficulty of obtaining a well crystallized sample.
If the preliminary XRD powder study allows the preservation of an average cubic structure, further characterization by electron diffraction shows a distorted cell with a long range. Numerous steps have been identified and some steps appear reversible as already observed for La2Mg17 [2]. In conclusion, hydration on Gd13Ni9.5Mg77.5 will be studied with attention because the nanostructuring of the sample should induce original sorption properties.
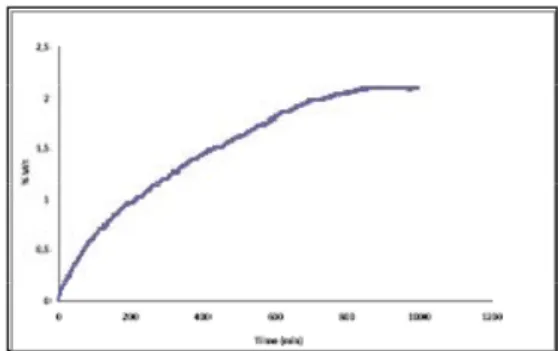
Effect of Severe Plastic Deformation on Hydrogen Storage Properties of Magnesium-Based Alloys
A New Ternary Phase Destabilised Hydrogen Storage System
MgH 2 : LiAlH 4
Properties
Visualising Hydrogen Absorption/Desorption in a MH Storage Tank Using Neutrons
Effect of Mechanically Induced Modification on TiH 2 Thermal Stability
The quaternary system Li-B-N-H shows a rich and complex phase behavior along the bond line connecting LiBH4 and LiNH2. Surprisingly, Li4BN3H10 is so stable with respect to LiNH2 and LiBH4 that it forms spontaneously from intimately mixed powders of LiNH2 and LiBH4 even at room temperature; in situ X-ray diffraction (XRD) confirms complete transformation to the α phase at slightly elevated temperature (73 °C). We recently observed that ball-milled Li3BN2H8 is unstable even at room temperature and spontaneously transforms over time into a mixture of β-Li2BNH6 and quaternary Li-B-N-H with the structure of the α phase, but with a composition enriched in LiNH2 intermediate between that of Li3BN2H8 and Li4BN3H10.
Recent thermal decomposition studies of Li3BN2H8 and Li4BN3H10 reveal three independent gas release events. By adding NiCl3 catalyst, however, we can demonstrate that these latter two processes are actually independent; The Ni nanocatalyst lowers the hydrogen release temperature by more than 100 °C, whereas the NH3 release temperature is unaffected, reducing the amount of NH3 released by more than an order of magnitude. For example, we have obtained similar hydrogen release characteristics by combining the two stable phases a-Li4BN3H10 and LiBH4 in a ratio of 1/0.5.
Improving Hydrogen Storage in Magnesium: A First-Principles Study
Sodium Alanate as a Practical Automotive On-board Solid-state Hydrogen Storage Medium
Nanoscale Fullerenes and Fullerene Hydrides
Combined Effects of Nanoconfinement and Catalysis on the Hydrogen Sorption Properties of LiBH 4
Alkali and Alkaline-Earth Metal Dodecahydro-Closo-Dodecaborates
Probing Structural Variations via Neutron Vibrational Spectroscopy
Thermal Conductivity Characterization of Metal Hydrides
A Reversible Nanoconfined Chemical Reaction
Lithium-Silicon Alloy as Hydrogen Storage Material
Nanosized Complex Hydrides in Carbon Scaffolds
Magnesium-Boron-Titanium Hydride System by High Throughput Method
Enhanced Hydrogen Sorption Properties of NaAlH 4 by Confinement in Nanoporous Carbon
The Nature of Hydrogen Adsorption in MOFs
Reaction Kinetics of a FeTi Alloy with Nano-Structured Surface Layers
Hydrogen Sorption in Magnesium Nanoparticles decorated by Transition Metals
Effect of Different Carbon Allotropies on the Synthesis of Magnesium Hydride by the Reactive Ball Milling in Hydrogen
Tailoring Thermodynamics in NaAlH 4 - Activated Carbon Composites
The Combination Importance of Nanostructures and Lightweight Materials for Efficient Hydrogen Storage
Binding Energy Estimation of Hydrogen Storage Materials by All-Electron Mixed-Basis Program TOMBO
Hydrogen storage materials have attracted worldwide attention for increasing requirements of energy for environmental protection. Among these, Mg is considered a promising hydrogen storage material due to its high storage capacity (7.6wt %), light weight and low cost. However, thermodynamics indicate that hydrogen desorption from bulk MgH2 only occurs at temperatures above 600K, which limits its use in practical applications, especially as hydrogen storage material in mobile applications and fuel cells that must operate at moderate temperature and under low hydrogen pressure. bars.
Hydrogen in thin films provides an opportunity to examine a number of unusual features, which are not present in bulk systems, since the absorption, absorption and storage of hydrogen is essentially a surface phenomenon. In the present work, Mg-Ni and Mg-Mg2Ni thin films, sandwiched between Pd, were prepared on Si substrate by vacuum vapor deposition technique at 10-6 torr in a chamber equipped with both an electron gun and also with resistant heating for simultaneous and sequential deposition. The areal concentration of hydrogen (NH in atoms/cm2) in both as-deposited and hydrogenated films was measured by ERDA using 120 MeV Ag9+ beam.
Economical and Engineering Scalability of Complex Hydrides as New Promising Hydrogen Storage Materials
The Rate Limiting Step in the H 2 Reaction of Nanostructured Catalysed Magnesium
Mechanochemical Synthesis of Mg 2 FeH 6 -Based Nanocomposites and the Effects of Different Additives on its H-Sorption Properties
Cluster Size Dependence of Hydrogen Desorption from Alkali Metal Hydride and Ammonia
Mechanochemical Synthesis and Characterization of Aluminium Hydride
Can Reduced Size of Metals Induce Hydrogen Absorption: ZrAl 2 Case
Sorption Properties of Dispersed MBH 4 -MgH 2 (M=Na, Li) Systems in Nano-Mesoporous Scaffolds
Grain Refinement, Solubility Limits, and Low T Hydrogen Storage in TiF 3 Catalyzed MgH 2
Interaction with Hydrogen of Highly Dispersed Magnesium Eutectic Alloys
High Pressure In Situ Diffraction Studies of the Metal-Hydrogen systems
IMC Hydrides with High Hydrogen Dissociation Pressure
Magnetic and Electronic Properties of FeH Using Synchrotron Radiation Mössbauser Spectroscopy
Poster Presentations
An Investigation of Liquid Ammonia Electrolysis for Hydrogen Production
Reactivity of TiH 2 with Lithium Ion
New Conversion Mécanism
Industrial Production of MgH 2 and its Application
Hydrogen as Promoter and Inhibitor of Superionicity in Li-N-H Systems
Pd-alloy Based thin Films for Hydrogen Sensing and Purification Applications
Hydrogen Absorption and Desorption Characteristics of High Coercivity NdDyFeCoNbCuB Sintered Magnet
Low Temperature Hydrogen Decrepitation Treatments
Transparent Yttrium Hydride thin Films Prepared by Reactive Sputtering
Effect of Annealing on Hydrogen Permeability and Microstructure of Melt Spun Amorphous and Crystalline Nb-TiNi Alloys
HYDROGEN PENETRATION THROUGH PALLADIUM MEMBRANES FROM THE FLAME OF HYDROCARBON
MATERIALS
Hydrogen Production by Alkaline Electrolysis: Surface Investigations of Materials for Membranes
Electrochemical Study of Porous Diaphragms used in Alkaline Electrolysers for Hydrogen Production
Stucture, Morphology and Hydrogen Penetrability of Vacuum Capacitors Pd-Y Formed on Porous Membrane Surface
Influence of Temperature on the Processes of Hydrogen Mass Transfer in Pd-based Membrane Tubular Element
Effect of temperature on hydrogen mass transfer processes in tubular Pd-based membrane element. Application of Pd-based membranes for the separation of gas mixtures in pure silicon production processes.
Application of Pd-Based Membranes for Separation of Gas Mixtures in Pure-Silicon Production Processes
Hydrogen Solubility and Permeability of Nb-W-Mo Alloy Membrane
Evaluation of Various Thin Films as a Hydrogen Permeation Barrier
Induced By Hydrogen Reversible and Unreversible Structural Changes In Subsurface layers of Palladium and Its Alloys Of Hydrogen
Equal Channel Angular Pressing (ECAP), a Severe Plastic Deformation (SPD) Technique to Promote Fast Hydrogen Absorption in Mg Alloys
Mg-based Multilayers: Thermodynamic Effects at the Nanoscale
Hydrogen Effect on Dislocation Nucleation in Vanadium (100) Single Crystal Examined by Nanoindentation
Coupling of Manometric and Calorimetric Measurements to Probe Unique Charaterisation of Solid Hydrogen Storage Systems
System Analysis of Thermodynamic Characteristics of Complex Alumo- and Binary Hydrides of Alkaline Metals
Separation Factor (H/D) Pd /(H/D) gas for the Pd-D-H System at High Pressure
A Computational Study of Thermodynamic Properties of M-H-F Systems for Hydrogen Storage Applications
In-Situ Diffraction Evidence for Eutectic Formation in the Mg(NH 2 ) 2 /LiNH 2 System
Thermodynamic Investigations on the Mg-D System by Pressure Composition Isotherm Measurements
Lattice Dynamics and Stability of Co and Ni Monohydrides
Phase Diagrams of Hydrogen Clathrate Hydrates: Theoretical Aspects of Hydrogen Storage Application
Combined Optical and Electrical Analysis of Phase Boundaries of Transition Metal Hydride Films
Thermodynamics of Stable and Metastable Cu-O-H Compounds
Structure of Ti0.9Zr0.1Mn1.2V0.1 and Ti0.9Zr0.1Mn1.3V0.5 and their Hydrides and Features of Hydrogen Interaction with Intermetallic Compounds. We noticed that in the plots of the isotherms ΔH – X (ΔH – partial molar enthalpy of hydrogen desorption from the hydride phase, X = H/IMC) for IMCs such as ZrMn2, ZrMn2.7 and Ti0.9Zr0.1Mn1.1V0 . 1 the enthalpy values in the plateau region decreased with increasing hydrogen concentration in the metal matrix and in some cases at a certain temperature two regions with constant enthalpy values could be distinguished. However, for the compounds Ti0.9Zr0.1Mn1.3V0.5 and Ti0.9Zr0.1Mn1.5V0.8 we discovered opposite dependences of ΔH – X that is the values of the enthalpy of hydrogen desorption from the hydride phase increased with the increase of X, but the structure of the formed hydride remained the same as the initial IMC (MgZn2) only the volume of the crystal lattice expanded about 25%.
For this purpose, two alloys Ti0.9Zr0.1Mn1.2V0.1 and Ti0.9Zr0.1Mn1.3V0.5 were prepared and their hydrides and deuterides were synthesized. The Ti0.9Zr0.1Mn1.2V0.1 – H system was researched by the calorimetric method and P – X isotherms were measured at temperature range from 62 to 132ºC and hydrogen pressure up to 60 atm. The structural data show that there are differences in the distribution of metal atoms at positions of metal matrix.
Calorimetric Study of Hydrogen Interaction with CaSi
Thermodynamic Aspects of Hydrogen Interaction with Laves Phase Structured Intermetallic Compounds
Desolvation, Thermal Decomposition and Thermodynamic Properties of Lanthanide Borohydrides of Yttrium Subgroup
New Clathrate Phase in the Water-Hydrogen System
Hydrogen Interaction with Ti-Zr-Hf Alloys in the SHS Mode
The Effect of Anion Substitution in Metal Borohydrides
Electrochemical Hydrogen Charging of Sigma Phase Investigated by In-Situ Neutron Diffraction
The Tetragonal-to-Orthorhombic Phase Transformation in Ammonia Borane and in Its Deuterium Substituted Compounds
Novel Metal Borohydrides – Crystal Structures, Thermal Decomposition and Reactivity
Analysis of Processes in Metal Materials Caused by Presence of Hydrogen with the Help of Methods of Acoustomicroscopy Defectoscopy
Phase Transformation in Ti-V-Cr-H Composition
The Influence of Contamination by Light Elements on the Structural Stability of CoSn Under Compression
In this case, it is important to know the physical nature of the peaks observed in the spectra of hydrogen thermosorption of metals. With Ti, Pd as examples, the correlation was established between the peaks in deuterium thermodesorption spectra and the phase transformations in the metal-hydrogen system [1-3]. The results obtained in the studies indicate the conclusion that the TDS technique can be related to the methods that allow the estimation of the structural state of materials.
The presence of these peaks correlates with the information that the phase transformations that occur in the process of deformation follow the scheme γ ⇒ ε ⇒ α, where γ indicates the austenite with the fcc lattice, α is the martensite with the bcc lattice, and ε is the martensite with the hcp lattice, which is the intermediate phase. An increase in the percentage reduction (curve 3) is accompanied by an increase in the α-martensite concentration and a decrease in the ε-martensite with the result that at δ =44% the TD spectrum of deuterium has a single peak with Tm ~ displayed. 520 K. Note that the peaks in the spectra are broad, confirming a high degree of dispersion of the resulting phases.
In-Situ X-ray Powder Diffraction Cell for Hydrogen Absorption Studies
Hydrogenation of Pd-Ni and Pd-Cu Alloys
Effect on the Interatomic Interactions
The Role of Hydrogen and Vacancies in Structural Transformations of Palladium and Its Alloys
Synthesis of ZrCr
2 Laves Phases by Arc Melting in Ar/H
2 Plasmas
Thermal Decomposition and Reversibility of Ca(BH 4 ) 2
Hydrogen Dissolution in Pd Nanoparticles Effect on Their Structure in Metal-Carbon Nanocomposite
The aim of this research is to study the influence of partial substitution of Ho with other hydride-forming elements – Zr or Hf – on the structure and hydrogenability of the HoFe2 alloy. The ternary alloys Ho1-xZrxFe2 and Ho1-xHfxFe2 (0 ≤ x ≤ 0.2) are single-phase and adopt the structure of the parent binary compound HoFe2 (type C15). Partial substitution of Ho (rHo=1.76 Å) with smaller Zr (rZr=1.60 Å) or Hf (rHf=1.59 Å) linearly decreases the lattice parameter of the cubic Laves phase.
The hydrogenation ability and structural characteristics of Ho1-xZrxFe2 alloys and their hydrides are similar to those of Ho1-xHfxFe2 alloys. The specific increase in unit cell volume per absorbed hydrogen atom for the Zr-containing alloys increases linearly from 2.7 Å3 to 3.5 Å3, remaining constant for the Hf-substituted material. The results of refining the crystal structure of pseudobinary Ho1–xMxFe2 (M=Zr, Hf; . 0 ≤ x ≤ 0.2) compounds and their hydrides will be presented and discussed.
Lattice Strain Formation in Ti-V-Mn During the First Absorption and Desorption
Pressure-Induced Phase Transformation of LaH 3 Studied by Raman and UV-Visible Absorption Spectroscopy
Investigation of Interaction of Ti–Al Alloys with Ammonia
We find a formation of Re-hydride after decomposition of silane and reaction of hydrogen with the Re-packing. We also identify the recently reported metallic hcp phase of silane [5] as PtH [8], which is formed upon the decomposition of silane and reaction of released hydrogen with platinum metal present in the sample chamber. In this study, the influence of hydrogen on the martensitic transformation behavior of TiNi shape memory alloys produced by a combination of MA and DC sintering was investigated.
The martensitic transformation behavior was determined by differential scanning calorimetry (DSC), and the hydrogen desorption property was measured by thermal desorption spectroscopy (TDS). The martensitic and reverse transformations were observed after hydrogenation, but the enthalpy of the martensitic transformation was smaller than that of the reverse transformation. Finally, the enthalpy of the martensitic transformation turned to be similar to that of the reverse transformation after 1 cycle of the transformations.
Boron - Impurity Trap for Atomic Hydrogen in Nickel Alloys
Hydrogen Capacity of Structural Defects in the Nickel Coatings Doped with Boron
Influence of Hydrogen on Magnetic Arrangement of the Intermetallic Compounds RNi (R=Sm, Tb, Dy)
It is believed that the formation of NaBH4 does not occur directly, but follows the formation of NaMgH3. In order to determine the optimal conditions for the formation of 2NaBH4-MgH2 from 2NaH-MgB2 and to better understand the reaction mechanism, several experiments were performed. The absorption of hydrogen by the previous ball-milled compounds (2NaH-MgB2) was studied in isotherms under conditions at several temperatures C) at a H2 pressure of 100 bar.
Experimental results will be presented to discuss the optimal conditions for hydrogen absorption in the indicated system.
Modelling of Discrete TDS-Spectrum of Hydrogen Desorption
Nuclear Magnetic Resonance Study of Hydrogen Motion in A15-Type Ti 3 SbH x
Structural Phase Transitions and Dynamic Relaxation Processes in Calcium Borohydride
Rotational and Diffusional Dynamics in Calcium Borohydride from DFT and Quasi Elastic Neutron Scattering
Hydrogen Trapping Properties of Zr-based Intermetallic Compounds in the Presence of Contaminant Gases
Hydrogen Absorption Behavior of Nano-Crystalline Mg Thin Films
Synthesis of Si Nanoparticles to Improve Reaction Kinetics and Thermodynamic Properties of Magnesium Hydride
Low Temperatures Hydrogen Evolution from LiAlH 4 -Based Systems
The Effect of Surface Preparation on the Depth of Hydride Initiation at Lightly Oxidized Uranium Surfaces


![Fig. 1 Hydrogen desorption mechanisms at various hydrides surfaces/interfaces; (a) AlH 3 [1] (b) LiBH 4 [2] (c) NaBH 4 + MgH 2](https://thumb-eu.123doks.com/thumbv2/pdfplayernet/435720.50644/65.892.215.606.526.743/hydrogen-desorption-mechanisms-various-hydrides-surfaces-interfaces-libh.webp)