The
Brazilian
Journal
of
INFECTIOUS
DISEASES
w w w . e l s e v i e r . c o m / l o c a t e / b j i d
Original
article
Acute
exacerbation
of
chronic
hepatitis
B
virus
infection
in
renal
transplant
patients
Christini
Takemi
Emori
a,∗,
Renata
Melo
Perez
b,
Carla
Adriana
Loureiro
de
Matos
a,
Silvia
Naomi
Oliveira
Uehara
a,
Patricia
da
Silva
Fucuta
Pereira
a,
Ana
Cristina
Amaral
Feldner
a,
Roberto
José
de
Carvalho-Filho
a,
Ivonete
Sandra
de
Souza
e
Silva
a,
Antonio
Eduardo
Benedito
Silva
a,
Maria
Lucia
Gomes
Ferraz
aaDivisionofGastroenterology,UniversidadeFederaldeSãoPaulo(UNIFESP),SãoPaulo,SP,Brazil
bInternalMedicineDepartment,UniversidadeFederaldoRiodeJaneiro(UFRJ),RiodeJaneiro,RJ,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received5April2014 Accepted11June2014
Availableonline29August2014
Keywords:
Renaltransplantation HepatitisB
ALTflare Lamivudine
a
b
s
t
r
a
c
t
Introduction:ThereisscarceinformationregardingclinicalevolutionofHBVinfectionin renaltransplantpatients.
Aims: ToevaluatetheprevalenceofacuteexacerbationinHBV-infectedrenaltransplant patientsanditsassociationwiththetimeaftertransplantation,presenceofviralreplication, clinicalevolution,anduseofantiviralprophylaxis.
Materialsandmethods:HBVinfectedrenaltransplantpatientswhounderwentregular follow-upvisitsat6-monthintervalswereincludedinthestudy.Thecriteriaadoptedtocharacterize exacerbationwere:ALT>5×ULNand/or>3×baselinelevel.Predictivefactorsof exacerba-tionevaluatedwereage,gender,timeondialysis,typeofdonor,post-transplanttime,ALT,
HBeAg,HBV-DNA,HCV-RNA,immunosuppressivetherapy,anduseofantiviralprophylaxis.
Results:140HBV-infectedrenaltransplantpatientswereincluded(71%males;age46±10 years;post-renaltransplanttime8±5years).Duringfollow-up,25%(35/140)ofthepatients presentedexacerbation within3.4±3yearsafterrenal transplant.Viralreplicationwas observedinallpatientswithexacerbation.Clinicaland/orlaboratorysignsofhepatic insuf-ficiencywerepresentin17%(6/35)ofthepatients.Threepatientsdiedasaconsequenceof liverfailure.Inunivariateanalysisvariablesassociatedwithexacerbationwerelessfrequent useofprophylactic/preemptivelamivudineandofmycophenolatemofetil.Lamivudineuse wastheonlyvariableindependentlyassociatedwithexacerbation,withaprotectiveeffect. Conclusions: AcuteexacerbationwasafrequentandsevereeventinHBV-infectedrenal trans-plantpatients.Prophylactic/preemptivetherapywithantiviraldrugsshouldbeindicatedfor allHBsAg-positiverenaltransplantpatients.
©2014ElsevierEditoraLtda.Allrightsreserved.
∗ Correspondingauthorat:RuaPrimeirodeJaneiro,apto153,SãoPaulo,SP04044-060,Brazil.
E-mailaddress:christinisp@yahoo.com.br(C.T.Emori). http://dx.doi.org/10.1016/j.bjid.2014.06.004
Introduction
AccordingtotheWorldHealthOrganization,thenumberof
chronichepatitisBvirus(HBV)carriersexceeds350million
worldwide.1AmongrenaltransplantpatientsHBVinfection
continuestobeanimportantcauseofmorbidityand
mortal-ity,althoughitsincidencedeclinedaftertheintroductionof hepatitisBvaccinein1982andasaresultofimprovedoverall careduringhemodialysis.2Theprevalenceofchronichepatitis
Bafterkidneytransplantationrangesfrom2to21%according
togeographicregions.3
AlthoughdataaboutthenaturalcourseofHBVinfection
inrenaltransplantrecipientsare scarce,evidenceindicates
that viralreplication isaccelerated byimmunosuppression
andthatHBV-relatedliverdiseaseismoreaggressiveinrenal
transplantrecipients.4Somestudieshavedemonstratedthat
theprogressionofliverdiseasecouldoccurinmorethan80%
ofHBsAg-positiverenaltransplantpatients,withahigh
mor-talityrate5andhigherincidenceofgraftloss.6
Reactivation of HBV infection in immunosuppressed
patientscanbeseparatedintothreephases: (1)increasein
HBVreplication;(2)appearanceofhepaticinjury(ALTflares) and(3)recovery.7Biochemicalevidenceofreactivationis
char-acterizedbyALTflaresandsometimesassociatedlossofliver
functionfromranging30–70%indifferentcaseseries.8More
recently,Murakamietal.9reportedreactivationin45%(5/11)
ofrenaltransplantpatientswithhepatitisBsurfaceantigen
positiveandSavasetal.10observedreactivationin70%(14/20)
withinameanperiodof16.3±7.1monthsafter
transplanta-tion.
In view of the severity of reports, prophylaxis with
lamivudine, a nucleoside analog, has become common
practice to prevent reactivation of HBV infection after
renal transplantation.11 However,prolonged administration
oflamivudinemay resultinthedevelopment oftreatment
resistance.12Therateofemergenceofresistancemutations
progressivelyincreaseswithduration oftherapy,exceeding
30%withintwoyearsinimmunocompetentpatients.13
Unfor-tunately,resistanceisacceleratedaftertransplantationandits occurrenceishigherinrenaltransplantpatients(30–57%after 1–2years),reflectingsteroid-enhancedHBVreplication.14
InviewofthehighprevalenceofHBVinfectioninthis spe-cialgroupofpatientsandthescarcedataregardingthenatural
historyofinfection,abetterunderstandingoftheevolution
ofHBV inrenaltransplantrecipientsisnecessaryto
estab-lishthebestmanagementstrategyforthesepatientsandthe
indicationofantiviraltreatmentinthispopulation.
Theobjectivesofthepresentstudyweretoevaluatethe
prevalenceofbiochemical exacerbationin renaltransplant
patientschronically infectedwithHBV andtoevaluatethe
factorsrelatedtoitsoccurrence.
Materials
and
methods
Patients
Renal transplant patients followed-up ata post-transplant
outpatientclinicintheFederalUniversityofSaoPaulo,Brazil,
whowerepersistentlyHBsAgpositiveformorethan6months,
were referredtoliverevaluationattheHepatitisoutpatient
clinicofthesame institution.Thepatientswho underwent
regularfollow-upvisitsat6-monthintervalswereincludedin
thestudy.Patientsconsumingmorethan50gofalcoholper
dayandHIV-infectedpatientswereexcluded.
Method
Variablesanalyzed
All patients were evaluatedregarding age,gender, time on
dialysis,typeofdonor(cadavericvslivingdonor),timeof
post-transplantfollow-up, alanineaminotransferase(ALT)index,
HBeAg,quantitativeHBV-DNA(determinedbyreal-timePCR),
anti-HCV,HCV-RNA(determinedbyreal-timePCR),
immuno-suppressive therapy, and use or not of lamivudine, after
reviewingthedatafrommedicalcharts.Histologicalvariables
were also analyzedin patientssubmitted to aliver biopsy
afterkidneytransplantation.Aliverbiopsywasindicatedin
patientswithevidenceofviralreplication,irrespectiveofALT levels.Thepatientsweredividedintotwogroupsaccordingto thestageofhepaticfibrosisusingtheMETAVIRscoringsystem (F0–F2vs.F3–F4).15
Biochemicalandserologicaltests
Forbiochemicalanalysis,serumALTwasreportedasthe
quo-tientbetweenthemeanvalueobtainedandtheupperlimitof
normal(ULT)forgender.
HBeAgwasdeterminedusingtheHBeAgIMxassay(Abbott
Laboratories,Chicago,IL,USA).Anti-HCVreactivitywas deter-minedbytheIMxHCVassay,version3.0(AbbottLaboratories).
Moleculartests
HepatitisCvirus-RNA. HCV-RNAwasdeterminedinall
anti-HCVpositivesamplesbyqualitativePCRusingAmplicorkits
(RocheDiagnostics,Basel,Switzerland).Thelowerdetection
limitofthemethodwas50IU/mL.
Hepatitis B virus-DNA.Quantitative real-time PCR assays
wereperformedusingtheABIPRISM7700sequencedetection
system (Applied Biosystems). HBV-DNA was inconsistently
detectedindilutionscontaininglessthan50IU/ML,whichwas the3SDlimitofdetection(99.9%confidenceinterval).
Histologicalanalysis
Aliverbiopsywasindicatedinallpatientsshowingevidence
ofHBVreplication.Allbiopsyslideswerereviewedbyasingle
pathologist.Thestageoffibrosiswasanalyzed
semiquantita-tivelybasedontheMETAVIRclassification(F0–F4).15
Biochemicalexacerbation
Renal transplant patients under follow-up were evaluated
regarding the occurrenceof biochemicalexacerbation. The
followingcriteriawereadoptedforthecharacterizationof bio-chemicalexacerbation:ALT>5timestheupperlimitofnormal and/or>3timesthebaselinelevel.16Inordertoidentify
predic-tivefactorsofexacerbationthefollowingvariablesandthose
citedabovewereevaluatedinpatientswithandwithout
bio-chemicalexacerbation:intervalbetweentransplantationand
theoccurrenceofexacerbation,presenceofascites,jaundice
Table1–GeneralcharacteristicsoftheHBsAg-positive renaltransplantpatients(n=140).
Malegender(%) 99(71)
Age(years),mean±SD 46±10
Timeondialysis(years) 5.2±3.8
Cadavericdonor(%) 68(49)
Post-transplanttime(years),mean±SD 8±5
HCV-RNApositive(%) 28(20)
HBeAgpositive(%) 70(50)
HBV-DNA(log)a,mean±SD 6.64±2.09
HBV-DNA(%)a
<200IU/mL 10(9.5)
200–2000IU/mL 8(7.6)
≥2000IU/mL 87(83)
Fibrosis(F3–4)(%)b 21(23.1) Tripleimmunosuppression(%) 107(76%)
Immunosuppression(%)
RegimenincludingMMF 42/140(32) RegimenincludingAZA 89/140(63.6) RegimenincludingCSA 87/140(62)
MMF,mycophenolatemofetil;AZA,azathioprine;CSA,cyclosporine A.
a n=105.
b n=95.
albuminlevels,prothrombinactivity,serologicalormolecular
markersofviralreplication,andoutcome(spontaneouscure,
treatmentresponsewithlamivudine,ordeath).Thepresence
ofclinicalsignssuchasascites,encephalopathyorareduction
inserum albuminlevels (<3g/dL) andprothrombin activity
(<70%)wasdefinedassignsofhepaticinsufficiency.
Aliverbiopsywasobtainedatexacerbation,whenpossible, forstagingfibrosis,gradeofnecroinflammatoryactivity,and detectionofHBcAgintissue.15
ThestudywascarriedoutinaccordancewiththeHelsinki
Declaration.Allpatientsselectedforthestudygavewritten
informedconsent.Thestudyprotocol wasapprovedbythe
localEthicalCommittee(number2143/08).
Statisticalanalysis
TheChi-squaretestwasusedforcomparingcategorical
vari-ables and the Student t-test and Mann–Whitney test for
numericalvariables.Binarylogisticregression analysiswas
performedtoidentifythevariablesindependentlyassociated
tobiochemical exacerbation. Alevel ofsignificanceof0.05
(˛=5%)wasadopted.
Results
Atotalof140HBsAg-positiverenaltransplantpatientswere
followedupattheHepatitisoutpatientclinicofFederal
Univer-sityofSaoPaulo.Ninety-nine(71%)weremale,themeanage
was46±10years(range:17–74).Thepatientswereincluded
indifferenttimepointsafterrenaltransplantwithamean
timeofpost-transplantfollow-upof8±5years.Thegeneral
characteristicsofthepatientsareshowninTable1.
Duringfollow-up,25% (35/140)ofthe patientspresented
elevatedALT,characterizingbiochemicalexacerbation. This
eventwasobservedwithinameanperiodof3.4±3yearsafter
kidneytransplantation(medianoftwoyears).
Amongthepatients presentingexacerbation,viral
repli-cation(HBV-DNAand/orHBeAgand/orHBcAgintissue)was
observedinallpatientsinwhomthisvariablecouldbe
ana-lyzed(n=33).Viralloadwasdeterminedin20/35patientsby
real-time PCRand the median was29×106IU/mL inthese
patients.
Aliverbiopsywasobtainedfrom83%(29/35)ofthepatients
withbiochemicalexacerbation.Withrespecttofibrosis,76%
(22/29)ofthepatientshadfibrosisstage0–2and24%(7/29)had
stage3–4.Mildnecroinflammatoryactivitywasobservedin
27.5%(8/29)ofthepatients,moderateactivityin65.5%(19/29), andintenseactivityin7%(2/29).
Clinical and/or laboratory signs ofhepatic insufficiency
wereobservedin17%(6/35)ofthepatients,encephalopathy
in17%(6/35),ascitesin11.4%(4/35),andsignificantlaboratory abnormalitiesin14%(5/35).Threeofthesepatientsdiedasa
consequenceofliverfailuredespitetheuseoflamivudinein
twoofthesecases.
Amongthe35patientswithexacerbation,10/35werenot
treatedwithlamivudine.Spontaneousresolutionofthe
bio-chemical abnormalities without loss of liver function was
observedin9/10nottreatedpatientsandonepatientdiedwith
liverfailure.Accordingtolamivudineuse,inonly9%(3/35)
ofthepatientsthedrugwasusedaspreemptive/prophylactic
therapy.Treatmentwithlamivudineafteronsetof
exacerba-tionwasadministeredto22/35patients,withclinicalresponse
in18/22.ThemeantimetoALTnormalizationwas8.8months
inthesepatients.Whenlamivudinewasgivenatthetimeof
exacerbation,nodifferenceinmortalityduetohepatic
insuf-ficiencywasobservedbetweentreatedanduntreatedpatients
(9%vs.10%;p=0.69).
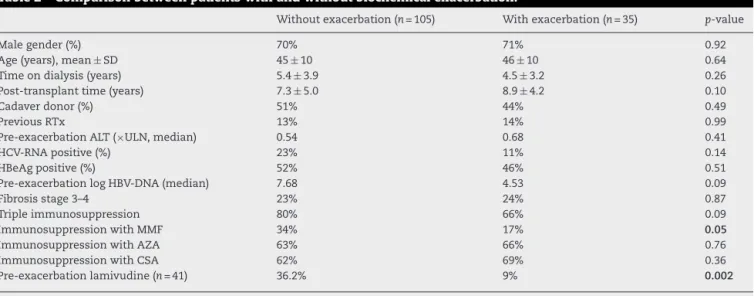
Table 2 lists the clinical and laboratory characteristics
associated with biochemical exacerbation. Variables
asso-ciated with the occurrence of biochemical exacerbation
were a less frequent use of mycophenolate mofetil in the
immunosuppressionregimenandalowerproportionof
pro-phylactic/preemptiveadministrationoflamivudine.
In the logistic regression model only pre-exacerbation
lamivudine use was found to beindependently associated
withbiochemicalexacerbation(Table3)showingaprotective effect.
Discussion
Chronic HBV infection presents an unfavorable course in
immunosuppressed patients. Faster progressionto hepatic
fibrosisandahigherfrequencyofcomplicationsofliver
dis-easehavebeendemonstratedinrenaltransplantrecipients.8
Additionally,casesofreactivationofHBVinfectionafterrenal
transplantationhavebeenreported,sometimespresentinga
fulminant course.17 However, dataregardingthe frequency
andseverityofepisodesofbiochemicalexacerbationinrenal
transplantpatientsinfectedwithHBVarescarce.
Inthepresentstudy,biochemicalexacerbationwasa
fre-quenteventinrenaltransplantpatientschronicallyinfected
withHBV andwasobservedin25%ofthepatientsstudied
Table2–Comparisonbetweenpatientswithandwithoutbiochemicalexacerbation.
Withoutexacerbation(n=105) Withexacerbation(n=35) p-value
Malegender(%) 70% 71% 0.92
Age(years),mean±SD 45±10 46±10 0.64
Timeondialysis(years) 5.4±3.9 4.5±3.2 0.26
Post-transplanttime(years) 7.3±5.0 8.9±4.2 0.10
Cadaverdonor(%) 51% 44% 0.49
PreviousRTx 13% 14% 0.99
Pre-exacerbationALT(×ULN,median) 0.54 0.68 0.41
HCV-RNApositive(%) 23% 11% 0.14
HBeAgpositive(%) 52% 46% 0.51
Pre-exacerbationlogHBV-DNA(median) 7.68 4.53 0.09
Fibrosisstage3–4 23% 24% 0.87
Tripleimmunosuppression 80% 66% 0.09
ImmunosuppressionwithMMF 34% 17% 0.05
ImmunosuppressionwithAZA 63% 66% 0.76
ImmunosuppressionwithCSA 62% 69% 0.36
Pre-exacerbationlamivudine(n=41) 36.2% 9% 0.002
Boldvaluesindicateslevelofsignificanceof0.05wasadopted.
MMF,mycophenolatemofetil;AZA,azathioprine;CSA,cyclosporine;RTx,renaltransplantation;ULN,upperlimitofnormal.
patients,biochemicalexacerbationisfrequentandgenerally relatedtoHBeAgseroconversion.16Yuenetal.followedupa
cohortof3063patientsandobservedbiochemical
exacerba-tionin35% ofpatientsover ameanfollow-up periodof29
months.18Inrenaltransplantpatientsexacerbationisrelated
toadistinctphenomenonandhasamarkednegativeimpact
becauseofthegreaterseverityoftheseepisodesinthisspecific groupofpatients.19
Inviewoftheimmunosuppressiontowhichtheyare
sub-mitted,renal transplant patients generally present intense
viremia,20withveryhighlevelsofHBV-DNAevenin
HBeAg-negativepatients.21Matosetal.4 demonstratedaviralload
higherthan2.000IU/mLin80%ofHBeAg-negativetransplant
patientswithchronichepatitisB.Mostofthesecases
proba-blycorrespondtomutationsinthepre-coreorcorepromoter
regionofHBVsincenoHBeAgwasdetectedandviral
replica-tionwasclinicallysignificant.
TheoccurrenceofbiochemicalexacerbationofHBV
infec-tioninrenaltransplantpatientsismorerelatedtotheimmune
reconstitutionobservedafterreductionof
immunosuppres-sion. Patients receive more intense immunosuppression
duringtheimmediatepost-transplantperiod,whichpromotes
asignificantincreaseinviralloadassociatedwithapattern
ofimmunotolerancetoHBV.Theprogressivereductioninthe
dose ofthe immunosuppressiveagents over time leads to
improvementintheimmunestatusandconsequentgreater
hepatocellulardamageduetothelossofimmunotolerance
tothevirus.7Inaddition,exacerbationmightbemediatedby
othermechanisms,suchashepatotoxicityofthe
immunosup-pressivedrugs.Inthisrespect,azathioprineandcyclosporine
havebeenshowntocauseliverinjuryaccompaniedbya
sig-nificantincreaseinaminotransferases.22
Inthepresentstudy,viralreactivationwasobservedwithin anaverage3.4±3years(medianof2years)afterrenal
trans-plantation, incontrasttoother studiesinwhichthis event
usuallyoccurred withinthefirst post-transplantyear.9–11,20
Thisfindingsuggeststhatexacerbationmaynotbesuchan
earlyevent,andmaybepossiblyrelatedtomodificationsofthe
immunosuppressiveregimenastimegoesby.Thisshouldbe
consideredintherecommendationsregardingthedurationof
prophylacticantiviraltreatment,whichshouldbeprolonged
duringthepost-transplantperiodandshouldnotberestricted tothefirst12or24monthspost-transplant.
In additiontoits high frequency,biochemical
exacerba-tionwasaseriouseventandwasassociatedwithclinicaland
laboratorysignsofhepaticinsufficiencyin17%(6/35)ofthe cases.Threeofthesepatientsdiedasaconsequenceofhepatic
failure. Another study involving the same type ofpatients
alsoreportedahighrateofliverdysfunction(30%)associated withreactivationofHBVinfection.11Inthepresentstudy it
wasnotpossibletoidentifythefactorsrelatedtothe
sever-ityofreactivation,duetothesmallnumberofpatientswith
hepatic failure. Thus, all HBsAg-positive kidney transplant
patientsshouldbecarefullymonitored.Biochemical
exacer-bationneedstoberapidlyrecognizedandcontrolmeasures
shouldbereadilyadoptedinviewofthehighmorbidityand
mortalityrelatedtothisevent.
In view of the high frequency and severity of the
bio-chemicalexacerbationthatoccurinrenaltransplantpatients
infectedwithHBV, itwould beimportanttodeterminethe
associatedfactorsinordertoallowforearlyidentificationof patientsatriskofthisevent.Nodemographic,epidemiological orlaboratoryvariablescouldpredicttheoccurrence biochemi-calexacerbation.Intheunivariateanalysis,theonlyvariables
Table3–Logisticregression(finalmodel).
p-value OR 95%CI
thatwere associatedwith this eventwere the inclusion of
mycophenolatemofetilintheimmunosuppressionregimen,
whichwaslessfrequentamongpatientswithexacerbation,
andpreemptiveorprophylacticadministrationoflamivudine,
whichwasalsolessfrequentamongpatientswith
exacerba-tion.
One possible explanation for the less frequent use of
mycophenolatemofetilinthe immunosuppressionregimen
amongpatientswithexacerbationmighthavebeenthepotent
immunosuppressiveeffectofthisdrugonthehostimmune
response,whichwouldeventuallyreduceimmunomediated
hepatocellulardamagebymoreefficientlypreventingimmune
reconstitutionovertime.Anotherpossibilityisrelatedtothe
antiviraleffect ofmycophenolate mofetilininhibiting HBV
replication,whichhasbeendemonstratedinvitro.23
However,themostimportantobservationinthisstudywas
thatlamivudinewaseffectiveinthepreventionof
exacerba-tionasdemonstratedbythesignificantlyhigherproportion
ofpatientsusingthisdruginthegroupwithoutexacerbation
whencomparedtothegroupwithexacerbation(36%vs.9%;
p=0.002).Thiswastheonlyvariableindependentlyassociated
withexacerbationinthis study,supportingthe
recommen-dationofpreemptive/prophylacticadministrationofantiviral
prophylaxistoallpatientswithchronicHBVinfection receiv-ingarenaltransplant.
Ontheotherhand,whenlamivudinewasinitiatedatthe
timeofexacerbation,nodifferenceinmortalityduetohepatic
insufficiency was observedbetween treated and untreated
patients.InthestudyofHanetal.,11althoughlamivudine
pro-motednormalizationofALTlevelsand suppressionofviral
replicationinalltreatedcases,itdidnotpreventthe progres-sionofhistologicalinjury.Thesedatasuggestthatlamivudine haspoorefficacyasarescuedrugincasesofexacerbationand
thattreatmentshouldpreferentiallyandideallybeinitiated
beforetransplantationsinceimmunosuppressionhasnotyet
beeninstitutedandthepatientthereforepresentslowerviral loads.Nevertheless,administrationofantiviraldrugsasearly
aspossibleshouldbeconsideredevenforpatientswhohave
notreceivedpre-transplantprophylactic/preemptivetherapy.
Finally,sofartherearenostudiesevaluatingmorepotent antiviraldrugswithahighergeneticbarriertoresistancein thisparticularsubgroupofpatients.However,thisnew gener-ationofdrugswillprobablybecometheidealoptiontoprevent exacerbationofHBVinfectioninrenaltransplantpatients.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
TheauthorswishtothankDr.ValériaPereiraLanzoni, Depart-mentofPathology,FederalUniversityofSãoPaulo,SãoPaulo,
BrazilandDr.JoséOsmarMedinadeAbreuPestana,Division
ofNephrology,FederalUniversityofSãoPauloandHospitaldo RimeHipertensão,SãoPaulo,Brazil.
r
e
f
e
r
e
n
c
e
s
1.LavanchyD.HepatitisBvirusepidemiology,diseaseburden, treatment,andcurrentandemergingpreventionandcontrol measures.JViralHepat.2004;11:97–107.
2.CDC.Recommendationsforpreventingtransmissionof infectionsamongchronichemodialysispatients.MMWR RecommRep.2001;50:1–43.
3.TsaiMC,ChenYT,ChienYS,ChenTC,HuTH.HepatitisB virusinfectionandrenaltransplantation.WorldJ Gastroenterol.2010;16:3878–87.
4.MatosCA,PerezRM,LemosLB,etal.Factorsassociatedwith theintensityofliverfibrosisinrenaltransplantpatientswith hepatitisBvirusinfection.EurJGastroenterolHepatol. 2007;19:653–7.
5.ParfreyPS,ForbesRD,HutchinsonTA,etal.Theimpactof renaltransplantationonthecourseofhepatitisBliver disease.Transplantation.1985;39:610–5.
6.MathurinP,MouquetC,PoynardT,etal.ImpactofhepatitisB andCvirusonkidneytransplantationoutcome.Hepatology. 1999;29:257–63.
7.HoofnagleJH.ReactivationofhepatitisB.Hepatology. 2009;49:S156–65.
8.FornaironS,PolS,LegendreC,etal.Thelong-termvirologic andpathologicimpactofrenaltransplantationonchronic hepatitisBvirusinfection.Transplantation.1996;62: 297–9.
9.MurakamiR,AmadaN,SatoT,etal.Reactivationofhepatitis andlamivudinetherapyin11HBsAg-positiverenalallograft recipients:asinglecentreexperience.ClinTransplant. 2006;20:351–8.
10.SavasN,ColakT,SelcukH,YilmazU,HaberalM.Clinical courseofhepatitisBvirusinfectioninrenalallograft recipients.DigDisSci.2007;52:3440–3.
11.HanDJ,KimTH,ParkSK,etal.Resultsonpreemptiveor prophylactictreatmentoflamivudineinHBsag(+)renal allograftrecipients:comparisonwithsalvagetreatmentafter hepaticdysfunctionwithhbvrecurrence.Transplantation. 2001;71:387–94.
12.FontaineH,ThiersV,ChretienY,etal.HBVgenotypic resistancetolamivudineinkidneyrecipientsand hemodialyzedpatients.Transplantation.2000;69:2090–4. 13.LauDT,KhokharMF,DooE,etal.Long-termtherapyof
chronichepatitisBwithlamivudine.Hepatology. 2000;32:828–34.
14.Tur-KaspaR,BurkRD,ShaulY,ShafritzDA.HepatitisBvirus DNAcontainsaglucocorticoid-responsiveelement.ProcNatl AcadSciUSA.1986;83:1627–31.
15.BedosaP,PoynardT.Analgorithmforthegradingofactivity inchronichepatitisC.TheMETAVIRCooperativeStudy Group.Hepatology.1996;24:289–93.
16.LokAS,LaiCL.Acuteexacerbationsinchinesepatientswith chronichepatitisBvirus(HBV)infection.Incidence, predisposingfactorsandetiology.JHepatol.1990;10: 29–34.
17.LeeWC,WuMJ,ChengCH,ChenCH,ShuKH,LianJD. Lamivudineiseffectiveforthetreatmentofreactivationof hepatitisBvirusandfulminanthepaticfailureinrenal transplantrecipients.AmJKidneyDis.2001;38: 1074–81.
18.YuenMF,YuanHJ,HuiCK,etal.Alargepopulationstudyof spontaneousHBeAgseroconversionandacuteexacerbation ofchronichepatitisBinfection:implicationsforantiviral therapy.Gut.2003;52:416–9.
20.DegosF,LugassyC,DegottC,etal.HepatitisBvirusand hepatitisB-relatedviralinfectioninrenaltransplant recipients.Aprospectivestudyof90patients. Gastroenterology.1988;94:151–6.
21.NorderH,BrattstromC,MagniusL.Highfrequencyof hepatitisBvirusDNAinanti-HBepositiveseraon
longitudinalfollow-upofpatientswithrenaltransplantsand chronichepatitisB.JMedVirol.1989;27:322–8.
22.DePinhoRA,GoldbergCS,LefkowitchJH.Azathioprineand theliver.Evidencefavoringidiosyncratic,mixed
cholestatic-hepatocellularinjuryinhumans. Gastroenterology.1984;86:162–5.