Titanium
surface
topography
after
brushing
with
fluoride
and
fluoride-free
toothpaste
simulating
10
years
of
use
Laiza
M.G.
Fais
a,
Romeu
B.
Fernandes-Filho
a,
Marcelo
A.
Pereira-da-Silva
b,
Luis
G.
Vaz
a,
Gelson
L.
Adabo
a,*
aAraraquaraDentalSchool,UNESP-UnivEstadualPaulista,DepartmentofDentalMaterialsandProsthodontics,
RuaHumaita´,1680,Araraquara,Sa˜oPaulo14801-903,Brazil
bUniversityofSa˜oPaulo,DepartmentofPhysicsandMaterialScience,Av.TrabalhadorSa˜o-carlense,400,Sa˜oCarlos,Sa˜oPaulo13566-590,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received14April2011 Receivedinrevisedform 15December2011 Accepted3January2012
Keywords:
Titanium Sodiumfluoride Toothbrushing Surfaceproperties Microscopyatomicforce Corrosion
a
b
s
t
r
a
c
t
Objectives: Toconductacontrolledstudycontrastingtitaniumsurfacetopographyafter procedures that simulated 10 years of brushing using toothpastes with or without fluoride.
Methods:Commerciallypuretitanium(cpTi)andTi–6Al–4Vdisks(6mmØ4mm)were mirror-polishedandtreatedaccordingto6groups(n=6)asafunctionofimmersion(I)or brushing(B)usingdeionisedwater(W),fluoride-freetoothpaste(T)andfluoridetoothpaste (FT).Surface topography wasevaluatedatbaseline (pretreatment) andpost-treatment, usingatomic forcemicroscopeinorder toobtain three-dimensionalimages andmean roughness.Specimenssubmittedtoimmersionweresubmergedinthevehicleswithout brushing. Forbrushed specimens,procedures wereconducted usinga linear brushing machine witha soft-bristled toothbrush. Immersionand brushingwere performedfor 244h. IFT and BFT samples were analysed under scanning electron microscope with Energy-DispersiveX-raySpectroscopy(EDS).Preandpost-treatmentvalueswerecompared usingthepairedStudentT-test(a=.05).Intergroupcomparisonswereconductedusing
one-wayANOVAwithTukeypost-test(a=.05).
Results: cpTimeanroughness(innanometers)comparingpreandpost-treatmentwere:IW, 2.290.55/2.330.17; IT, 2.240.46/2.020.38; IFT, 2.220.53/1.950.36; BW, 2.220.42/3.760.45; BT, 2.270.55/16.053.25; BFT, 2.270.51/22.395.07. Mean roughness(in nanometers)measuredinTi–6Al–4V disks(pre/post-treatment)were:IW, 1.790.25/2.010.25; IT, 1.610.13/1.740.19; IFT, 1.920.39/2.290.51; BW, 2.000.71/2.050.43;BT,2.370.86/11.172.29;BFT,1.830.50/15.731.78.No signifi-cantdifferenceswereseenafterimmersions(p>.05).Brushingincreasedtheroughnessof
cpTiand ofTi–6Al–4V(p<.01); cpTihadtopographicchanges afterBW,BT andBFT
treatmentswhilstTi–6Al–4VwassignificantlydifferentonlyafterBTandBTF.EDShasnot detectedfluorideorsodiumionsonmetalsurfaces.
Conclusions: Exposuretotoothpastes(immersion)doesnotaffecttitaniumperse;theiruse during brushing affectstitanium topographyand roughness. The associatedeffectsof toothpasteabrasivesandfluoridesseemtoincreaseroughnessontitaniumbrushed sur-faces.
#2012ElsevierLtd.Allrightsreserved.
*Correspondingauthor.Tel.:+55162368395;fax:+551633016406. E-mailaddress:adabo@foar.unesp.br(G.L.Adabo).
Available
online
at
www.sciencedirect.com
journalhomepage:www.intl.elsevierhealth.com/journals/jden
1.
Introduction
Theuseoftitanium,andtitaniumbasedalloys,has signifi-cantly increased in dentistry with the broader use of osseointegratedimplants.Dentistshaveindeedbeen encour-agedtousethesemetalsinimplantedsupportedstructures, crowns,fixedandremovablepartialdentures,1,2orthodontic
wires and brackets,3 based on their favourable properties,
suchasbiocompatibility,lowdensity,lowthermal conduct-ibility,sufficientstrengthtowithstandhighstaticandcyclic stressesofthemasticatorysystem,lowweight,lowcostand goodresistancetocorrosion.4
Resistancetocorrosion andbiocompatibility aredirectly relatedtothepassiveoxidelayerformedontitanium’ssurface anditsalloys.4,5Theexposureoftitaniumtoairortosolutions
leads to spontaneous surface passivation6,7 and, within
nanoseconds,1a4–6nmthicknessfilmistypicallydeveloped,4
mainlyconsistingofamorphousorlow-crystallineTiO2.This
filmactsasakineticbarrieragainstcorrosion.7
Themerepresenceoftheoxidelayerdoesnotmaintainthe stability,sinceits surfacemaynotbefullyprotectedinthe verycomplexchemistryoftheoralcavity.Thislayermaybe mechanically or chemicallyremoved or destroyed.4 Whilst
titanium shows high resistance to corrosion in artificial saliva,8,9 0.9% NaCl,9 and physiological saline solution,9 it
hasbeensuggestedthatfluorideionsfromtoothpastes,dental gels, and mouthrinses can cause deleterious effects on commercially pure titanium (cp Ti), Ti–6Al–4V and Ni– Ti.1,3,6,8–14Theseionsmaydecreasethepolarisationresistance
whilstincreasinganodiccurrentonthetitaniumoxidelayer,11
makingitssurfacemorepronetocorrosion.9,10,3,15Associated
withmetalionrelease6thesechangescouldaffectchemical
composition,1,6,12,16 microstructure,8 surface topography,1,12
surfaceroughness1,11andmechanicalproperties.17
Moststudiesfocusedonthechemicaleffectsoffluoride,by submergingtitaniuminfluoridesolutions.1,3,6,8,12,16,18Inreal
life, however, removable prosthesis, implanted supported structures, brackets and orthodontic wires must be daily cleaned by brushing. Theeffects of this procedure on the titanium surface are not conclusive,2,14,19–21 with a single
study focusing on the relationship between the chemical actionsoffluorideandthemechanicalactionsof toothbrush-ingusingtoothpastes.14
Accordingly,itwasconductedacontrolledstudy contrast-ing titanium surface topography after procedures that simulated 10 years of brushing using toothpastes with or without fluoride.Furthermore,surfacesexposed tofluoride ionscontactwereanalysedunderscanningelectron micro-scope(SEM)withEnergy-DispersiveX-raySpectroscopy(EDS), inordertotestpossiblefluoridereactionswithcpTiandTi– 6Al–4V.Thenullhypothesiswasthatnosignificantdifference would befound after(1)chemical action ofthetoothpaste compoundsandtoothpastefluorideions,aswellafter(2)ofthe toothbrush bristles, toothpaste abrasives, and toothpaste abrasives+fluorideions.
2.
Materials
and
methods
Thestudy flowis summarisedin Fig.1. Seventy-two disks specimens(4mmthickand6mmindiameter)weremachined fromrodsofcpTiGrade2(Ti,99.76%;O,0.16%;Fe,0.06%;C, 0.01%; N,0.001%; H, 0.002%) and Ti–6Al–4V (Ti,89.78%; Al,
6.06%;V,4.0%;O, 0.10%;Fe,0.04%;C,0.007%;N,0.004%;H, 0.003%),bothprovidebyRealum(RealumInd.Com.demetais puroseligasLtda,Sa˜oPaulo,SP,Brazil).
2.1. Specimenpreparation
Specimens were mirror-polished using a polishing device (ArotecInd. eCom.Ltd, Cotia,SP, Brazil). Procedureswere conductedunderrunningwater,at600rpmand0.5kgf,using 320,400,600,800,1200,1500and2000gritSiCabrasivepapers (NortonAbrasivosBrasil,Sa˜oPaulo,SP,Brasil).Finalpolishing was performed with buffing cloth (Microcloth1, Buehler,
Illinois, EUA) using 3mm, 1mm and 0.25mm diamond
suspensions (Metadi Supreme, Buehler, Illinois, EUA), 0.06mm colloidal silica (Mastermet, Buehler, Illinois, EUA)
and0.05mmaluminasuspension(AlfaMicropolishII,Buehler,
Illinois, EUA).DisksofcpTi(N=36) andTi–6Al–4V (N=36) wereultrasonic cleaning(Thornton,Inpec Electronics, Vin-hedo, SP, Brazil) in isopropyl alcohol (JTBaker, Xalostec, Me´xico)for3cyclesof30mineach.Specimenswererandomly allocatedtothe6groups(n=6).
2.2. Toothpasteslurries
Fortheimmersionandbrushingtests,slurrieswereprepared with1partoftoothpaste(grams)to2partsofdeionisedwater (mL),2,21whichweremixedimmediatelybeforeusefor10min,
using a magneticdevice.22 Two types of toothpastes were
prepared:
(1) Fluoridefree-toothpaste –52.5%micronisedCaCO3,25%
C3H5(OH)3; 18% ethyl hydroxyethyl cellulose; 2%
C12H25SO4Naanddeionisedwater(qsp);23pHequalto6.3.
(2) Fluoride toothpaste – 52.5% micronised CaCO3, 25%
C3H5(OH)3; 18% ethyl hydroxyethyl cellulose; 2%
C12H25SO4Na, NaF (1500ppm) and deionised water
(qsp);23pHequalto6.3.
2.3. Immersiontest
Specimens were statically submerged18 in deionised water
(GroupIW), fluoride-freetoothpaste slurry (GroupIT) or in fluoridetoothpasteslurry(GroupIFT)for244h.Thistimeis equivalentto10yearsof2minbrushingpersession,twicea day.2,21,24Vehicles(waterorslurries)werechangedevery12h,
whenspecimenswerewashedunderrunningwaterfor30s. Attheendoftheimmersiontests,thediskswerecleanedfor 30mininisopropylalcohol(JTBaker,Xalostec,Me´xico)using ultrasound(Thornton,InpecElectronics,Vinhedo,SP,Brazil).
2.4. Brushingtest
Specimens werebrushedby amechanicaldevice equipped with6softbristletoothbrushesheads(OralB,straighthead #35;GillettedoBrazil,Sa˜oPaulo,Brazil)witheitherdeionised water (BW), fluoride-free toothpaste slurry (BT) or fluoride toothpasteslurry(BFT).Themachinewassettobrushatarate of60reciprocalstrokesperminute,andtoprovideavertical loadof200g25onthespecimens.Deionisedwater(150mL)or
toothpaste slurries (100mL of deionised water+50g of toothpaste) were inserted into the slurry bath, remaining thespecimensstaticallysubmergedduringbrushing.
Brushing lasted 244h with automatic linear move-ments2,21,24(amplitudeof10mm)ofthetoothbrushesheads.
Toothbrushesandvehicleswerechangedevery22,080strokes, or the equivalent of three months of use. At the end of brushing time, thedisks werecleaned in isopropylalcohol (JTBaker,Xalostec,Me´xico)usingultrasound(Thornton,Inpec Electronics,Vinhedo,SP,Brazil)for30min.
2.5. Characterisationoftitaniumsurfaces
Atomic force microscopy (AFM) was used to evaluate the surface topography and surface roughness at baseline (pretreatment) andpost-treatment(immersionorsimulated brushing).AFManalysiswasconductedinstandardcontact mode(NanoscopeIIIATM,DigitalInstruments,SantaBarbara,
USA). Images were analysed using Gwyddion 2.5 (Prague, Czech Republic)and3DimageswerenormalisedinscaleZ. Surfaceroughnesswasdefinedasthearithmeticalaverageof the surfaceheight relative to the mean height (Ra). Three surfacesof50mm2wererandomlychosenineachspecimen.
ThemeanvaluesofRawerecalculatedforeachspecimen. Foreachmetal,onespecimenofIFTandBFThaditssurface examined using ascanning electron microscope (SEM) (FEI Quanta400FEGESEM,FEICompany,Hillsboro,Oregon,USA) equippedwithanEnergy-DispersiveX-raySpectroscopy(EDS) (INCA 250 energy dispersive X-ray, Oxford Instruments, Concorde, NewHampshire, USA).Allanalyseswerecarried outat20kV,137eVresolution,and90mAbeamcurrent.Discs
wereplaceddirectlyontothestubandexaminedwithoutany preparation or manipulation (i.e. the sampleswere neither coatednordehydratedfortheanalysis).
2.6. Statisticalanalysis
Data were transformed using linear transformation. Ra measurementswereanalysedindividuallyforcpTiandTi– 6Al–4V,inthreesteps:(1)one-wayANOVA(a=.05)wasusedto
compare the sixpretreatmentgroups(baseline) inorderto verifythepolishing standardisation;(2) withingroups (pre-treatmentvs.post-treatment)analyseswereconductedusing thepairedStudentT-test(a=.05);(3)post-treatment
compar-isons wereconducted using one-wayANOVA (a=.05) with
Tukey post-test (a=.05), in order to identify differences
amongsttheexperimentaltreatments.
3.
Results
MeanRavalueswithstatisticalresultsaredisplayedinTable1
(cpTi)andTable2(Ti–6Al–4V).Nosignificantdifferenceswere foundatbaselineforroughness(cpTi–p=0.99;Ti–6Al–4V–
p=0.40), suggesting that groups had similar profiles at baseline.
roughnessofTi–6Al–4V(Table2).AsforTi–6Al–4V,significant differenceswereseenafterthetwobrushingtreatments(BT andBFT)(Table2).
Post-treatment comparisons showed significant differ-ences amongst the experimental treatments in cp Ti (p<0.001) and in Ti–6Al–4V (p<0.001). The Tukey HSD
showedthattheroughnessofcpTiimmersiongroups(Table 1)wassimilaracrossgroupsandlowerthanthoseofBW,BT andBFT(Table1);BFThadthehighestroughness,followedby BT and BW,i.e. the roughness increased fromBW toBFT. Similarly,forTi–6Al–4Vnosignificantdifferenceswereseen amongsttheimmersiongroups(Table2),althoughIW,ITand IFTwere no different than BW, which had,in turn, lower roughness than the brushed groups. BFT had the highest valuesofroughness.
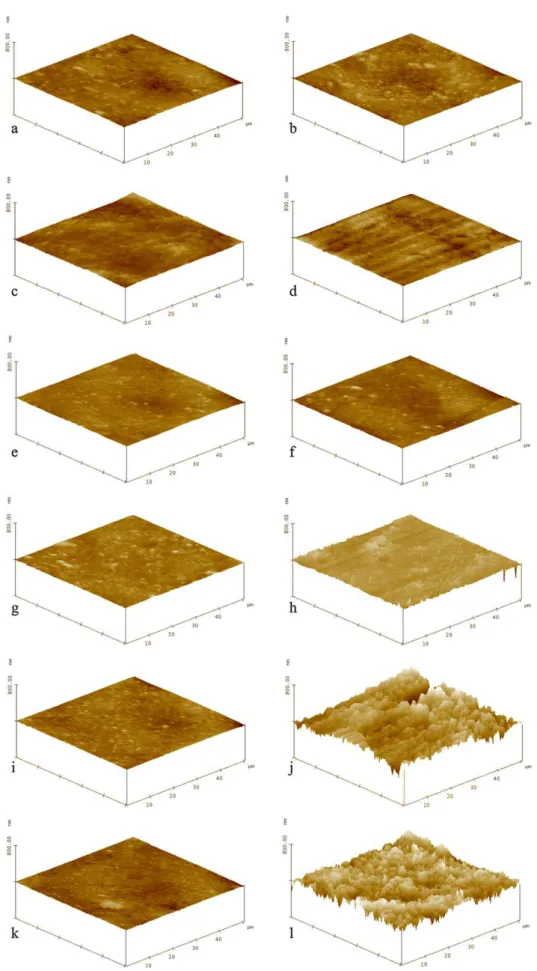
Three-dimensionaltopographies(Figs.2and3) corroborat-edtheresultsoftheRaanalyses.Pretreatmentsurfacesofcp Ti(Fig.2a,c,e,g,iandk)andTi–6Al–4V(Fig.3a,c,e,g,iandk) had homogeneous surfaces. No significant changes were observedinIW(Figs.2band3b),IT(Figs.2dand3d)andinIFT (Figs.2fand3f). Ti–6Al–4VsurfacesafterBWwerealsonot significantlychanged(Fig.3h).
ChangeswereseenonbrushedcpTi(Fig.2h,jandl).They wereminorinBW(Fig.2h),intermediateinBT (Fig.2j)and moreheterogeneousinBFT(Fig.2l).TopographyofTi–6Al–4V
showed important irregularities after BT (Fig. 3j) and BFT (Fig.3l).
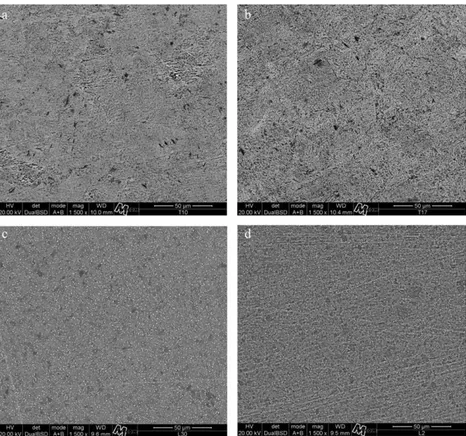
Post-treatment microstructures (1500 magnification) of theIFTandBFTsurfacesareshowninFig.4.Regardless of microstructuraldifferencesbetweencpTiandTi–6Al–4V,IFT surfaces(Fig.4aandc)wereevenandcompletelyfeatureless whilstBFTsurfaces(Fig.4bandd)exhibitedanunevensurface with significant grooves and some pores. The EDS point-analyses of these surfaces (600) did not detect traces of sodiumorfluorides(Figs.5–8).
4.
Discussion
The null hypothesis was rejected based on the data (3D topographies and Ra measurements) when metals were brushed, suggesting that the presence of abrasives on the toothpastesaffectedtheroughnessandtopographyof titani-umbasedmaterials.Majorchangeshappenedlikelyduetothe combinedeffectsoffluorideionsandtoothpasteabrasiveness duringbrushing.
Severalfactorsinfluencematerial’ssurfacesduring tooth-brushing and some may explain the differences found betweencpTiandTi–6Al–4V.Variablessuchas microstruc-tures,hardness,tensileproperties,fracturetoughness,fatigue
Table1–MeansandstandarddeviationsofRa(nm)obtainedfromatomicforcemicroscopyanalysisofcpTispecimens.
Groups Pretreatment Post-treatment
IW–Immersionindeionisedwater 2.29(0.55)Aa 2.33(0.17)Aa*
IT–Immersioninfluoride-freetoothpaste 2.24(0.46)Aa 2.02(0.38)Aay
IFT–Immersioninfluoridetoothpaste 2.22(0.53)Aa 1.95(0.36)Aaz
BW–Brushingwithdeionisedwater 2.22(0.42)Aa 3.76(0.45)Bb§
BT–Brushingfluoride-freetoothpaste 2.27(0.55)Aa 16.05(3.25)Cb#
BFT–Brushingfluoridetoothpaste 2.27(0.51)Aa 22.39(5.07)Db**
Differentuppercaselettersindicatesignificantdifferencesincolumnsobtainedbyone-wayANOVAfollowedbyHSDTukeytest.
DifferentlowercaselettersindicatesignificantdifferencesinrowsobtainedbyStudentT-test:
* p=0.66(pretreatmentIWvs.post-treatmentIW).
** p<0.0001(pretreatmentBFTvs.post-treatmentBFT).
y
p=0.16(pretreatmentITvspost-treatmentIT).
z
p=0.24(pretreatmentIFTvs.post-treatmentIFT).
§ p<0.0001(pretreatmentBWvs.post-treatmentBW).
# p<0.0001(pretreatmentBTvs.post-treatmentBT).
Table2–MeansandstandarddeviationsofRa(nm)obtainedfromatomicforcemicroscopyanalysisofTi–6Al–4V specimens.
Groups Pretreatment Post-treatment
IW–Immersionindeionisedwater 1.79(0.25)Aa 2.01(0.25)Aa*
IT–Immersioninfluoride-freetoothpaste 1.61(0.13)Aa 1.74(0.19)Aay
IFT–Immersioninfluoridetoothpaste 1.92(0.39)Aa 2.29(0.51)Aaz
BW–Brushingwithdeionisedwater 2.00(0.71)Aa 2.05(0.43)Aa§
BT–Brushingfluoride-freetoothpaste 2.37(0.86)Aa 11.17(2.29)Bb#
BFT–Brushingfluoridetoothpaste 1.83(0.50)Aa 15.73(1.78)Cb**
Differentuppercaselettersindicatesignificantdifferencesincolumnsobtainedbyone-wayANOVAfollowedbyHSDTukeytest.
DifferentlowercaselettersindicatesignificantdifferencesinrowsobtainedbyStudentT-test:
* p=0.22(pretreatmentIWvs.post-treatmentIW).
** p<0.0001(pretreatmentBFTvs.post-treatmentBFT).
y p=0.12(pretreatmentITvs.post-treatmentIT).
z p=0.08(pretreatmentIFTvs.post-treatmentIFT).
§ p=0.77(pretreatmentBWvs.post-treatmentBW).
Fig.4–Scanningelectronmicroscopeimages(1500T)ofsamplesexposedtofluorideions;cpTi–GroupIFT(a),GroupBFT (b);Ti–6Al–4V–GroupIFT(c)andGroupBFT(d).
Fig.6–Semi-quantitativecompositionofcpTiafterbrushingwithfluoridetoothpaste(GroupBFT).
resistance,andfracturebehaviour10seemtoberelatedtothe
materialsurface;abrasiveness,21viscosity,2fluoride
concen-tration and pH2 seem to be determined by the toothpaste
compounds. Variables related to the characteristics ofthe toothbrushes25mayalsobeofimportance.
Thecurrentstudysuggestthattheabrasiveactionofthe toothbrushbristlesdidnotchange theRaand the 3D-AFM topographyofTi–6Al–4V,andwasassociatedwithonlyslight changesincpTiBW.Alternatively,substantialchangeswere seen afterbrushing withfluoride-free toothpaste and with fluoridetoothpaste[about6.8and9.6timeshigherthanthecp Ticontrolgroup(IW)andabout5.5and7.8timeshigherthan theTi–6Al–4Vcontrolgroup(IW)].Furthermore,3D topogra-phy was significantlyaltered likely dueto the presence of fluoridesplusmechanicalactionofabrasives.
Theinfluenceofbrushingwithnon-fluoridetoothpasteon roughness and topography has been described,2,21 but
controversies exist about the effects of abrasiveness in fluoride toothpastes. Siirila and Kononem14 described that
toothbrushbristlesinfluencetitaniummoreextensivelythan fluorideabrasivetoothpastes.Noguesetal.20failedtodetect
significant differences on titanium roughness after using fluoridetoothpasteswithdifferentabrasiveness. Discrepan-ciesmaybeduetothedifferentmethodologiessincethefirst authorsperformedmanuallybrushing,whilsttheotherused electric toothbrushes with rotating oscillation, with lower abrasionpower.25
Available data suggest thatchanges on 3DAFM surface topography, Ra values and SEM micrographs in titanium immersiontests,wereassociatedwithhigherfluoride concen-trationsand/oracidicNaFsolutions,3,8,11,12,14,18likelybecauseof
thereactionsthatoccurwiththeNaFandtheprotectiveoxide layer.WhenNaFagentsareincontactwithtitanium,sodium and fluoridesionsare released. Fluorideionscombine with hydrogengeneratinghydrofluoricacid(HF),whichreactswith theoxidelayer,dissolvingtitanium.Inseverecorrosionrates, titaniumfluoride,titaniumoxidefluoride,orsodiumtitanium fluoridecompoundscouldbeformedonthesurface.1,6,12,16
In the currentstudy,the IFwas not associatedwith3D topographicchangesordifferentroughness.Sincesodiumor fluoridecompounds arenot detected onthe surface,it was suggest thatreactions werenot enoughtochangetitanium surfaces. These results may have been driven by the low concentrationoffluorideandbythepHvalue.8,9,13,15,18,26Inthis
study,fluoridetoothpastewith1500ppmF andpH=6.3was used. According to Nakagawa et al.9,13,15 titanium changes
induced by fluoride can be predicted by pH and fluoride concentration.Accordingtothispredictivemodel,forafluoride concentrationof1500ppm,corrosionshouldonlyoccurifpHis lowerthan4.7forcpTiand5.1forTi–6Al–4V.Accordingly,at least10,000ppmF seemtobenecessaryinordertocorrode titaniumwhentoothpasteswithpHequalto6.3areused.
The significant increase in roughness of BFT samples relativetoBTsuggestthatevenforpotentiallynon-corrosive
toothpastes(1500ppmF,pH=6.3),theactionoffluoridewas influencedbychangesinsurfaceswearcausedbytoothpaste abrasives,whichmaydestroytheoxidefilm21andmayalso
produceinternalstressthatincreasecurrentdensity,facilitating corrosion.19Thus,theinstantaneousrepassivationoftheoxide
layer,thatistypicallygraduallyreorganisedandstabilised5may
beaffectedbyfluorideions.4Nonetheless,EDSspectrafindings
(Figs.5–8)supporttheconceptthatfluoride(fromthetoothpaste usedinourstudy)didnotcauseseverecorrosionorformationof insoluble fluoride compounds on the surface of titanium, highlightingtheimportancenotonlyoffluorideconcentration, butalsoofthepH.ReclaruandMeyer26founddepositionof
fluoridewhentitaniumwasimmersedina1000ppmF solution atapH=3.5.TheformationofcrystalsofNa2TiF6,Na5Ti3F14and
Na2TiFwereobserved,respectively,byHuang,16Kanekoetal.27
and Mabilleau et al.,12 using solutions with 10,000ppm F/
pH=6.0, 9.000ppm F/pH=6.5 and 25,000ppmF/pH ranging from4.5to5.3.Sartorietal.18didnotfoundfluorideions or
crystals on thesurface of implants after immersion inNaF solution(1500ppmF andpHrangingfrom5.3to7.4).
In the present study, experimental treatments were definedinorder todisentanglethe effectsoffluoride from theeffectsofabrasivesandbrushingontitaniumtopography. Regardingtheuseoftitaniumanditsalloysasosseointegrated implantsindentistry,twoaspectsshouldbeconsidered:(1) areas in contact with bone tissue have typically higher roughness inorder to facilitate osseointegration,whilst (2) abutmentsandotherstructures thatwillbeexposedtothe oral environment must have smooth surfaces, crucial for controllingbiofilmformation.28,29Whilstimplantsand
com-ponentsusedasmedicaldevicesmusthaveroughnesslower than10nm,30nostandardisationhasbeendefinedregarding
surfaceroughnessofdentistryabutments.31
Thisstudyhassomelimitations.Forexample,althoughit simulated10yearsofexposure,itsinvitronatureshouldbe cautiouslyextrapolatedforclinicalpractice.Methodsusedto simulatebrushingforlongperiodsimplycontinuousbrushing forseveralhours,whichcouldaccentuateabrasiveeffectsand influence of fluoride. Future studies must test clinical conditions,focusingalsoonplaqueaccumulation,ionrelease, and mechanical strength, as well as on other oral cavity variables, such as presence of proteins, fluctuations on fluorideconcentrations,changesinpHandtemperature.
5.
Conclusions
Withinthelimitationsofthisstudy,itwasconcludethatafter simulated10years,exposuretotoothpastes(immersion)with fluorideconcentrationof1500ppmand apH=6.3doesnot affecttitaniumperse;theiruseduringbrushingaffecttitanium topographyandroughness.Theassociatedeffectsof tooth-pasteabrasivesandfluoridesseemtoincreaseroughnesson titaniumbrushedsurfaces.
Acknowledgements
ThisinvestigationwassupportedbyCNPQandFAPESP(State ofSa˜oPauloResearchFoundation),Grantno.08/028710.
r
e
f
e
r
e
n
c
e
s
1. StajerA,UngvariK,PelsocziIK,PolyankaH,OszkoA, MihalikE,etal.Corrosiveeffectsoffluorideontitanium: investigationbyX-rayphotoelectronspectroscopy,atomic forcemicroscopy,andhumanepithelialcellculturing.
JournalofBiomedicalMaterialsResearchPartA2008;87:450–8.
2. HossainA,OkawaS,MiyakawaO.Surfacetextureand compositionoftitaniumbrushedwithtoothpasteslurriesof differentpHs.DentalMaterials2007;23:186–92.
3. HuangHH.VariationinsurfacetopographyofdifferentNiTi orthodonticarchwiresinvariouscommercial fluoride-containingenvironments.DentalMaterials2007;23:24–33.
4. SivakumarB,KumarS,NarayananTSNS.Frettingcorrosion behaviorofTi–6Al–4Valloyinartificialsalivacontaining varyingconcentrationsoffluorideions.Wear2010;270:317–24. 5. HanawaT,AsamiK,AsaokaK.Repassivationoftitanium
andsurfaceoxidefilmregeneratedinsimulatedbioliquid.
JournalofBiomedicalMaterialsResearch1998;40:530–8. 6. MatonoY,NakagawaM,MatsuyaS,IshikawaK,TeradaY.
Corrosionbehaviorofpuretitaniumandtitaniumalloysin variousconcentrationsofAcidulatedPhosphateFluoride (APF)solutions.DentalMaterialsJournal2006;25:104–12.
7. EhrensbergerMT,SivanS,GilbertJL.Titaniumisnot‘‘the mostbiocompatiblemetal’’undercathodicpotential:the relationshipbetweenvoltageandMC3T3preosteoblast behavioronelectricallypolarizedcpTisurfaces.Journalof BiomedicalMaterialsResearchPartA2009;93:1500–9.
8. Al-MayoufAM,Al-SawayihAA,Al-MobarakNA,Al-Jabab AS.Corrosionbehaviorofanewtitaniumalloyfordental implantapplicationinfluoridemedia.MaterialsChemistry andPhysics2004;86:320–9.
9. NakagawaM,MatsuyaS,ShiraishiT,OhtaM.Effectoffluoride concentrationandpHoncorrosionbehavioroftitaniumfor dentaluse.JournalofDentalResearch1999;78:1568–72. 10.KononenMH,LavoniusET,KivilahtiJK.SEMobservations
onstresscorrosioncrackingofcommerciallypuretitanium inatopicalfluoridesolution.DentalMaterials1995;11:269–72. 11.PopaMV,VasilescuE,DrobP,VasilescuC,DemetrescuI,
IonitaD.Long-termassessmentoftheimplanttitanium material–artificialsalivainterface.JournalofMaterialsScience MaterialsinMedicine2008;19:1–9.
12.MabilleauG,BourdonS,Joly-GuillouML,FilmonR,BasleMF, ChappardD.Influenceoffluoride,hydrogenperoxideand lacticacidonthecorrosionresistanceofcommerciallypure titanium.ActaBiomaterialia2006;2:121–9.
13.NakagawaM,MatsuyaS,UdohK.Corrosionbehaviorof puretitaniumandtitaniumalloysinfluoride-containing solutions.DentalMaterialsJournal2001;20:305–14.
14.SiirilaHS,KononenM.Theeffectoforaltopicalfluorideson thesurfaceofcommerciallypuretitanium.International JournalofOral&MaxillofacialImplants1991;6:50–4. 15.NakagawaM,MatsuyaS,UdohK.Effectsoffluorideand
dissolvedoxygenconcentrationsonthecorrosionbehavior ofpuretitaniumandtitaniumalloys.DentalMaterialsJournal
2002;21:83–92.
16.HuangHH.Effectsoffluorideconcentrationandelastic tensilestrainonthecorrosionresistanceofcommercially puretitanium.Biomaterials2002;23:59–63.
17.RoselinoRibeiroAL,NoriegaJR,DamettoFR,VazLG. Compressivefatigueintitaniumdentalimplantssubmitted tofluorideionsaction.JournalofAppliedOralScience
2007;15:299–304.
18.SartoriR,CorreaCB,MarcantonioJrE,VazLG.Influenceofa fluoridatedmediumwithdifferentpHsoncommercially puretitanium-basedimplants.JournalofProsthodontics
19.MolinaC,NoguesL,Martinez-GomisJ,PeraireM,SalsenchJ, SevillaP,etal.Dentalcastingalloysbehaviourduringpower toothbrushingwithtoothpastesofvariousabrasivities.Part II:Corrosionandionrelease.JournalofMaterialsScience MaterialsinMedicine2008;19:3015–9.
20.NoguesL,Martinez-GomisJ,MolinaC,PeraireM,SalsenchJ, SevillaP,etal.Dentalcastingalloysbehaviourduringpower toothbrushingwithtoothpasteswithvariousabrasivities. PartI:Wearbehavior.JournalofMaterialsScienceMaterialsin Medicine2008;19:3041–8.
21. HossainA,OkawaS,MiyakawaO.Effectoftoothbrushingon titaniumsurface:anapproachtounderstandingsurface propertiesofbrushedtitanium.DentalMaterials2006;22:346–52. 22.HeintzeSD,ForjanicM,OhmitiK,RoussonV.Surface
deteriorationofdentalmaterialsaftersimulated
toothbrushinginrelationtobrushingtimeandload.Dental Materials2010;26:306–19.
23.WorschechCC,RodriguesJA,MartinsLR,AmbrosanoGM. Brushingeffectofabrasivedentifricesduringat-home bleachingwith10%carbamideperoxideonenamelsurface roughness.TheJournalofContemporaryDentalPractice
2006;7:25–34.
24.WatahaJC,LockwoodPE,NodaM,NelsonSK,Mettenburg DJ.Effectoftoothbrushingonthetoxicityofcastingalloys.
TheJournalofProstheticDentistry2002;87:94–8.
25.DyerD,MacDonaldE,NewcombeRG,ScratcherC,LeyF, AddyM.Abrasionandstainremovalbydifferentmanual toothbrushesandbrushactions:studiesinvitro.Journalof ClinicalPeriodontology2001;28:121–7.
26.ReclaruL,MeyerJM.Effectsoffluoridesontitaniumand otherdentalalloysindentistry.Biomaterials1998;19:
85–92.
27.KanekoEH,HatorriM,HasegawaK,YoshinariM,KawadaE, OdaY.Influenceoffinishontheelectrochemicalproperties ofdentalalloys.BullTokyoDentalCollection2000;41:49–57. 28.RimondiniL,FareS,BrambillaE,FelloniA,ConsoniC,
BrossaF,etal.Theeffectofsurfaceroughnessonearly invivoplaquecolonizationontitanium.Journalof Periodontology1997;68:556–62.
29.SubramaniK,JunghRE,MolenbergA,HammerleCH.Biofilm ondentalimplants:areviewoftheliterature.International JournalofOralMaxillofacialImplants2009;24:616–26.
30.TruongVK,LapovokR,EstrinYS,RundellS,WangJY,Fluke CJ,etal.Theinfluenceofnano-scalesurfaceroughnesson bacterialadhesiontoultrafine-grainedtitanium.Biomaterials
2010;31:3674–83.