HISTOLOGICAL STUDY OF HUMAN MAMMARY GLAND IN WOMEN
OF NORTH EASTERN REGION OF INDIA
Santona Thakuria1, Rajat Dutta Roy2, Debobani Bora3, Tarini Kanta Das4, K. L. Talukdar5
HOW TO CITE THIS ARTICLE:
Santona Thakuria, Rajat Dutta Roy, Debobani Bora, Tarini Kanta Das, K. L. Talukdar. “Histological Study of Human Mammary Gland in Women of North Eastern Region of India”. Journal of Evidence based Medicine and Healthcare; Volume 2, Issue 37, September 14, 2015; Page: 5898-5910,
DOI: 10.18410/jebmh/2015/813
ABSTRACT: INTRODUCTION: The “mamma” or “breast” is a modified, ectodermal, glandular
structure located in the superficial fascia of the anterior chest wall. In female they are evolved to secrete milk for the nourishment of their offspring. They are also present in a rudimentary form in males. MATERIALS AND METHODS: After obtaining institutional ethical clearance the specimens of human mammary gland were divided into two groups i.e. reproductive (14 to 49) and post-menopausal (50 and above) age. The specimens of both the age group were collected from fresh unembalmed human cadaver. The slides were prepared using the standard laboratory procedure. Stained slides were studied for different structures of the mammary gland such as the glandular structures, ducts and myoepithelial cells. Diameters of the ducts were measured in both age groups. The data were analyzed to calculate the mean and ‘t’ test was applied to find out the significant difference between mean values. RESULTS: The average diameter of the lactiferous duct as measured by micrometer scale was 2.0 mm and 0.83 mm in reproductive and post-menopausal age group respectively. Calculated value of t’= 4.68. d. f. (degree of freedom) =18, P <0.01. Therefore the diameter of lactiferous duct between reproductive and postmenopausal age group differs significantly. DISCUSSION: The results obtained in this study are compared with the available established findings of other workers to draw a definite conclusion in the histomorphological aspect of reproductive and postmenopausal age group. CONCLUSION:
Though different generations of sophisticated investigations such as molecular level studies have evolved for the early detection of breast carcinoma, the knowledge of the normal developmental histological changes is most important which will help to detect early abnormal changes of the breast if any.
KEYWORDS: Mammary gland, Alveoli, Lactiferous duct, Glandular epithelium.
INTRODUCTION: The “mamma” or “breast” is a modified, ectodermal, glandular structure
located in the superficial fascia of the anterior chest wall. In female they are evolved to secrete milk for the nourishment of their offspring. They are also present in a rudimentary form in males. In the adult female, the base of the breast, i.e. its attached surface, extends vertically from the second or third to the sixth rib, and in the transverse plane from the sternal edge medially almost to the midaxillary line laterally.1 The breast is made up of the mammary gland, the fatty
superficial fascia in which it is embedded and the overlying skin with the nipple and the surrounding zone of pigmented skin, the areola.
of the embryo.2 In human only two breasts develop normally but additional accessory nipples or
glandular masses are not uncommon.
AIM OF THE STUDY: To study & compare the histological pattern of human mammary gland in the reproductive and post-menopausal age groups.
MATERIALS AND METHODS: The present study on human mammary gland was conducted in the Department of Anatomy, Gauhati Medical College, Guwahati. After obtaining institutional ethical clearance the specimens of human mammary gland were divided into two groups i.e. reproductive (14 to 49) and post-menopausal (50 and above) age. Ten samples each from both the age group were collected from autopsies done in the department of Forensic Medicine, Gauhati Medical College, Guwahati. Breast with any pathology were excluded from the study. The subcutaneously removed breast tissue excluding the nipple and axillary tail were collected from the cadaver.
From the different dissected parts approximately 3-5 mm pieces were made and fixed in 10% formalin and labelled separately. Tissues were kept in 10% formalin for 24-48 hours. The fixed tissues were processed for embedding in paraffin and sectioned at 5 µm thickness in a ‘Rotary microtome’. Sections of tissues were stained by routine Haematoxylin and Eosin according to standard method of Carleton (1967).3
Stained slides were studied for different structures of the mammary gland. Then the glandular structures, ducts and myoepithelial cells were observed under both low and high power objective and oil immersion objective. Diameters of the ducts were measured with the help of a ‘Spencer ocular’lens and ‘objective micrometer’ scale. One division of the ocular micrometer scale was equivalent to 3µm at 400 times magnification.
Calculation of the Micrometer Scale: 3 divisions of ocular micrometer coincide with 1 division of objective micrometer. Therefore, 1 division of ocular scale = 1/3 division of objective micrometer scale.
As one division of objective micrometer scale measures 0.01mm, hence 1/3 division = 1/3 x 0.01 = 0.003 mm = 3µm (since 10-3 mm = 1µm)
Therefore, 1 division of ocular micrometer scale= 0.003mm.
Statistical Analysis: The data of luminal diameter (mm) of the duct were analyzed by standard statistical methods. The data were analyzed to calculate the mean and ‘t’ test was applied to find out the significant difference between mean values.
RESULTS: In the present study mammary glands were divided into two groups as given below:
Group A: Reproductive (14 years to 49 years).
Group B: Postmenopausal (50 years and above).
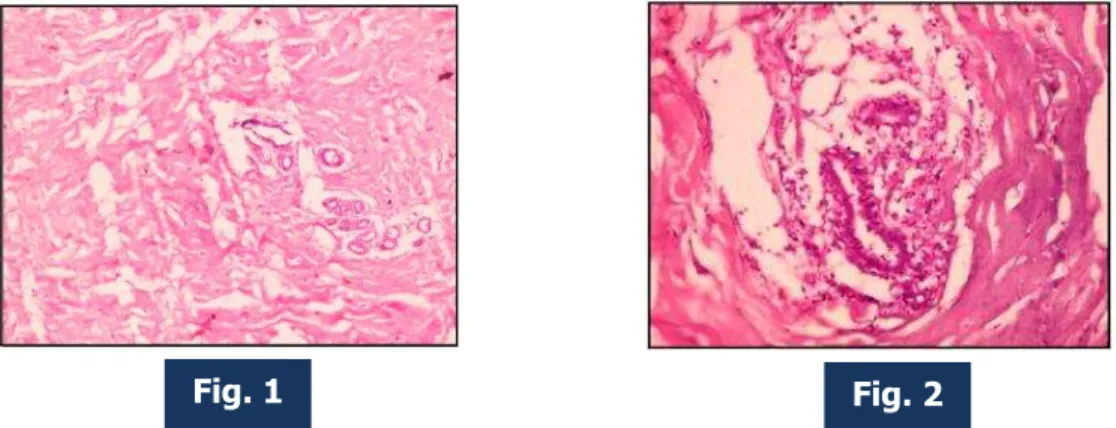
Each lobule consists of the portions of gland that have secretory potential (Fig. 2 & 3). The ducts are lined by columnar epithelium. In the larger ducts these are two cells thick, but in the smaller ones only a single layer of columnar or cuboidal cells is present. The bases of these cells are in close contact with numerous myoepithelial cells. Myoepithelial cells are numerous and they form a distinct layer surrounding the ducts and presumptive alveoli and give the epithelium a bilayered appearance (Fig. 4). The stratified cuboidal lining is replaced by keratinized stratified squamous epithelium, continuous with the epidermis, close to the openings of the lactiferous ducts on the nipple. The connective tissue stroma surrounding the lobules is dense and fibrocollagenous, whereas intralobular connective tissue has a loose texture. Fibrous condensations of stromal tissue extend from the ducts to the dermis. These are often well developed in the upper part of the breast as suspensory ligaments. The interlobar stroma contains variable amounts of adipose tissue.
In early reproductive age, the ducts undergo branching and the ends of the branches form solid, spheroidal masses of granular polyhedral cells, the potential alveoli (Fig.1). The bulk of the breast consists of connective tissue and fat that widely separate the glandular elements. The increased size of the glands is mainly from the deposition of fat.
In reproductive period, during pregnancy, the intralobular ductal epithelium proliferates and the number and lengths of their branches increase (Fig. 5). Alveoli develop at their terminal ends: with the synthesis and secretion of milk, the alveoli expand as their cells and lumens fill (Fig. 8). The intralobular ducts and alveoli are lined by two layers of cells, the luminal epithelium and a basal layer of flattened myoepithelial cells. The interlobular ducts lined with tall columnar cells, course in the interlobular connective tissue to join larger lactiferous duct that is usually lined with low pseudostratified columnar epithelium (Fig. 6 & 7). The myoepithelial cells, which are initially spindle-shaped, become highly branched stellate cells, especially around the alveoli. Adjacent myoepithelial cells intermesh to form a basket-like network around the alveoli and ducts. There is a concomitant reduction in adipose tissue in the stroma. During Later half of pregnancy, the alveolar cells become secretory and the ducts enlarge. The intralobular connective tissue decreases while the interlobular connective tissue increases (Fig. 8).
The gland undergoes full development during lactational period. During this period, the gland contains a large number of distended alveoli filled with secretions and vacuoles (Fig. 5 & 9). The active alveoli are lined with flat to low columnar epithelium and filled with milk that appears as eosinophilic (pink) material with large vacuoles of dissolved fat droplets. The interlobular connective tissue septa are reduced.
The average diameter of the lactiferous duct as measured by micrometer scale was 2.0 mm and 0.83 mm in reproductive and post-menopausal age group respectively (Table 1). The diameters of the lactiferous duct of each group (Fig. 14 & 15) and the inter-group (Fig. 16) variation have been shown with the help of bar diagram.
Calculated value of t’= 4.68. d. f. (degree of freedom) =18, P < 0.01.
Therefore the diameter of lactiferous duct between reproductive and postmenopausal age group differs significantly.
DISCUSSION: The results obtained in this study are compared with the available established findings of other workers to draw a definite conclusion in the histomorphological aspect of reproductive and postmenopausal age group.
The lobes contain a network of glandular tissue consisting of branching ducts and terminal secretory lobules in a connective tissue stroma. This finding is analogous to Foster et al (1964),4
Bloom et al (1978),5 Crouch J. E. (1985),6 Cunningham et al (2005),7 Johnson D. et al (2006).8
Brunicardi et al (2006)9 also recorded that the breast is composed of 15–20 lobes, each consists
of several lobules.
Each lobule consists of the portions of the gland that have secretory potential. Similar observation was made by Sutton D. (2003),10 Standring (2008)1 and Townsend et al (2009).11
The ducts were lined by columnar epithelium. In the larger ducts these were two cells thick, but in the smaller ones only a single layer of columnar or cuboidal cells is present. Similar observation was made by Haver et al (1967),12 Junqueira et al (1977),13 Ross & Reith (1985),14
Dey et al (1995),15 Damjana et al (1996).16
Present finding in respect of diameter of the lactiferous duct (average diameter 2.0mm) is compared with available research work (Table 2).
The bases of cells lining the alveoli and ducts were in close contact with numerous myoepithelial cells. Myoepithelial cells are numerous and they form a distinct layer surrounding the ducts and presumptive alveoli and give the epithelium a bilayered appearance. This finding is analogous to Lloyd et al (1964), Bloom et al (1978),5 Ross & Reith (1985),14 Tavassoli (1999).17
PUBERTY: In early reproductive age, the ducts undergo branching and the ends of the branches form solid, spheroidal masses of granular polyhedral cells, the potential alveoli. Similar finding is recorded by Ross & Reith (1985),14 Dey et al (1995),15 Damjana et al (1996),16 Tavassoli (1999),17
Beatrice et al (2000),18 Rosen (2001).19
Johnson D. et al (2006)8 stated that the interlobar stroma contains variable amounts of
adipose tissue, which contributes largely to the increase in breast size at puberty. This finding is similar to the present study.
Ham et al (1979),20 Standring S. (2008),1 Singh I.B. (2008),21 Eroschenko P. V. (2009)22
PREGNANCY: During pregnancy, the intralobular ductal epithelium proliferates and the number and lengths of their branches increase. This finding are similar to the Parks A.G. (1959),23
Junqueira et al (1977),24 Bloom et al (1978),5 Borysenko et al (1979).25
Alveoli develop at their terminal ends: with the synthesis and secretion of milk, the alveoli expand as their cells and lumens fill. This is similar to Borysenko et al (1979),25 Ross & Reith
(1985),14 Dey et al (1995),15 Johnson D. (2006).8
The intralobular ducts and alveoli are lined by two layers of cells, the luminal epithelium and a basal layer of flattened myoepithelial cells. Borysenko et al (1979),25 Eroschenko P. V.
(2009)22 also stated the similar findings.
It was noted that the intralobular connective tissue decreases while the interlobular connective tissue increases. These findings are analogous to Haver et al (1967),12 Junqueira et al
(1977),13 Borysenko et al (1979),25 Ross & Reith (1985).14
LACTATION: In present study it was found that the gland undergoes full development during lactational period. During this period, the gland contains a large number of distended alveoli filled with secretions and vacuoles. These findings are similar with Bloom W. (1978),5 Mitra (2000),26
Nelson et al (2005),27 Johnson D. et al (2006),8 Young et al (2006),28 Standring S. (2008),1
Eroschenko P. V. (2009).22
The active alveoli are lined with low columnar or cuboidal epithelium. Similar finding was noted by Foster et al (1964),4 Junqueira et al (1977),24 Bloom W. (1978),5 Gartner et al (2007),29
Eroschenko P. V. (2009).22
POSTMENOPAUSAL: In Postmenopausal age, the breast undergoes involution (atrophy), which is characterized by diminution and eventual atrophy of the parenchymal elements. This findings are similar to Cooper (1840), Foster et al (1964),4 Haver et al (1967),12 Bloom W. (1978),5 Ham
et al (1979),20 Sahana (1985),30 Ross & Reith (1985),14 Dey et al (1995),15 Damjana et al
(1996).16
Within the lobules, the ductular epithelial cells become attenuated, whereas the lumen is practically obliterated. Similar findings are noted by Parks A.G. (1959),23 Junqueira et al (1977),13
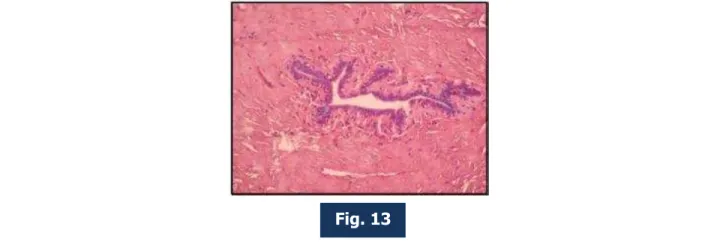
Dey et al (1995),15 Tavassoli (1999),17 Ranganathan (2002).31 The diameter of the lactiferous
duct as measured by micrometer scale was 0.83 mm in postmenopausal age group. But this finding needs further evaluation.
In present study it was noted that the breast stroma also undergoes involutional change. Alteration occurs in the elastic and collagen fibers, resulting in loss of supporting connective tissue. The adipose tissue on the other hand increases. These findings are analogous to Foster et al (1964),4 Junqueira et al (1977).13 Similar findings were noted by Bloom W. (1978),5 Ham et al
(1979),20 Sahana (1985),30 Ross & Reith (1985),14 Dey et al (1995),15 Damjana et al (1996),16
Tavassoli (1999),17 Beatrice et al (2000),18 Nelson et al (2005),27 Johnson D. (2006),8 Townsend
et al (2009).11
hormonal changes during pregnancy and the lactational period are basically for preparation of breast milk for the neonate. So any pathology developed in the breast not only affects the women herself but also hampers the breast feeding to her baby if it occurs during lactational period. Among the various pathological conditions breast carcinomas are the common and major clinical entities in present era.
Though different generations of sophisticated investigations such as molecular level studies have evolved for the early detection of breast carcinoma, the knowledge of the normal developmental histological changes is most important which will help to detect early abnormal changes of the breast if any.
This study has revealed several points of interest having marked importance in practical life with the hope that it will help in the investigation and management of various pathological conditions related to the breast.
Fig. 1: Photomicrograph of mammary gland lobule with surrounding stroma of early reproductive breast under low power (100X magnification).
Fig. 2: Photomicrograph of mammary gland lobule with surrounding stroma of early reproductive breast under high power (400X magnification).
Fig. 3: Photomicrograph of mammary gland alveoli of early reproductive breast under high power (400X magnification).
Fig. 4: Photomicrograph of mammary gland alveoli of early reproductive breast (under Oil Immersion), ME CELL – Myoepithelial cell.
Fig. 1 Fig. 2
Fig. 5: Photomicrograph of mammary gland lobule with surrounding stroma of reproductive breast under low power (100X magnification).
Fig. 6: Photomicrograph of lactiferous duct with its secretion in reproductive mammary gland under low power (100X magnification).
Fig. 7: Photomicrograph of lactiferous duct with its secretion in reproductive mammary gland under high power (400X magnification).
Fig. 8: Photomicrograph of alveoli & fatty tissues of reproductive mammary gland under low power (100X magnification).
Fig. 9: Photomicrograph of alveoli, lactiferous duct & fatty tissues of reproductive mammary gland under low power (100X magnification).
Fig. 10: Photomicrograph of mammary gland lobule with surrounding stroma of postmenopausal breast under low power (100X magnification).
Fig. 5 Fig. 6
Fig. 7 Fig. 8
Fig. 11: Photomicrograph of mammary gland lobule of post-menopausal breast under high power (400X magnification).
Fig. 12: Photomicrograph of lactiferous duct in post-menopausal mammary gland under low power (100X magnification).
Fig. 13: Photomicrograph of lactiferous duct in Post-menopausal mammary gland under high power (400X magnification).
Fig. 11
Fig. 12
Fig. 14: Bar diagram showing Diameters of lactiferous duct in reproductive age group (group A).
Fig. 15: Bar Diagram showing Diameters of Lactiferous duct in Post-menopausal age group (group B).
Fig. 14
Fig. 16: Bar Diagram showing inter-group variation of lactiferous duct of mammary gland. No. of Specimen Reproductive Age (Group A) Post-menopausal (Group B) Age in Years Diameter of lactiferous duct (mm) Age in Years Diameter of lactiferous duct (mm) 1 2 3 4 5 6 7 8 9 10 18 20 22 25 27 32 35 38 40 42 2.0 1.5 1.05 2.5 1.8 2.2 2.7 1.5 2.7 3.0 50 55 58 60 62 65 68 70 75 82 0.3 1.2 0.4 1.0 0.9 0.4 1.5 1.0 1.1 0.5
Mean x 2.0 y 0.83
Table 1: Comparison of Diameters of lactiferous duct of Mammary Gland in Reproductive and Postmenopausal age groups
Authors Diameter of lactiferous duct (mm)
Bloom et al (1978)5 2 to 4.5
Ross & Reith (1985)14 2 to 4
Brunicardi et al (2006)9 2 to 4
Geddes (2007)32 1.2 to 2.5
Present Study 2.0
Table 2: Diameters of lactiferous duct (Reproductive age) noted by different authors and present study
REFERENCES:
1. Standring S. Chest wall and breast, In: Susan Standring, Gray’s Anatomy. 40th Edition,
Churchill Livingstone Elsevier. London, 2008, Page: 929-936.
2. Sadler TW.: Ch- 21, Integumentary System. Langman’s Medical Embryology. 12th ed.
Lippincott Williams & Wilkins, Philadelphia, 2012: 342-343.
3. Drury R. A. B., Wallington E. A., Carleton’s Histological Technique,4th Edition, Oxford
University Press.
4. Foster C.L., Heinemann W.: Hewer’s Textbook of Histology for Medical Students, 8th Edition,
London, 1964, Page: 380.
5. Bloom W., Fawcett D. W.: A textbook of Histology, 10thEdition,W.B. Saunders Co. 1978,
Page: 907.
6. Crouch J. E.: Functional Human Anatomy, 4thEdition, Lea & Febiger Philadelphia, 1985,
Page: 574.
7. Cunningham G.F., Leveno J. K., Bloom L. S., Hauth C.J. : William’s Obstetrics, 22nd Edition,
McGraw-Hill Medical Publishing Division, 2005, Page: 699.
8. Johnson D., Ellis H.: Gray’s Anatomy, edited by Berkovitz B.K.B., Borley N.R., Crossman A.R., Davies M.S., Fitzgerald M.J.T, Glass J., Hackney C. M., Mundy A.R., Ind T., Newell R.L.M., Shah P., RuskelG.L. Editor in chief- Standring S, 39th Edition,2006, Page: 969-75.
9. Brunicardi C.F., Anderson K.D., Billar R. T., Dunn L.D., Hunter G. J., Pollock E.R.,: Schwartz’s Manual of Surgery, McGraw-Hill, Medical Publishing Division, 8th Edition, 2006,
Page: 344.
10.Sutton D.: Textbook of Radilogy and Imaging, 7th Edition, Vol-2, Churchill Livingstone, 2003,
Page: 1454.
11.Townsend C.M. Beauchamp R.D., Evers B. M., Mattox K.L.: Sabiston Textbook of Surgery, 18th Edition, Vol-1, Elsevier, 2009, Page: 853-5.
12.Haver C., Kelly E.D., Wood L.R.: Bailey’s Textbook of Histology, 17th Edition, 1967, Page:
682.
13.Junqueira I.C., Carneiro J., Contopoulos A.: Basic Histology, 2nd Edition, Lange Medical
Publications, 1977 Page: 449-52.
14.Ross M.H., Reith E.J.: Histology: A Text and Atlas, Harper & Row, Publishers, J. B. Lippincott Company, 1985, Page: 693-97.
15.Dey N.C. and Dey T.K: A Textbook of Pathology, New Central Book Agency (P) Ltd, 1995, Page: 31.3.
16.Damjana I. and Linder J.: Anderson’s Pathology, 10th Edition, Vol.2, 1996.
18.Beatrice A., Howard Barry A. Gusterson: Journal of Mammary Gland Biology and Neoplasia, Human Breast Development, Vol. 5, No. 2, 2000, Page: 124.
19.Rosen P.P.: Breast Pathology, 2nd Edition, Lippincott William & Wilkins, 2001, Page: 2.
20.Ham A. W., Cormack D. H.: Histology, 8th Edition, J. B. Lippincott, 1979, Page: 867.
21.Singh I.B.: Text Book of Human Histology, 5th Edition, Jaypee Brothers Medical Publishers
(P) Ltd, 2008, Page: 303.
22.Eroschenko P. V.: diFiore’s Atlas of Histology with Functional Correlations, 11th Edition,
Lippincott Williams & Wilkins, 2009, Page: 469, 482-6.
23.Parks A.G.: The Micro-Anatomy of the Breast, Hunterian Lecture delivered at the Royal College of Surgeons of England, 1959, Page: 237-238.
24.Junqueira I.C., Carneiro J., Contopoulos A.: Basic Histology, 2ndEdition, Lange Medical
Publications, 1977, Page: 449-52.
25.Borysenko M. And Beringer T.: Functional Histology, Little, Brown & Company, 1979, Page: 461.
26.Mitra: Anatomy, Part-2, 5th Edition, Academic Publishers, 2000, Page: 4.9-10.
27.Nelson C.M, Bissell M.J: Modeling dynamic reciprocity :Engineering three- dimensional culture models of breast architecture, function, and neoplastic transformation, Life Sciences Division, Lawrence Berkeley National Laboratory, Cyclotron Road, MS 977-225A, Berkeley, CA 94720, USA, 2005, Page 344.
28.Young B. Lowe J. S., Stevens A. and Heath J. W.: Functional Histology, 5th Edition, Churchill
Livingstone, 2006, Page: 386-89.
29.Gartner L. P., Hiatt J. L.: Color Textbook of Histology, 3rd Edition. Saunders, Elsevier, 2007,
Page: 485-88.
30.Sahana S. N.): Human Anatomy (Described and Applied), Vol. II, 4th Edition, 1985, Page:
422.
31.Ranganathan: A textbook of Human Anatomy, 6th Revised Edition, S.Chand & Company LTD. Ramnagar, New Delhi-110055, 2002, Page: 59.
4. Professor & HOD, Department of Anatomy, Jorhat Medical College, Jorhat.
5. Professor, Department of Anatomy, Gauhati Medical College.
NAME ADDRESS EMAIL ID OF THE CORRESPONDING AUTHOR: Dr. Santona Thakuria,
Assistant Professor, Department of Anatomy, Jorhat Medical College, Jorhat. E-mail: drsam2912@gmail.com
Date of Submission: 02/09/2015. Date of Peer Review: 03/09/2015. Date of Acceptance: 09/09/2015. Date of Publishing: 14/09/2015. AUTHORS:
1. Santona Thakuria 2. Rajat Dutta Roy 3. Debobani Bora 4. Tarini Kanta Das 5. K. L. Talukdar
PARTICULARS OF CONTRIBUTORS: 1. Assistant Professor, Department of
Anatomy, Jorhat Medical College, Jorhat.
2. Assistant Professor, Department of Anatomy, Jorhat Medical College, Jorhat.