ContentslistsavailableatSciVerseScienceDirect
Carbohydrate
Polymers
jo u r n al h om ep a ge : w w w . e l s e v i e r . c o m / l o c a t e / c a r b p o l
Structural
characterization
of
a
new
dextran
with
a
low
degree
of
branching
produced
by
Leuconostoc
mesenteroides
FT045B
dextransucrase
Mary
Helen
Palmuti
Braga
Vettori
a,
Sandra
Mara
Martins
Franchetti
b,
Jonas
Contiero
a,∗aUnesp–Univ.EstadualPaulista,BiologicalSciencesInstitute,DepartmentofBiochemistryandMicrobiology,LaboratoryofIndustrialMicrobiology,24AAve.,BelaVista,IBRC,Rio
Claro,SP,CEP13506-900,Brazil
bUnesp–Univ.EstadualPaulista,BiologicalSciencesInstitute,DepartmentofBiochemistryandMicrobiology,LaboratoryofPolymerBiotreatments,24AAve.,BelaVista,IBRC,Rio
Claro,SP,CEP13506-900,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received11October2011
Receivedinrevisedform14February2012 Accepted18February2012
Available online 25 February 2012
Keywords:
LeuconostocmesenteroidesFT045B dextransucrase
Dextran
Structuralcharacterization ComparisonwithcommercialB-512F dextran
a
b
s
t
r
a
c
t
This paper offers thephysical and chemical characterizationof a new dextran producedby
Leu-conostocmesenteroidesFT045B.ThechemicalstructurewasdeterminedbyFourierTransformInfrared
spectroscopyand1HNuclearMagneticResonancespectroscopy.Thedextranwashydrolyzedby
endo-dextranase;theproductswereanalyzedusingthinlayerchromatographyandcomparedwiththoseof
commercialB-512Fdextran.Thenumber-averagemolecularweightanddegreeofpolymerizationofthe
FT045Bdextranweredeterminedbythemeasurementofthereducingvalueusingthecopper
bicinchon-inatemethodandthemeasurementoftotalcarbohydrateusingthephenol–sulfuricacidmethod.The
datarevealedthatthestructureofthedextransynthesizedbyFT045Bdextransucraseiscomposedof
d-glucoseresidues,containing97.9%␣-(1,6)linkagesinthemainchainsand2.1%␣-(1,3)branchlinkages
comparedwiththecommercialB-512Fdextran,whichhas95%␣-(1,6)linkagesinthemainchainsand
5%␣-(1,3)branchlinkages.
© 2012 Elsevier Ltd. All rights reserved.
1. Introduction
Dextran is an extracellular bacterial homopolysaccharide (Sidebotham, 1974; Monsan et al., 2001), the main chain of
whichiscomposedofcontiguous␣-(1,6)-linkedd-glucopyranose residuesand branches, stemmingmainlyside-chains of ␣-(1,6)
glucose units attached by ␣-(1,3) branch linkages to ␣
-(1,6)-linkedchains(Buchholz&Monsan,2001;Dols,Remaud-Simeon, Willemot,Vignon,&Monsan,1998;Naessens,Cerdobbel,Soetaert, &Vandamme,2005;Robyt,1995;Seymour&Knapp,1980).Some dextranshave ␣-(1,2)or ␣-(1,4)branchlinkages, dependingon
thebacterialsource.Theexactstructureofeachtypeofdextran dependsonthedegreeofbranching,involving␣-(1,2),␣-(1,3)and ␣-(1,4)linkages(Seymour&Knapp,1980),whichdependsonthe
specificmicrobialstrainand,hence,onthespecificityof dextran-sucrase(s)EC.2.4.1.5involved(Jeaneset al.,1954).Dextranscan beproducedbygrowingvariousbacterialstrainsofLeuconostoc mesenteroidesandStreptococcusspeciesonamediumcontaining
Abbreviations: DPn,numberaveragedegreeofpolymerization;FTIR,Fourier TransformInfrared;1HNMR,1HNuclearMagneticResonance;MWn, number-averagemolecularweight;NCBI,NationalCenterforBiotechnologyInformation; Pyr/Ac,Pyridine/Acetate;TFA,trifluoroaceticacid;TLC,thinlayerchromatography.
∗Correspondingauthor.Tel.:+551935264180;fax:+551935264176.
E-mailaddresses:marypalmuti@bol.com.br(M.H.P.B.Vettori),
samaramf@rc.unesp.br(S.M.M.Franchetti),jconti@rc.unesp.br(J.Contiero).
sucrose(Kim,Robyt,Lee,Lee,&Kim,2003).Dextranproductionis influencedbyanumberoffactors:physicalandchemicalproperties (Jeanes,1965),suchassolubility,viscosity,specificopticalrotation andthecontentofnitrogen,phosphorusandashinthemedium, whichfurtherdependsonthespecificmicroorganismsinvolved (Jeanesetal.,1954).Asingleenzymecancatalyzethesynthesisof severaltypesofdextranlinkages,therebypermittingthe forma-tionofabranchedpolymer(Neely&Nott,1962;Smith,Sahnley,& Goodman,1994).Ontheotherhand,certainbacterialstrainshave beenshowntoproducedextransofdifferentstructures,whichhave beenattributedtotheexcretionofdifferentdextransucrases(Côté &Robyt,1982;Figures&Edwards,1981;Zahley&Smith,1995). Thus,thestructureofeachdextranisacharacteristicofthespecific dextransucrase(Jeanesetal.,1954).Thepresentstudydiscussesthe structureofawater-solubledextranproducedbyadextransucrase elaboratedbyL.mesenteroidesFT045B.FourierTransformInfrared (FTIR) spectroscopy,1H Nuclear Magnetic Resonance(1HNMR)
spectroscopyandTLC(ThinLayerChromatography)of oligosaccha-ridesobtainedfrompolymerhydrolysisbyendodextranaseisolated fromPenicilliumspwereusedforthedeterminationofthe struc-ture.
Thenumberaveragemolecularweight(MWn)and degreeof polymerization(DPn)weredeterminedbythemeasurementofthe reducingvalueusingthecopperbicinchoninatemethodandthe measurementoftotalcarbohydrateusingthephenol–sulfuricacid method.
2. Experimental
2.1. Organism
L.mesenteroidesFT045Bwasisolatedfromanalcoholandsugar millplantandwasprovidedbytheMicrobiologyIndustrialProcess ControlDivision ofFermentec(Brazil).Thenucleotidesequence wassubmittedtotheGenBanksequencedatabase(GenBankID: JF812153.1).
2.2. Enzymes
(a)L. mesenteroides B-512FMC dextransucrase (heretofore B-512FMC dextransucrase) was obtained from a constitutive mutant of L. mesenteroides B-512FMC (Kim & Robyt, 1994; Zahley&Smith,1995)whichwasprovidedbytheLaboratory ofCarbohydrateChemistryandEnzymology,IowaState Univer-sity(USA).Theculturesupernatantwasconcentratedbypassing itthroughapolysulfoneultrafiltrationhollowfibercartridge (0.1m×1.0m,H5P100-43,Amicon,Inc.,Beverly,MA,USA)ata
flowrateof4Lmin−1at21◦C.Whenthevolumeofthe
concen-tratewasapproximately1L,smallmoleculeswereremovedby passing,(10times)through500mLof20mMpyridine/acetic acidbuffer,pH5.2,containing0.04%PVA10Kand1mMCaCl2.
Theconcentratedenzymewasthencollectedandthehollow fibercartridgewaswashedwithapproximately400mLofthe bufferwhichwasaddedtotheconcentrate(Kitaoka&Robyt, 1998).B-512FMCdextransucrase activitywas89IUmL−1,as
determined by the 14C-sucrose assay (Vettori, Mukerjea, & Robyt,2011),inwhich1.0IU=1.0molof␣-d-glucose incor-poratedintodextranpermin.
(b) L.mesenteroides FT045B dextransucrase (hereinafter FT045B dextransucrase)wasobtainedfromL.mesenteroides FT045B. The growth medium for L. mesenteroides FT045 B was sucrose(40gL−1),yeastextract(20gL−1),K2HPO4 (20gL−1),
CaCl2 (0.02gL−1), MgSO4 (0.2gL−1),NaCl (0.01gL−1), FeSO4
(0.01gL−1)andMnSO
4 (0.01gL−1).Thestrainwasstoredat −20◦Cinacryogenicsolutionuntilpreparationofthe
inocu-lumfor fermentation.Thefermentationinoculum accounted for up to5% (v/v) of the total volume of the medium. The microorganismwastransferredfromthestockculturetothe samemediumandwasgrownfor18hat25◦Cand150rpm.
The culturesupernatant wasobtained by centrifugation for 30min at 13,000×g and stored at 4◦C during the
execu-tionoftheexperiments.FT045B dextransucraseactivitywas 2IUmL−1,asdeterminedbythe14C-sucroseassaydescribed
above.
(c)Dextranase from Penicillium sp was obtained from Sigma [lyophilized powder, 10–25unitsmg−1 of solid]; one unit
releases1.0moleofisomaltose(measuredasmaltose)permin
atpH6.0and37◦Cusingdextranassubstrate.Thepowderwas
dissolvedinabuffersolution(about4mLof20mMPyr/Ac,pH 5.5for1mgofpowder)toobtain5UmL−1.
2.3. Dextranreference
TheB-512FdextranwaspurchasedfromSigmaChemicalCo.
2.4. Dextranproduction
Theenzymedigestsolutionwaspreparedwiththeadditionof 40mLofbuffersolution(20mMPyr/Ac,pH5.2)to50mLof400mM sucroseandpreheatedto30◦Cfor10min.Theenzymereactionwas
startedbyadding10.0mLofthedextransucrase(2UmL−1)
pro-ducedbyL.mesenteroidesFT045Bandincubatedat30◦Cfor33h
(2conversionperiods).Attheendofthereaction200mLofcold
ethanolwasaddedtoprecipitatethesynthesizeddextranandthe solutionwaskeptat4◦Cfor24h.Theprecipitateddextranwas
cen-trifugedat13,000×gfor30minanddissolvedin100mLofwater.
Aftercomplete solubilization,thesolutionwascooledin icefor 10min.Thedextranwasprecipitatedagainwiththeadditionof 200mLofethanol,whichwasmixedandcentrifugedfor30min at13,000×g.Solubilizationandprecipitationwererepeatedonce
moretoensurethattherewasnoremainingfructoseorsucrose. Thedextranwastreatedfivetoseventimeswithacetone(30mL) andoncewithanhydrousethanol(30mL)andthenplacedintoa vacuumovenat40◦Cfor18h.
2.5. Thenumber-averagemolecularweight(MWn)anddegreeof polymerization(DPn)
TheMWnandDPn(Jane&Robyt,1984)ofthedextranwere determined by the measurement of the reducing value using thecopperbicinchoninatemethodandthemeasurementoftotal carbohydratesusingthephenol–sulfuricacidmethod(Fox&Robyt, 1991);DPn=[(totalcarbohydratesingof d-glucose)/(reducing valueingofmaltose)]×1.9;MWn=[(DPn)×162]+18.
2.6. Dextranhydrolysisbyendodextranase
Thedextran(10mg)wasdissolvedin960Lofbuffersolution
(20mMPyr/Ac,pH5.5);analiquotof40LofPenicilliumsp
endo-dextranase(5UmL−1)wasaddedandincubatedat37◦Cfor14h.
Aliquotsof100Lweretakenat10,20,30,40,50,60and840min.
FiveLofconcentratedtrifluoroaceticacid(TFA)wereaddedand
thealiquots(finalconcentrationof6.14MTFA)wereheatedto50◦C
for10mintostopthereaction.
2.7. Thinlayerchromatography
Analiquotof1Lofeachsampleandoftheisomaltodextrin
andmaltodextrinstandardswasapplied15mmfromthebottom ofathinlayerchromatography(TLC)plate(Whatman5K)using a 5L Hamiltonmicrosyringe pipette. Eachspot sizewas kept
between2and3mmdistantfromeachotherbyadding1Lat
atime,withintervalsfordrying.Theplatewasirrigatedwiththree ascentstothetopat20–22◦C using,85:20:50:70(v/v/v/v)
ace-tonitrile/ethylacetate/1-propanol/water. Afterdevelopment,the carbohydrateswerevisualizedby dippingtheTLCplateintoan ethanolsolutioncontaining0.5% (w/v)␣-naphthol and5%(v/v)
H2SO4.Afterairdrying,theTLCplatewasplacedintoanovenat
120◦Cfor10min.Thedensitiesofthecarbohydratespotsonthe
TLCplateweredeterminedusinganimagingdensitometer (Bio-Raddensitometer,ModelGS-670)(Robyt&Mukerjea,1994;Robyt, 2000).
2.8. 1HNMRspectroscopy
OnemilligramofdextranfromB-512Fand1mgofdextranfrom FT045Bweredissolvedseparatelyin0.5mLofpureD2Oandthen
placedinto5mmNMRtubes.1HNMRspectrawereobtainedfroma
BrukerDRX500spectrometer,operatingat500MHzat25◦C.
Ace-tone(2.22ppm)wasusedasthestandardforadjustingthe1HNMR
chemicalshifts.
2.9. Fourier-TransformInfraredspectrometricanalysis
Fig.1. TLCanalysisofhydrolysisofdextranFT045Bwithendodextranase(5U/mL) at37◦C.Lane1,2,3,4,5,6,and7refertosamplestakenat10,20,30,60,90, 120and840min,respectively;IM=isomaltodextrinstandards(IM=isomaltose, IM3=isomaltotriose,and soforth);MS=maltodextrin standards(G2=maltose; G3=maltotriose,andsoforth).
3. Resultsanddiscussion
ThedextranproducedbyL.mesenteroidesFT045Bhadanumber average molecular weight of 91,536Da and DPn of 565. Dex-tranhydrolysisbyendodextranaserequiresaminimumofsixor sevennon-substitutedcontiguouslylinked␣-(1,6)glucoseresidues
(Boune,Huston,&Weigel,1962;Côté&Robyt,1984;Richards& Streamer,1978).Thus,thedecisionwasmadetomakecertainthat L.mesenteroidesFT045Bwasproducingarealdextranwith predom-inantly␣-(1,6)-glucopyranosidiclinkageswithinthemainchains
priortocarryingouttheothercharacterizationmethods(Buchholz &Monsan,2001;Dolsetal.,1998;Seymour&Knapp,1980).
ThemixtureofoligosaccharidesobtainedfromFT045Bdextran hydrolysiswereappliedtoTLC(Fig.1)anddensitieswere deter-minedtofindthequantitativeamountofeachproductbyscanning imagingdensitometry,asdescribedbyRobytandMukerjea(1994). Thelimitofhydrolysisbyendodextranasewasexpressedasthe apparent conversioninto isomaltose.The major productsafter 840minofhydrolysiswithPenicilliumspdextranase(Fig.1,lane 7)wereisomaltose(33.2%)andisomaltotriose(24.6%).Thesewere derived fromthelinear regionsof the␣-(1,6)glucanbackbone
chains.Moreover,thebranchedoligosaccharides,representing sig-nificantbranches in dextran, branched isomaltotetraose (0.4%), isomaltopentaose (3.9%) and higher branched isomaltodextrins
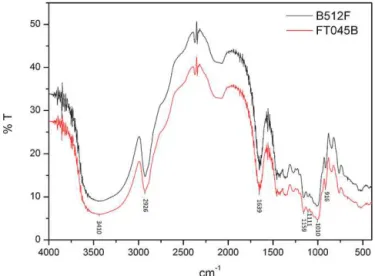
Fig.2. FTIRspectraforcommercialdextranfromB512FfromFT045B.
wereobtained,indicatingFT045Bdextranbranching.Thetypeof thebranchingwasthenconfirmedbyNMRas␣-(1,3)linkage.
The type of linkages and thefunctional groups of the com-mercialdextranfromL.mesenteroidesNRRLB-512Fanddextran fromFT045BwerecharacterizedbyFTIRspectroscopicanalysis. Fig.2showstheFTIRspectraofcommercialdextranfromL. mesen-teroidesB-512FanddextranfromFT045B.Bothdextransgavevery similarspectra,indicatingasimilarstructure,althoughwith differ-entdegreesof␣-(1,3)branchlinkages.DextranfromFT045Band
B-512Fcontain␣-(1,6)linkages,asdemonstratedbythepeakat
1010cm−1,whichcharacterizestheconsiderablechainflexibility
presentindextranaroundthistypeofglycosidicbond(Shingel, 2002).The ␣-glycosidicbond is also confirmed by thepeak at
916cm−1andthebandat1159cm−1causedbycovalentvibrations
oftheC O Cbondandglycosidicbridge.Thepeakat1111cm−1
wasduetothevibrationoftheC ObondattheC-4positionofthe glucoseresidue(Shingel,2002).Thehydroxylstretchingvibration ofthepolysaccharidecausedthebandintheregionof3410cm−1
andtheC Hstretchingvibrationcausedthebandintheregionof 2926cm−1 (Capeketal.,2011;Liu,Lin,Ye,Xing,&Xi,2007).The
bandintheregionof1639cm−1 wasduetoboundwater(Park, 1971).The␣-(1,6)linkages werefurtherconfirmedby1HNMR
analysis.
The1HNMRspectraaffordcompellingevidenceforthemain
structuralfeaturesofdextran.Theindividualassignmentsderived fromthepresentexperimentsandthosereportedintheliterature aredisplayedinTable1.
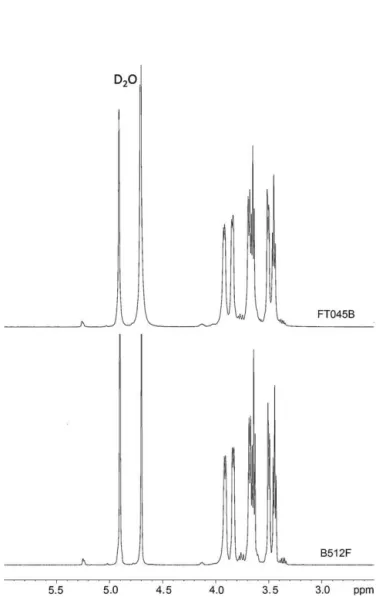
Fig.3displaysthe1HNMRspectraofthenativedextranfromL. mesenteroidesB-512FandFT045BinD2O.Theresultsshowthat
the dextran from L. m. B-512F and L.m. FT045B have a simi-lar structure, although the␣-(1,3) branch linkages and ␣-(1,6)
Table1
Assignmentsofindividualsignalsin1HNMRspectraofthenativedextranfrom B-512F(usedasstandard)andfromFT045Bdextran.
B-512F(reported values)
B-512F (experimental)
FT045B (experimental)
1H(ppm)a 1H(ppm) 1H(ppm)
C1 H 4.99 4.91 4.90
C2 H 3.6 3.50 3.51
C3 H 3.76 3.65 3.63
C4 H 3.52 4.45 3.42
C5 H 3.88–4.04 3.85 3.85
C6 H 3.88–4.04 3.93 3.95
C6′ H 3.81–3.86 3.70 3.70
Table2
Differentlinkagestructuresfoundinalldextranseriesandreportedinliterature.
Percentofglycosidiclinkagesa
␣-1,6 ␣-1,3 ␣-1,3branch ␣-1,4branch ␣-1,2branch
Leuconostocmesenteroides
FT045B Sb 97.9e 2.1e
B-512F S 95 5
B-742 Lc 87 13
B-742 S 50 50
B-1355 L 95 5
B-1355 S 54 35 11
B-1299 L 66 7 27
B-1299 S 65 35
B-1498 S 50 50
B-1501 S 50 50
Streptococcusdownei
Mfe28 12 88
Mfe28 90 10
Streptococcusmutans
GS5 13 87
GS5 15 85
GS5 70 30
6715 S 64 36
6715 Id 4 94 2
aAdaptedfromMonsanetal.(2001). b S=soluble.
c L=lowsoluble.
d I=insoluble.
eDeterminedinthisstudy.
Fig.3.1HNMRspectraofnativedextranfromLeuconostocmesenteroidesB512-F andFT045BinD2O.
linkagesdifferintermsofpercentage.Dextransofdifferent struc-turescanbeproducedbyspecificdextransucrases,eachexcreted byacertainbacterialstrain(Zahley&Smith,1995).Table2 dis-playsthedifferentlinkagestructuresfoundinthedextranseries producedbyLeuconostocmesenteroides,Streptococcusdownei,and StreptococcusmutansaswellasthecomparisonbetweenFT045B dextran,with97.9%␣-1,6linkagesand2.1%␣-1,3branchlinkages,
andB-512Fdextran, whichhasaboutmorethantwiceasmany
␣-1,3branchlinkages(5%).
The three signals that appear centered at ∼5.25ppm (not
shown)representimportantstructuralcharacteristicsof␣
-(1,3)-linked-d-glucosyl residues. The peak at 4.90ppm is due tothe H-1ofthe␣-(1,6)glucosylresiduesofthemainchain(Purama,
Goswami,Khan,&Goyal,2009;Seymour,Knapp,&Bishop,1979). Theintegrationofsignals5.25and∼4.90givesthepercentageof
␣-(1,3)-linkedd-glucosyland ␣-(1,6)-linkedd-glucosyllinkages, respectively(Table2).
The FTIR and 1H NMR spectral analysesconfirmed that the
polysaccharideproduced fromL.mesenteroides FT045Bis an␣
-(1,6) low-branched dextran, with2.1% ␣-(1,3)branch linkages,
givinganaveragechainlengthof(1/2.1)×100=48glucoseunits
betweenbranchlinkages,with97.9%␣-(1,6)linkages.The
com-mercialB-512FdextranfromSigmaexhibits5.0%branching,giving (1/5)×100=20glucoseunitsbetweenbranchlinkages,whichisa
significantdifference.Thereare21branchlinkagesforevery1000 glucoseunitsofFT045Bdextranand50branchlinkagesforevery 1000glucoseunitsofB-512Fdextran,indicatingasignificant dif-ferenceinstructure.B-512Fdextranhas5%␣-(1,3)branchlinkages
oneofthese,inwhichthebranchingislessthanhalfthatofthe B-512Fcommercialdextranoranyotherdextranthat hasbeen characterizedsofar.Thisproducesachainlengththatismorethan twicethelength(48vs.20glucoseunits,forFT045Bdextranand B-512Fdextran,respectively).Thisincreasedchainlengthshould givedifferentchemicalandphysicalproperties,incomparisonto otherdextrans.
Thediscoveryof newdextransthathave differentstructures and,hence,potentiallydifferentproperties,resultingfromdifferent structures,is important withregardto potentialnovel applica-tionsandthepotentialimprovementinoralterationstopreviously establishedapplications.
4. Conclusions
Thepresentexperimentsdemonstratedthatthedextran pro-ducedbyL.mesenteroidesFT045Bhasastructuresimilartothat ofL.mesenteroidesB-512Fdextran,butwith97.9%␣-1,6linkages
and 2.1% ␣-1,3 branch linkages, as compared to B-512F
dex-tran,whichhasmorethantwiceasmany␣-1,3branchlinkages
(5%).TheFT045Bdextranhasanaveragebranchchainlengthof 48 d-glucopyranose residues/molecule, as compared to B-512F dextran,whichthathasanaveragebranchchainlengthof20d -glucopyranoseresidues/molecule.
ThestructureoftheFT045Bdextran, with2.1%branching,is obviouslynotlinearandwouldhave12branchlinkagesper565 d-glucoseresiduesinadextranwithaMWnof91,536Da,as deter-minedinthepresentstudy.
Acknowledgements
TheauthorsaregratefultoProf.JohnF.RobytforallowingMary HelenP.B.Vettoritohaveanexternalresearchexperienceinthe LaboratoryofCarbohydrateChemistryandEnzymology, Depart-mentofBiochemistry,Biophysics,andMolecularBiology,atIowa StateUniversity,USA.
Financial support: The authors thank the FAPESP-São Paulo Research Foundation for financial support (grant number 2008/07410-1).
References
Boune,E.J.,Huston,D.H.,&Weigel,H.(1962).Theactionofmoulddextranases onmodifiedisomaltodextrinsandtheeffectofanomalouslinkagesondextran hydrolysis.BiochemicalJournal,85,158–162.
Buchholz,K.,&Monsan,P.(2001).Dextransucrases.InJ.R.Whitaker(Ed.),Handbook ofFoodEnzymology(pp.589–603).NewYork:MarcelDekker.
Capek,P.,Hlavonová,E.,Matulová,M.,Mislovicová,D.,Ruzicka,J.,Koutn ´y,M.,etal. (2011).Isolationandcharacterizationofanextracellularglucansproducedby LeuconostocgarlicumPR.CarbohydratePolymers,83,88–93.
Côté,G. L.,& Robyt, J. F. (1982).Isolationand partialcharacterization of an extracellularglucansucrasefromLeuconostocmesenteroidesNRRLB-1355that synthesizes alternating 1-6, 1-3-alfa-d-glucan. CarbohydrateResearch, 101, 57–74.
Côté,G.L.,&Robyt,J.F.(1984).Theformationofalfa-d-1-3branchlinkagesbya d-glucansucrasefromStreptococcusmutans6715producingasolubled-glucan.
CarbohydrateResearch,127,95–107.
Dols,M.,Remaud-Simeon,M.,Willemot,R.M.,Vignon,M.R.,&Monsan,P.F.(1998). Characterizationofthedifferentdextransucraseactivitiesexcretedinglucose, frutoseorsucrosemediumbyLeuconostocmesenteroidesNRRLB-1299.Applied andEnvironmentalMicrobiology,64,1298–1302.
Figures,W.R.,&Edwards,J.R.(1981).Alfa-d-glucosyltranferaseofStreptococcus mutansisolationoftwoformsoftheenzymethatbindtoinsolubledextran.
CarbohydrateResearch,88,107–117.
Fox,J.D.,&Robyt,J.F.(1991).Miniaturizationofthreecarbohydrateanalysesusing amicrosampleplatereader.AnalyticalBiochemistry,195,93–96.
Gil,E.C.,Colart,A.I.,ElGhzaoui,A.,Durand,D.,Delarbre,J.L.,&Bataille,B. (2008).Sugarcanenativedextranasaninnovativefunctionalexcipientfor thedevelopmentofpharmaceuticaltablets.EuropeanJournalPharmaceutics Bio-pharmaceutics,68,219–329.
Jane,J.L.,&Robyt,J.F.(1984).Structurestudiesofamylose-Vcomplexesand ret-rogradedamylosebyactionof␣-amylasesandanewmethodforpreparing amylodextrins.CarbohydrateResearch,132,105–118.
Jeanes,A.(1965).PreparationofdextransfromgrowingLeuconostoccultures. Meth-odsCarbohydrateChemistry,5,118–126.
Jeanes,A.,Haynes,W.C.,Wilham,C.A.,Rankin,J.C.,Melvin,E.H.,Austin,M.J.,etal. (1954).Characterizationandclassificationofdextransfromninetysixstrainsof bacteria.JournalofTheAmericanChemicalSociety,76,5041–5052.
Kim,D.,&Robyt,J.F.(1994).ProductionanselectionofmutantsofLeuconostoc mesenteroidesconstitutiveforglucansucrases.EnzymeandMicrobialTechnology,
16,659–664.
Kim,D.,Robyt,J.F.,Lee,S.Y.,Lee,J.H.,&Kim,Y.M.(2003).Dextranmolecularsizeand degreeofbranchingasfunctionofsucroseconcentrationpHandtemperatureof reactionofLeuconostocmesenteroidesB-512FMCM.CarbohydrateResearch,338, 1183–1189.
Kitaoka,M.,&Robyt,J.F.(1998).Useofamicrotiterplatescreeningmethodfor obtainingLeuconostocmesenteroidesmutantsconstitutiveforglucansucrase.
EnzymeandMicrobialTechnology,22,527–531.
Liu,C.,Lin,Q.,Ye,L.,Xing,Y.,&Xi,T.(2007).Characterizationandantitumoractivity ofpolysaccharidefromStrongylocentrotusnuduseggs.CarbohydratePolymers,
67,313–318.
Monsan,P.,Bozonnet,S.,Albenne,C.,Joucla,G.,Willemot,R.M.,&Remaud-Siméon, M.(2001).Homopolysaccharidesfromlacticacidbacteria.InternationalDairy Journal,11,675–685.
Naessens,M.,Cerdobbel,A.,Soetaert,W.,&Vandamme,E.J.(2005).Review Leu-conostocdextransucraseanddextran:productionpropertiesandapplications.
JournalofChemicalTechnology&Biotechnology,80,845–860.
Neely,W.B.,&Nott,J.(1962).DextransucraseaninducedenzymefromLeuconostoc mesenteroides.Biochemistry,1,1136–1140.
Park,F.S.(1971).ApplicationofI.R.spectroscopyinbiochemistry,biology,andmedicine. NewYork:Plenum.,pp.100–140)
Purama,R.K.,Goswami,P.,Khan,A.T.,&Goyal,A.(2009).Structuralanalysisand propertiesofdextranproducedbyLeuconostocmesenteroidesNRRLB-640. Car-bohydratePolymers,76,30–35.
Richards,G.N.,&Streamer,M.(1978).ModeofactionofdextranaseD2from Pseu-domonasUQM733onoligosaccharides.CarbohydrateResearch,62,191–196. Robyt,J.F.(1995).Mechanismintheglucansucrasesynthesisofpolysaccharidesand
oligosaccharidesfromsucrose.AdvancesinCarbohydrateChemistryBiochemistry,
51,133–168.
Robyt,J.F.(2000).Carbohydrates/thin-layer(planar)chromatography.InI.D. Wil-son, M.Cooke, &C. F.Poole (Eds.),Encyclopediaof separationscience(pp. 2235–2244).London:AcademicPress.
Robyt,J.F.,&Mukerjea,Ru.(1994).Separationandquantitativedeterminationof nanogramquantitiesofmaltodextrinsandisomaltodextrinsbythin-layer chro-matography.CarbohydrateResearch,251,187–202.
Seymour, F. R., & Knapp, R. D. (1980). Structural analysis of dextran from strainsofLeuconostocandrelatedgenera,thatcontain3-O-␣
-glucosylated-d-glucopyranosylresiduesatthebranchedpoints,orinconsecutive,linear position.CarbohydrateResearch,81,105–129.
Seymour,F.R.,Knapp,R.D.,&Bishop,S.H.(1979).Correlationofthestructureof dextrantotheir1H-NMRspectra.CarbohydrateResearch,74,77–92.
Shingel,K.I.(2002).Determinationofstructuralpeculiaritiesofdextranpullulanand gama-irradiatedpullulanbyFourier-transformIRspectroscopy.Carbohydrate Research,337,1445–1451.
Sidebotham,R.L.(1974).Dextrans.AdvancesinCarbohydrateChemistryBiochemistry,
30,371–444.
Smith,M.R.,Sahnley,J.,&Goodman,N.(1994).Glucosyltransferasemutantsof Leu-conostocmesenteroidesNRLB-1355.AppliedandEnvironmentalMicrobiology,60, 2723–2731.
Vettori,M.H.P.B.,Mukerjea,Ru.,&Robyt,J.F.(2011).Comparativestudyofthe efficaciesofnineassaymethodsforthedextransucrasesynthesisofdextran.
CarbohydrateResearch,346,1077–1082.