89
The effect of pH level on corrosion rate of Aluminium and copper
Zainab R. Muslim
1, Harith.I.Jaafer
2, and Mohanad Q.Fahem
31,2,3University of Baghdad, College of Science ,Physics Dept.
mohanad.physics@yahoo.com
Abstrak – The effect of weight loss of Aluminium and copper on corrosion rate was studied using two different temperatures (25 and 50)oC and different pH (4.8,7 and 8.2 ).It was shown that the weight loss was decreased with the increasing in pH ,this effect on the corrosion rate which decrease with increasing in pH . Temperature effect on weight loss, higher weight loss was observed with the increasing in temperature, higher weight loss in (pH=4.8) in Aluminium & Copper.
Key Words –Corrosion, Corrosion rate, Weight loss.
1
Introduction
The deterioration of materials of construction has a severe economic impact on world-wide activities;
practically all materials of construction undergo degradation or deterioration in a natural environment. Control or prevention of such damage can be a discouraging and complex problem, so that maintenance and repair, or replacement, of materials that have undergone severe deterioration is a matter of great concern. [1]
A mechanism of corrosion is the actual atomic, molecular, or ionic transport process that takes place at the interface of a material. These processes usually involve more than one definable step, and the major interest is directed toward the slowest step that essentially controls the rate of the overall [2]. The corrosion rate depends on both the metal type and corrosive media. The corrosion rate value has much importance in the mechanical parts choice or the interval between the beginning of their use [2]. In 2009 ABDULKARIM et .al . study (the effects of pH on Aluminium & galvanized steel Galvanized steel , the results shows that galvanized steel will most definitely corrode faster than Aluminium alloy under acidic conditions and the rate of this corrosion will increase with increasing acidity[5].
In 2012 AbdulkhaliqA. study the effect of sea water on Corrosion / Erosion Rate and the hardness of commercial Aluminium alloy. The Weight loss per unit area increase at the first hours of exposure time to the sea water ( till 3.5hrs ) , then be constant at the remaining time the corrosion rate was maximum after ( 0.5 hr) then decrease gradually , and be at low rate after more than 10 hrs of exposed time[4].
2
Experiment Part
2.1 Materials
International Journal of Basic and Applied Science,
Vol. 02, No. 04, April, pp. 89-92 Muslim, et. al
90 Insan Akademika Publications
three types of water of different pH (4.8 , 7 and 8.2).
2.2 Metal Description and Preparation
The test pieces, of two wire metal (pure Aluminium 99.9%,pure copper 99.57% ) were cut into wires rolled to as a spring of diameter of 2 cm, and were polished with zero emery paper for obtaining smooth surface . The surface of each specimens was cleaned by distilled water, and finally cleaned with ethanol to degreased. The metal were air dried and stored in a desiccator . These coupons were weighed and separately immersed in 100 ml beakers containing three types of water according to pH (4.8 , 7 and 8.2) in 50 oC by using water bath (Branson 3200) and in room temperature .
The simple immersion test continued for a total time period of 168 hours the corrosion rate was measured using the weight loss versus time for each metal.
2.3 Methods
2.3.1 Weight Loss
The simplest, and longest-established method of estimating corrosion losses in plant and equipment is
weight loss analysis. A weighed sample of the metal under consideration is introduced into the solution, and later removed after a reasonable time interval. The metal is then cleaned of corrosion product and is reweighed. The process was repeated for one week, the weight loss which represent the weight loss between the initial and final reading was used to estimate the corrosion rate, Equation (1) used to calculate the corrosion rate[3]:
Corrosion rate= ����ℎ � � � � … (1)
2.3.2 Temperature effect
The two metals were rinsed in water of different pH (4.8,7 and 8.4) and then were immersed bath of temperature 50 oC ,reading of weight loss were recorded every four hour until 168 hrs reached.
3
Results and Discussion
Weight loss as loss a function of time was studied for Aluminium &Copper. Copper shows higher weight loss than Aluminium, but higher weight loss was observed for (pH=4.8) for both of metals ,because the weight loss was decreasing with the increasing in pH, and that effect on the value of corrosion rate of Copper , table (1) and (2).
Temperature play a significant role in the corrosion rate, higher corrosion rate was observed in (50 0C)
as compared to room temperature , table (3) and (4).
Muslim, et. al International Journal of Basic and Applied Science, Vol. 02, No. 04, April 2014, pp. 89-92
www.insikapub.com 91
The temperature effect on weight loss of Aluminium for (pH= 4.8), which give higher corrosion rate as
compared to other specimens ,because temperature effect on the hydrogen ions and the presence of O2
in water can multiply H2 ion concentration many time, so that acids have the capability of being
corrosion.
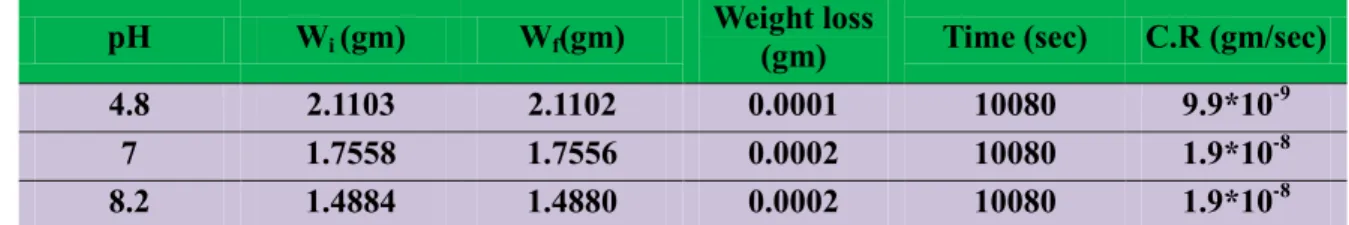
Table (1)Show the measurement of weight loss and corrosion rate for Copper in water with different
pH (4.8, 7, and 8.2) in R.T0C
pH Wi (gm) Wf(gm) Weight loss (gm) Time (sec) C.R (gm/sec)
4.8 2.1103 2.1102 0.0001 10080 9.9*10-9
7 1.7558 1.7556 0.0002 10080 1.9*10-8
8.2 1.4884 1.4880 0.0002 10080 1.9*10-8
Table (2) Show the measurement of weight loss and corrosion rate for Aluminium in water with
different pH (4.8, 7, and 8.2) in R.T0C.
pH Wi (gm) Wf(gm) Weight loss (gm) Time (sec) C.R (gm/sec)
4.8 3.4659 3.4638 0.0021 10080 2.08*10-7
7 3.9022 3.902 0.0002 10080 1.98*10-8
8.2 2.9052 2.9050 0.0002 10080 1.98*10-8
Table (3)Show the measurement of weight loss and corrosion rate for Copper in water with different
pH (4.8, 7, and 8.2) in 50 0C.
pH Wi (g) Wf(g) Weight loss (gm) Time (sec) C.R (g/sec)
4.8 3.9076 3.8825 0.0251 10080 24.9*10-7
7 3.7143 3.7109 0.0034 10080 3.37*10-7
8.2 3.7482 3.7455 0.0037 10080 3.67*10-7
Table (4) Show the measurement of weight loss and corrosion rate for Aluminium in water with
different pH (4.8, 7, and 8.2) in 50 0C.
pH Wi (g) Wf(g) Weight loss (gm) Time (sec) C.R (g/sec)
4.8 1.2528 1.2475 0.0053 10080 5.25*10-7
7 1.3334 1.3314 0.002 10080 1.98*10-7
8.2 1.1501 1.1469 0.0032 10080 3.17*10-7
4
Conclusions
The result shows that corrosion rate of Copper in 50 0C was higher than corrosion rate of Aluminium
International Journal of Basic and Applied Science,
Vol. 02, No. 04, April, pp. 89-92 Muslim, et. al
92 Insan Akademika Publications
Higher weight loss for Copper as compared to Aluminium.
Higher corrosion rate were observed for Copper in (50 0C) as to room temperature.
The corrosion rate was increase with the increasing in acidity.
Higher pH means these are power free H2 ions.
Low pH acid waters clearly accelerate corrosion by providing supply of H2 ion.
References
Showard A. Porte ,"THE EFFECT OF ENVIRONHEAT ON THE CORROSION OF METALS Yn4
IN SEA WATER--A LITEATURE SURVEY", Technical Note,1967,N-907.
E.E.Stansbury, R.A.Buchanan, "Fundamentale of electrochemical corrosion", first printing , July 2000.
AKINPELUMI,K-F-,TO investigate the effect of pH on corrosion rate ,chemical Engineering
Laboratory ,University of Lagos, Lagos June 28,2012.
A.A. Hasan, “The Effect of Sea water on the Corrosion Resistance of Commercial Aluminum
Alloys” Basrah Journal of Science, Vol.30(1),26-33, 2012.
B. I. ABDULKARIM1*,Yusuf Ahmed ABDULLAHI2, Kamoru Adio SAlAM3,”CORROSION
RESISTANCE OF COMMERCIAL ROOFING SHEETS TO ACID RAIN WATER IN ELEME, RIVERS, NIGERIA”, International Journal of ChemTech Research, Vol.1, No.4, pp