MINISTÉRIO DA EDUCAÇÃO
UNIVERSIDADE FEDERAL DO RIO GRANDE DO NORTE CENTRO DE CIÊNCIAS DA SAÚDE
PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS DA SAÚDE
EFEITO IMUNOMODULADOR E ATIVIDADE ANTIMICROBIANA DE HETEROFUCANAS DE Sargassum filipendula
CINTHIA BEATRICE DA SILVA TELLES
EFEITO IMUNOMODULADOR E ATIVIDADE ANTIMICROBIANA DE HETEROFUCANAS DE Sargassum filipendula
Tese apresentada ao Programa de Pós-Graduação em Ciências da Saúde da Universidade Federal do Rio Grande do Norte como requisito para a obtenção do título de Doutor em Ciências da Saúde.
Orientador: Prof. Dr. Hugo Alexandre de O. Rocha
iii
MINISTÉRIO DA EDUCAÇÃO
UNIVERSIDADE FEDERAL DO RIO GRANDE DO NORTE CENTRO DE CIÊNCIAS DA SAÚDE
PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS DA SAÚDE
Coordenador do Programa de Pós-Graduação em Ciências da Saúde:
iv
CINTHIA BEATRICE DA SILVA TELLES
EFEITO IMUNOMODULADOR E ATIVIDADE ANTIMICROBIANA DE HETEROFUCANAS DE Sargassum filipendula
Aprovada em: 26/06/ 2015
Banca Examinadora:
Presidente da Banca:
Prof. Dr. Hugo Alexandre de Oliveira Rocha (UFRN)
Membros da Banca
v
Dedico esta obra
A Deus. Pela minha existência e por me oferecer condições para realizar essa conquista.
Ao meu filho, Davi. Você é minha VIDA, tem me ensinado a cada dia o que é o amor incondicional, a minha maior alegria é a sua felicidade. É o seu sorriso, a sua alegria de viver, seus beijos e abraços apertados que me proporcionam força para continuar nessa caminhada. Te amo muito.
Ao meu marido, Jean. Não sei se somos almas gêmeas, afinal temos tantas diferenças, mas tenho a certeza
vi
Dedico esta obra
Aos meus pais, Ana e Carlos.
Por serem exemplo de amor incondicional, presentes em todos os momentos da minha vida, com certeza essa conquista foi construída graças a todo amor, apoio e incentivo dedicado a mim durante todos esses anos. Espero poder retribuir todo o investimento, e ainda proporcionar muito orgulho.
Ao meu irmão, Agusto.
Exemplo de homem… hoje tenho certeza que o que sou devo muito a você, meu
irmão mais novo, mas que sempre foi o mais maduro de nós dois… Agradeço o seu amor, carinho, compreensão, apesar de não usarmos muito as palavras para expressar todo o nosso amor, nos amamos muito.
A Hugo Rocha,
vii
Agradecimentos especiais
Ao meu marido. Jean Gouveia, meu amigo e companheiro. Obrigada por dividir a sua vida comigo, que nosso amor supere todos os obstáculos.
Ao meu filho Davi, que Deus abençoe seus caminhos, e lhe dê muita saúde e sabedoria, estarei sempre ao seu lado, lutando pela sua felicidade. Te amo muito.
À minha família, em especial, meus pais Ana Lúcia e Carlos Roberto e ao meu irmão Augusto César que me incentivaram em todos os momentos;
Ao amigo e orientador, Hugo Rocha, por estar ao meu lado nessa caminhada.
À UFRN, à Pós-graduação em Ciências da Saúde e ao Departamento de Bioquímica pela oportunidade de concluir esse curso de Pós-graduação, assim como as agências
Financiadoras CAPES e CNPq.
A todos os professores do CCS (UFRN) e do DBQ (UFRN), aos coordenadores do programa de pós-graduação (PPGCSa) e às secretárias do programa.
Aos membros da banca de qualificação: Profa. Luciana Guimarães, que desde a qualificação do mestrado ofereceu valiosas sugestões para melhorar a minha pesquisa e a
e a Jailma Almeida uma profissional exemplar e sempre dedicada.
Aos meus amigos de laboratório pela colaboração e ajuda nos meus experimentos e pelos momentos de descontração:
A Jadrilmaminha amiga, madrinha, anjo… obrigada por fazer parte da minha vida profissional e pessoal, devo muito da minha “transformação” ao seu carinho e presença na
minha vida e na vida do meu filho… obrigada por tudo.
A Dayn exemplo de perseverança, mãe, mulher e profissional exemplar. Desejo muito que o seu futuro profissional seja brilhante, tenho um grande carinho por você.
A Ruth e a Rafael, amigos que admiro muito, a Leandro (ou será rosa) hoje faz parte da família, você é um exemplo de profissional.
A Mariana ou Santana, amiga do lab, da turma do mestrado, hoje amiga para a vida, você é um exemplo de dedicação, de amor pelo que faz obrigada pela ajuda sempre que
precisei te adoro!
Agradeço muito à família BIOPOL, todos vocês são essenciais nessa trajetória: Gabriel, Moacir, Joanna, Pablo, Raniere, Ivan, Leonardo Nobre (Leo), Letícia, Arthur, Vinicius,
viii
Ao Prof. Marcelo Silva e a amiga Sônia Pestana do Instituto de Higiene e Medicina Tropical, Universidade Nova de Lisboa que durante os seis meses vividos em Lisboa,
Portugal, compartilharam comigo seus conhecimentos e proporcionaram meses maravilhosos;
A Profa. Tiana Tasca e todas as alunas da Faculdade de Farmácia da Universidade Federal do Rio Grande do Sul, em especial a aluna Amanda Picolli, pelo acolhimento e
disposição na realização dos ensaios.
Agradeço a Carol pela ajuda na realização dos experimentos com Leishmania e todos do Instituto de Medicina Tropical da UFRN que ajudaram na realização desse trabalho.
Agradeço, de forma especial, àqueles que contribuíram de forma diferencial para minha formação como pessoa, os meus amigos de SEMPRE: Tathiana, Raiane, Gabriela, Ana Paula, Isabel, Deborah, Nilmara e Isabelli. Sem o apoio de vocês tudo seria mais difícil
Agradeço aos meus sogros: Benigna e Gouveia. Sem a dedicação incondicional de vocês a criação do meu filho, nosso bebe, a realização dessa tese seria bem mais difícil. Muito
obrigada pela ajuda e pelo carinho. Também dedico esta tese a vocês!
Agradeço a todos os amigos que trabalham comigo na EAJ: Lígia, Welson, Ângela, André, Afrânio, Rose, etc. vocês são exemplos de profissionais, tenho aprendido muito ao lado de vocês. E, em especial, agradeço as substitutas que são insubstituíveis, Maiara e Louize, uma das conquistas durante esse período na EAJ com certeza foi a amizade de
vocês.
Agradeço aos professores e amigos do IFRN campus Currais Novos, em especial, a Andreilson, Itála, Jane, Jana, Dayana, Maura, Mayara, Lívia, Jonas, Marcelo, Danúbia, Cris, dentre tantos outros que mesmo com uma palavra de incentivo me deram força para a conclusão desse trabalho.
ix
“A vida é um sonho, torne-o real”.
x
RESUMO
Macroalgas marinhas constituem uma fonte extremamente rica de compostos bioativos, dentre eles, polissacarídeos sulfatados. Sargassum filipendula (SF),
alga pertencente à ordem Phaeophycea, é fonte de heterofucanas conhecidas pela capacidade de modular uma série de funções biológicas. Considerando a necessidade de encontrar fármacos mais eficientes no combate a infecções microbianas, as heterofucanas de SF foram avaliadas como agentes imunomoduladores e antimicrobianos. As heterofucanas SF0.5V, SF0.7V e SF1.0V apresentaram uma forte atividade imunomoduladora intensificando a liberação de óxido nítrico (NO) por macrófagos murinos (RAW 264.7), bem como, por macrófagos originados de monócitos primários humanos. Além disso, quando macrófagos humanos foram infectados por Leishmania infantum
e tratados com SF0.5V, SF0.7V e SF1.0V ocorreu um aumento significativo na liberação extracelular de NO. Como a citotoxicidadede de macrofágos contra a forma intracelular de leishmanias é mediada pela produção de NO, avaliamos a atividade leishmanicida sobre a forma amastigota intracelular de L. infantum e observamos que macrófagos infectados e tratados com SF0.5V, SF0.7V e SF1.0V se tornaram menos susceptíveis à infecção. As heterofucanas que se mostraram com capacidade de induzir a atividade anti-leishmania também apresentaram melhor taxa de produção de NO, porém os dados de correlação levaram a observação que este não é o principal mecanismo de ação das fucanas de SF no combate a esse protozoário. A heterofucana SF0.5V também apresentou atividade inibitória da formação de biofilme (~ 50%) frente a bactéria Staphylococcus epidermidis. Já SF0.7V e 1.0V inibiram quase que totalmente a replicação do protozoário Trichomonas vaginalis. Resultados como esse refletem o espectro de ação desses polissacarídeos sulfatados obtidos de SF e mostram o seu potencial como agentes imunomoduladores e microbicidas.
Palavras chaves: Imunomodulação; Oxido nítrico; Leishmania infatum,
xi
LISTA DE ABREVIATURAS E SIGLAS
µL Microlitros
g Grama
h Hora
kDa Kilodalton
KH2PO4 Fosfato monopotássico
M Molar
mg Miligrama Min. Minutos mL Mililitros mM Milimolar mm Milímetros NaCl Cloreto de sódio NaOH Hidróxido de sódio nm Nanômetros PA Para análise
xii
LISTA DE FIGURAS
xiii
SUMÁRIO
RESUMO... x
LISTA DE ABREVIATURAS E SIGLAS... xi
LISTA DE FIGURAS... xiii
1. INTRODUÇÃO... 15
1.1. SISTEMA IMUNOLÓGICO……… 15
1.2. PARASITAS VERSUS SISTEMA IMUNOLÓGICO………... 16
1.2.1. Leihmania sp……… 16
1.2.2. Trichomonas vaginalis……… 17
1.2.3. Staphylococcus epidermidis e Klebsiella pneumina………... 18
1.3. MODIFICADORES DA RESPOSTA BIOLÓGICA………. 19
1.4. FUCANAS: POLISSACARÍDEOS SULFATADOS DE MACROALGAS MARRONS………... 19
2. JUSTIFICATIVA... 20
3. OBJETIVOS... 21
3.1. OBJETIVO GERAL... 21
3.2. OBJETIVOS ESPECÍFICOS... 21
4. MÉTODOS... 22
4.1. MATERIAIS BIOLÓGICOS... 22
4.1.1. Algas... 22
4.2. OBTENÇÃO DAS HETEROFUCANAS DE Sargassum filipendula... 22
4.2.1. Obtenção do pó cetônico... 22
4.2.2. Proteólise... 22
4.2.3. Fracionamento do extrato bruto com concentrações crescentes de acetona... 23
4.3. ANÁLISES QUÍMICAS... 23
4.3.1. Dosagem de açúcares totais... 23
4.3.2. Dosagem de sulfato………... 23
4.3.3. Dosagem de proteínas………... 24
4.3.4. Determinação da composição monossacarídica…….... 24
5. ARTIGOS PRODUZIDOS... 26
5.1. ARTIGO 1 (SUBMETIDO)... 27
5.2. ARTIGO 2 (PUBLICADO)…... 53
6. COMENTÁRIOS, CRÍTICAS E SUGESTÕES... 72
Telles, C.B.S. PPGCSA/CCS
1. INTRODUÇÃO
1.1. Sistema imunológico
O sistema imunológico é uma rede interativa de órgãos linfóides, células, fatores humorais, citocinas, quimiocinas, dentre outros elementos, que trabalham em conjunto para defender o organismo do ataque desses
“invasores” [1]. A resposta imunológica é dividida em dois tipos de acordo com a velocidade e especificidade da reação, respostas inatas e adaptativas. A imunidade inata abrange os elementos do sistema imune (Neutrófilos, monócitos, macrófagos, complemento, citocinas e proteínas de fase aguda) que fornecem a defesa imediata do hospedeiro [2]. A adaptativa consiste de reações específicas contra o antígeno através do recrutamento de linfócitos T e linfócitos B [2].
Telles, C.B.S. PPGCSA/CCS
diferentes tipos de células efetoras: células Th1, pró-inflamatórias; Th2, anti-inflamatórias; células T regulatórias, Tregs; e células Th17 [7].
As células Th1 são críticas durante a resposta pró-inflamatórias, induzem a liberação de INF- , IL-2 e TNF-α, fudamentais na ativação de macrófagos. As células Th2 regulam a resposta imune humoral, proliferação de células B, respostas alérgicas e proteção contra infecções de helmintos, promovendo a produção de citocinas anti-inflamatórias IL-4, IL-5, IL-10 e IL-13. As células Treg promovem a supressão da ativação, proliferação e funções efetoras de várias células imunes como as células T, células NK, células B e células apresentadoras de antígeno [8]. Secretam citocinas imunossupressoras IL-10 e TGF- que inibem a proliferação da resposta Th1 e Th2 para minimizar o dano
tecidual [9]. As células Th17 estão envolvidas na resposta contra microparasitas extracelulares que necessitam de uma forte resposta inflamatória e que não são adequadamente tratadas pelas respostas Th1 ou Th2 [10].
Apesar de o sistema imunológico atuar fielmente na defesa do organismo, alguns agentes invasores podem transpor a resposta imune e, na ausência de um sistema imunológico eficaz, mesmo infecções menores podem se estabelecer e tornarem-se fatais.
1.2. Parasitas “versus” Sistema Imunológico
Os parasitas são um grupo altamente diversificado de organismos que desenvolveram diferentes estratégias para infectar seus hospedeiros.
1.2.1. Leishmania sp.
Telles, C.B.S. PPGCSA/CCS
flageladas dentro fagócitos de mamíferos [14], sendo considerado um parasita intracelular obrigatório.
Os macrófagos, células alvo das formas amastigotas de Leishmania, possuem diversas formas de combater microorganismos invasores, dentre elas, a ativação da enzima NADPH oxidase que induz a formação de espécies reativas do oxigênio; a alteração do pH das vesículas fagocíticas levando a desnaturação proteica [15]; a ativação da enzima óxido nítrico sintase induzida (iNOS), que quando ativada sintetiza óxido nítrico (NO), molécula efetora no combate às infecções [16]. Porém, mesmo diante do arsenal imunológico disponível contra microorganismos invasores, as espécies de Leishmania são capazes de evadir a resposta imunológica do hospedeiro vertebrado desde o momento da infecção. A primeira resposta evitada é a lise mediada pelo sistema complemento, as metaloproteses de superfície majoritárias (MSP), presente na superfície das formas promastigotas infectantes, atuam clivando o componente C3 em seus subprodutos e convertendo C3b em sua forma inativa C3bi, que permanecem aderidas a superfície do protozoário, opsonizando-as e facilitando sua fagocitose através da ligação aos receptores CR1 e CR3 dos macrófagos essa ligação leva à diminuição da expressão de INF- , IL-12 e, aumento na secreção de IL-4, contribuindo para a sobrevivência das promastigotas [17]. Os macrófagos, definem o curso da infecção dependendo da maneira como são ativadas. As células T auxiliares apresentam papel fundamental nessa ativação, a expansão de clones Th1 leva a um controle da doença, enquanto a expansão de clones Th2 leva a uma piora no quadro da doença [15].
O tratamento das leishmanioses é baseado em antimoniais pentavalentes (PVAs), cuja ação curativa foi descoberta pelo brasileiro Gaspar Vianna em 1912 [18]. No entanto, estes fármacos, que vêm sendo utilizadas há mais de seis décadas, estão longe de serem satisfatórios devido aos seus altos custos de produção, toxicidade, surgimento de resistência medicamentosa frente a todas as espécies de leishimania em várias partes do mundo [11], além de apresentarem reconhecidos efeitos colaterais [19].
Telles, C.B.S. PPGCSA/CCS
Trichomonas vaginalis é um protozoário flagelado parasita que causa tricomoníase, a mais comum doença sexualmente transmissível não viral [20]. A aderência e a citotoxicidade exercidas pelo parasita sobre as células do hospedeiro são controladas por fatores de virulência, como adesinas, cisteino-proteinases, integrinas, cell-detaching factor (CDF) e glicosidases. A aderência do parasita as células epiteliais modula a expressão gênica de proteínas funcionais das células do hospedeiro, como aquelas associadas à manutenção da estrutura da matriz extracelular, moléculas pró apoptóticas e pró-inflamatória. Um mecanismo de escape do sistema de defesa do hospedeiro é a capacidade de T. vaginalis se auto-revestir de proteínas presente no plasma do hospedeiro, impedindo, assim a identificação deste pelo sistema imune [21].
Metronidazole e Tinidazole são os únicos fármacos recomendados para o tratamento da infecção por T. vaginalis [22]. No entanto, alguns estudos vêm mostrando a resistência do T. vaginalis a esses fármacos [23], o que dificulta ainda mais o controle da infecção.
1.2.3. Staphylococcus epidermidis e Klebsiella pneumonia
As bactérias são os microorganismos que mais frequentemente causam infecções no homem [24]. Estes microorganismos são capazes de se replicar tanto no interior das células do hospedeiro como em ambientes extracelulares [25]. As infecções causadas por bactérias extracelulares são as mais frequentes. Nesses casos os mecanismos de defesa estão relacionados principalmente com as barreiras naturais do hospedeiro, a resposta imune inata, a ativação do complemento e a produção de anticorpos. Nas bactérias intracelulares a característica principal é a capacidade de sobrevivência dentro dos macrófagos, podendo estimular as células TCD4+ através da expressão de antígeno associado ao MHC classe II, os quais produzem citocinas ativadoras de TCD8+ que reconhecem e destroem as células infectadas que expressam antígenos associados a moléculas do MHC classe I. A ativação de células TCD4+ leva à secreção de IFN- , que ativa os macrófagos levando à produção
aumentada de NO e destruição da bactéria [24].
Telles, C.B.S. PPGCSA/CCS
portanto, torna-se um alvo importante para o desenvolvimento de estratégias de tratamento. Dentre as abordagens incluem estratégias como vacinação terapêutica com o designer de adjuvantes para conduzir determinados tipos de resposta imune [26] e a busca por moléculas imunomoduladoras [27].
1.3. Modificadores da resposta biológica
O crescimento dos conhecimentos de imunologia clínica tem revelado que a fisiopatologia de doenças pode ser causada tanto por exacerbação como imunodeficiências da resposta imune. A terapia imunológica moderna é dividida em dois grupos de imunomoduladores: o dos imunoestimuladores, que conduzem ao aumento da imunidade inata e adaptativa, e o dos imunossupressores, que diminuem a atividade do sistema imune [28].
Modificadores da resposta biológica (BRMs) são moléculas que atuam como ativadores ou supressores da resposta de células do sistema imune [29]. Os principais efeitos biológicos promovidos pelos BRMs são atividades anticoagulante, antitumoral, antifúngica, antibacteriana e antiparasitária [30-35]. Polissacarídeos de diferentes origens e com características estruturais variadas vêm sendo estudados quanto as suas potenciais aplicações biológicas [36-40].
1.4. Fucanas: Polissacarídeos sulfatados de macroalgas marrons
As fucanas são polímeros que têm como característica principal à presença da L-fucose sulfatada na sua estrutura [39,41], são os principais polissacarídeos sulfatados obtidos das Phaeophyceas ou algas marrons.
O gênero Sargassum C. Agardh (Sargassaceae) constitui um dos mais representativos dentre os 41 gêneros da ordem Fucales (Phaeophyceae, Heterokontophyta), é amplamente distribuído nas regiões tropicais e subtropicais do globo e é considerado um importante componente da flora marinha [42]. As espécies desse gênero são reconhecidas fontes de fucanas com uma variedade de atividade biológica: Sargassum horneri [43]; S. tenerrimum [44]; S. patens [45]; S. stenophyllum [46], S. wightii [47], S. vulgare
Telles, C.B.S. PPGCSA/CCS
cosméticos [50], apresenta fucanas ampla capacidade antioxidante e antimural [51,52].
2. JUSTIFICATIVA
Diante da patogenicidade das infecções causadas pelos microorganismos avaliados no presente trabalho e, devido ao limitado arcenal terapêutico no controle desses patógenos, além do efeito adverso dos fármacos disponíveis, que possuem um alto custo e o surgimento de resistência medicamentosa, vem sendo travada uma verdadeira batalha entre a criatividade humana na produção de drogas antimicrobianas cada vez mais potentes e de amplo espectro de ação.
Substâncias produzidas a partir de produtos naturais, como as macroalgas marinhas, podem oferecer uma oportunidade para superar esses efeitos adversos, pois apresenta como vantagens o suprimento sustentável e baixo custo.
Macroalgas marinhas do grupo das Phaeophyceas são potentes fontes de moléculas bioativas, dentre elas, destacam-se as fucanas. O gênero
Telles, C.B.S. PPGCSA/CCS
3. OBJETIVOS
3.1. GERAL
Avaliar o potencial imunomudulador e antimicrobiano das heterofucanas de S. filipendula frente aos microorganismos Leishmania infantum, Trichomonas vaginalis, Staphylococcus epidermidis e Klebsiella pneumonia (KPC).
3.2. ESPECÍFICOS
• Extrair polissacarídeos sulfatados da alga marrom Sargassum filipendula;
• Caracterizar quimicamente as heterofucanas obtidas;
• Avaliar o efeito das heterofucanas sobre macrófagos murinos (Raw 264.7) quanto à produção de NO, Il-6 e TNF-α;
• Investigar a atividade anti-leishmania das heterofucanas sobre macrófagos infectados com Leishmania infatum.
Telles, C.B.S. PPGCSA/CCS
4. MÉTODOS
Serão descritos a seguir apenas os métodos que necessitam de um maior detalhamento para compreensão, os demais métodos encontram-se claramente descritos nos artigos.
4.1. MATERIAIS BIOLÓGICOS
4.1.1. Algas
A alga marinha marrom Sargassum filipendulafoi coletada na Praia de Búzios, município de Nísia Floresta (litoral sul do Rio Grande do Norte), em marés baixas entre 0,0 a 0,2 metros a uma temperatura situada entre 28-30 °C.
Após serem coletadas, as algas foram trazidas ao laboratório de Biotecnologia de Polímeros Naturais (BIOPOL) da Universidade Federal do Rio Grande do Norte (UFRN), onde foram lavadas e retiradas as epífitas e inclusões calcárias. Em seguida foram secadas em estufa a 45 ºC, trituradas e guardadas em recipientes apropriados.
4.2. OBTENÇÃO DAS HETEROFUCANAS DA ALGA Sargassum filipendula
4.2.1. Obtenção do pó cetônico
A alga seca e pulverizada foi suspensa em dois volumes de acetona PA, durante 24 horas para despigmentação e delipidação do material. A mistura foi decantada e o resíduo colocado para secar a 45 °C sob aeração e denominado de “pó cetônico”. Esse pó foi utilizado em seguida na proteólise.
4.2.2. Proteólise
Telles, C.B.S. PPGCSA/CCS
permaneceu em banho-maria a 60 °C por 24 h. Depois, foi filtrado e o sobrenadante submetido a uma centrifugação 10.000 x g por 15 minutos a temperatura de 4 °C. Após a centrifugação, o sobrenadante foi denominado de extrato bruto.
4.2.3. Fracionamento do extrato bruto com concentrações crescentes de acetona
O extrato bruto obtido foi fracionado com volumes crescentes de acetona, obtendo-se as frações polissacarídicas. Adicionou-se um volume de acetona, sob agitação leve, necessário para que se visualizasse uma turvação da solução, essa solução foi mantida em repouso a 4 ºC durante 18 h, o precipitado foi coletado por centrifugação a 8.000 x g por 15 minutos a 4 ºC e seco a pressão reduzida.
Em seguida, esse procedimento foi repetido até que não se visualizasse mais a formação de precipitado [37]. As frações obtidas foram denominadas conforme o volume de acetona no qual foram precipitadas (SF0.5V, SF0.7V, SF1.0V, SF1.5V e SF2.0V) (Figura 01).
4.3. ANÁLISES QUÍMICAS
4.3.1. Dosagem de açúcares totais
Açúcares totais de cada extrato polissacarídico e de cada fração polissacarídica obtida foram determinados pelo método do fenol/ácido sulfúrico, utilizando-se como padrão galactose, sendo as leituras realizadas a 490 nm [53].
4.3.2. Dosagem de sulfato
Telles, C.B.S. PPGCSA/CCS
4.3.3. Dosagem de proteínas
O teor de proteína correspondente de cada extrato polissacarídico e de cada fração polissacarídica obtida foi determinado com o reagente de comassie blue R 250 e a leitura realizada a 595 nm [55].
4.3.4. Determinação da composição monossacarídica
Telles, C.B.S. PPGCSA/CCS
Telles, C.B.S. PPGCSA/CCS
5. ARTIGOS PRODUZIDOS
5.1. Artigo 1 (SUBMETIDO)
Immunomodulatory effect and antimicrobial activity of Sargassum filipendula heterofucanas
Periódico: Marine Drugs
Fator de impacto (2015): 3.512 ISSN: (Printed version)
ISSN: (Online version) Qualis: Medicina II – A2
Indexada: PubMed – indexado por MEDLINE
5.2. Artigo 2 (PUBLICADO)
Methanolic Extracts from Brown Seaweeds Dictyota cilliolata and Dictyota menstrualis Induce Apoptosis in Human Cervical Adenocarcinoma HeLa
Cells
Periódico: Molecules
Molecules. 2015 Apr 13;20(4):6573-91
Fator de impacto (2015): 2.095 ISSN: 1420-3049 (Printed version) ISSN: 1420-3049 (Online version) Qualis: Medicina II – A2
Indexada: PubMed – indexado por MEDLINE
5.3. Capítulo de livro
Chapter: Carrageenans
Periódico: Biochemistry and Molecular Biology in the Post Genomic Era Binding: ebook
Pub. Date: 2015
Telles, C.B.S. PPGCSA/CCS
5.1. ARTIGO 1 (SUBMETIDO)
Mar. Drugs 2015, 13, 1-x manuscripts; doi:10.3390/md130x000x
marine drugs
ISSN 1660-3397 www.mdpi.com/journal/marinedrugs
Article
Immunomodulator effects and antimicrobial activity of
heterofucanas from Sargassum filipendula
Cinthia Beatrice Silva Telles 1,2, Carolina Mendes-Aguiar 3, Gabriel Pereia Fidelis1, Amanda Piccoli Frasson4, Leandro Silva Costa 1,5, Tiana Tasca3,; Selma Maria Bezerra Jeronimo1,2 and Hugo Alexandre Oliveira Rocha 1,2,*
1
Laboratório de Biotecnologia de Polímeros Naturais (BIOPOL), Departamento de Bioquímica, Centro de Biociências, Universidade Federal do Rio Grande do Norte (UFRN), Natal,
Rio Grande do Norte-RN 59078-970, Brasil; E-Mails: cinthiatelles@yahoo.com.br (C.B.S.T.); leandro-silva-costa@hotmail.com (L.S.C.), gabrielfideliss@gmail.com (G.P.F.); hugo@cb.ufrn.br (H.A.O.R)
2
Programa de Pós-graduação em Ciências da Saúde, Universidade Federal do Rio Grande do Norte (UFRN), Natal, Rio Grande do Norte-RN 59078-970, Brasil.
3
Instituto de Medicina Tropical do Rio Grande do Norte, Departamento de Bioqiímica Universidade Federal do Rio Grande do Norte (UFRN), Natal, Rio Grande do Norte-RN 59078-970, Brasil; E-Mail: carolinamaguiar@gmail.com (C.M.A.);
selma.b.jeronimo@gmail.com (S.M.B.J.)
4
Faculdade de Farmácia da Universidade Federal do Rio Grande do Sul, Av. Ipiranga, 2752, Porto Alegre, RS, 90610-000, Brasil. E-Mails: tiana.tasca@ufrgs.br (T.T); amanda.pf@hotmail.com (A.P.F.)
5
Intituto Federal de Educação, Ciência e Tecnologia do Rio Grande do Norte (IFRN), Santa Cruz, Rio Grande do Norte-RN 59200-000, Brasil.
* Author to whom correspondence should be addressed; E-Mail: hugo@cb.ufrn.br; Tel.: +55-84-3215-3416 (ext. 207); Fax: +55-84-3211-9208.
Academic Editor:
Received: / Accepted: / Published:
Telles, C.B.S. PPGCSA/CCS
Abstract: Fucans, sulphated polysaccharides that contain L-fucose in its constitution, obtained from species of Phaeophyceae of the Sargassum kind, display several biological activities. Heterofucans from Sargassum
filipendula are bioactive molecules that contain strong antiproliferative and
antioxidant activity. However, their immunomodulatory and antimicrobial activities have not yet been examined. In this context, the aim of this research was to evaluate the heterofucans as for their immunomodulatory capacity and antimicrobial action against Leishmania infantum, Trichomonas vaginalis, Staphylococcus epidermidis and Klebsiella pneumonia (KPC). The five heterofucans obtained from S. filipendula show
activities that are distant as stimulants of the immune system and microbial agent. The SF0.5V, SF0.7V amd SF1.0V heterofucans were capable of acting in the activation of murine and human macrophages. In addition to that, SF0.5V has shown antibiofilm activity of S. epidermides and SF0.7V and 1.0V almost completely inhibited the survival of the protozoan T.
vaginalis. Results such as this one, reflect the broad range of action of the
sulphated polysaccharides obtained from seaweeds, especially from the species S.filipendula
Keywords: Immunomodulation; Nitric oxide; Leishmania infatum,
Trichomonas vaginalis, antibiofilm; macrophages; fucans.
1. Introduction
The immune response performs a fundamental role in the defense against infectious agents and it constitutes the most important prevention against the occurrence of infection spreads that are normally associated with a high mortality rate [1]. The establishment of an infection, in a vulnerable host, covers several mechanisms; one of the most relevant is the way that the microorganism interacts with the immune system and its response against the invader [2].
Telles, C.B.S. PPGCSA/CCS
in fighting the bacterial infections and caused by protozoans vary; generally, intracellular parasites are eliminated through mechanisms mediated by cell and the extracellular ones via mechanisms that mainly involve the complement system and antibodies [3, 4].
The drugs available for the treatment of parasite related diseases are far from satisfactory due to their high production costs, toxicity, as well as the appearance of resistance [5-8].
The increase in knowledge about clinical immunology has been revealing that the physiopathology of illnesses can be caused by exacerbation, as well as by immunodeficiencies of the immune response. The modern immunologic therapy is divided in two basic groups of immunomodulators: the immunostimulants, which lead to the increase of innate and adaptive immunity, and the immunosuppressives that diminish the activity on the immune system [9].
Natural products are important sources of innovative therapeutic agents for infectious diseases, cancer, lipid disorders and immunomodulation [10].
Sulphated polysaccharides are a complex group of bioactive macromolecules in which some of the hydroxyl groups from sugar waste are replaced by sulphate groups. Seaweeds are the main non-animal sources of obtainment of these anionic polysaccharides; in this group, we find the Fucans, which are a family of polysaccharides that contain L-fucose in its constitution [11].
Over the last few years, a variety of groups reported that sulphated polysaccharides obtained from Phaeophytas species, from the genus Sargassun showcase various biological activities: Sargassum horneri [12]; S. tenerrimum [13]; S. patens [14]; S.
stenophyllum [15], S. wightii [16], S. vulgare [17], S. siliquosum [18]. Our group
assessed the heterofucans from Sargassum filipendula, common seaweed along the northeastern cost of Brazil, and demonstrated that these polymers are bioactive molecules presenting strong antiproliferative and antioxidant activity. However, the immunomodulator and antimicrobial purified activities of the sulphated polysaccharides from S. filipendula have not yet been examined. In this context, the objective of this study was to obtain sulphated polysaccharides from S. filipendula and evaluate their immunomudulating and antimicrobial activities facing Leishmania infantum,
Trichomonas vaginalis, Staphylococcus epidermidis and Klebsiella pneumonia (KPC).
2. Results and Discussion
Telles, C.B.S. PPGCSA/CCS
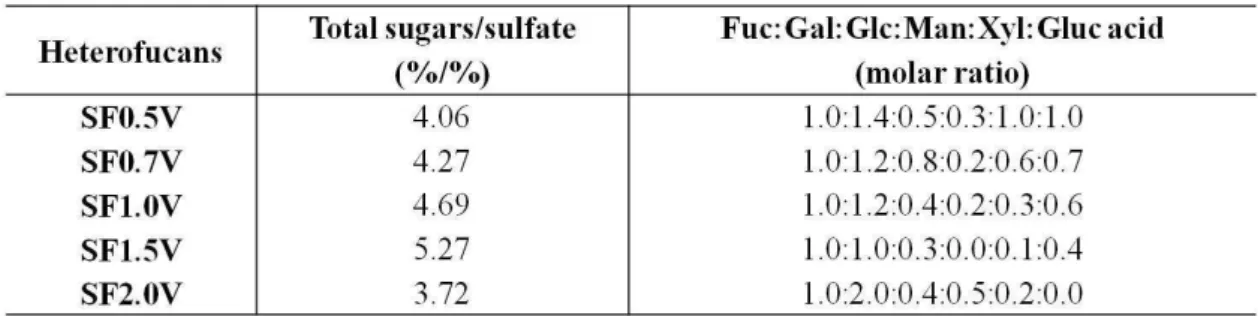
compared to the other heterofucans. The monosaccharides fucose, galactoses, glycoses and xyloses were found in different amounts in all polysaccharides. Mannoses and glucuronic acid are also present in almost all of the heterofucans, except for SF1.5V, that does not have mannoses residues and SF2.0V that does not have glucuronic acid in its structure. Comparing the data described on table 1 with the data published by Costa and collaborators [19], it is possible to verify that both are very similar, which indicates that the heterofucans extracted are the same obtained by Costa and collaborators [19]. These fucans present antioxidant and antitumor activities, however other activities have not been assessed yet, therefore, we verified weather these fucans had immunomodulator and antimicrobial effect.
Table 1. Chemical characteristics of the heterofucans from S.filipendula.
2.1. Imunomodulator effect of the sulphated heterofucans from S. filipendula in murine macrophages (RAW 264.7)
Macrophages are essential for the maintenance of homeostasis and play a main role in the defense of the host against pathogens. In this process, activated macrophages release some immunomodulator factors, such as NO, IL-6, TNF-α, amongst others [21]. Therefore, initially, we evaluated the effect of heterofucans in murine macrophages (RAW 264.7) in culture and isolation conditions.
2.1.1. Production of NO
Telles, C.B.S. PPGCSA/CCS
extracellular environment of the cells that were treated with positive control, which suggests a strong immunostimulating action of the macrophages by this heterofucan.
Other groups also proved that different fucans have distinct potentials as immunostimulating agents, e.g. fucans obtained from the weeds Ascophyllum nodosum and Fucus vesiculosus increased the amount of NO released to the extracellular environment when in contact with RAW cells. However, the Ascophyllum fucana was six times more potent than Fucus [22]. The presented information clearly indicates that the potency of stimulation of the RAW cells carried out by the fucans in order to release NO is not the same and depends on the properties of each fucana, as was observed with the heterofucans from S filipendula.
Amongst the properties featured as important for a fucan to interfere in the amount of NO releases, it is important to mention the degree of sulphatation. Nevertheless, the results obtained with the heterofucans (SF0.5V and SF2.0V) were intriguing; despite the two present similar ratio sugar/sulphate, they presented different effects in the immunostimulation of macrophages and releasing of NO, which means that one of them is a stimulator (SF0.5v), whereas the other one (SF2.0v) does not affect the amount of NO in the environment.
According to Leiro and collaborators [23] the sulphate groups are important points so that a sulphated polysaccharide is able to stimulate RAW 264.7 cells to release NO to the extracellular environment. These authors presented that sulphated polysaccharides of the Ulva rígida weed were stimulating agents of the RAW cells, however when they were disulphated, their activity was lost. Jiang and collaborators [24] also demonstrated that when disulphating ascophylan, a homofucan obtained from the weed Ascophyllum
nodosum, its RAW macrophages stimulating activity was significantly decreased when
compared to the native ascoplylan.
Telles, C.B.S. PPGCSA/CCS
Figure 1. Effect of the heterofucans from S. filipendula over the release of NO by RAW264.7 cells. The data are presented as average ± standard deviation (n = 3). The letters a, b indicate a significant difference (p <0,05) between the concentration of the heterofucans. # Indicates the significant difference (p <0,05) between the concentration of the heterofucana and positive control. * Indicates significant difference (p <0,05) between the concentration of the heterofucana e negative control. NC: Negatice control; PC: Positive Control (LPS 2 µg/mL).
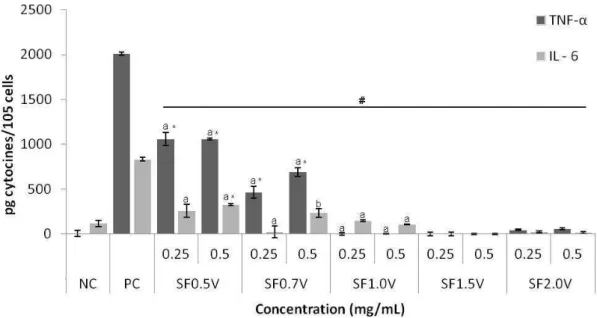
2.1.2. Production of the cytokines TNF-α and IL-6
On Figure 02 we observe that the cells RAW 264.7, when exposed to the heterofucans SF1.5V and SF2.0V, did not promote alteration on the level of TNF-α and IL-6 in the extracellular environment, proving, alongside with the previous data, that these polymers possibly do not act as immunomodulator agents.
The heterofucana SF1.0V also did not induce the cells RAW 264.7 to produce and release to the extracellular environment significant amount of TNF-α and IL-6. This characteristic is not entirely of these fucans, other heterofucan, extracted from the seaweed Dictyota menstrualis, was also not able to interfere on the production of these two cytokines [29]. The presented data lead to the observation that the immunomodulator mechanism of SF1.0V would be centred in its capacity to interfere on the production and release of NO.
Telles, C.B.S. PPGCSA/CCS
of this fucan increased in about a thousand times the amount of TNF-α in the extracellular environment in comparison with the amount of TNF-α found in the control group. This result was very expressive, since other fucans of Fucus vesiculosus [22] and
Ascophyllum nodosum [30], in the concentration of 0.2 mg/mL, were only capable of
increasing the amount of TNF-α in the extracellular environment in 20 times.
Figure 2. Effect of the heterofucans from S. filipendula over the release of TNF-α and IL-6 by RAW264.7 cells. The data are present as average ± standard deviation (n = 3). The letters a, b indicate a significant difference (p <0,05) between the concentration of the heterofucans. # Indicates significant difference (p <0,05) between the effect of the heterofucana and positive control. * Indicates significant difference (p <0,05) between the effect of the heterofucan and negative control. NC: Negative control; PC: Positive control (LPS 2µg/mL).
Telles, C.B.S. PPGCSA/CCS
Different groups that study fucans state that they are immunomodulators because they induce the activation in vitro of murine macrophages (RAW 264.7) leading to the increase of the production of NO and cytokines such as TNF-α and IL-6 [22,32-34]. Which leads us to propose that S. filipendula synthetizes imunomodulator fucans.
2.1.3. Cytotoxicity of the heterofucans related to RAW 264.7 cells
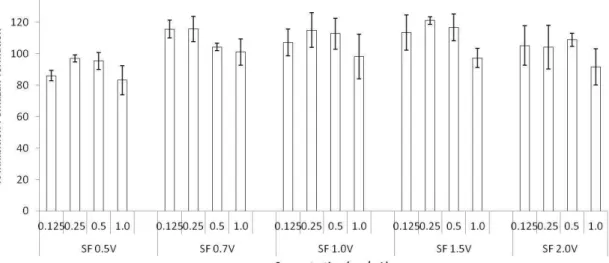
With the objective of evaluating weather the increase in the production of these chemical mediators, NO and the cytokines (TNF-α and IL-6) was not a response arising from the toxicity of the heterofucans, we evaluated the cytotoxicity of these heterofucans facing the same lineage used in previous trials. The data are on Figure 4.
Figure 4. Effect of the heterofucans from S. filipendula over the proliferation of RAW264.7 cells. The data are present as average ± standard deviation (n = 3). There were no meaningful differences in all of the tested concentrations.
By analysing Figure 04, it was possible to observe that under all of the conditions tested, the heterofucans did not compromise the cellular viability of the phagocytic cells, suggesting that these polymers do not have cytotoxic effect over RAW264.7 cells. Considering the cited aspects, we suggest that the increase in the release of NO, TNF-α and IL-6 result from the immunomodulating capacity of the heterofucans facing the line of macrophages.
2.2. Antimicrobial activity
Telles, C.B.S. PPGCSA/CCS
parasites, for instance: Leishmania infatum; trichomonas vaginalis; Klebsiella
pneumoniae (KPC) and Staphylococcus epidermidis.
2.2.1. Infection caused by Leishmania infatum in human macrophages
Considering that our group have not yet standardized the methodology for infection of RAW cells with leishmania, we chose to work with macrophages originated from primary human monocytes, as described in methods.
2.2.1.1. Production of NO
Macrophages originated from primary human monocytes exposed for 24 and 48 hours to the heterofucans from S. filipendula, in the concentration of 0.5 mg/mL, increased distinctly the amount of NO in the extracellular environment (Figure 5A). The heterofucans SF0.5V, SF0.7V and SF1.0V (Fig 5A) promoted meaningful increase in the release of NO to the extracellular environment in all of the periods analysed when compared to the untreated macrophages. In addition, the release of NO by macrophages stimulated by SF0.5V and SF0.7V in the period of 48 hours of exposure was about 2 times higher than that presented by untreated macrophages. The other polysaccharides (SF1.5V and SF2.0V) did not present meaningful effect over the production of NO by macrophages. These data is similar to that obtained during the treatment of murine macrophages (RAW 264) (Figure 02), that demonstrated the polysaccharides SF0.5V, SF0.7V and SF1.0V presented an effective immunomodulating action.
A second cultivation of phagocytic human cells was carried out. However, this time these cells were initially infected with the amastigote form (intracellular form of leishmania) and posteriorly incubated with the heterofucans from S. filipendula with the intent of evaluating the effect of the fucans in the release of NO by the infected cells.
On Figure 5B we can observe that the human macrophages infected and incubated with the heterofucans from S. filipendula (0.5 mg/mL), also distinctly increased the amount of NO in the extracellular environment. The production of NO after the treatment for 24 and 48 hours with the heterofucana SF2.0V was not altered; it kept the level of NO similar to that observed with the untreated observed cells. On the other hand, all of the other heterofucans induced an increment in the production of NO (Figure 5B). The heterofucans SF0.5V and SF0.7V were the best activators of the immune response, and presented time depending effect. Within 48 h these fucans elevated the amount of NO in about five times, comparing to the control.
Telles, C.B.S. PPGCSA/CCS
Figure 5. Effect of the heterofucans from S. filipendula over the release of NO by human macrophages. Production of NO by human macrophages after treatment with the heterofucans (A); Production of NO by macrophages infected by L. infatum and treated with the heterofucans (B). The data are presented as averages ± standard deviation (n = 3). The letters a, b indicate a significant difference (p <0,05) in the release of NO on different periods. * Indicates significant difference (p <0,05) between different heterofucans and the negative control.
2.2.1.2. Leishmanicidal activity
After the evaluation of the effects of the heterofucans over the production of NO, we evaluated the leishmanicidal activity over the amastigote intracellular form of
Leishmania infantum.
Telles, C.B.S. PPGCSA/CCS
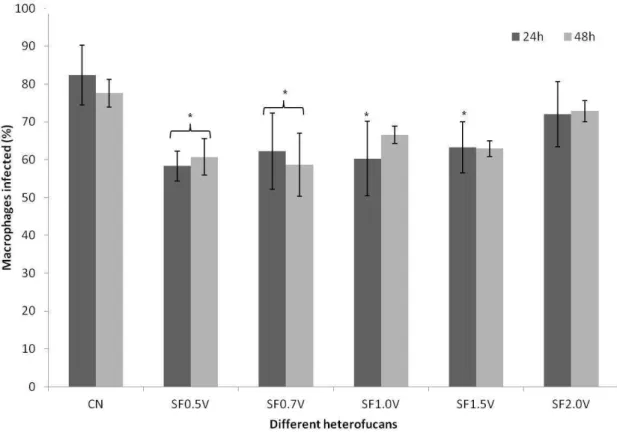
Figure 6. Leishmanicidal activity of the heterofucans from S. filipendula. The results are expressed in average ± standard deviation (n = 3) of the percentages of the macrophages infected with L. infantum * Indicates significant difference (p <0,05) between the concentration of the heterofucan and the CN. CN: Negative control – Untreated macrophages infected with L. infatum.
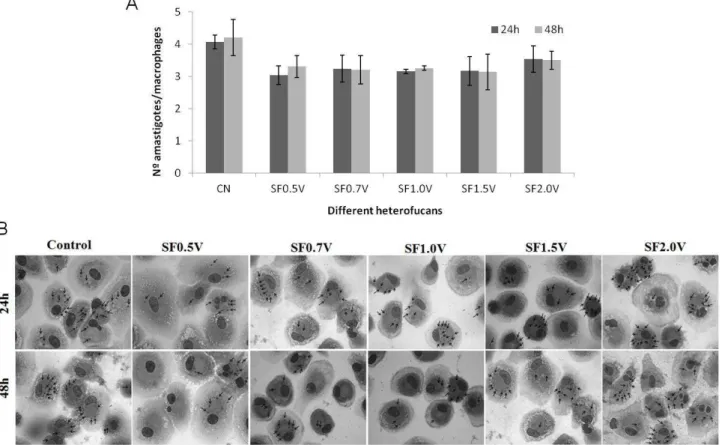
In a second experiment, the macrophages were plated over a cover slip and infected in the presence or not of the heterofucans (0.5 mg/mL), posteriorly they were fixed and coloured, and the number of amastigotes that were inside the cells was determined. The data are on Figure 7. In the control group and in the group treated with SF2.0V, the number of intracellular parasites was similar and corresponded to approximately 4 amastigotes per macrophage. During the treatment with the other heterofucans (SF0.5V, SF0.7, SF1.0V and SF1.5V) the number of amastigotes was smaller, about 3 amastigotes/macrophages. (Figure 07A).
Telles, C.B.S. PPGCSA/CCS
Figure 07B illustrates micrographs that represent the preparation of macrophages infected by Leishmania (L.) infatum, treated or not with the heterofucans from S.
filipendula. On all of the preparations, we observed the presence of amastigotes in the
interior of the cells, indicated by arrows. We observed a slight decrease in the number of amastigotes after treatment with the heterofucans when compared to the untreated cells (control group)
Altogether, the presented data demonstrates that the fucans (mainly SF0.5V, SF0.7V and SF1.0v) decreased in approximately 20% the percentage of macrophage infection (Figure 06). This percentage is low if compared to the activity of fucoidan of F.
vesiculosus, which promotes a decrease of about 90% of the number of human
macrophages infected by Leishmania [35]. That indicates that the fucans from
Sargassum are not candidates for the treatment of leishmaniasis, but, due to their
immudomodulator capacity, they can act as supporting agents in the treatment of this illness, and is raw material for the production of nanoparticles that carry antileishmanicidal drugs.
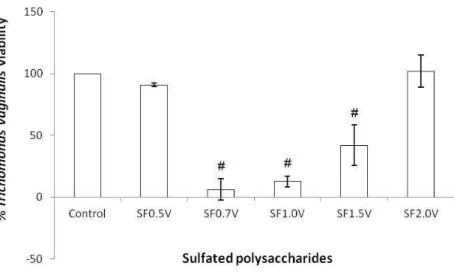
2.2.2. Anti-trichomonas vaginalis activity
Heterofucans from the seaweed S. filipendula were also analysed against trophozoites of T. vaginalis (Figure 08). Triage revealed that the heterofucans SF0.5V and SF2.0V did not present inhibitory activity facing trophozoites. Whereas SF0.7V, SF1.0V and SF1.5V presented anti-T. vaginalis activity after 24h of treatment.
Telles, C.B.S. PPGCSA/CCS
The heterofucans SF0.7V and SF1.0V that presented best inhibitory capacity facing trophozoites of T. vaginalis (approximately 90% of inhibition) present ratio sugar/sulphate of approximately 4.5, in counterpart, SF0.5 and SF2.0V present smaller values (4,06 and 3,72) did not have any activity in this essay, which leads us to suggest that the degree of sulphatation should not be a preponderant factor to a satisfactory
anti-T. vaginalis activity.
The action of the heterofucans as strong inhibitory agents of the flagellate protozoan
Trichomonas vaginalis is of key importance since trichomoniasis, sexually transmitted
disease (STD), is an infirmity present all over the world [36] and its treatment is essentially based in the use of the drug 5-nitroimidazole [37]. However, some reports have demonstrated the appearance of resisting strains [38]. Therefore, it is necessary to search for a new therapeutic arsenal. Besides, there is not a study that has demonstrated the activity of polysaccharides in the fighting of T. vaginalis, so this is the first study that brings up that polysaccharides obtained from seaweed (SF0.7V, SF1.0V and Sf1.5V) have cytotoxic action against this pathogenic protozoan.
2.2.3. Antibacterial and Antibiofilm activity
All of the heterofucans from S. filipendula were evaluated as to their antibacterial and inhibitory capacity concerning the formation of bacterial biofilms (Table 02). In this study, no heterofucana of S. filipendula was proved effective in the fighting of bacterial growth, also did not considerably inhibit the formation of biofilms, promoted by the association of bacteria of the species Klebsiella pneumoniae (KPC). The heterofucans also did not have antibacterial activity against Staphylococcus epidermidis. However, SF0.5V presented antibiofilm activity, inhibiting about 50% of biofilm formation by these bacteria.
Table 2. Antibacterial and antibiofilm activity of the heterofucans from S.filipendula. a The data are the average value of three determinations ± DP; b The results were obtained in the concentration of 2 mg/mL, other tested concentrations did not have significant activity; c rifampicin (Sigma-Aldrich Co., St. Louis, MO, USA) was used as
Telles, C.B.S. PPGCSA/CCS
The difference in the activity of SF0.5V in the formation of biofilms between the two species of bacteria studied, may result from the structural difference presented by this two bacterial groups; since the bacterial strains of the species S. epidermides are gram positive, whereas KPC is a gram negative bacteria and, therefore, has a membrane external to the bacterial cellular wall, this way they have binders distinct from the ones presented by S. epidermides.
Considering that the heterofucana SF0.5V does not have bactericidal activity (Table 02), its antibiofilm activity of S. epidermidis should be mediated by other mechanism unrelated to the inhibition of bacterial growth. Some studies show that the charge of the surface is an important parameter to the formation of biofilms. Positively charged surfaces promote a stronger bacterial adhesion, probably due to attractive electrostatic forces [39]. This way, sulphated polysaccharides or with carboxylic groups, when bond to the surfaces, would provide negative charges to the surface and would affect the bacterial adhesion by electrostatic repulsion. As an example we have the effect of two ulvanes (sulphated polysaccharides) extracted from the green seaweeds Ulva rotundata and Ulva compressa. They were efficient in the reduction of the colonization of titanium substrate by S. epidermides, reducing in 96% the initial adhesion [40]. However, we do not completely agree with this hypothesis, since only one of the five tested heterofucans presents antibiofilm activity. Probably, as in other sulphated polysaccharides act ivities, the distribution of negative charges by the molecule is a factor that is more important than the simple fact of having negative charge for a polysaccharide to present antibiofilm activity.
Telles, C.B.S. PPGCSA/CCS
This antibiofilm activity presented by the heterofucana SF05V is fundamentally important since biofilms hamper the arrival of antimicrobial drugs and even phagocytic cells to the infection site. It can be harmful to health, as in the case of bacterial pellicles that develop on teeth - originating cavities - and other problems related to mouth, lungs, urinary catheters and contact lenses, which can originate serious infections on tissues (osteomyelitis and endocarditis) and rejection to prosthetic material[42,43].
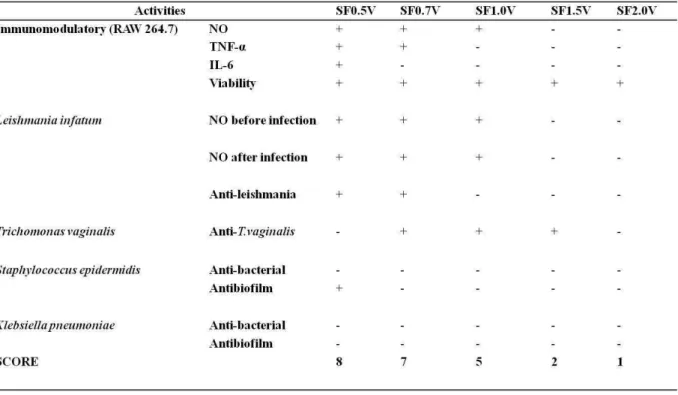
2.3. Heterofucans from S.filipendula versus biological activity
In this study we analysed five heterofucans from the seaweed S. filipendula regarding their immunomodulating and antimicrobial capacity. On table 3, we attributed positive (+) and negative (-) scores to identify which heterofucan presented the best performance on the trials carried out in this project. We observed that the heterofucan SF2.0V followed by SF1.5 presented the worst responses on the tests performed. On counterpart, SF0.5V and SF0.7V were the most promising. In addition, we noticed that none of the fucans is effective in all kinds of tests because each polymer presents a unique structure responsible for the specificity of a response during the different trials. In the future, it is intended to structurally characterize these fucans and propose correlations between their structures and the activities they perform.
Telles, C.B.S. PPGCSA/CCS
3. Experimental Section
3.1. Materials
Bromide of 3- (4,5-dimetiltiazol-2-il) -2-5-diphenyltetrazoliumbromide (MTT), Griess reagent, Histopaque 1077, Methanol PA, MCSF (Stimulating factor of macrophages colony), medium of bacterial cultivation Luria Bertani (LB), Serum AB (SIGMA) and metronidazole were acquired from Sigma Chemical Company, St. Louis, MO, USA. Medium of cellular culture (RPMI 1640) (Developed by Roswell Park Memorial Institute) and DMEM (Dulbecco's Modified Eagle's Medium), trypsin and Bovine Fetal Serum (BFS), were obtained from CULTILAB (Campinas, Brazil). L-glutamine, gentamicin, penicillin, streptomycin, sodium bicarbonate, HEPES, sodium pyruvate and saline solution tamponed with phosphate buffered saline (PBS) were acquired from Invitrogen Corporation (Burlington, ON, USA). ELISA Kits (for the TNF-α, and IL-6) were acquired from BD Biosciences. Ficoll-Hypaque was acquired from GE Healthcare. Panoptic dye was acquired from NewProv. Entellan was acquired from Merck. Half Schneider’s insect was obtained from Gibco. All of the other solvents and chemical products were obtained from analytic degree.
3.2. Biological material
The cell line of murine macrophages RAW 264.7 (ATCC number TIB-71) was donated by Dr. Carmen Ferreira (Biochemistry department, UNICAMP, Brazil). The protozoan Trichomonas vaginalis (ATCC number 30236) and Leishmania infatum were given, respectively, by Dr. Tiana Tasca (Clinical Analysis and Toxicology Laboratory, Faculdade de Farmácia, UFRGS, Brazil) and by Dr. Selma Jerônimo (Institute of Tropical Medicine of Rio Grande do Norte, Biochemistry Department, UFRN, Brazil).
The bacterial strains Staphylococcus epidermidis (ATCC 35984) and the clinical isolate of Klebsiella pneumoniae were given by Dr. Alexandre Jose Macedo (Biotecnology center and Faculdade de Farmácia, UFRGS, Brazil).
3.3. Maintenance of cell lineages
The lineage of murine macrophages (RAW 264.7) were cultivated in supplemented DMEM with 10% of Bovine Fetal Serum (BFS) and antibiotics (100 U / mL of penicillin and 100 µg / mL of streptomycin). The cells were maintained as cultures in monolayers at a humidified atmosphere of 5% of CO2 at 37 ° C.
Tricomonas vaginalis were cultivated in vitro in the TYM (trypticase-yeast
Telles, C.B.S. PPGCSA/CCS
more than 95% of viability and normal morphology were retracted, centrifuged and suspended again in medium TYM for utilizing in tests.
The promastigotes of Leishmania Infantum were kept at 25oC in Schneider’s insect medium, supplemented with 10% BFS, 200 IU/mL of penicillin and 200 µg/mL of streptomycin, and grew until stagnant phase.
Staphylococcus epidermidis and the clinic isolate of Klebsiella pneumoniae (KPC)
were utilized as biofilm former bacterial models. S. epidermidis and KPC were cultivated in Luria Bertani medium (LB) at 37 ° C under agitation of 150 rpm (Shaker Série Excella E25; New Brunswick Scientific) and adjusted to an OD600 equivalent to 108 CFU / mL for utilization in antibacterial and antibiofilm trials.
3.4. Obtainment of peripheral human blood mononuclear cells
For the obtainment of peripheral human blood mononuclear cells (PBMC), 15 mL of venous blood from six donors were obtained aseptically in heparinized tube. The tubes containing the blood were diluted in sterile saline solution (v/v). The PBMCs were obtained through the Ficoll-Hypaque gradient and adjusted to the concentration of 2 x 106 cells/mL in RPMI 1640 complete (100 L/mL of gentamicin, L-glutamine 2mM, 30mM HEPES), containing 10% of AB Serum (Life technologies GIBCO BRL, Gaithersburg, MD).
After that the PBMCs were incubated at 37 ° C, 5% CO2 for 4 h in 6 and 24 well
plates, for the adhesion of the monocytes. To the 6 and 24 well plates, were added, respectively, 2 and 3 mL/well of cell suspension. The 6 well plates were prepared with 4 cover slips round/well, for posterior microscopic analysis of the cells. After 4 h of incubation, the medium - with the non-adherent cells - was removed. The wells were washed with RPMI medium at room temperature. The adherent cells were incubated in complete RPMI medium (10% of serum AB, 5 ng/mL of MCSF (Macrophage Colony Stimulating Factor) for 6 days at 37 ° C, 5% of CO2.
3.5. Extraction of sulphated polysaccharides (Heterofucans)
The Phaeophyta Sargassum filipendula was collected at Búzios beach, Nísia Floresta, Rio Grande do Norte, Brazil. The heterofucans SF0.5V, SF0.7V, SF1.0V, SF1.5V and SF2.0V of S. filipendula were obtained utilizing the methodology described by Costa and collaborators. [19].
3.6. Production of nitric oxide (NO)
Telles, C.B.S. PPGCSA/CCS
from supernatants of the cultures to be dosed - were incubated with equal volume of Griess reagent and were incubated at room temperature for 10 minutes. The analysis was carried out in the microplate reader Multiskan Ascent (Thermo Labsystems, Franklin, MA, USA) with absorbance to 540 nm. Utilizing as standard curve the generated per NaNO2.
3.7. Cytokine analysis
RAW 264.7 (3 × 105 cells/mL) cells treated with the different heterofucans in the concentrations of 0.125, 0.25, 0.5 mg/mL, were cultivated in 24 well plates. After 24 h, the supernatants of the culture were collected. Following, they were centrifuged at 4000 rpm for 5 min. The levels of TNF-α and IL-6 were determined utilizing specific ELISA kits (immunoabsorbent enzymatic test), the negative control consists of untreated cells with the heterofucans and positive control those in the presence of LPS (2µg/mL). The plate was read at 450 nm, with remediation at 570 and 590 nm.
3.8. Cytotoxicity tests in macrophages
The cytotoxicity in RAW 264.7 cells were measured through the MTT test as described previously by Telles and collaborators [26]. The cells were cultivated in 96 well plates to a density of 5 x 103 cells/well with the heterofucans in different concentrations (0.125, 0.25, 0.5 and 1.0 mg/mL) for 24 hours at 37 ° C and 5% of CO2.
After the incubation, 100 uL of MTT were added to each well, incubated during 4 h at 37 ° C and 5% of CO2, in the dark. The product MTT-formazano, dissolved in 100 mL
of ethanol was estimated through the measurement of the absorbance to 570 nm Multiskan Ascent (Thermo Labsystems, Franklin, MA, USA) microplate reader.
3.9. Leishmanicidal activity
Cultures of Leishmania infantum at stagnant phase were resuspended in 1 mL of complete RPMI medium and the concentration was adjusted to 107 parasites/mL. 6 well plates cultivated with human macrophages were incubated with 3 mL of suspension of parasite/well at 37 ° C, 5% of CO2 for 2 h for the infection of macrophages. The
medium containing parasites that did not adhere was retracted and the wells were washed with RPMI medium at room temperature. The different heterofucans (0,5 mg/mL) were added in supplemented RPMI medium (10% of AB serum) to the wells with macrophages infected with the parasite and the treatment was held for 24h.
Telles, C.B.S. PPGCSA/CCS
clean plate. 200 total macrophages were counted and the quantity of amastigotes per infected macrophage was counted.
3.10. Anti-T. vaginalis assay
Heterofucans from S. filipendula were analysed against T. vaginalis trophozoites (ATCC 30236). In 96 well microplates, 50 µL/well of solutions containing the different heterofucans and150 µL/well of the suspension of trophozoites were added, resulting in a final volume of 200 µL containing 2,5 x 105 trophozoite/mL and 2.0 mg/mL of the heterofucan to be tested. In control cultures, heterofucans samples were substituted by distilled water. The plates were incubated for 24 h at 37 ° C. After that period, 20 µL of a solution of resaurzurin at 0.1 mg/mL in Phosphate Buffered Saline was added in each well. After 1 hour of incubation at 37° C the fluorescence of each well was read in a fluorescence spectrophotometer (Spectramax Gemini XS – Molecular Devices Cooperation, Sunnyvale, CA, USA), the quantification of viable trophozoites was carried out as described by Duarte and collaborators [46].
3.11. Antibiofilm assay
Antibiofilm activity was measured as described by Melo-Silveira and collaborators [47]. 80 uL of bacterial suspension (Staphylococcus epidermidis ATCC-35984 and a clinic isolate of KPC Klebsiella pneumonia type 174), 80 µl of the heterofucans (0,5; 1,0; 1,5; and 2,0 mg/mL) and 40 mL of tryptone soy broth (TSB) (Oxoid Ltd., England) were added to the 96 well plate and incubated (37 °C for 24 h). The rest of the adhered bacteria was fixed at 60 ° C for 1h. The biofilm formed was dyed with 0.4% of crystal violet for 15 minutes at room temperature. The crystal violet bonded to the cells/biofilm was solubilized with 99,5% of DMSO (Sigma-Aldrich Co., St. Louis, MO, USA) and read at 570 nm (Spectramax M2e multimode Microplate Reader, Molecular Devices, Sunnyvale, CA , USA). The controls were considered as 100% of the formation of biofilm and the values obtained for the extract were the average of three experiments.
3.12. Bacterial inhibition assay
Telles, C.B.S. PPGCSA/CCS
3.13. Statistical analysis
All the data were expressed in average ± standard deviation. The analysis was carried out by analysis of variance. Student-Newman-Keuls post-tests were done for multiple comparison by group. In all the cases, statistical significance was established at p <0,05.
4. Conclusions
The heterofucans from S. filipendula presented distant activities as stimulators of the immune system and antimicrobial agents. The heterofucans SF0.5V, SF0.7V and SF1.0V were able to act in the activation of murine and human macrophages promoting increase in the release of the chemical mediators that are important for fighting intracellular parasites. In addition, SF0.5V presented antibiofilm activity facing the strain of S. epidermides whereas SF0.7V and 1.0V inhibited almost completely the survival of the protozoan T. vaginalis.
All five heterofucans obtained from the seaweed S. filipendula presented different/specific levels of activities in the trials carried out, making again evident that their biological activities depend on their structural characteristics. Results such as this reflect the great spectrum of action of these sulphated polysaccharides, but also intensify the need of posterior studies looking to elucidate the complete structure of these polysaccharides, configuration of glycosidic bonds, their position as well as the position of sulphate groups and ramification points.
Acknowledgments
Research was supported by Ministério de Ciência, Tecnologia e Informação (MCTI), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq), Brazil. Hugo A O Rocha is a CNPq fellowship honored researcher. Cinthia Telles had a Ph.D. scholarship from CAPES.
Author Contributions
Conceived and designed the experiments: C.B.S.T, T.T, L.S.C and H.A.O.R. Performed the experiments: C.B.S.T., C.M.A and A.P.F. Analyzed the data: C.B.S.T. and H.A.O.R. Contributed reagents/materials/analysis tools: G.P.F, L.S.C., T.T., S.J., L.F.D.P and H.A.O.R. Wrote the paper: C.B.S.T. and H.A.O.R.
Conflict of Interest
Telles, C.B.S. PPGCSA/CCS
References
1. Machado PRL, Carvalho L, Araújo MIAS, Carvalho EM. Immune response mechanisms to infections. An bras Dermatol, Rio de Janeiro, 2004; 79(6):647-664.
2. Coelho-Castelo AAM, Trombone APF, Rocha CD, Lorenzi JCC. Immune response to infectious diseases. Medicina (Ribeirão Preto) 2009;42(2): 127-42. 3. Abbas A K, Lichtman A H, Pober JS. Imunologia celular e molecular. 5a ed.,
Rio de Janeiro, Elsevier, 2005. 580 p.
4. Janeway C A, Jr, Travers P, Walport M, Shlomchik M J. The Immune System in Health and Disease. Immunobiology, 5th edition. 2001. New York: Garland Science.
5. Chouhan G, Islamuddin M, Sahal D, Afrin F. Exploring the role of medicinal plant-based immunomodulators for effective therapy of leishmaniasis. Front Immunol. 2014; 5:193.
6. Helms D.J., Mosure D.J., Secor W.E., Workowski K.A. Management of
Trichomonas vaginalis in women with suspected metronidazole hypersensitivity.
Am. J. Obstet. Gynecol. 2008; 198: 370–7.
7. Innocente AM, Vieira Pde B, Frasson AP, Casanova BB, Gosmann G, Gnoatto SC, Tasca T. Anti-Trichomonas vaginalis activity from triterpenoid derivatives. Parasitol Res. 2014; 113:2933-40.
8. Limban C, Chifiriuc MC. Antibacterial activity of new dibenzoxepinone oximes with fluorine and trifluoromethyl group substituents. Int J Mol Sci. 2011;12(10):6432-44.
9. Uthaisangsook S, Day NK, Bahna SL, Good RA, Haraguchi S. Innate immunity and its role against infections. Ann Allergy Asthma Immunol. 2002 Mar;88(3):253-64; quiz 265-6, 318.
10. Altmann KH. Microtubule-stabilizing agents: a growing class of important anticancer drugs. Curr Opin Chem Biol. 2001 5: 424-431.
11. Albuquerque I R, Queiroz K C, Alves LG, Santos EA, Leite EL, Rocha H A. Heterofucans from Dictyota menstrualis have anticoagulant activity. Braz J Med Biol Res. 2004, 37, 167-71.
12. Hoshino T, Hayashi T, Hayashi K, Hamada J, Lee JB. An antiviral active sulfated polysaccharide from Sargassum horneri (Tuner) C. Agardh. Biol. Pharm. Bull. 1998, 21, 730–734.
13. Sinha, S.; Astani, A.; Ghosh, T.; Schnitzler, P.; Ray, B. Polysaccharides from
Sargassum tenerrimum: structural features, chemical modification and anti-viral
Telles, C.B.S. PPGCSA/CCS
14. Zhu, W.; Ooi, V.E.C; Chan, P.K.S.; Ang, P.O.J. Isolation and characterization of a sulfated polysaccharide from the brown alga Sargassum patens and determination of its anti-herpes activity. Biochem. Cell Biol. 2003, 81, 25–33. 15. Stevan, F.R.; Oliveira, M.B.; Bucchi, D.F.; Noseda, M.D.; Iacomini, M., Duarte,
M.E. Cytotoxic effects against HeLa cells of polysaccharides from seaweeds. J.
Submicrosc. Cytol. Pathol. 2001, 33, 477–484.
16. Josephine, A.; Veena, C.K.; Amudha, G.; Preetha, S.P.; Sundarapandian, R.; Varalakshmi, P. Sulphated polysaccharides: new insight in the prevention of cyclosporine A-induced glomerular injury. Basic Clin. Pharmacol. Toxicol. 2007, 101, 9–15.
17. Dore CM, das C Faustino Alves MG, Will LS, Costa TG, Sabry DA, de Souza Rêgo LA, Accardo CM, Rocha HA, Filgueira LG, Leite EL. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr Polym. 2013 Jan 2;91(1):467-75.
18. Vasquez RD, Ruby SP, Garcia-Meim, Ramos JDA. Polysaccharide extracts from sargassum siliquosum j.g. Agardh modulates production of pro-inflammatory cytokines in lps-induced pbmc and delays coagulation time in-vitro. Jour. Harmo. Res. Pharm., 2014, 3(3), 101-112
19. Costa LS, Fidelis GP, Telles CB, Dantas-Santos N, Camara RB, Cordeiro SL, Costa MS, Almeida-Lima J, Melo-Silveira RF, Oliveira RM, Albuquerque IR, Andrade GP, Rocha HA. Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar Drugs. 2011;9(6):952-66.
20. Costa LS, Telles CB, Oliveira RM, Nobre LT, Dantas-Santos N, Camara RB, Costa MS, Almeida-Lima J, Melo-Silveira RF, Albuquerque IR, Leite EL, Rocha HA. Heterofucan from Sargassum filipendula induces apoptosis in HeLa cells. Mar Drugs. 2011;9(4):603-14.
21. Gamal-Eldeen AM, Amer H, Helmy WA, Talaat RM, Ragab H. Chemically-modified polysaccharide extract derived from Leucaena leucocephala alters Raw 264.7 murine macrophage functions. Int Immunopharmacol. 2007 Jun;7(6):871-8.
22. Jiang Z, Okimura T, Yamaguchi K, Oda T. The potent activity of sulfated polysaccharide, ascophyllan, isolated from Ascophyllum nodosum to induce nitric oxide and cytokine production from mouse macrophage RAW264.7 cells: Comparison between ascophyllan and fucoidan. Nitric Oxide. 2011 Nov 30;25(4):407-15.