UNIVERSIDADE FEDERAL DO RIO GRANDE DO NORTE CENTRO DE CIÊNCIAS DA SAÚDE
PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS DA SAÚDE
AVALIAÇÃO DE EFEITOS BIOLÓGICOS DE ANTIINFLAMATÓRIOS DERIVADOS
DO ÁCIDO PROPIÔNICO ATRAVÉS DE MODELOS EXPERIMENTAIS EM NÍVEL
MOLECULAR E CELULAR.
ii
MARCIA DE OLIVEIRA PEREIRA
AVALIAÇÃO DE EFEITOS BIOLÓGICOS DE ANTIINFLAMATÓRIOS DERIVADOS
DO ÁCIDO PROPIÔNICO ATRAVÉS DE MODELOS EXPERIMENTAIS EM NÍVEL
MOLECULAR E CELULAR.
Dissertação apresentada à Universidade Federal do Rio Grande do Norte- UFRN, para a obtenção do título de Mestre em Ciências da Saúde pelo programa de Pós- graduação em Ciências da Saúde.
Orientador: PROF. DR. MARIO BERNARDO-FILHO
iii
UNIVERSIDADE FEDERAL DO RIO GRANDE DO NORTE
CENTRO DE CIÊNCIAS DA SAÚDE
PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS DA SAÚDE
Coordenadora do Programa de Pós Graduação em Ciências da Saúde Profa. Dra. Técia Maria de Oliveira Maranhão
iv
Dados Internacionais de Catalogação-na-Publicação (CIP)
P436 Pereira, Márcia de Oliveira.
Avaliação de efeitos biológicos de antiinflamatórios derivados do ácido propiônico através de modelos experimentais em nível molecular e celular / Márcia de Oliveira Pereira – Rio Grande do Norte, Natal 2010.
70 f; 30cm.
Dissertação (Mestrado em Ciências da Saúde) - Universidade Federal do Rio Grande do Norte, 2010.
Orientador: Prof. Dr. Mario Bernardo Filho
1. Ciência da saúde 2. Antiinflamatórios 3. Constituintes sanguíneos 4. DNA 5. Escherichia coli I.Título.
v
MARCIA DE OLIVEIRA PEREIRA
AVALIAÇÃO DE EFEITOS BIOLÓGICOS DE ANTIINFLAMATÓRIOS DERIVADOS
DO ÁCIDO PROPIÔNICO ATRAVÉS DE MODELOS EXPERIMENTAIS EM NÍVEL
MOLECULAR E CELULAR.
PRESIDENTE DA BANCA: Prof. Dr. Mario Bernardo-Filho (UERJ) BANCA EXAMINADORA
Prof. Dr. Irami Araújo Filho Prof. Dra. Patrícia Froes Meyer
SUPLENTES
vi
DEDICATÓRIA
Dedico este trabalho:
A meu esposo Elcio que me ensinou a ter paciência, a aceitar as minhas limitações e me deu um incrível desejo de crescer como ser humano, além de que me ensinar que o limite entre o possível e o impossível é diretamente proporcional ao nosso nível de esforço.
A minha filha Jéssica que compreendeu minha ausência, me apoiou e incentivou em todos os momentos.
vii AGRADECIMENTOS
Agradeço ao meu orientador Mario Bernardo-Filho pelo profissionalismo e ética, virtudes que a cada dia fui tendo a oportunidade de vivenciá-las.
A amiga Gabrielle, que me despertou o interesse pela pesquisa e sempre esteve ao meu lado.
Ao professor Adenilson Fonseca, no que diz respeito ao desempenho, ao compromisso, ao esforço, e a dedicação ele que foi fundamental para a existência desse trabalho.
Ao Programa de Pós-graduação em Ciências da Saúde pela oportunidade de fazer parte de um processo que hoje me faz ser uma nova profissional. Com uma visão crítica da saúde e de todas as suas manifestações no indivíduo e no mundo.
As funcionárias da Secretaria do Programa de Pós-graduação em Ciências da Saúde, que me auxiliaram e estiveram sempre solícitas.
A Deus por estar sempre presente na minha vida, e tornar tudo possível e me fazer sentir sempre que toda dificuldade é um novo desafio para um novo começo.
Ao professor Sebastião Santos Davi que foi muito solícito e disponível em todos os momentos.
viii SUMÁRIO
Sumário...viii
Lista de abreviações siglas e símbolos...ix
Resumo………...………...………...x
1. Introdução...1
2. Revisão de literatura...3
3. Artigos anexados...7
3.1. Artigo publicado...7
3.2. Artigo submetido I...12
3.3. Artigo submetidoII...27
4. Comentários, críticas e conclusões...44
5. Anexos...46
6. Referências...52
ix
LISTA DE ABREVIAÇÕES, SIGLAS E SÍMBOLOS.
BC Blood Cell (célula sanguínea)
CAPES Coordenação de Aperfeiçoamento de Pessoal de Nível Superior CNPQ Conselho Nacional de Desenvolvimento Científico e Tecnológico FAPERJ Fundação de Amparo a Pesquisa do Rio de Janeiro
FI-C fração insolúvel da célula FS-P fração solúvel da célula FI-P fração insolúvel do plasma FS-P fração solúvel do plasma
IBRAG Instituto de Biologia Roberto Alcantara Gomes LRE Laboratório de Radiofarmácia Experimental Mo molibdênio
P plasma
% ATI porcentagem de radioatividade incorporada rpm rotações por minuto
SnCl2 cloreto estanoso
99mTc tecnécio-99m
Na99mTcO4 pertecnetato de sódio
TCA ácido tricloroacético
x RESUMO
Os derivados do acido propiônico são antiinflamatórios não esteroidais inibidores irreversíveis da enzima cicloxigenase amplamente utilizados. O objetivo deste trabalho foi avaliar, através de diferentes modelos experimentais, efeitos biológicos de derivados do ácido propiônico (fenoprofeno, naproxeno, ibuprofeno e cetoprofeno) em nível celular e molecular. A marcação de constituintes sanguíneos com tecnécio–99m (99mTc) e a análise morfológica de hemácias de sangue de ratos Wistar, bem como, o crescimento, sobrevivência de culturas de Escherichia coli (E. coli) e a avaliação do perfil eletroforético plasmídios bacterianos, foram modelos experimentais utilizados para avaliação de possíveis efeitos biológicos dos antiinflamatórios. Os resultados obtidos demonstram que, de modo geral, os antiinflamatórios avaliados não foram capazes de alterar a marcação de constituintes sanguíneos com 99mTc, a morfologia de hemácias de sangue de ratos Wistar, assim como, o crescimento de culturas de E. coli e o perfil eletroforético de plasmídios. Entretanto, o naproxeno parece apresentar efeito citotóxico em culturas bacterianas, efeito genotóxico em plasmídios e diminuição da ação do cloreto estanoso em culturas de E. coli. A utilização de modelos experimentais de rápida realização e baixo custo se mostrou importante para avaliação de efeitos biológicos, contribuindo para uma melhor compreensão das propriedades dos derivados do ácido propiônico estudados. Esse trabalho teve caráter multidisciplinar e na vigência dos auxílios concedidos pela CAPES, FAPERJ e CNPq.
1. INTRODUÇÃO
Os antiinflamatórios não-esteroidais são fármacos de diferentes classes químicas, mas que apresentam propriedades terapêuticas similares, com atividade inibitória da enzima ciclooxigenase 1.
Os derivados do acido propiônico são antiinflamatórios não-esteroidais prescritos no mundo inteiro, sendo muito utilizados para doenças músculo-esqueléticas e reumatológicas 2. Apesar de alguns possíveis efeitos biológicos não terem ainda sido descritos, estes fármacos são muito utilizados para tratamento da dor e inflamação 3.
Fármacos inibidores das enzimas cicloxigenase são amplamente utilizados no mundo inteiro, e devido a ações atribuídas ao uso desses fármacos, alguns já foram retirados de circulação devido a identificação de efeitos biológicos deletérios 4,5.
Modelos experimentais têm sido utilizados para avaliar efeitos biológicos de produtos naturais ou sintéticos. A marcação de constituintes sanguíneos com tecnécio– 99m (99mTc), a morfologia de hemácias 6-9, o crescimento 10 e a sobrevivência de
culturas bacterianas de Escherichia coli 11, tem sido usados para avaliar estes efeitos em nível celular e, o perfil eletroforético de plasmídios bacterianos, para avaliação destes efeitos em nível molecular 12.
O objetivo deste estudo foi avaliar efeitos biológicos de antiinflamatórios derivados do ácido propiônico (ibuprofeno, naproxeno, cetoprofeno e fenoprofeno) através de modelos experimentais em nível molecular e celular.
2
Janeiro. Os experimentos foram possíveis através de convênio firmado entre a Universidade do Estado do Rio de Janeiro e a Universidade Federal do Rio Grande do Norte, sob a orientação do Professor Doutor Mario Bernardo Filho e na vigência dos auxílios concedidos pela CAPES, FAPERJ e CNPq.
3
2. REVISÃO DE LITERATURA
Drogas antiinflamatórias não esteroidais derivadas do ácido propiônico, como o ibuprofeno, naproxeno, cetoprofeno e fenoprofeno, estão entre as drogas mais amplamente prescritas para o tratamento de dor, febre e inflamação 1,11. Estes
fármacos são extensamente utilizados para o tratamento de doenças músculo-esqueléticas, tais como traumatismos, artrite reumática, espondilite anquilosante e artrite gotosa, bem como outras enfermidades, com a dismenorréia 12.
As propriedades farmacodinâmicas dos derivados do ácido propiônico são semelhantes às propriedades de outros antiinflamatórios não esteroidais. Estes fármacos são inibidores eficazes da ciclooxigenase, embora exista variação considerável em sua potência 3,11,12. O naproxeno, por exemplo, é vinte vezes mais
potente do que a aspirina. Enquanto o ibubrofeno, o fenoprofeno, e a aspirina são eqüipotentes como inibidores da ciclooxigenase alterando a função plaquetária e prolongando o tempo de sangramento 3.
Estudos demonstraram que efeitos biológicos de produtos naturais e sintéticos podem ser avaliados através de modelos experimentais simples, de rápida realização e baixo custo 4,6,8.
O 99mTc é o radionuclídeo mais utilizado para obtenção de imagens cintilograficas do tipo SPECT (single photon emission computed tomography) devido às propriedades de emissão de um fóton de 140 keV, de possuir meia vida física de 6 horas, impacto ambiental desprezível e ser facilmente obtido em gerador13. Esse radionuclídeo também
4
A marcação de constituintes com tecnécio-99m (99mTc) tem sido utilizada como um modelo experimental para avaliação de transporte de íons (pertecnetato e estanoso) estrutura e função da membrana plasmática 14-17. Essa técnica é de grande relevância na medicina nuclear 18-20.
Constituintes sanguíneos marcados com 99mTc são radiobiocomplexos que podem ser utilizados na detecção de hemorragias gastrintestinais 21, hemangiomas 22
na avaliação da função cardíaca 23, bem como na medida do fluxo sanguíneo em artérias periféricas 24.
Tem sido proposto que no processo de marcação de constituintes sanguíneos, o íon pertecnetato atravessaria a membrana celular por troca com o íon cloreto e/ou bicarbonato através do canal de ânions (banda-3) 25, e o íon estanoso, através dos
canais de cálcio 26.
Autores têm descrito que produtos naturais e fármacos sintéticos podem interferir na radiomarcação dos constituintes sanguíneos com 99mTc 27,4,7 comprometendo a
interpretação de resultados clínicos ou a repetição de exame em Medicina Nuclear 28. A alteração da marcação de constituintes sanguíneos com Tc-99m por fármacos poderia ocorreria por: (i) formação de complexos com os íons estanoso e pertecnetato; (ii) alterações morfológicas (qualitativas) e morfométricas (quantitativa) membrana eritrocitária; (iii) competição com os referidos íons pelos mesmos sítios de ligação nas proteínas plasmáticas e celulares e/ou (iv) oxidação direta do íon estanoso ou através da (v) geração de radicais livres 27,29.
5
para microscopia de luz 30. As hemácias são células que sofreram o processo de
extrusão de seu núcleo durante a diferenciação celular, o que facilita sua visualização na microscopia óptica (análise qualitativa), visto que não apresenta sistemas intracelulares de membranas 31. Da mesma forma, as análises de parâmetros, como a
relação perímetro/área, são de grande utilidade para avaliação de efeitos de fármacos na morfologia de hemácias 6,7.
O crescimento de uma cultura bacteriana pode ser avaliado através da medida da variação do número de células viáveis presentes na cultura com o tempo. O aumento do número de células na cultura pode ser acompanhado através da titulação da cultura em um meio não nutritivo (solução salina 0,9%, por exemplo) ou através da densidade óptica da cultura, obtida em espectrofotômetro, utilizando comprimento de onda de 600 nm 8. Pode-se, então, utilizar o crescimento de uma cultura bacteriana como modelo experimental para avaliar a citotoxicidade de uma substância química através da avaliação do crescimento da cultura incubada com esta substância 8,32.
A marcação de estruturas celulares ou moleculares de interesse biológico com 99mTc envolve, de modo geral, a utilização de um agente redutor 13,33. O cloreto
estanoso (SnCl2) tem sido largamente utilizado com essa finalidade 25,26. Entretanto,
têm sido descritas as ações citotóxica e mesmo genotóxica associada com essa substância química 34,38. A citotoxicidade e genotoxicidade do SnCl
2 parecem estar
associadas com a geração de radicais livres 35, embora uma ação direta sobre o sistema biológico também tenha sido sugerida 36,38-40. Além disso, foi demonstrado que
6
ilicifolia e Peumus boldus) pode aumentar a sobrevivência de culturas bacterianas, proficientes e deficientes nos mecanismos de reparo de lesões no DNA, ao tratamento com SnCl2 40-43.
A avaliação do perfil eletroforético de plasmídios bacterianos pode ser utilizada para o estudo do potencial genotóxico de substâncias químicas 6,8,10. A incubação de plasmídios bacterianos com o SnCl2 pode alterar banda referente à forma circular
7
3- Indexação de Artigos 3.1- Artigo Publicado FENOPROFEN EFFECTS ON THE LABELING OF BLOOD CONSTITUENTS WITH
TECHNETIUM-99m, ON THE MORPHOLOGY OF RED BLOOD CELLS AND ON THE PLASMID DNA
Marcia de Oliveira Pereira1, 2,Gabrielle de Souza Rocha1, 2, Simone dos Santos Lombardi2, Mauro Geller4, Mário José Pereira4, Sebastião David Santos-Filho2, Adenilson de Souza da Fonseca2,4,* and Mario Bernardo-Filho2,5.
1Programa de Pós-Graduação em Ciências da Saúde, Centro de Ciências da Saúde, Universidade Federal do Rio Grande do Norte, Avenida General Gustavo Cordeiro de Farias, s/n, 59010180, Natal, Rio Grande do Norte, Brasil; 2Departamento de Biofísica e Biometria, Instituto de Biologia Roberto Alcantara Gomes, Universidade do Estado do Rio de Janeiro, adenilso@uerj.br; 3Departamento de Fisiologia, Avenida 28 de setembro, 87, 20551030, Rio de Janeiro, Brasil; 4Centro de Ciências da Saúde,Centro Universitário Serra dos Órgãos, Avenida Alberto Torres, 111, 25964004, Teresópolis, Rio de Janeiro, Brasil; 5Instituto Nacional do Câncer, Coordenadoria de Pesquisa Básica, Praça Cruz Vermelha, 23, 20230-130, Rio de Janeiro, Brasil.
ABSTRACT
The aim of this work was to evaluate the effect of fenoprofen on the labeling of blood constituents with technetium-99m, on the morphology of red blood cells and on the plasmid DNA. Blood samples from Wistar rats were incubated with fenoprofen and the assay of labeling of blood constituents with technetium-99m(99mTc) was performed. Blood cells, plasma, soluble and insoluble fractions of blood cells and plasma were separated. The radioactivity in each fraction was counted and percentage of incorporated radioactivity (%ATI) was determined. Blood smears were prepared, fixed, stained and the qualitative and quantitative morphology of the red blood cells (RBC) was evaluated. Plasmid (pBSK) was incubated with fenoprofen with stannous chloride, and agarose gel electrophoresis procedure was carried out to evaluate genotoxic and the protection of this drug against stannous chloride effect on DNA. In conclusion, under the conditions used in this work, our data suggest that fenoprofen would not (i) affect the fixation of the 99mTc on the blood constituents, (ii) alter the RBC membrane and (iii) present genotoxic and redox effects.
Key words: technetium-99m, blood, morphology, plasmid, fenoprofen.
INTRODUCTION
Nonsteroidal antiinflammatory drugs are used for treatment of rheumatic and other inflammatory, degenerative, and articulate diseases (Insel, 2001). The action mechanism of these drugs results from the inhibition of cyclooxygenase activity, with a consequent reduction of the synthesis of prostaglandin, one of the main mediators of the inflammatory process (Poggi et al., 2006). Fenoprofen is a nonselective cyclooxygenase inhibitor commonly used for the treatment of acute and chronic pain (Insel, 2001).
In vitro red blood cells (RBC) labeled with technetium-99m (99mTc) has been proposed as an assay to assess biological effects of natural and synthetic drugs (Fonseca et al., 2007; Benarroz et al., 2008). Morphogical analysis of RBC has been utilized as another method to evaluate effects of drugs (Frydman et al., 2008). Electrophoretic profile of bacterial plasmids has also been used as
a reliable assay to evaluate genotoxic effect of drugs (Ferreira-Machado et al., 2004).
The aim of this work was to evaluate the effect of fenoprofen on the labeling of blood constituents with 99mTc, on the morphology of RBC and on the plasmid DNA.
MATERIALS AND METHODS
Drugs
Fenoprofen used in this study was purchased from Biolab Sanus Farmacêutica Ltda (São Paulo, Brazil, lot 601034) and stannous chloride (SnCl2)
was purchased from Sigma Chemicals Co (St Louis, USA).
Animals
8 Experimental protocols were approved by the Ethical Committee of the Instituto de Biologia Roberto Alcantara Gomes, Universidade do Estado do Rio de Janeiro (protocol number CEA/203/2007).
In vitro radiolabeling of blood constituents
Samples of whole blood (n=7, for each fenoprofen concentration) were incubated with this drug at different concentrations (0.0, 0.1, 1.0, 10, 100,
1000 μg/mL; 1 hour). After that, SnCl2 (1.2
µg/mL, 1 hour) was added and, in sequence, 99mTc (3.7 MBq, 10 minutes) as sodium pertechnetate (Na99mTcO4), recently milked from a
99
Mo/99mTc generator (Instituto de Pesquisas Energéticas e Nucleares, Comissão Nacional de Energia Nuclear, São Paulo, Brazil). These samples were centrifuged (1500 rpm, 5 minutes) and plasma (P) and blood cells (BC) were separated. Aliquots of P and BC were also precipitated with trichloroacetic acid (5 %) and soluble (SF) and insoluble (IF) fractions were obtained. The radioactivity (% ATI) in P, BC, IF-P, SF-P, IF-BC and SF-BC was determined in a well gamma counter (Packard, model C5002, Illinois, USA). The %ATI was calculated as described previously (Bernardo-Filho et al., 1983).
Morphological evaluation
Smears were prepared from blood samples incubated with fenoprofen at different
concentration (0.0, 0.1, 1.0, 10, 100, 1000 μg/mL;
5 slides for each concentration) and stained by May-Grünwald-Giemsa (Barcia, 2007). The slices were analyzed by optical microscopy and for morphometric measurements a total of five fields per each slide were evaluated. A spherical shape and normal size distribution were assumed to RBC on control samples. Area and perimeter of RBC were measured (Software image pro plus, media Cibernetics, USA) and perimeter/area ratio was calculated.
Plasmid DNA
Plasmid (pBSK) was obtained by alkaline cell lysis method (Sambrook et al,. 1989) from Escherichia coli DH5aF’Iq (rec-) strain hosting this plasmid.
Plasmid treatment with fenoprofen
Plasmids were incubated with fenoprofen at different concentrations (3.0, 30, 300 µg/mL). To assess the action of fenoprofen on effects of SnCl2,
plasmids were incubated with fenoprofen, at the same concentrations, in the presence of SnCl2 (200
g/mL). Plasmid incubated only with SnCl2 was
used as positive control and, as negative control, plasmid incubated at 10 mM Tris buffer (vehicle, pH 7.4). The incubations were carried out at room temperature for 40 minutes. After that, each sample was mixed with loading buffer (0.25% xylene cyanol, 0.25% bromophenol blue and glycerol in water) and applied in 0.8% agarose horizontal gel electrophoresis chamber in Tris-acetate-EDTA buffer (pH 8.0, 7 V/cm). The gel was stained with ethidium bromide (0.5 g/mL) and the DNA bands were visualized by fluorescence under an ultraviolet transilumination system. The assay was repeated at least four times, the results were digitalized (Kodak Digital Science 1d, EDAS 120) and the bands semiquantified using the computer program Image J for Windows.
Statistical analysis
Data are reported as (means ± SD) of the %ATI, the perimeter/area ratio and the percentual of plasmid forms. The One-way analysis of variance– ANOVA test was performed to verify possible statistical differences p<0.05 as less significant level.
RESULTS
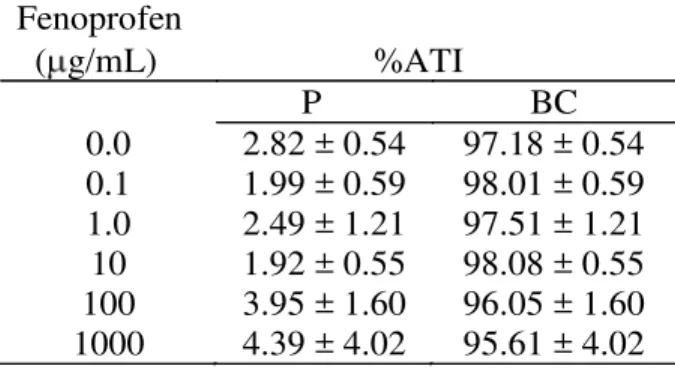
Table 1 presents the effects of fenoprofen on the radioactivity distribution between cellular and plasma compartments. This data indicates no alteration (p<0.05) of 99mTc distribution in these compartments.
Table 2 presents the effect of fenoprofen on the fixation of 99mTc on insoluble and soluble fractions plasma proteins. This data indicates that the
fenoprofen was not capable to interfere on the fixation of the radioactivity on the insoluble and soluble fractions of plasma.
9
Table 1
Effect of fenoprofen on the radioactivity distribution on the cells and plasma compartments labeled with 99mTc.
Fenoprofen ( g/mL) %ATI
P BC
0.0 2.82 ± 0.54 97.18 ± 0.54
0.1 1.99 ± 0.59 98.01 ± 0.59
1.0 2.49 ± 1.21 97.51 ± 1.21
10 1.92 ± 0.55 98.08 ± 0.55
100 3.95 ± 1.60 96.05 ± 1.60
1000 4.39 ± 4.02 95.61 ± 4.02
Table 2
Effect of fenoprofen on the fixation of 99mTc on soluble and insoluble fractions of plasma.
Fenoprofen ( g/mL) %ATI
SF-P IF-P
0.0 24.19 ± 2.67 75.81 ± 2.67
0.1 31.90 ± 3.87 68.10 ± 3.87
1.0 26.00 ± 4.00 74.00 ± 4.00
10 25.99 ± 6.18 74.01 ± 6.18
100 25.31 ± 5.55 74.69 ± 5.55
1000 25.87 ± 7.78 74.13 ± 7.78
Table 3
Effect of fenoprofen on the fixation of 99mTc on soluble and insoluble fraction of blood cells.
Fenoprofen ( g/mL) %ATI
SF-BC IF-BC
0.0 19.56 ± 2.77 80.44 ± 2.77
0.1 19.81 ± 2.76 80.19 ± 2.76
1.0 18.34 ± 3.96 81.66 ± 3.96
10 18.84 ± 2.30 81.16 ± 2.30
100 20.63 ± 2.66 79.37 ± 2.66
1000 18.58 ± 3.05 81.42 ± 3.05
Photomicrographs of RBC from blood incubated with 0.9% NaCl or fenoprofen (1000 g/mL) under optical microscopy is shown in the figures 1 and 2. Qualitative evaluation of these figures indicates no alterations on the shape of the RBC incubated with fenoprofen.
Table 4 presents the perimeter/area ratio of RBC from blood samples incubated with fenoprofen. The results indicate that the perimeter/area ratio of RBC was not significantly (p>0.05) altered by
fenoprofen at the concentrations used.
(a) (b)
Figure 1 - Photomicrography of blood smear from blood incubated with 0.9% NaCl (control) (a) and Photomicrography of blood smear from blood incubated with fenoprofen (1000 g/mL) (b).
Table 4
Effect of fenoprofen the perimeter/area ratio of RBC.
Fenoprofen ( g/mL)
Perimeter/area ratio (1/ m)
0.0 0.62 ± 0.01
0.1 0.64 ± 0.01
1.0 0.65 ± 0.02
10 0.66 ± 0.02
100 0.64 ± 0.01
1000 0.63 ± 0.01
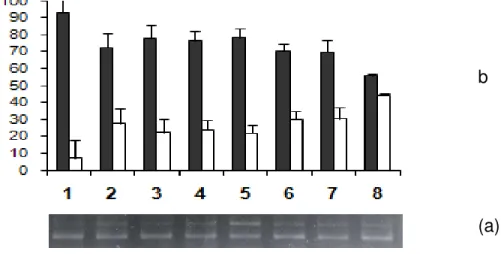
The Figure 2 shows the photograph of agarose gel electrophoresis of pBSK plasmid treated with fenoprofen in presence and absence of SnCl2. This
figure indicates that fenoprofen is not capable to induce alterations on the electrophoretic profile of plasmids (lanes 3, 4 and 5) when compared with negative control (lane 1). Also, figure 2 indicates that the effect of SnCl2 (lane 2) is not altered by
fenoprofen at concentrations used (lanes 6, 7 and 8). These results were confirmed by semiquantitative analyses of the percentages of supercoiled (SC) and open circle (OC) plasmid forms (Figure 3) indicating no alteration on the electrophoretic profile.
DISCUSSION
Blood constituents labeled with 99mTc have been used in several clinical examinations (Saha, 2004) and also as an experimental assay to verify the effect of drugs on radiopharmaceuticals (Fonseca
et al., 2007). This experimental model has
10 incubated with fenoprofen (tables 1, 2 and 3). Despite the absence of effects of the fenoprofen on radiolabeling of blood constituents, it has described drug-related immune hemolytic anemia after use of fenoprofen in human beings (Shirey et al., 1988). Other data has indicated that fenoprofen is almost completely bond to plasma proteins (Insel, 2001).
0 20 40 60 80 100
1 2 3 4 5 6 7 8
LANE P E R C E N TU A L F O R M (a)
1 2 3 4 5 6 7 8 (b)
Figure 2: Percentage of topological forms (a) and photograph (b) of agarose gel electrophoresis of plasmid pBSK treated with fenoprofen in presence and absence of SnCl2. Lanes: (1) pBSK + buffer (negative
control); (2) pBSK + SnCl2 (positive control); (3) pBSK
+ fenoprofen (300 µg/mL); (4) pBSK + fenoprofen (30 µg/mL); (5) pBSK + fenoprofen (0.3 µg/mL); (6) pBSK + fenoprofen (300 µg/mL) + SnCl2; (7) pBSK +
fenoprofen (30 µg/mL)+ SnCl2; (8) pBSK + fenoprofen
(3.0 µg/mL) + SnCl2. (■) OC (open circle); (□) SC
(supercoiled).
Morphological analysis has been used to demonstrate effects of salicylic acid derivatives on membrane of RBC (Li et al., 1999). On the other hand, our data indicates that fenoprofen would not alter the morphology of RBC (Figure 1 and table 4). As morphological analysis of RBC has been used as complementary technique, these results could confirm the data obtained with fenoprofen on the labeling of blood constituents with 99mTc.
The genotoxic effect of stannous chloride on DNA has been demonstrated by different experimental models and the mechanism action has been so far related to free radical generation (Melo et al. 2001, Dantas et al. 2002). In fact, the presence of free
radicals scavengers could reduce the changes of electrophoretic profile of plasmid DNA induced by stannous chloride decreasing the DNA strand breaks (Dantas et al. 1999, de Mattos et al., 2000). Fenoprofen has been suggested to be scavenger of free radicals (Costa, et al., 2006). However, at conditions used in this work, fenoprofen did not seem to protect plasmid DNA against the effects of stannous chloride. In addition, fenoprofen could not present genotoxic effect because no alteration on the electrophoretic profile of plasmids was observed (Figure 2).
In conclusion, under the conditions used in this work, our data suggest that fenoprofen would not (i) affect the fixation of the 99mTc on the blood constituents, (ii) alter the RBC membrane and (iii) present genotoxic and redox effects.
ACKNOWLEDGEMENTS
This study was supported by grants and financial support from CAPES, CNPq and FAPERJ.
RESUMO
O objetivo deste trabalho foi avaliar o efeito do fenoprofeno na marcação de constuintes sanguíneos com tecnécio-99m (99mTc), na morfologia de hemácias e no DNA plasmidial. Amostras de sangue de ratos Wistar foram incubadas com fenoprofeno e a marcação de constituintes sangüíneos com 99mTc foi realizada. Células sangüíneas (CS) e plasma (P) foram isolados. Alíquotas de CS e P foram precipitadas, frações insolúvel e solúvel foram separadas. A radioatividade em cada fração foi contada e o percentual de radioatividade incorporada (%ATI), determinada. Distensões sangüíneas foram preparadas, fixadas, coradas e análise morfológica, qualitativa e quantitativa, de hemácias foi realizada sob microscopia óptica. Plasmídios pBSK foram incubados com fenoprofeno na presença e ausência de cloreto estanoso, e o procedimento de eletroforese em gel de agarose realizado para avaliar o efeito genotóxico deste fármaco e seu efeito sobre a ação do cloreto estanoso no DNA. Os resultados obtidos sugerem que, nas condições utilizadas nesse estudo, o fenoprofeno não poderia: (i) afetar a fixação do
99m
11
REFERENCES
Benarroz, M. O.; Fonseca, A. S.; Rocha, G. S.; Frydman, J. N.; Rocha, V. C.; Pereira, M. O.;
Bernardo-Filho, M. (2008), Cinnamomum zeylanicum extract on the radiolabelling of blood constituents and the morphometry of red blood cells: In vitro assay. Appl Radiat Isot., 66, 139-146. Barcia, J. J. (2007), The Giemsa stain: its history and
applications. Int. J. Surg. Pathol., 15, 292-296. Bernardo-Filho, M.; Santos-Filho, S. D.; Moura, E. G.;
Maiworm, A. I.; Orlando, M. M. C.; Penas, M. E.; Cardoso, V. N.; Bernardo, L. C.; Brito, L. C. (2005), Drug Interaction with Radiopharmaceuticals: a Review. Braz. Arch. Biol. Technol., 48, 13-28. Bernardo-Filho, M.; Moura, I. N. S.; Boasquevisque,
M. (1983), 99m technetium –labeled red blood “in
vitro”. Arq. Biol. Technol., 4,455-461.
Costa, D.; Moutinho, L.; Lima J. L. F.; Fernandes, E. (2006), Antioxidant activity and inhibithion of human neutrophil oxidative burst mediated by arylpropionic acid non-steroidal anti-inflamatory drugs. Phamaceut. Soc. Japan, 29, 1659-1670.
Dantas, F. J. S.; De Mattos, J. C. P.; Viana, M. E.; Lage, C. A. S.; Cabral-Neto, J. B.; Leitão, A. C.; Bernardo-Filho, M.; Bezerra; R. J. A. C.; Carvalho; J. J.; Caldeira-de-Araújo, A. (2002), Genotoxic effects of stannous chloride (SnCl2) in K562 cell line. Food
Chem Toxicol., 40: 1493-1498.
Dantas, J. S. F.; Morais, O.; Mattos, C. P. J.; Bezerra, J. A. C. R.; Carvalho, F. E.; Bernardo-Filho, M.; Araújo, C. A. (1999), Stannous chloride mediates single strand breaks in plasmid DNA through reactive oxygen species formation. Toxicol Lett., 110, 129- 136.
Ferreira-Machado, S. C.; Rodrigues, M. P.; Nunes, A. P.; Dantas, F. J.; De Mattos, J. C.; Silva, C. R.; Moura, E. G.; Bezerra, R. J.; Caldeira-de-Araujo, A. (2004), Genotoxic potentiality of aqueous extract prepared from Chrysobalanus icaco L. leaves. Toxicol Lett., 151, 481-488.
Fonseca, A. S.; Frydman, J. N.; Rocha, V. C.; Bernardo-Filho, M. (2007), Acetylsalicylic acid decreases the labeling of blood constituents with technetium-99M. Acta Biol Hung., 2, 187-198. Freitas, R. S.; Moreno, S. R. F.; Lima-Filho, G. L.;
Fonseca, A. S.; Bernardo-Filho, M. (2007), Effect of a comercial extract of Paulinia cupana (guaraná) on the biding of 99mTc-DMSA on blood constituents: An in vivo study. Appl Radiat Isot., 65,528-533. Hladik III, W. B.; Saha, G. B.; Study, K. T. (1987),
Essentials of nuclear medicine science. Williams and Wilkins, Baltimore, London.
Insel, P. A. (2001), Analgesic-antipiryretic and antiinflamatory agentes and drugs employed in the treatment of gout. In: Harman, J.G., Limbird, L. E., Gilman, A.G. (eds) The Pharmacological Baisis of
Therapeutics. 10th McGraw/Hill, New York; pp. 617-657.
Li, A.; Seipelt, H.; Muller, C.; Artmann, M.; (1999), Effects of salicylic acid derivatives on red blood cell membranes. Pharmacol Toxicol., 85, 206-211. Melo, S. F.; Soares, S. F.; da Costa, R. F.; da Silva, C.
R.; de Oliveira, M. B.; Bezerra, R. J.; Caldeira-de- Araujo, A.; Bernardo-Filho, M. (2001), Effect of the Cymbopogon citratus, Maytenus ilicifolia and Baccharis genistelloides extracts against the stannous chloride oxidative damage in Escherichia coli. Mutat Res., 496, 33-38.
Moreno, S. R. F.; Rocha, E. K.; Pereira, M.; Mandarim- Lacerda, C.; Freitas, R. S.; Nascimento, A. L. R. Carvalho, J. J.; Lima-Filho, G. L.; Diré, G.; Lima, E. A. C.; Bernardo-Filho, M. (2004), Ginkgo biloba extract: experimental model to evaluate its action on the labeling of blood elements with Technetium-99m and on the morphometry of red blood cells. Pak J Nutr., 3, 68-71.
Oliveira, J. F. F.; Brito, L. C.; Frydman, J. N. D.; Santos-Filho, S. D.; Bernardo-Filho, M. (2005), An aqueous extract of Pfaffia sp. does not alter the labeling of blood constituents with technetium-99m and the morphology of the red blood cells. Braz J Pharmacol., 15, 126-132.
Poggi, J. C.; Barissa, G. R.; Donadi, E. A.; Foss, M. C. Cunha, F. Q.; Lanchote, V. L.; Reis, M. L. (2006), Pharmacodynamics, Chiral Pharmacokinetics, and Pharmacokinetic-Pharmacodynamic Modeling of Fenoprofen in Patients With Diabetes Mellitus. J Clin Pharmacol., 46; 1328.
Santos-Filho, S. D.; Bernardo-Filho, M. (2005), Effect of Hypericum perforatum extract in vitro labeling of blood elements with technetium-99m and on biovailability of sodium pertechentate in Wistar rats. Acta Cir Bras., 1, 76-80.
Saha, G. B. (2004), Fundamentals in Nuclear Pharmacy. Springer-Verlag, New York.
Sambrook, J.; Fritsch, E. F.; Maniatis, T. (1989), Molecular cloning: a laboratorial manual. Cold Spring Harbor Laboratory Press, New York.
Shirey, R.S.;Morton; S. J.; Lawton, K. B., Lowell, C.;Kickler, T. S.; Ness, P. M. (1988), Fenoprofen-induced immune hemolysis. Difficulties indiagnosis and complications in compatibility testing. Am J Clin Pathol., Mar., 89, 410-4.
12
3.2- Artigo Submetido I
Evaluation of biological effects of the naproxen
Marcia de Oliveira Pereira1,2,Gabrielle de Souza Rocha1,2, Aldo Cunha Medeiros1,
Adenilson de Souza da Fonseca2,3,*, Mario Bernardo-Filho2,4.
1Programa de Pós-Graduação em Ciências da Saúde, Centro de Ciências da Saúde,
Universidade Federal do Rio Grande do Norte, Avenida General Gustavo Cordeiro de Farias, s/n, 59010180, Natal, Brasil
2Departamento de Biofísica e Biometria, Instituto de Biologia Roberto Alcantara Gomes,
Universidade do Estado do Rio de Janeiro, Avenida 28 de Setembro, 87, Vila Isabel, 20551030, Rio de Janeiro, Brasil
3Departamento de Ciências Fisiológicas, Instituto Biomédico, Universidade Federal do
Estado do Rio de Janeiro, Rua Frei Caneca, 94, 20211040, Rio de Janeiro, Brasil
4Instituto Nacional do Câncer; Praça Cruz Vermelha, 23, 20230130, Rio de Janeiro,
Brasil Abstract
The aim of this work was to evaluate biological effects of the naproxen through of the labeling of blood constituents with technetium-99m, survival of bacterial cultures and electrophoresis profile of plasmid DNA. Blood samples from Wistar rats were incubated with naproxen or with saline (0.9% NaCl), as control, and radiolabeling of blood constituents was performed. Influence of naproxen on the E. coli AB1157 culture growth and survival in presence or absence of SnCl2 was used to assess cytotoxic effect.
Electrophoretic profile in agarose gels of bacterial plasmids in presence or absence of SnCl2 was used to evaluate antioxidant and genotoxic potential of naproxen. Results
obtained suggest that naproxen could not interfere on the labeling of blood constituents with 99mTc but it could present cytotoxic effect and protect E. coli cultures of the lethal effect of stannous chloride at low concentrations. Moreover, naproxen could present genotoxic effect in isolated plasmid DNA.
13 Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are a heterogeneous group of compounds that exhibit anti-inflammatory, analgesic, and antipyretic properties. These drugs can be separated into three groups: salicylates, represented by aspirin; propionic acid derivatives, including ibuprofen and naproxen sodium; and the para-aminophenols, represented by acetaminophen (Abramson et al., 2001). NSAIDs are the most commonly prescribed analgesic medications worldwide, and their efficacy for treating acute pain has been well demonstrated (Van Tunder et al., 2000; Warner et al., 2006). They reversibly inhibit cyclooxygenase (prostaglandin endoperoxide synthase), the enzyme mediating production of prostaglandins and thromboxane A2 (Fitzgerald and Patrono, 2001).
Naproxen is a NSAID used for treatment of rheumatic as osteoarthritis (Fendrick and Greenberg, 2009), other inflammatory degenerative and articulate diseases (Costa
et al., 2006; Insel, 2001). Clinicians prescribe NSAIDs on a routine basis to treat of mild-to-moderate pain using doses ranging 440 up to 660 mg day (Schiff and Minic, 2004). However nor all their biological effects have been well established and the use of different experimental models could be worthwhile.
Blood constituents are labeled with technetium-99m (99mTc) using stannous chloride (SnCl2) as reducing agent and they are utilized in nuclear medicine to aid in the
14
transmembrane transport of stannous and pertechnetate ions into internal compartment of RBC; (ii) reduction of 99mTc (99mTcO4) by the SnCl2 and (iv) binding of the reduced
99mTc to hemoglobin (Callahan and Rabito, 1990). Based on these sequential steps, blood constituents from Wistar rats have been proposed as experimental model to evaluate redox properties and possible interactions of drugs on cellular membrane (Abreu et al., 2006; Fonseca et al., 2007; Benarroz et al., 2008; Frydman et al.,2008).
Some experimental models to evaluate genotoxic, citotoxic and potential redox, involving SnCl2, have been suggested (Pungartnik et al., 2007 and Almeida et al., 2005).
Escherichia coli (E. coli) cultures have been used to evaluate the citotoxic effect of extract of medicinal plants (Almeida et al., 2005; Mello et al., 2001). Assays based on these cultures are fast, easy and very cheap to perform. Electrophoretic profile of bacterial plasmids have been used to assess the ability of natural drugs to induce single strand breaks in DNA through generation of free radicals in vitro (Ferreira-Machado et
al., 2004; Presta et al., 2007). Moreover, bacterial cultures and plasmid DNA samples treated with SnCl2 has been also proposed as models to evaluate redox of drugs
(Almeida et al., 2005; Pereira et al., 2008).
15 Materials and Methods
Drugs
Naproxen used in this study was purchased from Laboratory Teuto Brasileiro S/A. (Goiás, Brazil, lot 601034) and SnCl2 was purchased from Sigma Chemicals Co (St
Louis, USA). Animals
Adult male Wistar rats (3-4 months, 250-300g) were maintained in a controlled environment: normal light/dark cycle conditions (12-h light/12-h dark; lights at 6 am), free access to water and food, room temperature was kept at 25±2 ºC. Experimental protocols were approved by the Ethical Committee of the Instituto de Biologia Roberto
Alcantara Gomes, Universidade do Estado do Rio de Janeiro (protocol number CEA/203/2007).
In vitro radiolabeling of blood constituents
Samples of whole blood (n=7, for each naproxen concentration) were incubated with this drug at different concentrations (0.1, 1.0, 10, 100, 1000 μg/mL; 1 hour). Blood samples incubated with saline solution (0.9% NaCl). After that, SnCl2 (1.2 μg/mL, 1
hour) was added and, in sequence, 99mTc (3.7 MBq, 10 minutes), as sodium pertechnetate (Na99mTcO4), recently milked from a 99Mo/99mTc generator (Instituto
16
and SF-BC was counted in a well gamma counter (Packard, model C5002, Illinois, USA) and the percentage of radioactivity incorporated (%ATI) on each fraction was calculated as described previously (Bernardo-Filho et al., 1983). Briefly, %ATI for each fraction was obtained by ratio between the radiation counting for a fraction and the sum of the radiation counting for this fraction and the complementary fraction multiplied by 100. Bacterial growth assay
From a stock (in glycerol 50% v/v) of E. coli AB1157, a wild-type strain proficient in repairing DNA damage, an aliquot was grown in liquid LB (Luria and Burrous, 1957) medium at 37 °C overnight up to stationary growth phase. Samples of these cultures were grown in presence of naproxen at 3 and 30 μg/mL. After that, these samples were allowed to grow for up to 4 hours. The growth of bacterial cultures was evaluated by the optic density at 600 nm. As controls, bacterial cultures were grown in presence of saline solution.
Bacterial survival assay
From cultures of E. coli AB1157, in stationary growth phase, aliquots were taken and further incubated under the same conditions to reach exponential growth (108
17
fraction was calculated as described before (Almeida et al., 2005). Experiments were carried out in triplicate and the results presented are the average mean of three independent assays.
Plasmid treatment with naproxen and electrophoretic profile assay
Bacterial plasmids (pBSK) were obtained by alkaline cell lysis method (Sambrook
et al., 1989) from E. coli DH5aF’Iq (rec-) strain hosting this plasmid. Plasmid samples were incubated with naproxen at different concentrations (3.0, 30, 300 g/mL). To assess the action of naproxen on effects of SnCl2, plasmids were incubated with
naproxen, at the same concentrations, in the presence of SnCl2 (200 g/mL) (Sambrook
et al., 1989). Plasmids incubated with 10 mM Tris buffer (vehicle, pH 7.4) or SnCl2 alone
were used as positive control. The incubations were carried out at room temperature for 40 minutes. After that, each sample was mixed with loading buffer (0.25% xylene cyanol, 0.25% bromophenol blue and glycerol in water) and applied in 0.8% agarose horizontal gel electrophoresis chamber in Tris-acetate-EDTA buffer (pH 8.0, 7 V/cm). The gel was stained with ethidium bromide (0.5 g/mL) and the plasmids forms (supercoiled and open circle) were visualized by fluorescence under an ultraviolet transilumination system. The assay was repeated at least three times, the results were digitalized (Kodak Digital Science 1d, EDAS 120) and the plasmid forms semiquantified using the computer program Image J for Windows.
Statistical analysis
18 Results
In vitro radiolabeling of blood constituents
Table 1 presents the effects of naproxen on the radioactivity distribution between cellular and plasma compartments. These data indicate no alteration (p>0.05) of 99mTc distribution into these compartments.
Table 2 presents the effect of naproxen on the fixation of 99mTc on insoluble and soluble fractions plasma proteins. Similarly to present in Table 1, naproxen was not capable to interfere significantly (p>0.05) on the fixation of the radioactivity on the insoluble and soluble fractions of plasma.
No significant (p>0.05) alteration on the fixation of radioactivity on proteins of blood cells from blood samples incubated with naproxen (Table 3) was also found. Table 1. Effect of naproxen on the radioactivity distribution between cells and plasma compartments.
Naproxen
( g/mL) %ATI
P BC
0.0 3.44 ± 2.04 96.56 ± 2.04
0.1 1.73 ± 1.29 98.27 ± 1.29
1.0 2.22 ± 1.20 97.78 ± 1.20
10.0 4.48 ± 2.69 95.52 ± 2.69
100.0 2.51 ± 1.72 97.49 ± 1.72
1000.0 3.53 ± 3.09 96.47± 3.09
Samples of whole blood from Wistar rats were treated with naproxen at different concentrations
19
Table 2. Effect of naproxen on the fixation of 99mTc on soluble and insoluble fractions of plasma.
Naproxen
( g/kg) %ATI
SF-P IF-P
0.0 31.72 ± 4.96 68.28 ± 4.96
0.1 29.83 ± 4.22 70.17 ± 4.22
1.0 34.89 ± 8.17 65.11 ± 8.17
10.0 27.61 ± 4.01 72.39 ± 4.01
100.0 27.86 ± 3.95 72.14 ± 3.95
1000.0 28.62 ± 2.80 71.38 ± 2.80
Samples of whole blood from Wistar rats were treated with naproxen at different concentrations.
After that, labeling of blood constituents with 99mTc was carried out. Plasma (P) was separated from blood cells by centrifugation and soluble (SF) and insoluble (IF) fractions of plasma were isolated by precipitation in trichloroacetic acid and centrifugation. Radioactivity in BC and P was counted and the %ATI was calculated. P <0.05, compared to control group of IF-P.
Table 3. Effect of naproxen on the fixation of 99mTc on soluble and insoluble fraction of blood cells.
Naproxen
( g/kg) %ATI
SF-BC IF-BC
0.0 19.71 ± 1.75 80.29 ± 1.75
0.1 18.48 ± 2.50 81.52 ± 2.50
1.0 19.03 ± 2.90 80.97 ± 2.90
10.0 20.09 ± 2.57 79.91 ± 2.57
100.0 17.96 ± 0.92 82.04 ± 0.92
1000.0 17.85 ± 2.17 82.15 ± 2.17
Samples of whole blood from Wistar rats were treated with naproxen at different concentrations.
After that, labeling of blood constituents with 99mTc was carried out. Blood cells (BC) were separated from plasma by centrifugation and soluble (SF) and insoluble (IF) fractions of BC were isolated by precipitation in trichloroacetic acid and centrifugation. Radioactivity in each fraction was counted and the %ATI was calculated. P <0.05, compared to control group of IF-BC.
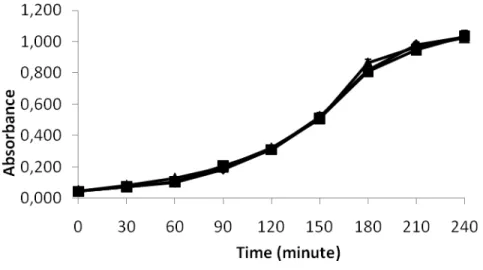
Bacterial growth assay
Figure 1 presents the optic density of E. coli AB1157 cultures grown in presence and absence of naproxen. Data in this figure suggest that the treatment with naproxen, at concentrations used (3 and 30 g/mL) would not present effects on the growth of E.
20
Figure 1: Growth curves of E. coli AB1157 in presence and absence of naproxen. From a culture
in stationary growth phase, aliquots of E. coli AB1157 cultures were put in rich medium at 37 °C
in presence an absence of naproxen at different concentrations (3, 30 μg/mL) and allowed to grow for up to 4 hours. Growth of bacterial cultures was evaluated by the optic density at 600 nm. Cultures grown in presence of saline solution (0.9% NaCl) were used as controls. (■) control, (▲) naproxen 3 g/mL ( ), naproxen 30 g/mL.
Bacterial survival assay
Figure 2 represents the survival graphic of E. coli AB1157 in presence and absence of SnCl2. Data in this figure suggest that the treatment with naproxen could
present cytotoxic effects on E. coli AB1157 cultures at concentrations used (3 and 30 g/mL). Other data in figure indicate that the lethal effect of SnCl2 on E. coli cultures
21
Figure 2: Bacterial survival curves of E. coli AB1157 treated with naproxen in presence and
absence of SnCl2. E. coli AB1157 cultures, in exponential growth phase, were centrifuged (3000
rpm, 20 minutes) and suspended in saline solution (0.9% NaCl). Samples of these bacterial suspensions were incubated (0, 30 and 60 minutes, 37 oC) with naproxen at different
concentrations (3, 30 μg/mL) in the presence and absence of SnCl2 (25 μg/mL). Aliquots were
diluted in saline and spread onto Petri dishes. After overnight incubation (37 oC), colony forming
units were counted to determine survival fractions. As controls, bacterial samples incubated with saline (negative control) or SnCl2 (positive control) alone. ( ) saline; (■) SnCl2; (□) naproxen
3μg/mL; (▲) naproxen 30 μg/mL; (∆) SnCl2 +naproxen 3 μg/mL; (●) SnCl2 +naproxen 30 μg/mL.
Plasmid treatment with naproxen and electrophoretic profile assay
The Figure 3b shows the photograph of agarose gel electrophoresis of bacterial plasmids treated with naproxen in presence and absence of SnCl2. The results shown in
this figure indicate that naproxen is capable to induce alterations on the electrophoretic profile of plasmids (lanes 3, 4 and 5) when compared with negative control (lane 1). Also, the results of the figure 3b indicate that the effect of SnCl2 (lane 2) is increased by
22
Figure 3: Percentage of bacterial plasmid forms (a) and photograph (b) of agarose gel after electrophoresis of plasmid pBSK treated with naproxen in presence and absence of SnCl2.
Samples of bacterial plasmids were incubated with naproxen (0.3, 30 and 300 μg/mL) in presence or absence of SnCl2 (200 μg/mL). After that, agarose gel electrophoresis procedure
(0.8%, 7 V/cm) was performed, gels were stained with ethidium bromide (0.5 μg/mL), plasmid forms were visualized by fluorescence and digitalized to obtain the percentage of each plasmid forms. As controls, plasmids samples incubated with buffer (negative control) or SnCl2 (positive
control) alone. Lanes: (1) pBSK + buffer; (2) pBSK + SnCl2; (3) pBSK + naproxen (300 μg/mL);
(4) pBSK + naproxen (30 μg/mL); (5) pBSK + naproxen (0.3 μg/mL); (6) pBSK + naproxen (300 μg/mL) + SnCl2; (7) pBSK + naproxen (30 μg/mL) + SnCl2; (8) pBSK + naproxen (3.0 μg/mL) +
SnCl2. (■) OC (open circle); (□) SC (supercoiled).
Discussion
Data obtained in this work indicate that there was not alteration on the labeling of the blood constituents with 99mTc when the blood was incubated with naproxen (tables 1, 2 and 3). Despite the absence of effects of the naproxen on radiolabeling of blood constituents, it has been reported hemolysis after use of naproxen in human beings (Orhan and Sahin, 2001). Other data has indicated that naproxen is almost completely bond to plasma proteins (Insel, 2001) and suffers reduction from its effectiveness when it has reduction of the concentration of plasma proteins (Warner et al., 2006). These findings could aid to understand the reason to the naproxen was not capable to interfere on the labeling of the blood constituents with 99mTc. The importance of these founds is relate to comprehension of possible interactions of medicines with radiopharmaceuticals.
b
23
Cytotoxic effect of some drugs has been demonstrated by different experimental models (Hassan et al., 1999). Anti-inflammatory drugs have been reported to decrease the survival of cancer cells (Ricchi et al., 2002). In our study, naproxen decreases the survival of E. coli AB1157 cultures suggesting a cytotoxic effect (Figure 2). This cytotoxic effect of naproxen is in agreement with data of other authors that have demonstrated cytotoxic effect of this drug on intestinal mucosa cells (Oh et al., 2005). These considerations are very important due to the level of biological organization is different in
E. coli and intestinal mucosa cells.
Stannous chloride has been suggested to decrease the survival of bacterial cultures by free radical generation (Bernardo-Filho et al., 1994; Agostinho et al., 2008) Natural products could abolish the effects of SnCl2 decreasing the free radical
production or as scavengers of these species (Almeida et al., 2007). Naproxen could protect bacterial cultures of the lethal effect of SnCl2, probably, decreasing the free
radical production (figure 2). Although the data obtained with association of SnCl2 and
naproxen could be paradoxical, these results are in agreement with data that have suggested that naproxen presents antioxidant property in non cellular and cellular experimental models (Costa et al., 2006).
The genotoxic effect of SnCl2 on DNA has been suggested to occur by
mechanisms related to free radical generation (Pereira et al., 2008; Dantas et al., 1996; Assis et al., 2002). These effects could, at last in part, to be attributed to the reactive oxygen species, generated during the SnCl2 treatment (Dantas et al., 2002; Mattos et
24
breaks (Dantas et al., 1999; de Mattos et al., 2000). Naproxen has been suggested to be scavenger of free radicals (Costa et al., 2006), in agreement with the results gotten in this work, but this was not obtained with another NSAIDs (Pereira et al., 2008). However, under the experimental conditions used in this work, naproxen did not seem to protect plasmid DNA against the effects of SnCl2. On the other hand, naproxen seems to
present genotoxic effect in consequence of the alterations in the electrophoretic profile of plasmids were found (Figure 3). This finding is very relevant, due to the importance of this NSAID and the results were obtained wit isolated plasmid DNA. However, further studies about this effect would be stimulated in specialized laboratories.
In conclusion, results obtained in this study suggest that naproxen could not interfere on the labeling of blood constituents with 99mTc but it could present cytotoxic effect and protect E. coli cultures of the lethal effect of stannous chloride at low concentrations suggesting antioxidant effect. Moreover, naproxen could present genotoxic effect in isolated plasmid DNA.
References
Abramson SB, Amin AR, Clancy R, Attur M (2001) Nitric oxide and inflammatory mediators in the osteoarthritis. Current Rheumatology Reports 6:535-544.
Abreu PRC, Almeida MC, Bernardo RM, Bernardo LC, Brito LC, Garcia EAC, Fonseca AS, Bernardo-Filho M (2006) Guava extract (Psidium gaujava) alters the labeling of blood constituents with technetium-99m. Journal of Zheijiang University Science B 7:429-435.
Agostinho RT, Santos-Filho SD, Fonseca AS, Missailidis S, Bernardo-Filho M (2008) The effect of an extract from ganoderma lucidum (reishi) on the labeling of blood constituents with technetium-99m and on the survival of Escherichia coli. Brazilian Archives of Biology and Techonology 51:157-162.
25
submitted to the lethal action of stannous chloride. Cellular and Molecular Biology (Noisy-le-grand-France) 53: 923-92.
Assis ML, De Mattos JC, Caceres MR, Dantas FJ, Asad LM, Asad NR, Bezerra RJ, Caldeira-de-Araújo A, Bernardo-Filho M (2002) Adaptive response to H2O2 protects against SnCl2 damage: the OxyR system involvement. Biochimie 84:291-294.
Benarroz MO, Fonseca AS, Rocha GS, Frydman JN, Rocha VC, Pereira M O, Bernardo-Filho M (2008) Cinnamomum zeylanicum extract on the radiolabelling of blood constituents and the morphometry of red blood cells: In vitro assay. Applied Radiation and Isotopes 66:139-146.
Bernardo-Filho M, Moura INS, Boasquevisque M (1983) 99m technetium – labeled red blood “in vitro”. Brazilian Archives of Biology and Techonology 4:455-461.
Bernardo-Filho M, Cunha MC, Valsa JO, Araujo AC, Silva FC, Fonseca AS (1994) Evaluation of potential genotoxicity of stannous chloride: inactivation, filamentation and lysogenic induction of Escherichia coli. Food Chemistry and Toxicology 32:477-479. Callahan RJ, Rabito CA (1990) Radiolabeling of erythrocytes with technetium-99m: role of band-3 protein in the transport of pertechnetate across the cell membrane. Journal of Nuclear Medicine 31: 2004-2008.
Costa D, Moutinho L, Lima JLF, Fernandes E (2006) Antioxidant activity and inhibithion of human neutrophil oxidative burst mediated by arylpropionic acid non-steroidal anti-inflamatory drugs. Biological & Phamaceutical Bulletin 29:1659-1670.
Dantas FJS, De Mattos JCP, Viana ME, Lage CAS, Cabral-Neto JB, Leitão AC, Bernardo-Filho M, Bezerra RJAC, Carvalho JJ, Caldeira-de-Araújo A (2002) Genotoxic effects of stannous chloride (SnCl2) in K562 cell line. Food Chemistry Toxicology
40:1493-1498.
Dantas FJS, Moraes MO, Carvalho EF, Valsa JO, Bernardo-Filho M, Caldeira-de-Araújo A (1996) Lethality induced by stannous chloride on Escherichia coli AB1157: participation of reactive oxygen species. Food Chemistry and Toxicology 34:959-962. Dantas FJS, Moraes O, Mattos CPJ, Bezerra JACR, Carvalho FE, Bernardo-Filho M, Araújo CA (1999) Stannous chloride mediates single strand breaks in plasmid DNA through reactive oxygen species formation. Toxicology Letters 110:129-136.
26
Fendrick AM, Greenberg BP (2009) A review of the benefits and risks of nonsteroidal anti-inflammatory drugs in the management of mild-to-moderate osteoarthritis. Osteopathic Medicine and Primary Care 6:3.
Ferreira-Machado SC, Rodrigues MP, Nunes AP, Dantas FJ, De Mattos JC, Silva CR, Moura EG, Bezerra RJ, Caldeira-de-Araujo A (2004). Genotoxic potentiality of aqueous extract prepared from Chrysobalanus icaco L. leaves. Toxicology Letters 151:481-488. Fitzgerald GA, Patrono C (2001). The coxibs, selective inhibitor of cyclooxygenase-2. New England Journal of Medicine 345:433-444.
Fonseca AS, Frydman JN, Rocha VC, Bernardo-Filho M (2007) Acetylsalicylic acid decreases the labeling of blood constituents with technetium-99M. Acta Biologica Hungarica 2: 187-198.
Frydman JNG, Rocha VC, Benarroz MO, Rocha GS, Pereira MO, Fonseca AS, Bernardo-Filho M (2008) Assessment of effects of a Cordia salicifolia extract on the radiolabeling of blood constituents and on the morphology of red blood cells. Journal of Medicinal Food 11:767–772.
Hassan HN, Barsoum BN, Habid IH (1999) Simultaneous spectrophotometric determination of rutin, quercetin and ascorbic acid in drugs using a Kalman Filter approach. Journal of Pharmaceutical and Biomedical Analysis 20:315-320.
Insel PA (2001) Analgesic-antipiryretic and antiinflamatory agentes and drugs employed in the treatment of gout. In: Harman, J.G., Limbird, L. E., Gilman, A.G. (eds) The Pharmacological Baisis of Therapeutics. 10th McGraw/Hill, New York, 617-657.
Luria SE, Burrous JW (1957) Hybridization between E.coli and Shigella. Journal of Bacteriology 74:461-476.
Mattos JPC, Dantas FJS, Bezerra RJA, Bernardo-Filho M, Cabral-Neto JB, Lage C, Leitão AC, Caldeira-de-Araújo A (2000) Damage induced by stannous chloride in plasmidial DNA. Toxicology Letters 116:159-163.
Melo SF, Soares SF, da Costa RF, da Silva CR, de Oliveira MB, Bezerra RJ, Caldeira-de- Araujo A, Bernardo-Filho M (2001) Effect of the Cymbopogon citratus, Maytenus ilicifolia and Baccharis genistelloides extracts against the stannous chloride oxidative damage in Escherichia coli. Mutation Research496:33-38.
27
Orhan H, Sahin G (2001) In vitro effects of NSAIDS and paracetamol on oxidative stress-related parameters of human erythrocytes. Experimental Toxicology and Pathology 53:133-140.
Pereira MO, Rocha GS, Lombardi SS, Geller M, Pereira MJ, Santos-Filho SD, Fonseca AS, Bernardo-Filho M (2008) Effects of fenoprofen on the labeling of blood constituents with technetium-99m, the morphology of red blood cells and the plasmid. Braz Arch Biol Technol 51:135-141.
Pungartnik C, Viau C, Picada J, Caldeira-de-Araujo A, Henriques JA, Brendel M (2005) Genotoxicity of stannous chloride in yeast and bacteria. Mutation Research 583:146-157.
Presta GA, Fonseca AS, Bernardo-Filho M (2007)A Chrysobalanus icaco extract alters the plasmid topology and the effects of stannous chloride on the DNA of plasmids. Brazilian Journal of Pharmacognosy 17:331-335.
Ricchi P, Di Matola T, Ruggiero G (2002) Effect of non-steroidal anti-inflammatory drugs on colon carcinoma Caco-2 cell responsiveness to topoisomerase inhibitor drugs. British Journal of Cancer 86:1501–1509.
Saha GB (2004) Fundamentals in Nuclear Pharmacy. Springer-Verlag, New York. Sambrook J, Fritsch EF Maniatis T (1989) Extraction and purification of plasmid DNA. In: Molecular cloning. A laboratory manual. New York: Cold Spring Harbour Laboratory Press.
Schiff M, Minic M (2004) Comparison of the analgesic efficacy and safety of nonprescription doses of naproxen sodium and ibrupofen in the treatment of osteoarthritis the knee. Journal of Rheumatology 31:1373-1383.
Van Tunder MW, Sholten RJ, Koes BW, Deyo RA (2000) Nonsteroidal anti-inflamatory drugs for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine 25:2501-2513.
28
3.3- Artigo submetido II
EVALUATION OF BIOLOGICAL EFFECTS OF THE IBUPROFEN AND KETOPROFEN
Marcia de Oliveira Pereira 1, 2,Gabrielle de Souza Rocha 1, 2, Adenilson de Souza da Fonseca 2,3,* and Mario Bernardo-Filho 2,4.
1Programa de Pós-Graduação em Ciências da Saúde, Centro de Ciências da Saúde,
Universidade Federal do Rio Grande do Norte, Avenida General Gustavo Cordeiro de Farias, s/n, 59010180, Natal, Brasil. 2Departamento de Biofísica e Biometria, Instituto de
Biologia Roberto Alcantara Gomes, Universidade do Estado do Rio de Janeiro, Avenida 28 de Setembro, 87, Vila Isabel, 20551030, Rio de Janeiro, Brasil. 3Departamento de Ciências Fisiológicas, Instituto Biomédico, Universidade Federal do Estado do Rio de Janeiro Rua Frei Caneca, 94, 20211040, Rio de Janeiro, Brasil. 4Instituto Nacional do
Câncer; Praça Cruz Vermelha, 23, 20230130, Rio de Janeiro, Brasil.
Abstract
29
the fixation of the 99mTc on the blood constituents, (ii) alter the RBC membrane and (iii)
present genotoxic and redox effects.
Key words: ibuprofen, ketoprofen, technetium-99m, morphology, plasmid. Introduction
Non-steroidal anti-inflammatory drugs widely used for the treatment of pain and inflammation and represent the drugs of choice commonly used in the management of musculoskeletal traumatisms, rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, acute gouty arthritis and dysmenorrhoea (Costa, 2006).
The arylpropionic acid derivatives constitute an important group of non-steroidal anti-inflammatory drugs widely used and with action similarly and very effective for treatment of pain and inflammation as irreversible cyclooxygenase enzyme inhibitors (Insel, 2001). Ibuprofen and ketoprofen are a peripherally acting non-steroidal anti-inflammatory drug indicated for analgesia, antipyresis, and various arthritic conditions (Olson, 2007). Clinicians prescribe NSAIDs on a routine basis to treat of mild-to-moderate pain using doses ranging 200mg up to 800mg of ibuprofen and 150mg up to 300mg doses of ketoprofen (Korolkovas, 1999, Insel, 2001).
Nor all biological effects were evaluated to these anti-inflammatory drugs justifying that experimental models can be used for evaluation of their effects.
Antioxidant action has been described for a number of chemical substances and drugs (Pereira et al, 2008) and interest on them is explained because some chronic diseases could be prevented when antioxidants are used (Valko, et al, 2006).
30
to cellular structures, including lipids and membranes, proteins and nucleic acids (G. Poli,1996). There is compelling evidence that ROS mediated oxidative stress is involved in a vast number of biological responses causing DNA modification, lipid peroxidation, and production of inflammatory cytokines (Brigantini, S. 2003). This could contribute to the pathogenesis of many inflammatory diseases (Zhou et al., 2009).
The aim of this work was to evaluate biological effects of the ibuprofen and ketoprofen through experimental models at cellular and molecular level.
MATERIALS AND METHODS
Drugs
Ibuprofen and ketoprofen used in this study was purchased from EMS Farmacêutica Ltda (São Paulo, Brazil, lot 12678) and Medley Farmacêutica (São Paulo, Brazil, lot 06110713), respectively, and SnCl2 were purchased from Sigma Chemicals
Co (St Louis, USA).
Animals
Adult male Wistar rats (3-4 months, 250-300g) were maintained in a controlled environment: normal light/dark cycle conditions (12-h light/12-h dark; lights at 6 am), free access to water and food, room temperature was kept at 25±2 ºC. Experimental protocols were approved by the Ethical Committee of the Instituto de Biologia Roberto
31
In vitro radiolabeling of blood constituents
Samples of whole blood (n=7, for each ibuprofen and ketoprofen concentration) were incubated with this drug at different concentrations (0.1, 1.0, 10.0, 100.0, 1000.0 μg/mL; 1 hour). Blood samples incubated with saline solution (0.9% NaCl). After that, SnCl2 (1.2 μg/mL, 1 hour) was added and, in sequence, 99mTc (3.7 MBq, 10 minutes)
as sodium pertechnetate (Na99mTcO4), recently milked from a 99Mo/99mTc generator (Instituto de Pesquisas Energéticas e Nucleares, Comissão Nacional de Energia
Nuclear, São Paulo, Brazil). These samples were centrifuged (1500 rpm, 5 minutes) and plasma (P) and blood cells (BC) were separated. Aliquots of P and BC were also precipitated with trichloroacetic acid (5 %) and soluble (SF) and insoluble (IF) fractions were obtained. The radioactivity (% ATI) in P, BC, IF-P, SF-P, IF-BC and SF-BC was counted in a well gamma counter (Packard, model C5002, Illinois, USA). The %ATI was calculated as described previously (Bernardo-Filho et al., 1983).
Morphological evaluation
32 Plasmid DNA
Plasmid (pBSK) was obtained by alkaline cell lysis method (Sambrook et al,. 1989) from E. coli DH5aF’Iq (rec-) strain hosting this plasmid.
Plasmid treatment with ibuprofen and ketoprofen and electrophoretic profile
assay
Plasmids were incubated with ibuprofen and ketoprofen at different concentrations (3.0, 30.0, 300.0 g/mL). To assess the action of ibuprofen and ketoprofen on effects of SnCl2, plasmids were incubated with ibuprofen and ketoprofen,
at the same concentrations, in the presence of SnCl2 (200.0 µg/mL). Plasmid incubated
only with SnCl2 was used as positive control and, as negative control, plasmid incubated
33 Statistical analysis
Data are reported as (means ± SD) of the %ATI, morphological analysis, growth bacterial of E. coli cultures and optic density and percentual of plasmid forms. The One-way analysis of variance (ANOVA) test was performed to verify possible statistical differences p<0.05 as less significant level.
Results
In vitro radiolabeling of blood constituents
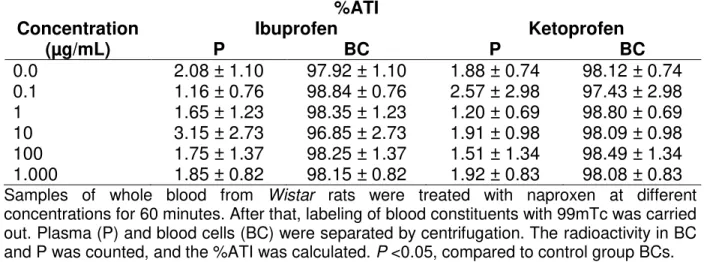
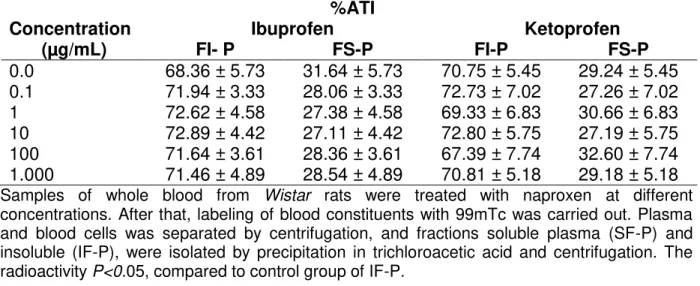
Table 1 presents the effects of ibuprofen and ketoprofen on the radioactivity distribution between cellular and plasma compartments. These data indicate no alteration (p<0.05) of 99mTc distribution in these compartments.
Table 1- Effect of ibuprofen and ketoprofen on the radioactivity distribution on the cells and plasma compartments labeled with 99mTc.
%ATI
Concentration Ibuprofen Ketoprofen
(µg/mL) P BC P BC
0.0 2.08 ± 1.10 97.92 ± 1.10 1.88 ± 0.74 98.12 ± 0.74 0.1 1.16 ± 0.76 98.84 ± 0.76 2.57 ± 2.98 97.43 ± 2.98 1 1.65 ± 1.23 98.35 ± 1.23 1.20 ± 0.69 98.80 ± 0.69 10 3.15 ± 2.73 96.85 ± 2.73 1.91 ± 0.98 98.09 ± 0.98 100 1.75 ± 1.37 98.25 ± 1.37 1.51 ± 1.34 98.49 ± 1.34 1.000 1.85 ± 0.82 98.15 ± 0.82 1.92 ± 0.83 98.08 ± 0.83
Samples of whole blood from Wistar rats were treated with naproxen at different
concentrations for 60 minutes. After that, labeling of blood constituents with 99mTc was carried out. Plasma (P) and blood cells (BC) were separated by centrifugation. The radioactivity in BC and P was counted, and the %ATI was calculated. P <0.05, compared to control group BCs.