Pore structure and mechanical properties of
directionally solidified porous aluminum alloys
* Hao Hai
Male, born in 1969, Ph.D., Professor. His research interests mainly focus on solidification control, casting technology of light metals, porous materials. He has published more than ninety technical papers.
E-mail: haohai@dlut.edu.cn
Received: 2013-08-15 Accepted: 2013-11-12
Komissarchuk Olga 1
, Xu Zhengbin1
, *Hao Hai 1
, Zhang Xinglu 1
, Vladimir Karpov2 1. School of Materials Science and Engineering, Dalian University of Technology, Dalian 116024, China 2. Department of Materials Science and Metal Processing, National Metallurgical Academy of Ukraine, Ukraine
P
orous metals are new engineering materials withlower density, higher specific strength, larger surface
area, good permeability and biological compatibility compared with dense metals, and have been used as
important materials for structural light-weight parts, heat-exchanger, filter, sound absorption, bioengineering etc [1, 2]
.
The different manufacturing methods of porous
materials in current use can be divided into two main groups: porous materials produced by sintering method,
and porous materials manufactured by the casting process. Based on the solubility difference of gas
between liquid and solid metals, regular porous metals
with oriented pores could be fabricated by directional solidification of molten metals saturated with gas (such as hydrogen), which is called directional solidification or metal-gas eutectic method, or GASAR process [3-5].
Abstract:
Porous aluminum alloys produced by the metal-gas eutectic method or GASAR process need to beperformed under a certain pressure of hydrogen, and to carry over melt to a tailor-made apparatus that ensures directional solidification. Hydrogen is driven out of the melt, and then the quasi-cylindrical pores normal to the solidification front are usually formed. In the research, the effects of processing parameters (saturation pressure, solidification pressure, temperature, and holding time) on the pore structure and porosity of porous aluminum alloys were analyzed. The mechanical properties of Al-Mg alloys were studied by the compressive tests, and the advantages of the porous structure were indicated. By using the GASAR method, pure aluminum, Al-3wt.%Mg, Al-6wt.%Mg and Al-35wt.%Mg alloys with oriented pores have been successfully produced under processing conditions of varying gas pressure, and the relationship between the final pore structure and the solidification pressure, as well as the influences of Mg quantity on the pore size, porosity and mechanical properties of Al-Mg alloy were investigated. The results show that a higher pressure of solidification tends to yield smaller pores in aluminum and its alloys. In the case of Al-Mg alloys, it was proved that with the increasing of Mg amount, the mechanical properties of the alloys sharply deteriorate. However, since Al-3%Mg and Al-6wt.%Mg alloys are ductile metals, their porous samples have greater compressive strength than that of the dense samples due to the existence of pores. It gives the opportunity to use them in industry at the same conditions as dense alloys with savings in weight and material consumption.
Key words:
porous aluminum alloys; GASAR; solidification pressure; pore structure; directional solidificationCLC numbers: TG146.21 Document code: A Article ID: 1672-6421(2014)01-001-07
This method belongs to the group of casting porous
materials. This method was developed by the researchers
of that National Metallurgical Academy in Ukraine [3].
Most metal-hydrogen binary systems also have eutectic
decomposition as the classic eutectic systems. These metals can be melted, saturated with hydrogen, and
then directionally solidified into a porous structure. During solidification, solid metal and hydrogen are simultaneously formed by a gas-eutectic reaction,
resulting in a porous structure with elongated gaseous
pores filled with hydrogen. Compared with traditional fabrication methods for porous metals, this process allows for an effective control of porosity, pore-size and orientation. A porous metal with oriented pore structure has attractive mechanical and functional properties [6].
One such property is a significantly improved thermal
conductivity over conventionally processed porous
materials. Moreover, these materials possess good mechanical properties with less weight. Gas pores of
regular shape do not cause stress concentration during the stretching process [7]. Due to all these well-combined
Fig. 1: Experimental apparatus for GASAR manufacture
applications and they have raised extensive research interest [8, 9]. It
is possible to produce GASAR materials on the basis of different metals and alloys. Although there have been many studies on the GASAR process, most of these studies have shown that it is easy to fabricate porous pure metals with oriented pores [10-13], but
difficult to create similar porous alloys [14-16], which have greater
industrial potential than porous pure metals.
1 Experiment
1.1 Experimental equipment
Two different kinds of self-developed apparatus for fabrication of porous alloys by directional solidification were utilized in the present experiment, as shown in Fig.1 (a, b). The preliminary experiments with pure Al (Figs. 2, 3) were carried out using the apparatus with 180° rotation mechanism [Fig.1 (a)]; the rest of the experiments were done using the apparatus with 90° rotation mechanism [Fig.1 (b)]. The apparatus with 90° rotation mechanism has some benefits compared to the 180° rotation
one and can be considered as a new generation machine. In our research, the new equipment with improved design has been
made. The primary difference between our new equipment and
that in current use is a system of heating. The 180° apparatus
uses resistance heating, but in the new equipment it is replaced
with induction heating. This significantly speeds up the velocity of heating, consequently, the productivity increases. Moreover, due to the induction furnace, the stirring of the melt is taking place that simplifies the saturation process of the melt with hydrogen. During the process of pouring metal from the crucible to the mold, the mixing of liquid metal inside the mold was taking place. This happens because the distance the liquid
metal overcame while pouring at the apparatus with the rotation
mechanism of 180° is longer than that in the case of the 90° apparatus and the speed is faster as well. This phenomenon leads to uneven temperature distribution in the crystallizing metal. The uneven temperature distribution will lead to non-uniform structure. During the pouring of metal using the apparatus with the rotation mechanism of 90°, the melt is poured more
evenly, because the distance the liquid metal travels between the crucible and the mold is shorter, the pouring goes more
smoothly and the process of mixing is expressed much less. The homogeneity of the melt is much better in this case which allows for obtaining more qualitative samples with uniform structure.
The apparatus consists of a crucible (1) surrounded by an induction coil (2) and a mold (3) with a water-cooled copper chiller (4). These are all installed in a high-pressured chamber (5).
1 – crucible; 2 – induction coil; 3 – mold; 4 – water-cooled copper chiller; 5 – water-cooled chamber; 6 – gas valve; 7-
cooling water valve; 8 – swivel; 9 – melt; 10 – thermocouple.
1.2 Experimental procedure
General conditions for carrying out the experiments were similar for the different aluminum alloys used. In this experiment, high pure Al (99.9%), industrial pure aluminum (0.3% of impurities), Al-3wt.%Mg, Al-6wt.%Mg and Al-35wt.%Mg alloys were used as the tested materials and were melted at 750℃ and held
for some time in a crucible heated by the induction furnace.
Hydrogen was injected into the chamber through the gas valve
depicted in the Fig.1(b)-10 and the holding was used to allow the hydrogen to saturate the melt to a certain extent (further will
use the term saturation pressure).
The main principle of operation for these two apparatus was similar: during the experiment, first the air from the working volume of apparatus was pumped out with the backing pump to
create a vacuum inside the chamber. The furnace was preheated
to 200-300 ℃ to remove adsorbed moisture from the coating of
furnace, tap-hole and mold, then a certain amount of high-purity 99.9% hydrogen was introduced into the chamber to create a pressure equal to the saturation pressure (Psat), which is the
pressure initially set while the metal is melting in order to allow
the desired amount of hydrogen to be introduced into the liquid metal. The higher the pressure, the higher the final porosity of
specimens.
The mold in the experiment was made of steel 0.3 mm in thickness. A thin layer of non-stick coating was applied to the mold and the funnel to prevent direct contact with the metal. A funnel was installed between the crucible and the mold. The
mold was attached to the copper chiller.
(a) Apparatus with 180° rotation mechanism (b) Apparatus with 90° rotation mechanism
1 - crucible; 2 - induction coil; 3 – mold; 4 - water-cooled copper
Fig. 2: Macrostructure of high pure aluminum with pores formed under different parameters (bottom part of the sample was in contact with the chiller)
The furnace was heated up to the temperature of 750℃,
or in other words, above the melting point. After the desired temperature was reached, it was held for around 30 min (for Al-Mg alloys the time was shortened to 15 min), allowing for setting the balance between gas and molten substance. The furnace temperature was controlled by tungsten-rhenium thermocouple with a wire diameter of 0.35 mm. An automatic
potentiometer was the main device recording the temperature.
After this, the desired value of the solidification pressure was set before the apparatus rotation. The solidification pressure (Psol)
is a pressure set before the solidification begins. It is responsible for the size of pores. As it was mentioned before, metal melting
in the crucible is going under the certain saturation pressure
(pressure initially set while the metal is melting). After this was done and the holding was finished, we reduced the pressure in the chamber up to the point we called the solidification pressure. This is usually lower than saturation pressure, so their difference is the condition for the pore growth. The higher pressure of solidification tends to yield smaller pores in aluminum and its alloys. The control of the solidification pressure was carried out with the help of a high-pressure gauge and the backing pump. We set the desired solidification pressure using the pump and controlled its value by monitoring the scale of pressure gauge. After the metal was melted, the apparatus was rotated at 90° and the melt went from the crucible to the molding box through the funnel, where the solidification began from the copper chiller. The metal was directionally solidified upwards from the
water-cooled copper chiller.
After fabrication, the sample was extracted from the mold and the remains of non-stick coating were removed from its surface. The shape of type A and B samples and direction of crystallization were different. However, the geometrical size of the longitudinal section of the specimens was the same. Experimental samples of type A were made in a shape of rectangle with the length of 100 mm and height of 20 mm. Samples of type B were made in a cylinder with a diameter of 20 mm and length of 100 mm. Then it was sectioned into two kinds: longitudinal section and transverse section. For the convenience of examining the samples, longitudinal samples
were cut into two or three parts. For revealing macrostructure,
NaOH solution (15% -25%) was used. The macrostructure of GASAR samples after polishing and etching was studied under lower magnification (from ×5 to ×15). The structure and fractures of the pores were examined with a scanning electron microscope (SEM). The porosity and average pore diameter were evaluated through the image editing software.
2 Results and discussion
2.1 Porous high pure aluminum
A series of experiments with high pure Al was carried out using the apparatus shown in Fig.1 (a). Preliminary experimental results show that the pressure of hydrogen is perhaps the most fundamental parameter which affects the final formation of the
specimen structure.
The saturation pressure (Psat) is the pressure which is initially
set while the metal is melting in order to allow the desired
amount of hydrogen to be introduced into the liquid metal. The higher it is, the higher porosity the specimen will finally have.
The solidification pressure (Psol) is a pressure set before the
solidification begins. It is responsible for the size of pores. The higher pressure of solidification tends to yield smaller pores in
aluminum and its alloys. We can observe it in aluminum samples
[Fig. 2 (a), (b)]. It is visible that the central (on a vertical plane) part of a specimen has the most homogeneous structure.
(a) Psat = 0.3 MPa, T = 750 ℃, t = 25 min, Psol= 0.3 MPa
(b) Psat =0.3 MPa, T = 750°C, t = 25 min, Psol = 0.05 MPa
The overheating up to 750 ℃ and the holding time about
half an hour (shorter for Al-Mg alloys) were needed to create the favorable conditions for the melt saturation process. It is connected with the high viscosity of Al melt compared to the hydrogen one, and existence of oxide film. Theoretically, the necessity of a longer time and high temperature was proved. One can notice that if there is a big difference between the saturation and solidification pressure it facilitates the pore growth.
In Fig. 3, crystallization begins with the homogeneous porous structure (Zone1) that is followed by a layer of oxide films (Zone 2). This phenomenon reduces the output of porous aluminum and the mechanical properties of samples. Oxide films appear probably because of active boiling of liquid aluminum with hydrogen during solidification. The produced GASARs specimens had the porosity of about 15%-20%.
2.2 Porous pure aluminum
The experiments with industrial pure Al have been carried out by using the improved apparatus shown in Fig.1 (b). Figure 4 represents the macrostructure of a specimen manufactured under the following parameters: Psat = 0.2 MPa, T = 750 ℃, t =
25 min, Psol= 0.05 MPa. Experimentally, these parameters were
according to the literature data [17] the hydrogen solubility in
pure Al is very low, about 0.5 cm3 per 100 g under pressure of
1 atm and 660 ℃, but the experimental results show that in fact
the melt could be saturated with a larger amount of hydrogen [Fig. 4(a, b)]. Nevertheless, some problems were found, which, if solved, would give better results. In terms of experiments with pure Al, the problem of the dense oxide film remains number one to be solved. The oxide film on the surface of the melt prevents the free penetration of hydrogen into the liquid metal.
with extended axis-oriented pores similar in size to the previous ones. That is the expression of solidified oxide film, but not as clearly visible as in the short specimen (Fig. 3, zone 2). Figure 4(b) represents the cross section of the pure Al sample made at the height of 30 mm from the bottom. One can see that the pores have similar shape, but different size and non-uniform distribution.
The ability of hydrogen to saturate in liquid aluminum is significantly affected by the alloying elements. In the Al-Mg
alloys system, the hydrogen solubility increases monotonically
from pure aluminum to pure magnesium. At t = 660-700 ℃,
the hydrogen solubility in Al increases almost linearly with the increasing of magnesium content in it. Thus, for example, at t = 700 ℃ the hydrogen solubility in Al is 0.9 cm3
per 100 g, in the
Al-4%Mg alloy 1.6 cm3 per 100 g, in Al-6%Mg alloy 2.0 cm3
per 100 g [18] respectively.
Even though pure Al is not widely used in the industry because of its softness, it is important to learn its solidification behavior during the GASAR process, which could provide useful information to deal with aluminum alloys.
2.3 Porous Al-Mg alloys
Aluminum-based magnesium alloys are one of the most attractive materials for application in modern industry. GASAR experiments of Al-Mg alloys with different Mg content and mechanical experiments of those specimens have been done to
reveal their properties in this study. The theoretical data about
the improved saturation ability of hydrogen in the melt with the increasing of Mg content have been proved as well.
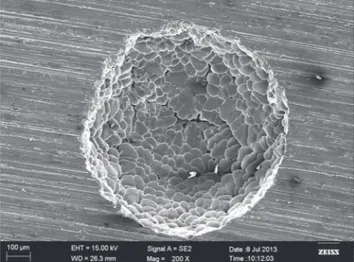
During the research of industrial alloy Аl-3wt.%Мg, the significant dependence of the structure on solidification pressure has been observed, which is similar to the results of the porous pure aluminum. The higher solidification pressure results in the smaller pore size. However, there was no oxide film observed during the crystallization of Аl-3wt.%Мg alloy, because Mg oxides are not as dense as Al oxides. Irregularity of the pore size along the solidification direction was quite obvious. Pores have irregular shapes, differing from spherical or elliptical (Fig. 6).
During the study of pore structure, dendrites which have sprouted into the pore (that make pores not very spherical in form)
were distinctly visible as shown in Fig. 7. It could be connected
Zone 1
Zone 2
Fig. 3: Type of porous high pure aluminum sample with oxide films (Zone 1: zone of directional crystallization, part
in contact with a chiller; Zone 2: area of oxide films)
Fig. 4: Macrostructure of industrial pure Al specimen made under following parameters: Psat = 0.2 MPa, T = 750 °C, t = 25 min, Psol = 0.05 MPa
From Fig. 4, it can be seen that in the bottom part of the sample, metal solidifies very fast so the pores have less time to form. Then on the height of 20 mm (from the bottom) pores begin their growth and the relatively uniform porous zone lasts till the height of 55 mm. The pores have elongated shape, 1.5 - 2.5 mm of length with diameter of 0.2-0.3 mm. The pore shape
is elliptical (Fig. 5). In some parts of the monolith the output of dendrites was observed [Fig.5 (c)]. The size and shape of the pores change a little. Radial pores are almost absent. In the upper part of the specimen (Fig. 4) there is a zone of large radial pores of 1-1.5 mm in diameter and 2-3 mm long. In the center of the sample there are sparse small spherical pores. This zone has a length of about 15 mm, and subsequently transferred to an area
(a) Longitudinal section
with the temperature interval in which the crystallization of the
samples was occurring.
The influence of Mg concentration on the final porous structure of aluminum-based magnesium alloy was also studied.
Fig. 5: Scanning electron micrographs of porous pure aluminum
Fig. 6: Scanning electron micrographs of porous Al-3wt.%Mg alloy
Fig. 7: SEM photo of pore of Аl-3wt.%Мg alloy with dendrites
(a) Position at upper part (b) Position at middle part (c) Position at bottom part
(a) Position at upper part (b) Position at middle part (c) Position at bottom part
It can be seen in Fig. 8, the structure is changing with the
increasing of the amount of Mg from 3wt.% to 35wt.%: the pore size considerably increases and pores acquire elliptical shape (the solubility of hydrogen increases due to Mg content). Al-35wt%Mg is an alloy that has a structure close to the eutectic one [Fig. 8(c)], so the crystallization occurs similarly to pure metals. The specimen has lots of elongated pores, due to the high content of Mg, and they have a relatively uniform structure. However, the mechanical properties of such an alloy sharply deteriorate, and the alloy becomes fragile and unsuitable for practical use (part 4).
3 Compressive tests of Al-Mg alloys
Cylindrical samples of the porous and dense Al-Mg alloy with a size of 20 mm × 20 mm were manufactured for the compressive tests. By doing these tests, it has been found that the porous
samples have greater compressive strength than the dense
Al-3wt.%Mg Al-6wt.%Mg Al-3wt.%Mg
Al-6wt.%Mg
worse results (in this case pores are smashed easier and deformation results in less strength). Meanwhile, the deformation of the dense samples
has the same strain in all directions.
The fact that 3wt.%Mg and Al-6%Mg alloys are ductile metals has been proved, however Al-3wt.%Mg is more ductile than Al-6wt.%Mg, as can be seen from Fig.10.
GASAR materials have well-defined anisotropic structure: soft and ductile along the pores; durable and resistant across the pores. Deformation is hindered by the movement of dislocations. As for porous materials,
dislocations are merged by pores
during deformation process, so it is necessary to apply more force to deform porous samples. The results of
the compression tests proved that the
(a) (b) (c) (d)
(e)
Fig. 8: Influence of Mg concentration on porosity of Аl-Мg alloys: (a) 3wt.% Mg; (b) 6wt.% Mg; (c) 35wt.% Mg; (d), (e) SEM photo of pores
Fig. 9: Compressive tests for porous and dense samples
Fig. 10: Comparison of Al-3wt.%Mg and Al-6wt.% Mg alloys (a) Al-3wt.%Mg
(a) Porous
(b) Al-6%wt.Mg
(b) Dense
difference between porous and dense samples is not very high. As it can be seen from Fig.10, the porous and dense specimens of Al-3wt.%Mg and Al-6wt.% Mg show similar results. So it gives the
opportunity to use them in industry at the same conditions as dense alloys with weight and material consumption savings.
4 Conclusions
(1) The directional solidified porous pure and industrial pure aluminum mostly have elliptical pores and have similar size along the center axis of casting. A layer of oxide film always exists in the upper part of the specimens, which sharply reduces the output of qualitative porous aluminum. This layer interferes with the process of fluent hydrogen penetration into the melt and
is obviously the biggest obstacle on the way to obtain qualitative
directional solidified pure aluminum.
(2) The ability of hydrogen to saturate in liquid aluminum is significantly affected by the alloying elements. The hydrogen solubility increases in liquid Al-Mg alloy with the increasing of Mg content. It could be explained that Mg oxides are not as dense as Al oxides. With the increasing of Mg content the quantity of pores significantly increases. Their shape also undergoes changes when the Mg content reaches eutectic point; in this case the solidification process goes similarly to pure metal.
(3) Compressive tests reveal that with the increasing of Mg
Fig. 11: Al-3wt.%Mg sample after (a) and before (b) compressive test
Fig.12: Al-35wt.%Mg sample after compressive test
content up to 35wt.%, mechanical properties of such alloy sharply deteriorate, and the alloy becomes fragile and unsuitable for practical use. However Al-3%Mg and Al-6wt.%Mg
alloys are ductile metals, their porous samples have greater compressive strength than the dense samples due to existence
of pores. It gives the opportunity to use them in industry at
the same conditions as dense alloys with weight and material consumption saving.
References
[1] Gibson L J, Ashby M F. Cellular Solids: Structure and Properties, UK; Cambridge University Press, 2nd edition 1999.
[2] Nakajima H. Progress in Materials Science, 2007; 52(7):1091-1173. [3] Shapovalov V I. Method of manufacture of porous articles. USA
patent, 5181549, 1993-06-23.
[4] Shapovalov V I, Boyko L. Gasar – A new class of porous materials. Advanced Engineering Materials, 2004, 6(6): 407- 410.
[5] Liu Yuan, Li Yanxiang. A theoretical study of Gasarite eutectic growth. Scripta Materialia, 2003, 49(5): 379-386.
[6] Jiang Guangrui, Li Yanxiang, Liu Yuan. Influence of solidification mode on pore structure of directionally solidified porous Cu-Mn alloy. Transactions of Nonferrous Metals Society of China, 2011, 21: 88-95 [7] Yamamura S, Shiota H, Murakami K. Evaluation of porosity in porous
copper fabricated by unidirectional solidification under pressurized hydrogen. Material Science Engineering, 2001, A318: 137–143. [8] Tane M, Ichitsubo T, Nakajima H, et al. Elastic properties of lotus-type
porous iron: acoustic measurement and extended effective-mean-field theory. Acta Materialia, 2004; 52(17): 5195-5201.
[9] Kovacik J. The tensile behaviour of porous metals made by GASAR process. Acta Materialia, 1998, 46(15): 5413-5422.
[10] Nakajima H, Hyun S K, Ohashi H, et al. Fabrication of porous copper by unidirectional solidification under hydrogen and its properties. Colloids and Surfaces A, 2001, 179(2-3): 209-214.
[11] Liu Yuan, Li Yanxiang, Zhang Huawei. Fabrication of lotus-structure porous magnesium with GASAR process. Acta Metallurgica Sinica, 2004, 40(11): 1121-1126.
[12] Hyun S K, Ikeda T, Nakajima H. Fabrication of lotus-type porous iron and its mechanical properties. Science and Technology of Advanced Materials, 2004, 5(1-2): 201-205.
[13] Hyun S K, Nakajima H. Fabrication of lotus-structured porous iron by unidirectional solidification under nitrogen gas. Advanced Engineering Materials, 2002, 4(10): 741-744.
[14] Ikeda T, Tsukamoto M, Nakajima H. Fabrication of lotus-type porous stainless steel by unidirectional solidification under hydrogen atmosphere. Materials Transactions, 2002, 43(11): 2678-2684. [15] Takuya I, Masakazu T, Hyun S K, et al. Fabrication of lotus-type
porous Ni3Al by unidirectional solidification/Nakajima H, Kanetake N.
Porous Metals and Metal Foaming Technology. Kyoto: The Japan Institute of Metals, 2005: 229-232.
[16] Park J S, Suzuki S, Nakajima H. Fabrication of lotus-type porous Al-Si alloys using continuous casting technique/Lefebvre L P, Banhart J, Dunand D C. Porous Metals and Metallic Foams. Montreal: DEStech Publications, 2007: 229-232.
[17] Gabidullin R M, Kolachev B A, Shvecov I B, et al. Metallurgy of non-ferrous metals and alloys. Science, 1992: 94-99 (in Russian) [18] Gabidullin R M, Kolachev B A, Shvecov I B. The structure, properties
and applications of metals. Science, 1994: 188-190 (in Russian)
The study was financially supported by Liaoning BaiQianWan Talents Program (No. 2011921065).
![Fig. 1: Experimental apparatus for GASAR manufactureapplications and they have raised extensive research interest [8, 9]](https://thumb-eu.123doks.com/thumbv2/123dok_br/15716193.122213/2.892.427.722.583.815/fig-experimental-apparatus-gasar-manufactureapplications-raised-extensive-research.webp)