Control Strategy Scenarios for the Alien
Lionfish
Pterois volitans
in Chinchorro Bank
(Mexican Caribbean): Based on
Semi-Quantitative Loop Analysis
Marco Ortiz1
*, Fabián Rodriguez-Zaragoza2, Brenda Hermosillo-Nu
ñez3, Ferenc Jordán4,5
1Instituto Antofagasta, Instituto de Investigaciones Oceanológicas, Facultad de Recursos del Mar, Universidad de Antofagasta, Antofagasta, Chile,2Laboratorio de Ecosistemas Marinos y Acuicultura, Departamento de Ecología, Centro Universitario de Ciencias Biológicas y Agropecuarias, Universidad de Guadalajara, Carretera a Nogales Km. 15,5, Las Agujas Nextipac, Zapopan C.P. 45110, Jalisco, México,
3Programa de Doctorado en Ciencias Aplicadas, Mención Sistemas Marinos Costeros, Facultad de Recursos del Mar, Universidad de Antofagasta, Antofagasta, Chile,4Centre for Ecological Research, Hungarian Academy of Sciences, Karolina ut 29, Budapest, Hungary,5The Microsoft Research–COSBI, Piazza Manifattura 1, Rovereto, Italy
*marco.ortiz@uantof.cl
Abstract
Ecological and eco-social network models were constructed with different levels of com-plexity in order to represent and evaluate management strategies for controlling the alien speciesPterois volitansin Chinchorro bank (Mexican Caribbean). Levins´s loop analysis was used as a methodological framework for assessing the local stability (considered as a component of sustainability) of the modeled management interventions represented by vari-ous scenarios. The results provided by models of different complexity (models 1 through 4) showed that a reduction of coral species cover would drive the system to unstable states. In the absence of the alien lionfish, the simultaneous fishing of large benthic epifaunal species, adult herbivorous fish and adult carnivorous fish could be sustainable only if the coral species present high levels of cover (models 2 and 3). Once the lionfish is added to the sim-ulations (models 4 and 5), the analysis suggests that although the exploitation or removal of lionfish from shallow waters may be locally stable, it remains necessary to implement additional and concurrent human interventions that increase the holistic sustainability of the control strategy. The supplementary interventions would require the implementation of pro-grams for: (1) the restoration of corals for increasing their cover, (2) the exploitation or removal of lionfish from deeper waters (decreasing the chance of source/sink meta-popula-tion dynamics) and (3) the implementameta-popula-tion of bans and re-stocking programs for carnivorous fishes (such as grouper) that increase the predation and competition pressure on lionfish (i.e. biological control). An effective control management for the alien lionfish at Chinchorro bank should not be optimized for a single action plan: instead, we should investigate the concurrent implementation of multiple strategies.
OPEN ACCESS
Citation:Ortiz M, Rodriguez-Zaragoza F, Hermosillo-Nuñez B, Jordán F (2015) Control Strategy Scenarios for the Alien LionfishPterois volitansin Chinchorro Bank (Mexican Caribbean): Based on Semi-Quantitative Loop Analysis. PLoS ONE 10(6): e0130261. doi:10.1371/journal.pone.0130261
Editor:John F. Valentine, Dauphin Island Sea Lab, UNITED STATES
Received:September 26, 2014
Accepted:May 19, 2015
Published:June 26, 2015
Copyright:© 2015 Ortiz et al. This is an open access article distributed under the terms of the
Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability Statement:All relevant data are within the paper and its Supporting Information file.
Funding:The authors received no specific funding for this work.
Introduction
In the last three decades, there has been a growing interest in the study of the wide and rapid spread of the two alien lionfish species,Pterois volitansandPterois miles, into the western Atlantic, Caribbean and Gulf of Mexico [1]. These are the first marine fish known to invade such large ecosystems [2]. The presence of this species seems to be the consequence of acciden-tal escape or intentional introduction from aquaria in Florida in the last decade [3].P.volitans is presently one of the most important predators in such ecosystems, reaching densities several orders of magnitude higher than observed in native environments [1]. Reports show that along the coast of North Carolina this alien species reaches an average density of 21 individuals ha-1[4], and estimates from the Bahamian coral reefs reaches a mean density of 390 individuals ha-1[5], which is nearly five times greater than have been reported from its native Pacific range [6].
For these reasons, the presence of this voracious, carnivorous fish could be considered as an additional perturbation factor to ecosystems already highly stressed by overexploitation, tour-ism, pollution, carbonate production decline and climate change [7]. Its presence could easily lead to a phase-shift transition from corals to fleshy macroalgae (as the dominant species) [8]. In addition to these disturbances, lionfish predation also decreases the overall biodiversity of coral reefs, as it consumes a high variety of invertebrate and vertebrate prey species [9,10,11]. Indirectly, this alien species favors the live coral cover loss because it also consumes herbivores, which reduces the grazing on algae, contributing to a shift to algal dominance [8]. Likewise, lionfish invasion is a potential human health risk due to its venomous fin spines [1]. These problems could produce negative impacts on fishery yields due to the predation and competi-tion of lionfish on the early history stages of targeted fish, which would reduce their recruit-ment [12]. Likewise, the attractiveness of scuba diving destinations on Caribbean reefs will be threatened due to scenic beauty loss, generated by extensive algal overgrowth on coral [13].
One of the most relevant characteristics of species introduced into a non-natural ecological system is to showr-type dynamics [14]. The lionfishP.volitansis a clear example because of its ability to spread in different environments, ranging from the outer margins of reefs to nursery habitats such as sheltered mangrove lagoons [15]. In the Atlantic, lionfish shows high individ-ual growth and reproductive rates [13] and a high population growth rate [8]. This behavior could be explained by at least the following two reasons: (1) lionfish present predatory features that are not recognized by the other species in invaded habitats, so that their prey cannot detect it as a potential threat, making it a more successful predator than native predators (i.e. native local community) [9]. This fish thus exhibits high predation efficiency (as an ambush-unknown predator) mainly upon reef-fish species, including economically important fish and crustaceans [16]; (2) this species shows a reduced mortality by predation, as most of its putative predators are present only in low densities as a consequence of intensive (historical) exploita-tion [17], even reaching larger sizes in invaded habitats compared to those recorded in its native ecosystems [9]. Under such a particular scenario, the receiving ecological system could be dominated by positive feedbacks at different levels of complexity (within the network), showing unstable states [18]. For this reason, there is a growing concern that lionfish will affect the structure and function of invaded marine ecosystems [1].
system, therefore we may introduce uncertainties [19]. Different research strategies, which are not mutually exclusive, can be used to study, assess, and attempt to predict the transformations in a natural system as a response to human interventions and/or introduction of exotic species. These strategies include (1) the reduction of objects of study to their small parts, assuming that this subsystem represents well the whole original ecosystem; (2) the statistical analysis of fac-tors (weighted by their relative importance); (3) quantitative simulation requiring fairly precise measurements of the variables and parameters and exact equations (this is quite difficult for variables that cannot be measured); and (4) semi-quantitative or qualitative models (Loop
Analysis) that do not need precise nor quantitative equations: these allow the integration of
non-measurable variables and physically different forms, focusing on the nature of the change (e.g. its sign) rather than its precise magnitude [20]. Likewise, it is a useful technique for esti-mating the local stability (as a component of sustainability) of systems [20] and assessing the propagation of direct and indirect effects as a response to external perturbations [21]. This approach has been applied widely in different fields of the natural sciences [19,22,23,24,].
Due to the difficulty in carrying out replicated experiments to estimate stability properties under different regimes of disturbances (such as fishing and pollution) [25,26], we chose loop models to capture the general ecological and eco-social interactions underlying the invasion and spread of the lionfishP.volitansin the Chinchorro bank coral reef ecosystem (Mexican Caribbean). Simultaneously, we assessed the local stability (as an approach to holistic sustain-ability) of a set of different ecological and eco-social models in response to alternative control management scenarios to reduce the abundance of this alien species. We note that our models should be considered as complementary and extended versions of the recently published age-structured population model for the Western Atlantic Ocean [27] and quantitative trophic models for the Caribbean coral reef ecosystem) [28] developed for lionfish recently.
Material and Methods
Ethic Statement
No protected and endangered species were involved in this study. No vertebrate neither inver-tebrate species were collected in the present work. No sampling program was performedin situ because the models were built taken information from scientific literature. No specific permis-sions were required for this study area neither for the intellectual work.
Study area
For this work, we modeled the ecological coral reef system of Chinchorro bank (18° 35’N; 87°
23’W), Mexican Caribbean, which is located 30.8 km to the southwest of the Yucatán
Penin-sula. It is separated from the continent by a channel nearly 500 m in depth [29]. Chinchorro bank is of ovoid shape, 43.2 km long and 18.0 km wide [30]. The reef lagoon is surrounded by a semi-continuous barrier reef with a perimeter of approximately 115 km, an area exceeding 500 km2, and depths between 1 and 9 m in the south, and 2 m in the north. The reef patches and coral knolls decrease in number and size from south to north [31].
Chinchorro bank is one of the largest platform coral reefs of the Caribbean Sea [29] and was
declared as a Biosphere Reserve in 1996 by the Mexican government [28]. Chinchorro bank
has received little impact from tourism and coastal human populations due to its isolation. Nevertheless, it has been subjected to intense fishing activities for over 40 years, mainly
target-ingPanulirus argus (Caribbean spiny lobster),Strombus gigas(queen conch), and various
also been permanent and historical. However, the reef fish harvest increased especially when the Mexican fishing authority (dependent on the federal government) implemented bans for the harvest on the queen conch (S.gigas) and the spiny lobster (P.argus). Likewise, the reef of Chincorro Bank includes four cays that cover 0.4% of the total reserve surface, named North Cay at the north, Central Cay in the center-east of the system, and Lobos Cay in the south. The seawater is oligotrophic with sea surface temperature ranging from 27 to 29° C, and salinity ranging from 36.6 to 36.9‰[30]. Trade winds dominate this coral reef across the year,
although northern winds predominate between October and May [31]. In summer, the reef is
exposed to tropical storms and hurricanes that can reach level 5 on the Saffir-Simpson scale, as was the case of Hurricane Dean in 2007.
Loop Analysis, a semi-quantitative modeling approach
Models of populations, communities or ecosystems represent only some selected relations of the studied ecosystems in a qualitative or quantitative way [20]. Loop models provide a qualita-tive or semi-quantitaqualita-tive framework for formulating the relationships between variables within a particular system. It is also possible to estimate the local stability properties of the system (sustainability) and to determinate the effects of external factors on the variables [20]. Loop models show only the sign of a relationship, which indicates the type of influence each variable has upon another (i.e., positive, negative or zero) (Fig 1). In ecological interactions, (+,-) denotes a predator-prey, parasite-host, or resource-consumer relationship, (-,-) represents competition between two species, whereas (+,+), (+,0), and (-,0) represent mutualism, com-mensalism and acom-mensalism, respectively. Each variable is represented by a node (large circle) and edges (lines) representing directions and types of relationships: an arrow at one end indi-cates a positive effect, a circle means the effect is negative and the lack of a symbol shows a null effect.Loop Analysisis based on the correspondence between systems of differential equations at equilibrium, community matrices and loop diagrams. Therefore, in the system, the element aijof the matrix and the loop diagram represent the effect of variablejon the growth of variable
iwhen the equation:
dXi
dt ¼fiðX1;X2;X3;. . .;Xn;C1;C2;C3;. . .;CnÞ ð1Þ
where the change in time of variableXi, is a functionfiof other interconnected variablesXnand
parametersCnand is solved at equilibrium (X). The link fromXjtoXiis similar to theαijin
[32], as follows:
a
ij¼
@ðdXi
dtÞ
@Xj
ð2Þ
whereXiis evaluated at a moving equilibrium for the system. The element of the graph
repre-senting the link fromjtoiis sign (αij)–whether positive, negative, or zero- where the function
sign (X) is 1 whenX>0, 0 whenX= 0, and -1 whenX<0.
Local stability, as determined by the Routh-Hurwitz criteria, translates into loop terms as Condition 1 whenFk<0 for allk; i.e.,Fkcorresponds to the negative feedback on every level of
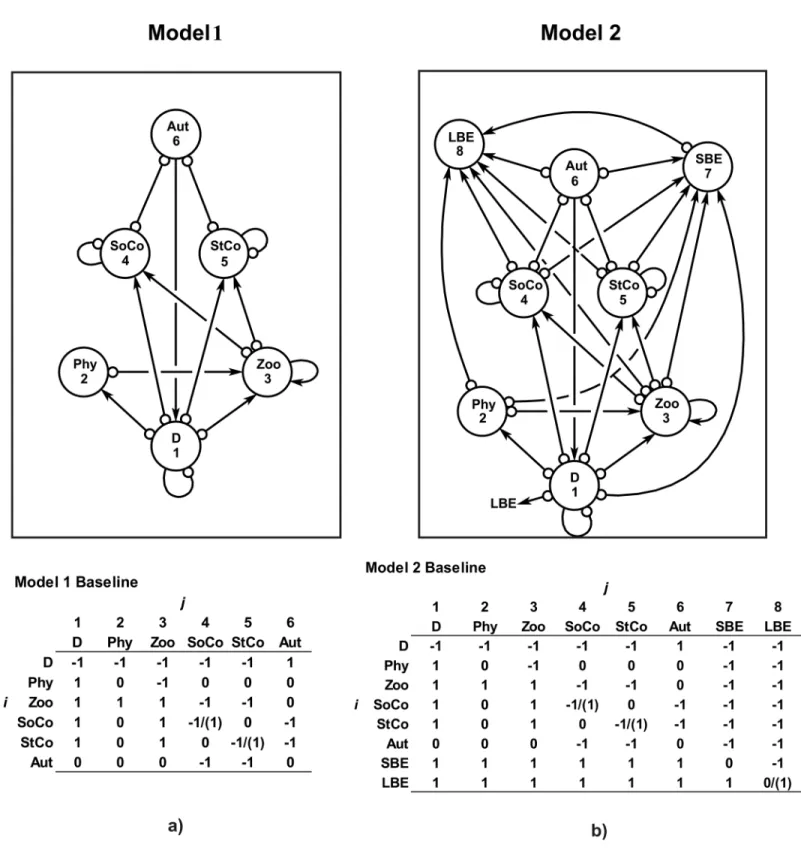
Fig 1. Models 1 and 2.Ecological models 1 and 2 for the coral benthic system of Chinchorro bank (México). The baseline community matrices with the nominal effect ofjvariable toiare also shown. The parenthesis shows the kind of intervention. For more explanation of the name of variables and interactions see the text.
feedback for each level can also be calculated by estimating the characteristic polynomial related to theJacobianinteraction matrix, in which the polynomial now can be written in terms of the feedback notation as:F0λn+F1λn-1+F2λn-2+. . ...+Fn-1λ+ Fn= 0, whereF0-1
and theFnis the feedback of the entire system (n= total number of variables in the system).
The Levins’s stability criterion assumes that the system is locally stable whenFnis negative.
The stronger the negative feedback (Fn) becomes, the greater the resistance will be to external
change [20]. Based on this local stability criterion, it is possible to estimate the degree of resis-tance to perturbations (as a measure of sustainability) of the system and, simultaneously, to explore strategies to increase this resistance. Likewise, the semi-quantitative loop models allow indicate what must be measured by identifying the self-dynamics and interactions that changes the local stability [20]. It is relevant to indicate that theLoop Analysisdoes not permit to assess the effect of the extinction of some variable on the model properties. In the present study, this framework helps us to determine what scenarios for reduction of lionfish density (as control mechanism) are locally stable (as an approach to holistically sustainable).
Selection of network boundaries, structure and assumptions
Five semi quantitative models were constructed considering the most important variables and interactions before and after the invasion of the lionfishP.volitans. In the simplest version (model 1), six variables are represented, and sequential models expand the boundaries until the eco-social version of the model (model 5) contains 19 variables. It is important to indicate that models 1, 2 and 3 represent different levels of ecological complexity before the invasion of lion-fish, whereas in models 4 and 5 we added lionfish and social variables.
Most of trophic relationships among the variables were taken from [28], It should be noted that, in all the models, some variables (the most relevant ones) were considered self-damping (density-dependent growth rates) due to higher density close to carrying capacity or self-enhanced (density-independent growth rates) as response to intensive harvest (reduction of abundance) [19].Both self-dynamics can be demonstrated using the following difference (Rick-er’s) equation:
fðxÞ ¼xexprð1 xkÞ ð
3Þ
wherexis the abundance of a variable (species or functional group),r= intrinsic growth rate andk= carrying capacity. This difference equation is considered the analogue of the logistic differential equation with similar parameters.
For restoration or re-stocking program of a variable (self-damped dynamics), consider the following extension ofEq 3:
fðxÞ ¼xexprð1 xkÞ þb
x; ð4Þ
whereb/x= the restoration or re-stocking rate ofx. The derivative of (4) with respects tox (self-damped dynamics) becomes as:
@fðxÞ
@x ¼exp
rð1 xkÞ xr
k exp
rð1 xkÞ b
x2 ð5Þ
therefore, the restoration or re-stocking ofxwill cause self-damping on itself.
By another side, the over-exploitation or destruction ofx(self-enhanced dynamics) becomes as:
fðxÞ ¼xexprð1 xk hxÞ
where the parameterh/xis the harvest (or destruction) rate ofx. The derivative of Eq (6) with respects tox(self-dynamics) becomes:
@fðxÞ
@x ¼exp
r 1 x k hx
ð Þ þxrh
x2 exp
r 1 x k hx
ð Þ xr
k exp
r 1 x k hx
ð Þ ð7Þ
thus, the over-exploitation or destruction ofxwill cause self-enhanced dynamics onx.
Model 1. This model includes six variables: detritus (D) (as a complex of microorganisms and nutrients), phytoplankton (Phy), zooplankton (Zoo), soft corals (SoCo) (constituted by all octocoral, such asPseudopterogorgiaspp.,Muriceaspp.,Plexauraspp.,Pterogorgiaspp., and others), stony corals (StCo) (formed by hermatypic corals [e.g.,Montastraeaspp.,Diploria spp.,Siderastreaspp.,Poritesspp.,Agariciaspp., and others] and hydrocorals [i.e.,Millepora spp.]), and a group of benthic autotrophs (Aut) (including fleshy macroalgae, crustose calcare-ous algae, articulated calcarecalcare-ous algae, and seagrass). Most of these groups were connected by prey-predator relationships. An exception was the Aut, with a positive impact to detritus, and the negative interaction (competition) between SoCo and StCo with the Aut. No interaction between SoCo and StCo was considered because rigorous information is limited. The D, SoCo and StCo were self-damped, that is, their abundance close to carrying capacity (healthy condi-tions), and Zoo is self-enhanced due to high mortality and low density by predation (Fig 1A). Table 1Ashows the scenarios considered for the sustainability (stability) assessment.
Model 2. This model is the extension of model 1, including two new variables: the small benthic epifauna (SBE) and the large benthic epifauna (LBE). The SBE comprises all small spe-cies that live on reef benthos as amphipods, bivalves, chitons and gastropods, crabs, shrimps, barnacles, bryozoans, hemichordates, isopods, polychaetes, tunicates, and other organisms. The LBE incorporated larger species as asteroids, holothurians, echinoids, ophiuroids, octo-puses, large gastropods (e.g.,S.gigas), lobsters (e.g.,P.argus), large crabs, sponges, and others. Both variables were connected by predation with the other groups (Fig 1B).Table 1B summa-rizes the scenarios simulated for the sustainability estimates.
Model 3. This model includes yet another six variables: (1) herbivore fish juveniles (HFj) and adults (HFa) principally corresponding to scarids (parrotfishes), acanthurids (surgeon-fish); (2) omnivore fish juveniles (OFj) and adults (OFa), which were mainly accounted for by damselfish; and (3) carnivore fish juveniles (CFj) and adults (CFa), representing groupers, snapper, jacks, barracudas, grunts and other reef carnivore fish. Within this model HFj and OFj have a positive influence on HFa and OFa, respectively. These relationships are similar to those described in population models [16]. CFj and CFa were connected by predation due to cannibalism (Fig 2). The CFa was considered as top predator, which preys on OFa, OFj, HFa, and HFj. The HFj and HFa have a negative (direct) influence on StCo as a consequence of an incidental depredation.The scenarios considered to evaluate stability are visualized in Table 1C.
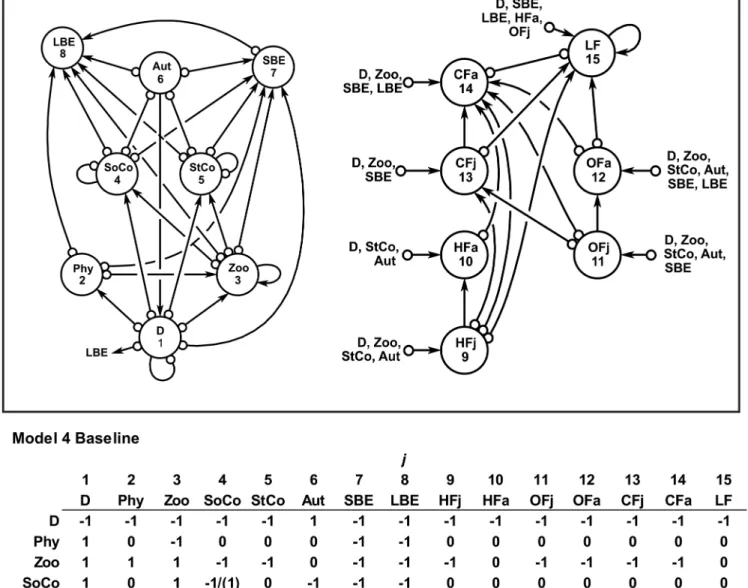
Model 4. This model is the extension of model 3, including also the alien lionfishP.
voli-tans(LF). This variable was connected as predator upon D, SBE, LBE, HFj, HFa, OFj, OFa, and
CFj. The lionfish and CFa were qualitatively simulated as competitors due to cross-predation (Fig 3). The scenarios considered for the assessment of sustainability are described inTable 1D.
variables related to fisheries were included: artisanal fishermen (F1) exploiting LBE, HFa, CFa, and LFs, and control fishermen (F2) harvesting exclusively on LFs. The F1 were limited (self-damping) in the number of boats permitted, and their harvest activities are controlled (by legis-lation), whereas the F2 were self-enhancing because their activity is promoted by the authority. F1 and F2 were connected to LFs and other groups by predation. The demand (DE) was also included, and it has a positive influence on fishermen (F1 and F2), and the fishermen have a negative effect on demand. This relationship represented a situation where the prices would be determined by local market [19]. All commercial groups stimulate demand positively. DE is self-enhancing because the market is dominated by positive feedbacks [33] (Fig 4).Table 1E shows the scenarios simulated for the sustainability estimate. Due to the high complexity
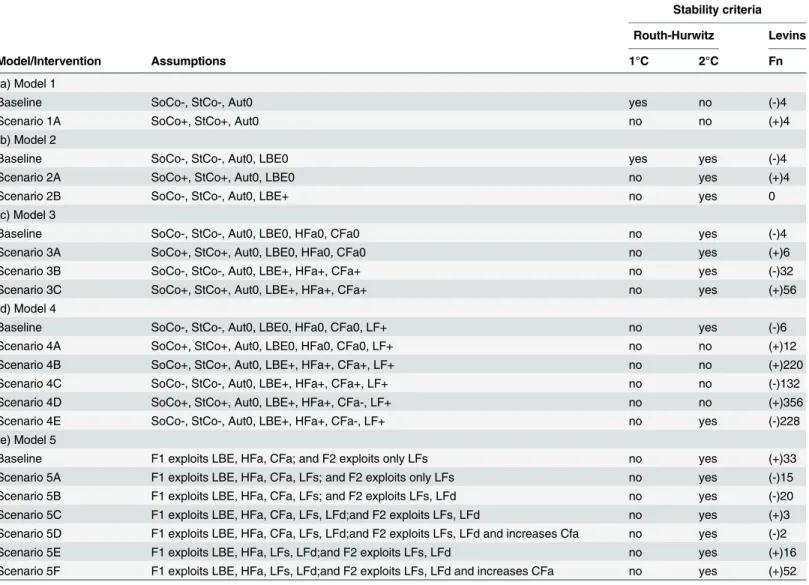
Table 1. Routh-Hurwitz and Levins´s stability criteria (as sustainability measure) for the different models and scenarios simulated.
Stability criteria
Routh-Hurwitz Levins
Model/Intervention Assumptions 1°C 2°C Fn
(a) Model 1
Baseline SoCo-, StCo-, Aut0 yes no (-)4
Scenario 1A SoCo+, StCo+, Aut0 no no (+)4
(b) Model 2
Baseline SoCo-, StCo-, Aut0, LBE0 yes yes (-)4
Scenario 2A SoCo+, StCo+, Aut0, LBE0 no yes (+)4
Scenario 2B SoCo-, StCo-, Aut0, LBE+ no yes 0
(c) Model 3
Baseline SoCo-, StCo-, Aut0, LBE0, HFa0, CFa0 no yes (-)4
Scenario 3A SoCo+, StCo+, Aut0, LBE0, HFa0, CFa0 no yes (+)6
Scenario 3B SoCo-, StCo-, Aut0, LBE+, HFa+, CFa+ no yes (-)32
Scenario 3C SoCo+, StCo+, Aut0, LBE+, HFa+, CFa+ no yes (+)56
(d) Model 4
Baseline SoCo-, StCo-, Aut0, LBE0, HFa0, CFa0, LF+ no yes (-)6
Scenario 4A SoCo+, StCo+, Aut0, LBE0, HFa0, CFa0, LF+ no no (+)12
Scenario 4B SoCo+, StCo+, Aut0, LBE+, HFa+, CFa+, LF+ no no (+)220
Scenario 4C SoCo-, StCo-, Aut0, LBE+, HFa+, CFa+, LF+ no no (-)132
Scenario 4D SoCo+, StCo+, Aut0, LBE+, HFa+, CFa-, LF+ no no (+)356
Scenario 4E SoCo-, StCo-, Aut0, LBE+, HFa+, CFa-, LF+ no yes (-)228
(e) Model 5
Baseline F1 exploits LBE, HFa, CFa; and F2 exploits only LFs no yes (+)33
Scenario 5A F1 exploits LBE, HFa, CFa, LFs; and F2 exploits only LFs no yes (-)15
Scenario 5B F1 exploits LBE, HFa, CFa, LFs; and F2 exploits LFs, LFd no yes (-)20
Scenario 5C F1 exploits LBE, HFa, CFa, LFs, LFd;and F2 exploits LFs, LFd no yes (+)3
Scenario 5D F1 exploits LBE, HFa, CFa, LFs, LFd;and F2 exploits LFs, LFd and increases Cfa no yes (-)2
Scenario 5E F1 exploits LBE, HFa, LFs, LFd;and F2 exploits LFs, LFd no yes (+)16
Scenario 5F F1 exploits LBE, HFa, LFs, LFd;and F2 exploits LFs, LFd and increases CFa no yes (+)52
Local stability measures Routh-Hurwitz and Levins (Fn) criteria in the models and scenarios simulated. First criterion (1°C) describes stability condition, and the second criterion (2°C) determines asymptotic or oscillation condition. The Levins’s (Fn) criterion can be used as an approach for holistic sustainability. The assumptions considered were changes in the self-dynamics (damped ´-´and/or enhanced ´+´) for the variables in each modelaaThe
names of the variables are described with details in Methods section.
Fig 2. Model 3.Ecological model 3 for the benthic-pelagic coral system of the Chinchorro bank (México). The baseline community matrix with the semi-quantitative effect ofjvariable toivariable is also shown. The parenthesis shows the kind of intervention. For details see section ofMethods.
Fig 3. Model 4.Benthic-pelagic ecological model 4, including the alien lionfish (LF) into the benthic-pelagic system of the Chinchorro bank (México). The baseline community matrix with the semi-quantitative effect ofjvariable toivariable is also shown. The parenthesis shows the kind of intervention. See Methodsfor more details.
represented by this model, the soft corals (SoCo) and stony corals (StCo) were only simulated under self-damping dynamics, which would be a consequence of a permanent restoration program.
Results
Model 1 shows that a perturbation impacting stony and soft-corals by reducing their abun-dances far below their carrying capacity would produce an unstable (unsustainable) ecological system under Routh-Hurwitz and Levins’s criteria (Table 1A). On the other hand, any
restora-tion program (baseline model) for corals that increases their abundances (near carrying capac-ity) would be stable or sustainable (Table 1A) (Fig 1A). In the case of model 2 (including two new variables) (Fig 1B), a similar pattern to model 1 is observed regarding the changes in coral cover (Table 1B); however, an intensive exploitation of large benthic epifaunal species (LBE) was found only to be asymptotically locally stable (scenario 2B) (Table 1B).
Table 1Cpresents the stability measurements from model 3 (Fig 2). In the case of the resto-ration program for corals, stability would be partially obtained (baseline model). It is important to indicate that under any restoration program for corals and an exploitation of LBE, HFa and CFa would also be partially sustainable (baseline model) (Table 1C). However, if any perturba-tion impacts stony and soft-corals negatively and simultaneously the fishermen intensively exploit LBE, HFa and CFa induced dynamics that produce an unsustainable (unstable) system (Table 1C).
The stability outcomes obtained from model 4 (Fig 3), which integrates the lionfish (LF) as a variable into the system, are summarized inTable 1D. It is relevant to note that the most sus-tainable scenario was obtained under the following simultaneous conditions: (1) restoration program for corals, (2) fishing on LBE, HFa and LF, and (3) implementation of bans for the fishery of carnivore fishes (CFa) (scenario 4E) (Table 1D). Likewise, any negative perturbation on corals would drive the system towards unstable (unsustainable) states, which agree with the results obtained in the model 1, 2 and 3 (Table 1A, 1B, 1C and 1D).
Table 1Eshows the results of stability for the eco-social model 5 (Fig 4). In this case the sys-tem reached the most highly partially stable state when the F1 exploits LBE, HFa, CFa and LFs, and F2 exploits LFs and LFd (scenario 5B) (Table 1E). Therefore, an exploitation program of lionfish inhabiting deeper waters should also be implemented. Likewise, a re-stocking program to increase the abundance of the carnivorous fish (CFa) (as grouper species) would also achieve the necessary stability (scenario 5D) (Table 1E). All characteristic polynomials regarded each
model/scenarios are summarized inS1 Text.
Discussion
It is widely recognized that accidental or planned introductions of alien species primarily moti-vated by commercial and recreational purposes have resulted in significant ecological distur-bances in aquatic and terrestrial systems, promoting the local reduction of native populations (in some cases to the point of extinction, resulting in biodiversity loss) [1].
The qualitative or semi-quantitative models built and analyzed herein correspond to a par-tial representation of the variables and interrelationships underlying the ecological and eco-social dynamics of the Chinchorro bank ecosystem. This caveat, however, is applicable to any type of model and independent of its level of complexity [34,35]). In this sense, we recognize
Fig 4. Model 5.Ecological and social model 5 for the Chinchorro bank (México). The lionfish is separated in two meta-populations (from shallow and deeper waters) and two kinds of fishers and the demand (from the market) are also integrated. The baseline community matrix with the semi-quantitative effect ofj variable toivariable is also shown. The parenthesis shows the kind of intervention. For more details of the variables and interactions see the text (Methods).
that the models presented have made use of at least five sources of simplifications: (1) we reduced the system complexity through the composition of functional groups for the most vari-ables of the model, an exception was the lionfishP.volitans; (2) only the native fishes were sep-arated in two class groups (juveniles and adults) without enough scientific information about their own dynamics, (3) we assumed the system (community-matrix) to be in a moving equi-librium, (4) we included only the fishermen and demand coming from socio-economics field, and (5) regardless of the well-known limitations of theLoop Analysistheoretical framework, the models constructed represent the processes underlying the systems analyzed when only considering short-term dynamics. Despite these limitations, we claim that the results obtained are sufficiently robust given the agreement among models of different levels of complexity, therefore permitting us both to compare different management strategies to control the alien lionfish and to assess their consequences in the local stability or sustainability of the Chinch-orro coral ecosystem. Moreover, for our purposes, the moving equilibrium is understood to be a trajectory path in a short dynamic that includes the set of variables of the system from which the system will not move unless perturbed.Loop Analysisis used to determine the direction of change of the equilibrium values of any variable in response to changes in any parameter [18]. Likewise, it is recognized for its high correspondence between model predictions and
observed-empirical responses [36,37,38,39], which is possible based on stable qualitative community matrices.
The results obtained in the models 1, 2, 3, 4, and 5 showed clearly that a restoration program for the soft- and hard corals, that is under self-damped dynamics, emerge as a locally stable or sustainable human intervention independent of the model complexity level. This result agrees with experimental studies in which the construction of artificial coral reefs improved the health (increasing biodiversity and ecological complexity) of historically highly perturbed ecosystems (i.e., that have suffered from stresses including fisheries, pollution, and eutrophication) [40]. Models 2 and 3 (before lionfish invasion) and model 4 withP.volitansshowed that the exploi-tation of species belonging to the LBE, HFa and CFa functional groups would be possible only if a restoration program for corals is implemented concurrently. Likewise, the harvest of lion-fish achieves the highest local stability or sustainable control management only when the CFa are not exploited, agreeing with the conclusions described by [41,42]. It is important to indi-cate that species of grouper (within CFa) such asEpinephelus striatusandMycteroperca tigris feed upon lionfish [43]. It has also been described that lionfish appeared to remain closer to ref-uges at places with high grouper densities, suggesting that the grouper may reduce both the abundance and consumption rates of lionfish [44]. However, it is not clear whether recruit-ment of lionfish in non-reef benthic habitats without large predatory groupers results in an increase (by migration) of lionfish in reef systems [45].
5D). In this sense, large-bodied predatory fishes could be capable of controlling the fast spread and population explosion of lionfish [4], although this opinion should be taken with caution as there is evidence that native predators would not influence invasion success (i.e., colonization or post-establishment population density) of lionfish on Caribbean reefs [47].
Our models show that an exploitation ofP.volitansfrom shallow and deeper waters seems to be a suitable control mechanism for this species at Chinchorro bank. However, this manage-ment strategy should be pursued with care because exploitation for the human consumption market could propagate unexpected negative long-term consequences (since this species could be introduced in many other places for commercial proposes [48]. Likewise, it is necessary to consider that complex ecosystems subjected to exploitation are continuously changing (exhib-iting oscillatory dynamics as a consequence of the relative dominance of positive feedbacks), which could promote negative effects on the health of the systems, reducing the effectiveness of this control mechanism forP.volitans.
Conclusions
The loop model outcomes obtained in this work help us to better understand the possible con-sequences of different control strategies forP.volitansat Chinchorro bank, especially when the population dynamics of any species depends on a complex network of intercactions among the biotic and abiotic components of the ecosystem [49]. The situation is even more complex if we also consider the populations as meta-populations heterogeneously scattered in an envi-ronment, which is also heterogeneous, where the extinction and colonization rates of an exotic species would depend on the superposition of different co-existing meta-populations [50]. Although the harvest on lionfish from shallow waters as a control mechanisms was found to be locally stable, agreeing in general terms with [25,26,51], it remains necessary to plan other additional human interventions (within an ecosystem-wide adaptive management program), which should include: (1) the restoration of corals, (2) the exploitation of lionfish from deeper waters, (3) the implementation of bans and re-stocking program for carnivore fishes (as grou-per), and (4) the exploitation of juvenile lionfishes, which in turn fed on recruits of native her-bivore, carnivore and omnivore fishes [12]. Nevertheless, these management strategies should be implemented simultaneously because implementation in isolation could have a negative impact on lionfishes only at a local spatial scale [46]. Even though the current contribution shows the importance of combining different types of studies that tackle the issue from several angles, thereby providing robust conclusions, however, additional explorations should be also performed in order to assess the impact of changes in the sign of interactions on the local sta-bility properties of the system. Therefore, we should seek to implement multiple and simulta-neous strategies to reduce the exotic lionfish population at Chinchorro bank (Mexico) and bring it within sustainable boundaries. Additionally, the approach developed here should be considered a general strategy for examining the consequences of natural changes and human interventions in ecosystems.
Supporting Information
S1 Text. Characteristic polynomials.Characteristic polynomials, p(λ), for each model and scenarios simulated. The polynomials were multiplied by (-1) due to F0-1 (for more details
Acknowledgments
The authors thank M.C. García-Rivas for her support with field data from Chinchorro Bank.
Author Contributions
Conceived and designed the experiments: MO FRZ. Performed the experiments: MO FRZ BHN. Analyzed the data: MO FRZ BHN FJ. Contributed reagents/materials/analysis tools: MO FRZ BHN FJ. Wrote the paper: MO FRZ BHN FJ.
References
1. Green SJ, Akins JL, Maljkovic A, Coté IM (2012) Invasive lionfish drive Atlantic coral reef fish declines. PLoS One. 7(3): e32596. doi:10.1371/journal.phone.0032596PMID:22412895
2. Whitfield PE, Gardner T, Vives SP, Gilligan MR, Courtenay WR Jr., Ray GC, et al. (2002) Biological invasion of the Indo-Pacific lionfishPterois volitansalong the Atlantic coast of North America. Marine Ecology Progress Series 235: 289–297.
3. Schofield PJ (2009) Geographic extent and chronology of the invasion of nonnative lionfishPterois voli-tans(Linnaeus 1758) andP.miles(Bennett 1828)) in the western north Atlantic and Caribbean Sea. Aquatic Invasions 4: 473–479.
4. Whitfield PE, Hare JA, David AW, Harter SL, Muñoz RC, Addison CM (2007) Abundance estimates of the Indo-Pacific lionfishPterois volitans/milescomplex in the Western North Atlantic. Biological Inva-sions 9: 53–64.
5. Green SJ, Côte IM (2009) Record densities of Indo-Pacific lionfish on Bahamian coral reefs. Coral Reefs 28: 107
6. Kulbicki M, Beets J, Chabanet P, Cure K, Darling E, Floeter SR, et al. (2012) Distributions of Indo-Pacific lionfishes Pterois spp. in their native ranges: implications for the Atlantic invasion. Marine Ecol-ogy Progress Series 446: 189–205.
7. Perry CT, Murphy GN, Kench PS, Smithers SG, Edinger EN, Steneck RS, et al. (2013) Caribbean-wide decline in carbonate production threatens coral reef growth. Nature Communications 4: 1402 doi:10. 1038/ncomms2409PMID:23360993
8. Albins MA, Hixon MA (2013) Worst case scenario: potential long-term effects of invasive predatory lion-fish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environmental Biology of Fishes 96: 1151–1157.
9. Albins MA (2013) Effects of invasive Pacific red lionfish Pterois volitans versus a native predator on Bahamian coral-reef fish communities. Biological Invasions 15: 29–43.
10. Côté IM, Green SJ, Hixon MA (2013) Predatory fish invaders: Insight from Indo-Pacific lionfish in the western Atlantic and Caribbean. Biological Conservation 164: 50–61.
11. Benkwitt CE (2014) Non-linear effects of invasive lionfish density on native coral-reef fish communities. Biological Invasions. doi:10.1007/s10530-014-0801-3
12. Frazer TK, Jacoby CA, Edwards MA, Barry SC, Manfrino CM (2012) Coping with the Lionfish Invasion: Can Targeted Removals Yield Beneficial Effects? Reviews in Fisheries Science 20(4): 185–191.
13. Morris JA Jr., Whitfield PE (2009) Biology, ecology, control and management of the invasive Indo-Pacific lionfish: An updated integrated assessment. NOAA Technical Memorandum NOS NCCOS 99: 57.
14. Levin S (2000) Multiple scales and the maintenance of biodiversity. Ecosystems 3: 498–506.
15. Jud ZR, Layman CA (2012) Site fidelity and movement patterns of invasive lionfish,Pteroisspp., in a Florida estuary. Journal of Experimental Marine Biology and Ecology 414–415: 69–74.
16. Valdez-Moreno M, Quintal-Lizama C, Gómez-Lozano R, García-Rivas MC (2012) Monitoring an Alien Invasion: DNA Barcoding and the Identification of Lionfish and Their Prey on Coral Reefs of the Mexi-can Caribbean. PLoS ONE 7(6): e36636. doi:10.1371/journal.pone.0036636PMID:22675470
17. Mumby PJ, Stenec RS, Edwards AJ, Ferrari R, Coleman R, Harborne AR, et al. (2012) Fishing down a Caribbean food web relaxes trophic cascades. Marine Ecology Progress Series 445: 13–24.
18. Ch Puccia, Levins R (1985) Qualitative modeling of complex systems: an introduction to loop analysis and time averaging. Harvard University Press, MA. 256 p.
20. Levins R., 1998. Qualitative mathematics for understanding, prediction, and intervention in complex ecosystems. In: Raport D, Costanza R, Epstein P, Gaudet C, Levins R, editors. Ecosystem Health. Blackwell, Boston, MA, pp. 178–204.
21. Ramsey D, Veltman C (2005) Predicting the effects of perturbations on ecological communities: what can qualitative models offer? Journal of Animal Ecology 74: 905–916.
22. Levins R, Vandermeer J (1990) The agroecosystem embebed in a complex ecological community. In: Carroll R, Vandermeer J, Rosset P, editors. Agroecology. McGraw-Hill, New York, pp. 341–362.
23. Ortiz M, Stotz W (2007) Ecological and eco-social models for the introduction of the abaloneHaliotis discus hannaiinto benthic systems of north-central Chile: sustainability assessment. Aquatic Conser-vation: Marine Freshwater Ecosystems 17: 89–105.
24. Dambacher J, Gaughan D, Rochet M, Rossignol P, Trenkel V (2009) Qualitative modelling and indica-tors of exploited ecosystems. Fish and Fisheries 10: 305–322.
25. Carpenter S (1989) Replication and treatment strength in whole-lake experiments. Ecology 70: 453–
463.
26. Lewontin R, Levins R (1998) How different are natural and social science? Capitalism Nature Socialism 9: 85–89.
27. Barbour AB, Allen MS, Frazer TK, Sherman KD (2011) Evaluating the Potential Efficacy of Invasive Lionfish (Pterois volitans) Removals. PLoS ONE 6 (5): e19666. doi:10.1371/journal.pone.0019666 PMID:21572951
28. Arias-González JE, González-Gándara C, Cabrera JL, Christensen V (2011) Predicted impact of the invasive lionfishPterois volitanson the food web of a Caribbean coral reef. Environmental Research 111: 917–925. doi:10.1016/j.envres.2011.07.008PMID:21840517
29. Vega-Zepeda A, Hernández-Arana H, Carricart-Ganivet JP (2007) Spatial and size-frequency distribu-tion of Acropora (Cnidaria: Scleractinia) species in Chinchorro Bank, Mexican Caribbean: implicadistribu-tions for management. Coral Reefs 26: 671–676.
30. INE (2000) Programa de Manejo Reserva Banco Chinchorro. Instituto Nacional de Ecología, México.
31. Jordán E, Martín E (1987) Chinchorro: morphology and composition of a caribbean atoll. Atoll Research Bulletin 310: 1–25.
32. Levins R (1968) Evolution in changing environments. Princeton Monograph Series. 120 p.
33. Agliardi E (1998) Positive feedbacks economics. MacMillan Press. 160 p.
34. Levins R (1966) The strategy of model building in population biology. American Scientific 54: 421–431.
35. Levins R (1994) Natural selection in pathogens. Annals of the New York Academy of Sciences 740: 260–270. PMID:7840456
36. Briand F, McCauley E (1978) Cybernetic mechanisms in lake plankton systems: how to control undesir-able algae. Nature 273: 228–230.
37. Lane P (1986) Symmetry, change, perturbation and observing mode in natural communities. Ecology 67: 223–239.
38. Hulot F, Lacroix G, Lescher-Moutoue F, Loreau M (2000) Functional diversity governs ecosystems response to nutrient enrichment. Nature 405: 340–344. PMID:10830961
39. Ortiz M (2008) The effect of a crab predator (Cancer porteri) on secondary producers versus ecological model predictions in Tongoy Bay (southeast Pacific coast). Implications for management and fisheries. Aquatic Conservation: Marine and Freshwater Ecosystems 18: 923–929.
40. Abelson A (2006) Artificial reefs vs coral transplantation as restoration tools for mitigating coral reef deterioration: benefits, concerns, and proposed guidelines. Bulletin of Marine Science 78 (1): 151–
159.
41. Coleman FC, Koenig CC, Huntsman GR, Musick JA, Eklund AM, McGovern JC, et al. (2000) Long-lived reef fishes: The grouper-snapper complex. Fisheries 25: 14–21.
42. Mumby PJ, Harborne AR, Brumbaugh DR (2011) Grouper as a Natural Biocontrol of Invasive Lionfish. PLoS ONE 6(6): e21510. doi:10.1371/journal.pone.0021510PMID:21731769
43. Maljkovic A, Van Leeuwen TE, Cove SN (2008) Predation on the invasive red lionfish,Pterois volitans (Pisces: Scorpaenidae), by native groupers in the Bahamas. Coral Reefs 27(3): 501.
44. Stallings CD (2008) Indirect effects of an exploited predator on recruitment of coral-reef fishes. Ecology 89: 2090–2095. PMID:18724719
46. Stallings CD (2009) Fishery-Independent Data Reveal Negative Effect of Human Population Density on Caribbean Predatory Fish Communities. PLoS ONE 4(5): e5333. doi:10.1371/journal.pone. 0005333PMID:19421312
47. Hackerott S, Valdivia A, Green SJ, Côte IM, Cox CE, Akins L, et al. (2013) Native Predators Do Not Influence Invasion Success of Pacific Lionfish on Caribbean Reefs. PLoS ONE 8(7): e68259. doi:10. 1371/journal.pone.0068259PMID:23874565
48. Núñez MA, Kuebbing S, Dimarco RD, Simberloff D (2012) Invasive species: to eat or not to eat, that is the question. Conservation Letters 5(5): 334–341.
49. Levins R, Lewontin R (1985) The dialectical Biologist. Harvard University Press, Cambridge, MA. 336 p.
50. Levins R (1969) Extinction. In: Gerstenhaber M, editor. Mathematical Problems in Biology. Lectures on mathematics in the Life Sciences, 2. American Mathematical Society, Providence, RI, pp. 77–107.