PRODUCTION AND
CHARACTERIZATION OF CELLULASE
BY LOCAL FUNGAL ISOLATE OF
INDIA USING WATER HYACINTH AS
CARBON SOURCE AND REUSE OF
FUNGAL BIOMASS FOR DYE
DEGRADATION
SACHIN TALEKAR*
Department of Biotechnology engineering, KIT’s college of engineering,
Kolhapur- 416234 Maharashtra, India. VISHAL GHODAKE
Department of Biotechnology engineering, KIT’s college of engineering,
Kolhapur- 416234 Maharashtra, India. SANDEEP CHAVARE Department of Biotechnology engineering,
KIT’s college of engineering, Kolhapur- 416234
ROHAN INGROLE
Department of Biotechnology engineering, KIT’s college of engineering,
Kolhapur- 416234 Maharashtra, India.
AJINKYA KATE
Department of Biotechnology engineering, KIT’s college of engineering,
Kolhapur- 416234 Maharashtra, India.
SACHIN MAGDUM
Department of Biotechnology engineering, KIT’s college of engineering,
Kolhapur- 416234 Maharashtra, India.
MEENA PILLAI
Department of Biotechnology engineering, KIT’s college of engineering,
ABSTRACT
The production of cellulase using Eichhornia crassipes (water hyacinth) as a carbon source and dye degradation potential of local fungal isolate of India was studied. The basal medium supplemented with water hyacinth blend in the proportion of 1:05(V/V) as carbon source and pH 5.0 showed maximum cellulase production after 6 days of incubation at 30º C with agitation speed of 150 rpm in rotary shaker. Effect on enzyme activity was investigated at different temperatures and pH. The optimum temperature and pH for the cellulase activity was 40ºC and 5.0. Kinetic investigations showed that KM and Vmax of
cellulase were 4.7 mg/ml and 58.3μmol/ml/min, respectively. The reuse of fungal biomass after cellulase production for colorization of methylene blue was studied. Methylene blue was completely de-colorized within 5 days of incubation at temperature 30º C and pH 5.0 with agitation speed of 150 rpm. This demonstrates reuse of fungal biomass for dye degradation after enzyme production.
Key words: Cellulase, water hyacinth, methylene blue, de-colorization
1.0 INTRODUCTION
Ponds, lakes, backwaters and canals, both natural and man-made play an important role in the cultural heritage and economic status of the nations. But now most of these aquatic systems are infested with one kind of aquatic weed Eichhornia crassipes (water hyacinth) also known as ‘blue devil’ [1]. In Kolhapur (Maharastra) the Rankala Lake suffers from the huge amounts of water hyacinth spread over large surface areas. As it is non endemic, there are no natural control mechanisms such as insects and fishes that feed on it [2]. Also other control mechanisms including physical, chemical and biological methods have failed miserably or are too expensive to carry out on a regular basis. Hence the concept of eradication through utilization is being adopted and researchers are focusing on new methods of utilizing these natural resources [3-4]. Water hyacinth comprises considerable amount of α-cellulose (~ 60%) and was thought to be used as a cheap source of cellulose for the production of cellulases by fungal isolates [5]. Cellulase is a complex enzyme system that is used to break down cellulose into glucose or other oligosaccharide compounds [6]. The cellulase system in fungi comprises three hydrolytic enzymes: endo-(1,4)-α-D-glucanase which cleaves α-linkages at random, commonly in the amorphous parts of cellulose, exo-(1,4)-α-D-glucanase which releases cellobiose from non reducing or reducing end, generally from the crystalline parts of cellulose and α-glucosidase which releases glucose from cellobiose and short-chain cellooligosaccharides [7]. Cellulases have wide range of industrial applications such as starch processing, animal feed production, grain alcohol fermentation, malting and brewing, extraction of fruit and vegetable juices, pulp and paper industry and textile industry [8-12]. Cellulase production using different agricultural wastes and water hyacinth by fungi was reported [13-17].
Colored industrial effluent from the dyeing industries is one more reason for pollution of aquatic systems which leads major environmental problems due to its impact on water bodies and growing public concern over its toxicity and carcinogenicity. So dye wastewater discharged from such industries has to be treated. Ligninolytic fungi like white rot fungi and Phanerochaete chyrsosporium has capacity to degrade synthetic dyes [18-19].So present study focuses on the production and characterization of cellulase by local fungal isolate using water hyacinth as carbon source and reuse of fungal biomass for dye degradation after cellulase production.
2.0 MATERIALS AND METHODS
2.1 Screening and isolation of cellulase producing fungus: Cellulase producing fungal strain was isolated from heaps of water hyacinth on the bank of Rankala Lake situated in Kolhapur (Maharashtra).Serially diluted sample prepared from the surface flora of water hyacinth was spread on surface of potato dextrose agar and incubated for 7 days at 30ºC. Colonies were picked and sub-cultured to obtain pure culture. Stock cultures were maintained on potato dextrose agar at 4ºC. Cellulase producing fungi were screened on selective carboxymetyl cellulose agar containing (gm/L): NaNO3 2.0, KH2 PO4 1.0, MgSO4 .7H2O 0.5, KCl 0.5, carboxymethyl cellulose sodium salt 2.0, peptone 0.2, agar 17.0.Plates were spot inoculated with spore suspension of pure culture and incubated at 30ºC.After 3 days, plates were flooded with 0.1% Congo red solution for 15 minutes then de-stained with 1M NaCl solution for 15 minutes. The diameter of zone of de-colorization around each colony was measured. The fungal colony showing largest zone of de-colorization was selected for cellulase production.
2.3 Cellulase assay: Cellulase activity was measured using carboxymethyl cellulose as substrate [20]. Reaction
mixture containing 2% carboxymethyl cellulose in 0.05 M sodium citrate buffer of pH 4.8 and culture supernatant was incubated for 30 min at 50°C. The formation of reducing sugar was measured by DNSA method. One international unit activity is one micromole of reducing sugar released per milliliter of culture supernatant per minute.
2.4 Optimization of carbon source for cellulase production: The basal medium used for enzyme production
under submerged fermentation comprised of (gm/L): NaNO3 2.0, KH2 PO4 1.0, MgSO4 .7H2O 0.5 and (mg/L) FeSO4 10.0. The isolated fungal strain was grown for 7 days in 250 ml Erlenmeyer flasks containing 25 ml of the basal medium supplemented with different volumes of water hyacinth blend as carbon source. The volume of water hyacinth bends showing highest activity of cellulase was selected as optimum amount of carbon source required for cellulase production.
2.5 Cellulase production: Local fungal isolate was used for cellulase production. It was grown on potato dextrose agar incubated at 30ºC for 7 days and harvested with sterile distilled water for inoculum preparation. The basal medium supplemented with optimized volume of water hyacinth bend was used as production medium. 100ml of production medium (pH 5) in 250ml shake flask was inoculated with 2 ml of fungal spore suspension containing 104 spores per ml. The flask was incubated at 30ºC with agitation speed of 150 rpm in rotary shaker incubator. After 6 days, culture was centrifuged at 4000 rpm for 20 min and supernatant was used as crude cellulase.
2.6 Effect of temperature and pH on cellulase activity: To determine the optimum temperature of cellulase, enzyme activities were measured in the temperature range of 30-80ºC. The effect of pH on enzyme activity was determined in the pH range of 3-8.In the range of 3-5, 0.05M sodium citrate buffer and in the range of 6-8, 0.05 M sodium phosphate buffer were used.
2.7 Kinetic analysis: Kinetic parameters of cellulase were estimated by measuring initial reaction rates using different carboxymethyl cellulose concentrations in the range of 1mg/ml-10mg/ml in 0.05 M pH 5 sodium citrate buffer at 50°C. Km, Vmax values of cellulase were calculated from nonlinear regression fitting of the initial reaction rates corresponding to different carboxymethyl cellulose concentrations with GraphPad Prism software.
2.8 Reuse of fungal biomass for dye degradation: Dye degradation in broth culture was done by the method described by Jothimani and Prabakaran [21]. After cellulase production, fungal biomass was recovered by centrifugation and inoculated into 250ml Erlenmeyer flask containing 100ml of basal medium with 3% sucrose as carbon source and 40 mg of filter sterilized methylene blue and incubated at 30ºC with agitation speed of 200 rpm for 5 days. De-colorization of methylene blue was observed by visual observation of flask.
3.0 RESULTS AND DISCUSSION
Fig.1: Effect of Basal medium/Water hyacinth on cellulase production.
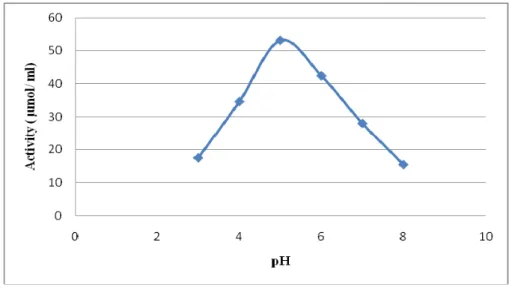
3.2 Effect of temperature and pH on cellulase activity: The effect of temperature on the activity of cellulase was determined in the temperature range of 30-80ºC. As given in (Fig.2), the optimum temperature recorded was at 40ºC. Exactly same optimum temperature 40ºC was obtained for cellulase from Tribolium castaneum [24]. Beyond 40ºC, thermostability decreased, may be due to thermal denaturation of enzyme. The pH effect on cellulase activity was determined in pH range of 3-8. Optimum pH value was 5.0 as shown in (Fig.3).This result resembles with that of maximum CMCase activity from Volvariella displosia at pH 5.4 [23] and Tribolium castaneum at pH 4.8 [24].
Fig.3: Effect of pH on cellulase activity.
3.3 Kinetic analysis: Kinetic parameters of cellulase were measured. Michaelis-Menten type kinetic behavior was observed. The Km and Vmax values obtained were 4.7 mg/ml and 58.3 μmol/min respectively (Table 1).Near about similar Km and Vmax values were obtained for thermophilic actinomycetes [25].
Table 1: Kinetic parameters of cellulase
Km (mg/ml) Vmax (μmol/min)
4.7 58.3
3.4 Reuse of fungal biomass for dye degradation: After 5 days of incubation, de-colorization of methylene blue was observed by visual observation of flask (Fig.4).Similar de-colorization of methylene blue was observed by fungal isolates from dye industry waste [26].This result demonstrates dye de-colorization potential of isolated fungus and reuse of fungal biomass for dye de-colorization after cellulase production.
(A) (B)
Fig.4: Photographs of de-colorization of methylene blue.
(A) Methylene blue dye bath solution containing fungal biomass
(B) De-colorization of methylene blue by local fungal isolate
4.0 CONCLUSION
after fermentation. Hence fungal biomass could be reutilized for de-colorization of textile effluent without dumping it after fermentation. This demonstrates the dye degrading capacity of isolated fungus.
ACKNOWLEDGEMENT
The authors thank the management of KIT’s college of engineering, Kolhapur for providing lab facilities and constant encouragement for this research work.
REFERENCES
[1] Harley, L. S., Julien, M. H and Wright, A. D. (1997).Water hyacinth: a tropical worldwide problem and methods for its control.Proceedings of the first meeting of the international water hyacinth consortium, World Bank.
[2] Gopal, B., (1987).Water hyacinth. Aquatic plant studies series. Hindasia Publishers. New Delhi.
[3] Nagendra Prabhu,G. Strategies for economic utilization of aquatic weeds of Kerala. Proceedings of the National Seminar on Kuttanad Development November, 2001, Edathua, Alleppey, India, pp: 22-24.
[4] Calvert, P, (2002).Water hyacinth: control and possible uses. Technical brief. International Technology Development centre. UK. [5] Abd-el-Naby, M.A., (1988).Biochemical study on fungal cellulase, Ph.D thesis. Faculty of science, Mansoura University.
[6] Chellapandi, P. and M.J. Himansu, (2008).Production of endoglucanase by the native strains of Streptomyces isolates in submerged fermentation.Braz.J.Microbiol., 39:122-127.
[7] Bhat, M.K. and S.Bhat, (1997).Cellulose degrading enzymes and their potential industrial applications. Biotechnol.Adv., 15:583-620. [8] Ögel, Z.B., K. Yarangümeli, H. Dürdar and I. Ifrij, (2001).Submerged cultivation of Scytalidium thermophilum on complex
lignocellulosic biomass for endoglucanase production. Enzyme and Microbial Technol., 28: 689-695.
[9] Roopesh, K., R.K. Sumetra, M.S. Nampoothiri, G. Szakacs and A. Pandey, (2006).Comparison of phytase production on wheat bran and oil cakes in solid-state fermentation by Mucor racemosus. Bioresour. Technol., 97: 506-511.
[10] Adsul, M.G., K.B. Bastawde, A.J. Varma and D.V. Gokhale, (2007). Strain improvement of Penicillium janthinellum NCIM 1171 for increased cellulase production. Bioresour. Technol., 98: 1467-1473.
[11] Kaur, J., B.S. Chadha, B.A. Kumar and H.S. Saini, (2007).Purification and characterization of two endoglucanases from Melanocarpus sp. MTCC 3922. Bioresour. Technol., 98: 74-81.
[12] Papinutti, V.L. and F. Forchiassin, (2007).Lignocellulolytic enzymes from Fomes sclerodermeus growing in solid-state fermentation. J. Food Eng., 81: 54-59.
[13] Kang, S.W., Y.S. Park, J.S. Lee, S.I. Hong and S.W. Kim, (2004). Production of cellulases and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass. Bioresour.Technol., 91: 153-156.
[14] Mukhopadhyay, S and Nandi, B, (1999). Optimization of cellulose production by Trichoderma reesei ATCC 26921 using a simplified medium on water hyacinth biomass J Sci Ind Res.,58:107-111.
[15] Ali, S., Sayed, A., Sarker, R.T and Akin, R., (1991).Factors affecting cellulose production by Aspergillus tarreus using water hyacinth. World J.Microbiol.Biotechnol., 7:62-66.
[16] M.Karamkar and R.R.Ray, (2010).Extracellular endoglucanase production by Rhizopus oryzae in solid and liquid state fermentation of agro waste. Asian journal of Biotechnology, 2(1):27-36.
[17] Kocher,G.S.,K.L.kalra and G.Banta,(2008).Optimization of cellulase production by submerged fermentation of rice straw by Trichoderma harziamm Rut-C 8230.The Internet J.microbiol.,Vol.5.Number 2.
[18] Pasczynski, A and Crawford, RL, (1995). Potential for bioremediation of xenobiotic compounds by the white rot fungus Phanerochaete chrysosporium. Biotechnol. Prog., 11: 368-379.
[19] Kirk, TK and Farrel, RL, (1987). Enzymatic combustion: The microbial degradation of lignin. Ann. Rev. Microbiol., 41: 465-505. [20] T. K. Ghose, (1987).Measurement of cellulase activities. Pure & Appl. Chem., 59(2):257-268.
[21] Jothimani, P. and J. Prabakaran, (2003).Dye factory effluent de-colorization by fungal cultures under static conditions. J.Ecobiol., 15:255-260.
[22] Usama F. Ali and Hala S. Saad El-Dein, (2008). Production and partial purification of cellulase complex by Aspergillus niger and A. nidulans grown on water hyacinth blend. Journal of Applied Sciences Research, 4(7): 875-891.
[23] Puntambekar U.S., (1995).Cellulase production the edible mashroom Volvariella diplasia.World J.Microbiol.Biotechnol., 11:695-695. [24] Fayyaz Ur Rehman, Mehwish Aslam, M. Ilyas Tariq, Ashraf Shaheen, Amtul Jamil Sami, Naima Huma Naveed and Aima Iram
Batool, (2009). Isolation of cellulolytic activities from Tribolium castaneum (red flour beetle). African Journal of Biotechnology, 8 (23):6710-6715.
[25] Dr. A. Aboul-Enein, Dr. F. Abou elalla, Dr. E. Serour, and Dr. T. Hussien, (2010). Purification and characterization of a novel thermoactive cellulase from thermophilic actinomycetes isolated from soil sample of Egypt. International journal of academic research, 2:81-86.