JPediatr(RioJ).2015;91(5):413---427
www.jped.com.br
REVIEW
ARTICLE
Probiotics
for
the
treatment
of
upper
and
lower
respiratory-tract
infections
in
children:
systematic
review
based
on
randomized
clinical
trials
夽
Georgia
Véras
de
Araujo
a,b,c,∗,
Mário
Henriques
de
Oliveira
Junior
a,d,
Décio
Medeiros
Peixoto
a,c,e,
Emanuel
Sávio
Cavalcanti
Sarinho
a,c,e,faUniversidadeFederaldePernambuco(UFPE),Recife,PE,Brazil
bHospitaldasClínicas,UniversidadeFederaldePernambuco(UFPE),Recife,PE,Brazil
cCentrodePesquisasemAlergiaeImunologia,UniversidadeFederaldePernambuco(UFPE),Recife,PE,Brazil dDepartmentofInternalMedicine,UniversidadeFederaldePernambuco(UFPE),Recife,PE,Brazil
eDepartmentofPediatrics,UniversidadeFederaldePernambuco(UFPE),Recife,PE,Brazil fConselhoNacionaldeDesenvolvimentoCientíficoeTecnológico(CNPq),Brazil
Received30January2015;accepted19March2015 Availableonline6June2015
KEYWORDS Children; Respiratorytract infections; Probiotics
Abstract
Objectives: Evaluatetheeffectofprobiotics onthesymptoms,durationofdisease,andthe
occurrenceofnewepisodesofupperandlowerrespiratoryinfectionsinhealthychildren.
Sources: In orderto identify eligible randomized controlled trials, two reviewers accessed
fourelectronicdatabases[MEDLINE/PubMed,Scopus(Elsevier),WebofScience,andCochrane (CochraneVHL)],aswellasClinicalTrials.govuntilJanuary2015.Descriptorsweredetermined byusingtheMedicalSubjectHeadingstool,followingthesamesearchprotocol.
Summaryofthefindings: Studiesshowedtobeheterogeneousregardingstrainsofprobiotics,
themodeofadministration,thetimeofuse,andoutcomes.Thepresentreviewidentified11 peer-reviewed, randomizedclinicaltrials,whichanalyzed atotalof2417children upto10 incompleteyearsofage.Intheanalysisofthestudies,reductioninnewepisodesofdisease wasafavorableoutcomefortheuseofprobioticsinthetreatmentofrespiratoryinfectionsin children.Itisnoteworthythatmostofthesestudieswereconductedindevelopedcountries, withbasicsanitation,healthcare, andstrict,well-establishedandwell-organizedguidelines ontheuseofprobiotics.Adverseeffectswererarelyreported,demonstratingprobioticstobe safe.
夽 Pleasecitethisarticleas:deAraujoGV,deOliveiraJuniorMH,PeixotoDM,SarinhoES.Probioticsforthetreatmentofupperandlower
respiratory-tractinfectionsinchildren:systematicreviewbasedonrandomizedclinicaltrials.JPediatr(RioJ).2015;91:413---27.
∗Correspondingauthor.
E-mail:georgiaveras@uol.com.br(G.V.deAraujo).
http://dx.doi.org/10.1016/j.jped.2015.03.002
414 deAraujoGVetal.
Conclusions: Despitetheencouragingresults---reducingnewepisodesofrespiratoryinfections
---the authors emphasizetheneed for further research,especially indeveloping countries, whereratesofrespiratoryinfections inchildren arehigherwhencompared tothehighper
capita-incomecountriesidentifiedinthisreview.
©2015SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Allrightsreserved.
PALAVRAS-CHAVE Crianc¸as;
Infecc¸õesdotrato respiratório; Probióticos
Probióticosnotratamentodasinfecc¸õesdotratorespiratóriosuperioreinferiornas crianc¸as:revisãosistemáticabaseadaemensaiosclínicosrandomizados
Resumo
Objetivos: Avaliaroefeitodousodeprobióticosnareduc¸ãodossintomas,dadurac¸ãodadoenc¸a
edaocorrênciadenovosepisódiosdeinfecc¸õesrespiratóriassuperioreinferioremcrianc¸as saudáveis.
Fontesdedados:Comafinalidadedeidentificarensaiosclínicosrandomizadoselegíveis,dois
revisoresacessaramquatrobasesdedadoseletrônicas[Medline/PubMed,Scopus(Elsevier),Web ofScienceeCochrane(TheCochraneLibrary)],alémdoClinicalTrials.gov,atéJaneirode2015. Foramutilizadosdescritores,pormeiodaferramentaMedicalSubjectHeadings,seguindoum mesmoprotocolodebusca.
Síntesedosdados:Osestudosapresentaramgrandeheterogeneidadeemrelac¸ãoàscepas
deprobióticos,àformadeadministrac¸ão,aotempodeusoeaosdesfechos.Identificamos11 ensaiosclínicosrandomizados,revisadosporpares,queanalisaramumtotalde2.417crianc¸as até10anosincompletos.Naanálisedosestudos,reduc¸ãodenovosepisódiosdedoenc¸afoio desfechofavorávelaousodosprobióticosnotratamentodasinfecc¸õesrespiratóriasnacrianc¸a. Importantesalientarqueessaspesquisasforamrealizadas,emsuamaioria,empaíses desen-volvidos,comcondic¸õesdesaneamento,deassistênciaàsaúdeederegulamentac¸ãorigorosa ao usodeprobióticosbemestabelecidoseorganizados.Quantoaosefeitosadversos,pouco relatados,configuramosprobióticoscomoseguros.
Conclusões:Apesardoresultadoencorajador-reduc¸ãodenovosepisódiosdeinfecc¸ões
res-piratórias - destacamos a necessidade de pesquisas futuras, principalmente em países em desenvolvimento, onde as taxas de infecc¸ões respiratórias na crianc¸a são maioresquando comparadasaosdospaísesdeelevadarendapercapitaidentificadosnestarevisão.
©2015SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Todososdireitos reservados.
Introduction
Respiratorytractinfectionsarecommoninchildrenand sig-nificantly contribute to pediatric morbidity and mortality worldwide.1Theeconomicandsocialimpactofthese infec-tionsissignificantandconstitutesanimportantchallengefor publichealth,duetohighcostsconcerningtreatment, hos-pitalizations,schoolabsenteeism,andlossofworkingdays byparentsandcaregivers.2
The greatvarietyof etiologicalagents, the inappropri-ateandlarge-scale useof antibiotics, increasedbacterial resistance, and reduced availability of vaccines for most virusesandbacteriachallengetheappearanceof efficient andadequatetherapiesforthetreatmentofthisdisease.3
Since their introduction by Metchnikoff in 1907,4 pro-bioticshave been increasingly usedto benefitthe human host’simmunesystem.5DefinedbytheWorldHealth Orga-nization(WHO)andtheFoodandAgricultureOrganization oftheUnitedNations(FAO)as‘‘livemicroorganismsthat, when administeredin adequate amounts aspart of food, conferbeneficialeffects tothehost throughhisintestinal flora,’’6probioticshavefoundwidespreaduseinthe respi-ratory,gastrointestinal,andurogenitaltracts;inallergicand autoimmunediseases;andincancer.7---11
Recent systematic reviews and meta-analyses have reported a positive, albeit modest, effect of probiotics inrespiratory tractinfectionprevention,12---17 but onlyone meta-analysisevaluatedtheeffectivenessofprobioticson thedurationofrespiratorydiseasesinchildrenandadults, restricted tothe randomizedclinicaltrials thatused only probioticsoftheLactobacillusandBifidobacteriumgenus.18 Thus, the objective of this systematic review was to explore and describe clinical trials that have as primary endpoint the effect of probiotics onthe reduction, dura-tion,and occurrenceof newepisodesof upper andlower respiratoryinfections,andasasecondaryoutcome,the pos-sibleadverseeventsduetotheuseofthesesupplementsin healthychildren,consideringdifferentprobioticstrains.
Methods
Researchprotocol
Probioticsandrespiratoryinfectionsinchildren 415
in accordance with the information items for systematic reviewsandmeta-analyses.20
Eligibilitycriteria
Eligiblestudiesforinclusioninthissystematicreviewwere randomizedclinicaltrials(RCTs) ofany duration(phaseIII studies)comparingstrainsofprobiotics,singleorcombined, consumedby any formofadministration, withplacebo or ‘‘notreatment’’inapparentlyhealthychildren(frombirth to10incompleteyearsofage),whodevelopedacuteupper orlowerrespiratoryinfectionatsomepointduringthestudy. Open or blind trials wereeligible, providedthat patients wererandomized.
Theprobioticstrainscouldbeadministeredatanydose, whetherornotcombinedwithotherfunctionalingredients (such as prebiotics and vitamins) or antibiotics, provided that the comparison included the same products, so that theoverall effectcouldbeattributedtotheused probio-tics.Tobeeligibleforinclusion,studieshadtobepublished inPortuguese,English,orSpanishlanguagesandtheresults hadtoshowoneormorethanoneofthestudyobjectives: decrease in disease symptoms, decrease in the duration, decreaseofoccurrenceofnewepisodes,andthepresence ofanyadverseevent.
Exclusion criteria were: clinical trials with follow-up losses >20%; animal studies; studies on respiratory infec-tionprevention;studiesinchildrenthathadsome typeof acquiredorcongenitalimmunedeficiency,orchronicillness; publicationssuchascomments,editorials,orletters; stud-ieswithresultsfromotheraffectedorgansother thanthe respiratorytract;duplicatedstudies,annalsofcongresses, inappropriate study designs (for instance: observational studies, non-randomizedstudies) andstudies in languages otherthanthosepreviouslymentioned.Eachidentified arti-cle was initially analyzed by title and abstract, and the eligiblearticleswereselectedforfullreading.
Definitionsofsearchterms
Initially,thefollowingtermsandkeywordswereused: (pro-biotics)AND(respiratorytractinfections)AND(infant)AND (children), withthe following definitions: probiotics --- all strainsofbacteriaand/oryeastpotentiallybeneficialtothe host,administeredby anyvehicle;respiratorytract infec-tions---upper(commoncold,otitismedia,pharyngitis,and sinusitis)andlower(bronchitisandpneumonia);infantAND children---allchildrenfrombirthto10yearsofage, char-acterizingtheexclusionofadolescents.
Studyresearchstrategy
TheelectronicsearchwascarriedoutinJanuary2015inthe following databases: MEDLINE/PubMed, Scopus (Elsevier), WebofScience(ThomsonReutersScientific),andCochrane VHL,withsearchstrategiesadaptedtoeachdatabase:
MEDLINE/PubMed
(‘‘probiotics’’[MeSH Terms] OR ‘‘probiotics’’[All Fields]) AND (‘‘respiratory tract infections’’[MeSH Terms] OR (‘‘respiratory’’[All Fields] AND ‘‘tract’’[All Fields] AND ‘‘infections’’[All Fields]) OR ‘‘respiratory tract infec-tions’’[All Fields]) AND (‘‘infant’’[MeSH Terms] OR ‘‘infant’’[All Fields]) AND (‘‘child’’[MeSH Terms] OR ‘‘child’’[AllFields]OR‘‘children’’[AllFields]).
Subsequently, to detail respiratory tract infections: (‘‘probiotics’’[MeSH Terms] OR ‘‘probiotics’’[All Fields]) AND (‘‘common cold’’[MeSH Terms] OR (‘‘common’’[All Fields] AND ‘‘cold’’[All Fields]) OR ‘‘common cold’’[All Fields])AND(‘‘child’’[MeSHTerms]OR‘‘child’’[AllFields] OR ‘‘children’’[All Fields]). The search for the other terms was carried out by sequentially substituting the word ‘‘common cold’’ with ‘‘otitis media,’’ ‘‘sinusitis,’’ ‘‘pharyngitis,’’‘‘bronchitis,’’and‘‘pneumonia.’’
Scopus(Elsevier)
(‘‘Probiotics’’ AND ‘‘respiratory tract infections’’ AND ‘‘infant’’AND‘‘children’’),followedbysequentially substi-tuting‘‘respiratorytract infections’’by ‘‘commoncold,’’ ‘‘otitismedia,’’‘‘sinusitis,’’‘‘pharyngitis,’’‘‘bronchitis,’’ and‘‘pneumonia.’’
WebofScience(ThomsonReutersScientific)
Articleswereselectedfrom1945toJanuary2015usingthe followingsearchstrategy:(Probiotics*ANDrespiratorytract infections*ANDinfant*ANDChildren*),followedby sequen-tiallysubstitutingrespiratorytractinfections*withcommon cold*,otitismedia*,sinusitis*,pharyngitis*,bronchitis*,and pneumonia*.
CochraneVHL
(Probioticsandrespiratorytractinfections andinfantAND children)followed by sequentially substitutingrespiratory tractinfections withcommoncold,otitismedia,sinusitis, pharyngitis,bronchitis,andpneumonia.
Atotalof52searcheswereperformedinthedatabases, thirteen in each, using the terms separately and sequen-tially,forbetteraccuracyandprecision.
Dataextraction
Two stages of the process were used to identify and select studies: first, two reviewers (GVA and MHO) inde-pendentlyidentifiedthetitles andabstractsof eachstudy toassesswhethertheymettheinclusioncriteria. Second, theselectedarticleswereobtainedasfull-textversionsand thenwereindependentlyreviewed,todeterminethe inclu-sionandexclusioncriteria.Anydiscrepancieswereresolved byconsensusand/orconsultingathirdreviewer.Whenever possible,theauthorswerecontactedbye-mail,incaseof doubt,intheabsenceofspecificdata,ortoobtainadditional information.
416 deAraujoGVetal.
literaturewasobtainedthroughresearchontrialregistries (ClinicalTrials.gov) and the main conference proceedings wereselected(threeyearsbeforetheresearchdate).
Additionally,amanualsearchwasperformedthroughthe referencesofthepre-selectedstudiesandreviewspublished onthesubject.
The following data were collected: characteristics of included studies, such asclinical condition, intervention, andcomparisondetails;riskofbiasassessment;andquality criteria of the selected studies. The decrease in symp-toms,duration of disease episodes,and the possibilityof reducing new episodesof respiratory tract diseases were analyzedasprimaryoutcomes;whethertheuseof probio-ticstriggeredanadverseeventwasanalyzedasasecondary outcome.
Qualityassessmentandriskofbias
Thestudieswerealsoevaluatedfortheoverallriskofbias (low,high, or unclear) basedon Cochrane Collaboration’s risk-of-biastool.21 Forthe purposeofthisreview, astudy wasconsideredashavinga‘‘lowriskofbias’’whenallmajor qualitycriteria(i.e.,randomizationmethod,allocation con-cealment,andmasking/blinding),aswellasotheradditional criteria(similaritybetweentheinterventionandcomparison groups,withdrawalofpatientsfromstudies,and intention-to-treatanalysis)wereadequatelymet;an‘‘unclearriskof bias’’when mostofthekeycriteriawerenotreportedor werenotclear;anda‘‘highriskofbias’’whenoneormore ofthemaincriteriawerenotproperlymet.The‘‘somerisk of bias’’category wasattributed when all aspectsof the keycriteria were adequate, but (1) an intention-to-treat analysiswasnot performed andwhen a criterionwasnot met,or (2)whentwokeycriteriawereadequate,butthe intention-to-treatanalysiswasnotperformed.
When analyzing the quality of the randomized studies, this review used GRADE (Grading of Recom-mendations Assessment, Development, and Evaluation) recommendations.22 Due to the great heterogeneity of theclinical trials, study data wereassessed qualitatively, without the use of meta-analysis, following the PRISMA (Preferred Reporting Items for systematic Reviews and Meta-Analyses) guidelines.23 To avoid publication bias, unpublishedstudies wereidentified,butdidnotmeetthe inclusioncriteriaofthissystematicreview.
Results
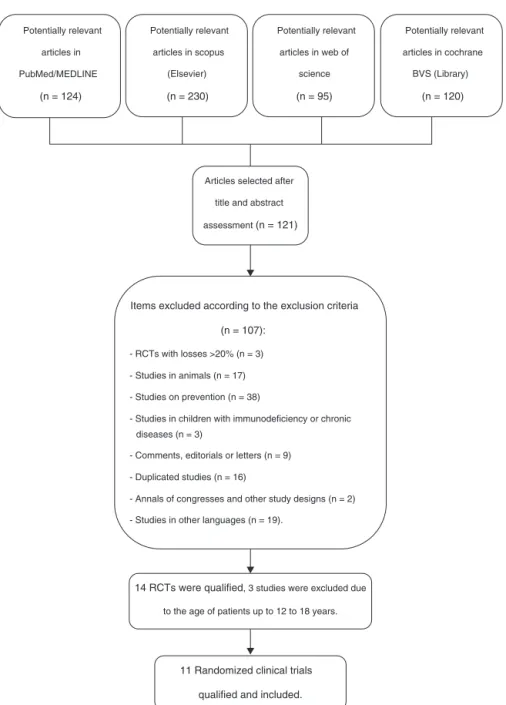
Of a total number of 569 citations identified in the four major electronic databases (PubMed/MEDLINE, Sco-pus [Elsevier], Web of Science, and Cochrane VHL), 11 peer-reviewed, randomized controlled trials (RCTs) were included,whichanalyzedatotalof2417childrenfrombirth to10incompleteyearsof age.Theinclusioncriteriawere metbyelevenRCTs,whichwereusedtoidentifytheprimary andsecondaryoutcomesofthissystematicreview.Thestudy selectionprocessisshowninFig.1.
ElevenRCTswereanalyzedfortheauthor/year,country ofstudy,ageofparticipants,clinicalcondition,intervention andcomparisondetails,randomizednumbers,andnumbers includedintheanalysisandadverseevents,asdescribedin Table1.The following clinicaltrials wereincluded inthis
systematicreview:Cohenetal.,24Hatakkaetal.,25Hatakka etal.,26Kumpuetal.,27Leyeretal.,28Rautavaetal.,29Roos etal.,30Skovbjergetal.,31Taipaleetal.,32Tanoetal.,33and Tapiovaaraetal.,34whichwereperformedintheirtotality in twocountries:Finland and Sweden,whereas theother trialswereperformedintwodifferentcountries:Chinaand France.The duration oftreatment withprobiotics ranged fromtendaysto12months,althoughmosttrialswere per-formed for approximatelysix toseven months during the wintermonths.
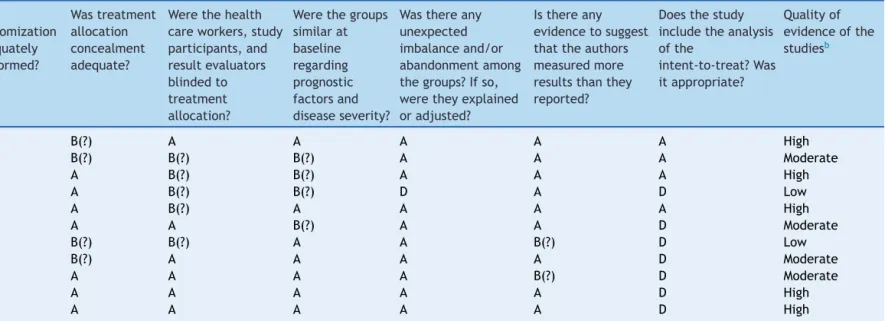
StudyqualityassessmentissummarizedinTable2, show-ing that all trials used correct randomization methods, such as a randomization list generated by computer or by a randomnumber. Appropriateallocationconcealment wasreportedbymost studies,includingtheuse ofsealed envelopes,24,25,27,30,31,33 and/or use of encoded contain-ers/packagesthatwereidenticalinappearance.26,28,29,32,34 Patientswereincluded sequentially, accordingtothe ran-domization list, and blinding was correctly performed in seven trials.26---29,32---34 Among the eleven trials that were identified as double-blinded, detailed descriptions of the blindingmethodswereprovidedbysixRCTs.24,29,31---34
Ingeneral,theelevenclinicaltrialswereconsideredas having a ‘‘low’’ risk of bias, with a few studies showing allocationconcealment andblindingwith‘‘low’’riskwith someareasofuncertainty.Asallqualitycriteriawere well-indicated, some studies showed that the intent-to-treat analysishadanunclearrisk,eitherbecauseitwasnot per-formedorbecauseitwasnotmentionedinmorethanhalf of thetrials.27,29---34 Notrial showed‘‘high’’risk ofbias in themainanalyzedcriteria.
Inthe clinical trialsincluding ‘‘commoncold,’’ ‘‘flu,’’ ‘‘respiratorytractinfections,’’and‘‘acuteotitismedia,’’ allauthorsofthestudiesreportedcleardescriptionsofthe signs,symptoms,anddiagnosesof theseconditions.Insix ofthetrials,24---26,28,32,34aphysicianconfirmedthediagnosis ofinfection,andintheother fivetrials,27,29---31,33thesigns and/or symptoms were reported by the participants in a diary,withthediagnosisconfirmedbyastudyinvestigator’s opinion.
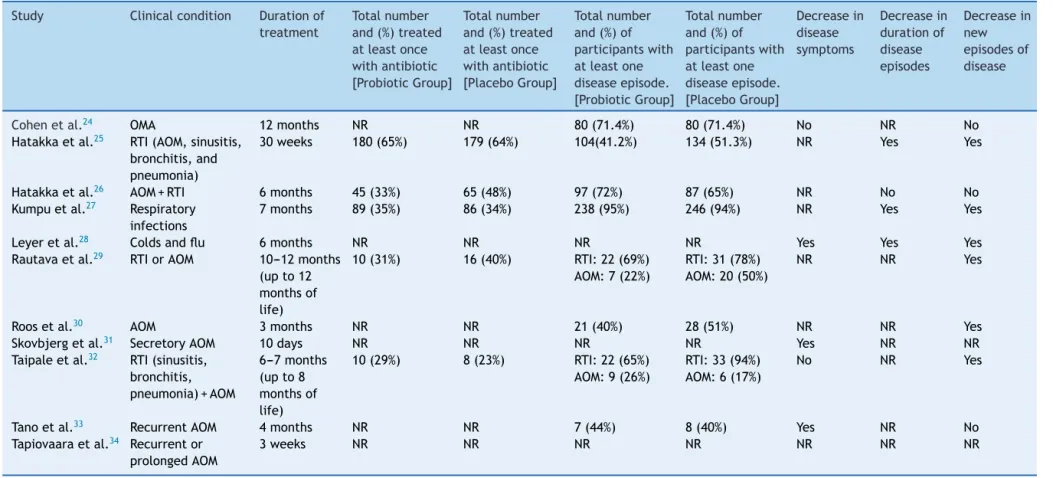
In the included trials, three main outcomes were reported:decreaseindiseasesymptoms,decreaseinthe durationofdiseaseepisodes,anddecreaseinnewdisease episodes.Theoutcomesofstudiesinchildrenarereported inTable3,whichalsoshowsthetotalnumberand percent-ageofpatientsthatusedantibioticsduringthestudy,both in theprobioticgroup andinthe placebogroups, andthe totalnumberandpercentageof patientsthathadat least onediseaseepisode.
Forbetterassessmentoftheprimaryoutcomes,Table4 adds valuesof oddsratios (OR),95% confidence intervals (95%CI),andp-valuesextractedfromtheselectedarticles thatreflectthepositiveresultsoftheprobioticgroupswhen comparedtoplacebogroupsintherandomizedclinical tri-als.
Evaluationofdiseasesymptomreduction
Probiotics
and
respiratory
infections
in
children
417
Table1 Characteristicsofrandomizedclinicaltrialsandadverseevents.
Author/year Countrywhere studywas performed
Ageofstudy participants
Clinical condition
Intervention Comparison
Details Randomized number (includedin theanalysis)
Details Randomized number (includedin theanalysis)
Adverseevents
Cohenetal.24
(2013)
France 7---13months
ofage
AcuteOtitis Media(AOM)
NAN®3withproB (Streptococcus
thermophilosNCC2496: 1×107cfu/g,
Streptococcussalivarius
DSM13084: 2.5×107cfu/g,
Lactobacillusrhamnosus
LPRCGMCC1.3724: 1×107cfu/g)andpreB
(Raftilose/Raftiline) ---300---630mL/day.
112(112) NAN®3follow-up
formulawithout probioticsor prebiotics.
112(112) --- Lossofappetite formilk
--- Regurgitation --- Dryskin
--- Chronicdiarrhea --- Abdominalpain --- Constipation
Hatakkaetal.25
(2001)
Finland 1to6years
ofage
ITR(otitis, sinusitis, bronchitis and pneumonia)
MilkwithLactobacillus
(Gefilus,Valio,Riihimäki, Finland)containing1%fat and5---10×105ufc/mLof
thestrainLactobacillus rhamnosusGG
(ATCC53103) ---200mL/day.
296(252) Milkwithout
probiotics.
298(261) None
Hatakkaetal.26
(2007)
Finland 10monthsto
6yearsof age
OMA Probiotic(L.rhamnosus
GG,ATCC53103;L. rhamnosusLC705;
Bifidobacteriumbreve99;
Propionibacterium freudenreichiissp shermaniiJS,
8---9×109cfu/gelcapsule.
155(135) Gelcapsule
containing microcrystalline cellulose.
154(134) NR
Kumpuetal.27
(2012)
Finland 2to6years
ofage
Respiratory infections
Milkcontaining1%fatand thestrainL.rhamnosus
GG(53103)6.7×105to
1.9×106cfu/mL,
400mL/day.
261(251) Milkwithout
probiotics.
262(250) ---Nausea
---Mildabdominal pain
418
de
Araujo
GV
et
al.
Table1(Continued)
Author/year Countrywhere studywas performed
Ageofstudy participants
Clinical condition
Intervention Comparison
Details Randomized number (includedin theanalysis)
Details Randomized number (includedin theanalysis)
Adverseevents
Leyeretal.28
(2009)
China 3to5years
ofage
Coldsandflu Twointerventions: 1.LacidophilusNCFM (ATCC700396)of 5.0×109cfu/g---sachet
addedto120mLofmilk. 2.50%ofeach:L. acidophilusNCFMandB. animalissubsplactisBi-07 (ATCCPTA-4802),witha doseof1.0×1010cfu/g
---sachetaddedto120mLof milk.
Group1:110 (110) Group2:112 (112)
Powderedsucrose sachet.
104(104) None
Rautavaetal.29
(2009)
Finland 2to65days
oflife
RTIorAOM Capsulescontaining between1×109and
1×1010cfu/gof
Lactobacillusrhamnosus
GG(53103)andB.lactis
Bb-12.
38(32) Follow-upformula
Enfamil®in capsules.
43(40) None
Roosetal.30
(2001)
Sweden 6monthsto
6yearsof age
AOM Threestrainsof
Streptococciatequal proportions:S.sanguis(2 strains),S.mitis(2 strains),andS.oralis(1 strain);5×106cfu/mL
---freeze-driedinskimmed milk,reconstitutedin 0.9%sodiumchloride beforeuseasnasalspray.
53(53) Skimmedmilk
powder reconstitutedin 0.9%sodium chlorideasnasal spray.
Probiotics
and
respiratory
infections
in
children
419
Skovbjerg etal.31(2009)
Sweden 1to8years
ofage
Secretory AOM
Twointerventions: 1.Ssanguinisstrain89a (NCIMB40104)lyophilized inskimmedmilkand resuspendedinsaline solutionwith 5×109cfu/mL.
2.Lrhamnosusstrain LB21,(NCIMB40564) lyophilizedinskimmed milkandresuspendedin salinesolutionwith 5×109cfu/mL--- asnasal
spray.
Group1:20 (19) Group2:20 (18)
Skimmedmilk powder reconstitutedin 0.9%sodium chlorideasnasal spray.
20(17) None
Taipaleetal.32
(2011)
Finland 1to2
monthsof age
RTIAOM+OMA Bifidobacteriumanimalis subsp.lactisBB-12(DSM 15954):
5×109cfu/g+xylitol
100mgto300mg ---administeredinapacifier containingapouchin whichthetabletis inserted.
55(34) Xylitol--- from
100mgto300mg --- administeredin apacifier
containingapouch inwhichthe tabletisinserted
54(35) None
Tanoetal.33
(2002)
Sweden 9monthsto
46monthsof age
Recurrent AOM
Asuspensionof10%skim milkand0.9%NaClwith fiveselectedstrainsof Streptococci
alpha-hemolytic
(containingmorethan 107cfu/mLofS.sanguis
(2strains),S.mitis(2 strains),andS.oralis(1 strain)---asnasalspray.
21(16) Skimmedmilk
powder reconstitutedin 0.9%sodium chlorideasnasal spray.
22(20) ---NonAllergic
Rhinitis ---Cough ---Skinrash ---Vomiting ---Mildepistaxis
Tapiovaara etal.34(2014)
Finland 1---5yearsof
age
Recurrentor prolonged AOM
CapsulescontainingL. rhamnosusGG(ATCC 53103)8-9×109cfu
---dissolvedindairyproduct.
20(14) Capsulecontaining
microcrystalline cellulose.
20(17) None
RTI,respiratorytractinfection;NR,notreported;AOM,acuteotitismedia;cfu,colony-formingunit.
420
de
Araujo
GV
et
al.
Table2 Biasariskandqualitycriteriaassessmentinselectedstudies.
Clinicaltrials Was
randomization adequately performed?
Wastreatment allocation concealment adequate?
Werethehealth careworkers,study participants,and resultevaluators blindedto treatment allocation?
Werethegroups similarat baseline regarding prognostic factorsand diseaseseverity?
Wasthereany unexpected imbalanceand/or abandonmentamong thegroups?Ifso, weretheyexplained oradjusted?
Isthereany evidencetosuggest thattheauthors measuredmore resultsthanthey reported?
Doesthestudy includetheanalysis ofthe
intent-to-treat?Was itappropriate?
Qualityof evidenceofthe studiesb
Cohenetal.24 A B(?) A A A A A High
Hatakkaetal.25 A B(?) B(?) B(?) A A A Moderate
Hatakkaetal.26 A A B(?) B(?) A A A High
Kumpuetal.27 A A B(?) B(?) D A D Low
Leyeretal.28 A A B(?) A A A A High
Rautavaetal.29 A A A B(?) A A D Moderate
Roosetal.30 A B(?) B(?) A A B(?) D Low
Skovbjergetal.31 A B(?) A A A A D Moderate
Taipaleetal.32 A A A A A B(?) D Moderate
Tanoetal.33 A A A A A A D High
Tapiovaaraetal.34 A A A A A A D High
a Cochranerisk-of-biastoolwasusedtoassesstheriskofbiasforeachstudy-A,lowrisk;B,lowriskwithsomeareasofuncertainty(?);C,highrisk;D,unclearrisk.
b GRADE---Highquality,itisveryunlikelythatconfidenceintheestimatewillchange;Moderate,Furtherresearchislikelytohaveasignificantimpactontheconfidenceoftheeffect;
Low,Furtherresearchislikelytohaveanimportantimpactonconfidenceintheestimateofeffectandislikelytochangetheestimate;Verylow,Thereisaveryhighlevelofuncertainty
Probiotics
and
respiratory
infections
in
children
421
Table3 Resultsoftheprimaryoutcomesofthestudies.
Study Clinicalcondition Durationof treatment
Totalnumber and(%)treated atleastonce withantibiotic [ProbioticGroup]
Totalnumber and(%)treated atleastonce withantibiotic [PlaceboGroup]
Totalnumber and(%)of participantswith atleastone diseaseepisode. [ProbioticGroup]
Totalnumber and(%)of participantswith atleastone diseaseepisode. [PlaceboGroup]
Decreasein disease symptoms
Decreasein durationof disease episodes
Decreasein new episodesof disease
Cohenetal.24 OMA 12months NR NR 80(71.4%) 80(71.4%) No NR No
Hatakkaetal.25 RTI(AOM,sinusitis,
bronchitis,and pneumonia)
30weeks 180(65%) 179(64%) 104(41.2%) 134(51.3%) NR Yes Yes
Hatakkaetal.26 AOM+RTI 6months 45(33%) 65(48%) 97(72%) 87(65%) NR No No
Kumpuetal.27 Respiratory
infections
7months 89(35%) 86(34%) 238(95%) 246(94%) NR Yes Yes
Leyeretal.28 Coldsandflu 6months NR NR NR NR Yes Yes Yes
Rautavaetal.29 RTIorAOM 10---12months
(upto12 monthsof life)
10(31%) 16(40%) RTI:22(69%)
AOM:7(22%)
RTI:31(78%) AOM:20(50%)
NR NR Yes
Roosetal.30 AOM 3months NR NR 21(40%) 28(51%) NR NR Yes
Skovbjergetal.31 SecretoryAOM 10days NR NR NR NR Yes NR NR
Taipaleetal.32 RTI(sinusitis,
bronchitis, pneumonia)+AOM
6---7months (upto8 monthsof life)
10(29%) 8(23%) RTI:22(65%)
AOM:9(26%)
RTI:33(94%) AOM:6(17%)
No NR Yes
Tanoetal.33 RecurrentAOM 4months NR NR 7(44%) 8(40%) Yes NR No
Tapiovaaraetal.34 Recurrentor
prolongedAOM
3weeks NR NR NR NR NR NR NR
422 deAraujoGVetal.
Potentially relevant
articles in
PubMed/MEDLINE
(n = 124)
Potentially relevant
articles in cochrane
BVS (Library)
(n = 120)
Potentially relevant
articles in web of
science
(n = 95)
Potentially relevant
articles in scopus
(Elsevier)
(n = 230)
Articles selected after
title and abstract
assessment (n = 121)
Items excluded according to the exclusion criteria
(n = 107):
- RCTs with losses >20% (n = 3)
- Studies in animals (n = 17)
- Studies on prevention (n = 38)
- Studies in children with immunodeficiency or chronic diseases (n = 3)
- Comments, editorials or letters (n = 9)
- Duplicated studies (n = 16)
- Annals of congresses and other study designs (n = 2)
- Studies in other languages (n = 19).
14 RCTs were qualified, 3 studies were excluded due
to the age of patients up to 12 to 18 years.
11 Randomized clinical trials
qualified and included.
Figure1 Flowdiagramoftheselectionprocessofrandomizedclinicaltrialsforinclusioninthesystematicreview.23
noimprovementofthedisease,observedbythephysician involvedintheresearchoranotherdoctor involvedinthe child’s care, and based upon the patient’s health diary, filledoutbyparentsorcaregivers.Datawerecategorizedas ‘‘yes’’whentherewasadecreaseinsignsandsymptoms, usuallyshown as percentages in the studies, ‘‘no’’ when theperceptionofdoctorsorparentsandcaregiversdidnot identifyimprovementindiseasepresentationpatterns,and ‘‘notreported’’(NR)whenthestudydidnotdescribethis outcome.
Considering that all studies were randomized in 1:1 ratiointheprobiotic andplacebogroups, thenumbers of participantsare similarand,therefore, the perception of symptom improvement approximately reflects the differ-ences in results in each group, considering that all had the same eligibility criteria defined in each study. The
systematicreviewshowedthattheprobioticgroupofthree RCTshaddiseasesymptomreduction.28,31,33
InthestudybyLeyeretal.,28inthesingleorcombined probiotic groups, therewasa reductionof fever of 53.0% and72.7%,ofcoughof41.4%and62.1%,andofrhinorrhea of 28.2% and 58.8%, respectively, when compared to the placebogroup.InthestudybySkovbjergetal.,31using pro-bioticspray,theprobioticgrouphadlessfluidandmoreairin themiddleear,i.e.,signsofimprovementorhealingin36.8%
vs.5.8%intheplacebogroup.InthestudybyTanoetal.,33 whichalsousedprobioticspray,therewasa10%reduction inotalgiaand12%reductioninmiddleearsecretioninthe probioticgroup,whencomparedtoplacebo.
Probioticsandrespiratoryinfectionsinchildren 423
Table4 Valuesoftheassociationmeasuresofpositiveprimaryendpointsintheprobioticgroupsoftheselectedstudies.
Primaryendpoints Oddsratio(OR) Confidenceinterval(95%CI) p-value
Symptomreduction
Leyeretal.28 0.55 (0.28---0.99) 0.045
Skovbjergetal.31 0.15 (0.08---0.65) 0.040
Tanoetal.33 0.83 (0.55---0.98) 0.050
Decreaseinepisodeduration
Hatakkaetal.25 0.86 (0.70---1.06) 0.160
Kumpuetal.27 0.83 (0.78---0.88) <0.001
Leyeretal.28 0.50 (0.26---0.98) 0.040
Decreaseinnewdiseaseepisodes
Hatakkaetal.25 0.75 (0.52---1.09) 0.130
Kumpuetal.27 0.97 (0.94---1.00) 0.098
Leyeretal.28 0.23 (0.10---0.45) <0.001
Rautavaetal.29 0.51 (0.27---0.95) 0.022
Roosetal.30 0.90 (0.60---1.85) 0.040
Taipaleetal.32 0.69 (0.53---0.89) 0.014
etal.)24,32 showedno differencein symptom reductionin theprobioticandplacebogroups.
Evaluationofdiseaseepisodeduration
Thiswasdefinedasthetotalsumofdiseaseepisode dura-tion(indays)dividedbythetotalnumberofdiseaseepisodes experiencedbythestudyparticipants.Theresultsshowed thatonlythreestudies25,27,28 included thisoutcomein the analysis,assevenRCTsdidnotreportthisoutcome.24,29---34 InthestudybyHatakkaetal.,25thedurationoftheepisodes was4.9days(95%CI:4.4---5.5)vs.5.8days(95%CI:5.3---6.4) in the probiotic and placebo groups, respectively; in the studybyKumpuetal.,27itwas4.7days(95%CI:4.5---4.9)vs. 5.6days(95%CI:5.4---5.9),respectively;andinthestudyby Leyeretal.,28thedecreasewas32%intheprobioticgroup withsinglestrainand48%intheprobioticgroupwith com-bined strains,whencomparedtothe placebogroup. Only onestudy,byHatakkaetal.,26 showedthatthedifference regardingthedurationofacuteotitismedia(AOM)episodes was5.6days(95%CI:3.5---9.4)vs.6.0days(95%CI:4.0---10.5) intheprobioticandplacebogroups,respectively,whichdid notreachstatisticalsignificance.
Assessmentofthedecreaseinnewepisodesofthe disease
Thiswascharacterizedas‘‘yes’’whentherewasadecrease in new episodes of the disease or reduction in disease incidence and‘‘no’’ when there wasnostatistical signif-icance. Among the studies included in this outcome, six RCTsshowed25,27---30,32intheirresultsthattheprobioticgroup favoredthedecreaseinnewepisodesofthediseasewhen statistically comparedto placebo. Two studies (Skovbjerg etal.andTapiovaaraetal.)31,34 didnotreportthesedata intheirconclusionsandthreestudies24,26,33showedthatthe probioticandplacebogroupsdidnotdifferinthedecrease ofoccurrenceofnewdiseaseepisodes.
When analyzing the need for antibiotic use during the occurrenceofassessedbacterialdiseases,fiveRCTs25---27,29,32 described the total number and percentage of patients treated at least once with antibiotics in the probio-tic and placebo groups. In two studies (Hatakka et al. and Kumpu et al.),25,27 there wasno difference between groups, in twoother studies (Hatakka etal. and Rautava etal.),26,29antibioticprescriptionwasmoreoftenobserved in the placebo group; six studies did not report this outcome.24,26,30,31,33,34
In addition to the five trials that showed more fre-quent use of antibiotics in the placebo group, a study32 thatcompared Bifidobacterium animalissubsp. lactis BB-12administeredastabletsinsertedintothepacifierwitha placebo,in69childrenaged1---2monthsofageinFinland, showedthat antibioticuse wasincreasedin the probiotic group,withtenpatients(29%),ratherthanintheplacebo group,witheightpatients(23%).Theauthorsreportedthat thisdifferencecanbeattributedtothefactthatexclusive breastfeedingwashigherintheplacebogroupthaninthe probioticgroup,resultingingreaterprotectionagainstthe riskofrespiratoryinfections.However,thisresultshouldbe consideredwithsomereserve.
Adverseevents
424 deAraujoGVetal.
Discussion
This reviewidentified anumber ofrandomizedcontrolled trials(RCTs),mostofmoderate-to-highquality,which eval-uatedtheuseofprobioticsinupperandlowerrespiratory tractinfections in children. The presentation, the doses, the different strains, the different mechanisms, and the timeofprobioticadministrationcausedthesestudiesto dis-playgreatheterogeneity and alterationsin thesensitivity analysis,makingitdifficulttoperformaconcomitant meta-analysis. Analyzing the primary outcomes of this review regardingsymptomreduction,timeofdiseaseduration,and ofnewepisodesofthedisease,thelatterwasshowntobe theobjectiveofmostRCTs,demonstratingthatinsixstudies therewasareductionofnewepisodesofrespiratory infec-tions,threeothersfoundnodifferenceinresults,andtwo didnotreportthisoutcome.Itwasalsofoundthatasmall numberofclinicaltrialsshowedadverseeventswiththeuse ofprobiotics,withmildcasesthatdidnotrequirehospital treatment.
Considering the values of the association measures of positiveprimaryoutcomes,thisreviewshowsthatregarding symptom reduction, three clinical trials28,31,33 showed a trendtowardstatisticalsignificance,withp-valuescloseto 0.05,despiteagreaterconfidenceintervalamplitude(95% CI)observedinstudiesbyLeyeretal.28andSkovbjergetal.31 Intheanalysisofthereductionindiseaseepisodeduration, onlythestudy byKumpuetal.27 showedstatistical signifi-cance;thestudybyLeyeretal.,28althoughwithasignificant
p-value of 0.04, demonstrated a wide 95% CI. Regarding the decrease of new disease episodes, of the six clinical trials,25,27---30,32 the studies by Leyer et al.28 and Taipale etal.32showedsignificantp-valuesandconfidenceintervals, twootherstudies25,27didnotshowsignificantdata,andthe studiesbyRautavaetal.29andRoosetal.30 showedhigher amplitudeoftheconfidenceinterval,althoughthep-values hadstatisticalsignificance.
Regarding the understanding of the term respiratory infection,itgenerallyreferstoupperandlowerrespiratory tract infections; however, term definitions showed varia-tionsbetweenstudies.InthestudybyHatakkaetal.,25acute otitismedia andsinusitiswere reportedasupper respira-tory infections, whereas acute bronchitis and pneumonia werereported aslower respiratory infections. In another study,Kumpuetal.27 considered sinusitis,otitis,common cold,pneumonia, andbronchitisasrespiratory infections, withoutspecifyingthefrequencyofeachoccurrence sepa-rately.
In many countries, children experience three to six episodesofrespiratoryinfectionsperyearand40%ofthem mayevensufferatleastoneepisodeofacuteotitismedia, whichisoneofthemostcommonbacterialinfectionsand complications,and oneof themain reasons totreat indi-viduals with antibiotics during early childhood.35,36 Thus, adecrease in newepisodes of respiratory infections with shorterdurationandsymptomreductioncouldbeof great clinicalimportance,withgreatimpactonpublichealthand positiveeconomicconsequences,particularlyindeveloping countries.
Probioticsarelivemicroorganismsofferedasnutritional supplements that act in the host organism’s intestine by
regulating the intestinal flora or modulating the micro-biota in other segments of the human body.37 Thus, they act by improving local andsystemic immunity, competing withpathogensinvadingthelocalintegrityandrestoringthe microorganismsthatprovidesafetyandmaintenanceofthe individual’shealth.Manystudieshave shownthereal ben-efitsandsafetyofprobioticuseinchildhood,38---40currently classified asbelonging tothe category ‘‘Generally Recog-nizedasSafe’’(GRAS)forconsumption,asclassifiedbythe FoodandDrugAdministration(FDA),androutinelyincluded inchildren’sformulainsomedevelopedcountries.41
Considering the complex results on probiotic use indi-cated in the scientific literature, it is emphasized that differentmechanismsofactionareexpectedinthehuman body: (a) microbiological functionality (due to competi-tiveexclusionoractivereductionofpathogensthroughthe production of short chain fatty acids and organic acids, andproductionofbacteriocinsandreactiveoxygenspecies suchashydrogenperoxide)aiming tostabilizeor improve microbial homeostasis in an area of the body andreduce the invasion and colonization by pathogens; (b) nutri-tional functionality (through the production of vitamins thatactthroughout thehumanhost’sorganism);(c) phys-iological functionality (through improvement of intestinal transitandrheologicalpropertiesofrespiratorysecretions), andd]immunologicalfunctionality(throughtheproduction of cytokines --- interleukin (IL)-10 and interferon (INF)-␥, which beneficially modulate immunity in the respiratory mucosa).42,43
Through pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs), pathogen-associated molecular patterns (PAMPs) generate immune responses in dendritic cells, especially in Th1 or Regulatory T-cells(Treg), withproduction of IL-12and IL-10, respectively, whichhave immune protectionfunctions againstviruses andbacteriaand includetolerogenic func-tions,sothereisnoinjurytothehumanhost.44---46
Recently,membersoftheEuropeanSocietyforPediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN)47 andtheAmericanAcademy ofPediatrics(AAP)48 reviewed theevidencefortheuseofprobioticsininfantsandchildren, andconcludedthattheprobioticformulasofferedas supple-mentstohealthyinfantsraisednosafetyconcernsregarding the growthin stature andadverse effects. However,they didnotobservedatarelatedtothesafetyofprobioticuse inthelongterm,anddidnotidentifyhomogeneityofdoses, strains,andthetimeofuseinRCTs.
Performingasearchofallsystematicreviewsand meta-analysesintheliteraturerelatedtotheuseofprobioticsand respiratorytractinfections,itwasfoundthatthereweresix systematicreviewstargetedforprevention,12---17whichwere veryheterogeneousregardingthestudypopulation(children and adults),the assessed respiratory segment(upper and lowerrespiratorytractinfections),andthetypeandstrains ofassessedprobiotics.
Probioticsandrespiratoryinfectionsinchildren 425
Among the reviews on prevention, Kang et al.12 con-cluded, based on the assessment of ten clinical trials in individuals of all ages, that there is a modest effect of probioticsonthepreventionofcommoncolds;Liuetal.13 analyzing four RCTs using only one probiotic strain, con-cludedthattheadministrationofLactobacillusrhamnosus GGhasthepotentialtoreducetheincidenceofacute oti-tismedia,upperrespiratoryinfections,andantibioticuse; in the review by Vouloumanou etal.14 when assessing 14 RCTs, they concluded that probiotics may have a benefi-cialeffecton symptom severityand duration,but do not seemtoreducetheincidenceofrespiratoryinfections;the meta-analysisbyHaoetal.15 alsowith14RCTsevaluating individualsofallages,concludedthatprobioticswere bet-terthanplaceboinreducingepisodesofupperrespiratory infectionsandantibioticuse.
In the recent study byOzen etal.17 analyzing 14 RCTs performedinthepediatricpopulation,theyconcludedthat a minimumreduction of 5---10% in the incidence of upper airwayinfectionswouldhaveasignificantclinicaland eco-nomicimpact.
Intheonlymeta-analysisthatevaluatedprobiotics exclu-sively for the treatment of respiratory infections, King etal.18 included children aged 1---12 years,in additionto adults andthe elderly, with this review analyzing studies withonlytwostrainsofprobiotics.ThereweretwentyRCTs, of whichten studieswere conductedin children,and the resultswereevaluatedinageneralizedway,witha reduc-tion of one day in disease duration. No comments from previous systematic reviews --- exclusively related to the treatment of respiratoryinfections in children--- provided summarized data on the reduction of disease symptoms, reduction in the duration of episodes, and new episodes ofrespiratoryinfections,whereasthisreviewprovidesnew evidencefortheseoutcomes.
Thisreviewaimedtoassessthebestcurrentlyavailable evidencein theliterature inordertoelucidate the bene-fitsofprobioticsinthetreatment ofrespiratoryinfections inhealthychildren.Itincludedcontrolledandrandomized clinicaltrialswithwell-definedprotocols,whileattempting tocontrolfor possiblebiasesasmuchaspossible.Quality ofthestudieswasassessedusingCochraneCollaboration’s risk-of-biastoolandGRADE,21,22currentlyconsideredamore appropriateandaccuratetoolthattheJadadscale.49
In spite of the care taken in constructing this system-atic review, some limitations have been identified: first, threeclinical trialsregistered inClinicalTrials.gov, includ-ingapproximately650patients,arestillintheongoingphase andcouldnotbeincludedintheevaluationduetolackof conclusive data.Theywillbeanalyzed ina futureupdate andthuswillhelpidentifytheactual benefitsobtainedso far;second,whilemostofthestudyauthorsreportedclear descriptionsofsignsandsymptoms,diagnosisconfirmedby adoctorwasattainedinonlyhalfof thetrials.Itis possi-blethatacuteinfectionsmayhavebeenunderdiagnosedor moreoftendiagnosedinsomeoftheseclinicaltrials;that is, as it occurs with all appraisals of systematic reviews, it is possible that the addition of future publications can change the results; the third aspect to be considered is thattheRCTsdifferedinrelationtodoses,thetimeofuse, andadministrationforms.Clinicalresponseswereobserved aftershort-timeuse,aswellasafterprolongedperiodsof
probioticuse,whichleadstotheconclusionthatthedesired effectdepends ontheinfectioncomplexity,theactivated site, the probiotic strains used, and the concentrations administeredascolony-formingunits(CFUs)ofprobiotics.
Althoughsomepublishedstudieshaveshownthat probio-ticadministrationpromotes abeneficialeffectinreducing theoccurrence of new episodesof respiratory infections, mainlyinthosepatientswithahistoryof recurrent infec-tions,it is observed thatthere arestillmany gapsin the knowledge,andthus,manyunansweredquestionsregarding themostappropriatestrainorstrainsofprobiotics,required dose,administrationregimens,optimaltimeofuse,andthe safetyof prolongeduse.Itis necessary,intheexerciseof pediatricpractice,to establishstandardized protocols for theuseofprobioticsinthetreatmentofmajorrespiratory infectionsinchildrenthroughguidelinesandscientific com-mittees.The authorsalsoemphasize the needfor further research,especially in developing countries, where rates ofrespiratoryinfectionsinchildhoodarehigherwhen com-paredtothehigherpercapitaincomecountriesidentified inthisreview.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.HardelidP,DattaniN,Cortina-BorjaM,GilbertR.Contribution ofrespiratorytractinfectionstochilddeaths:adatalinkage study.BMCPublicHealth.2014;14:1191.
2.Díez-DomingoJ,Pérez-YarzaEG,MeleroJA,Sánchez-LunaM, Aguilar MD, Blasco AJ, et al. Social, economic, and health impactoftherespiratorysyncytialvirus:asystematicsearch. BMCInfectDis.2014;14:544.
3.Andrews T, Thompson M, Buckley DI, Heneghan C, Deyo R, Redmond N, et al. Interventionsto influence consulting and antibiotic use for acute respiratory tract infections in chil-dren: a systematic review and meta-analysis. PLoS ONE. 2012;7:e30334.
4.MetchnikoffE.Theprolongationoflife:optimisticstudies.New York,NY:GP.Putman’sSons;1908.p.161---83.
5.LillyDM,StillwellRH.Probiotics:growth-promotingfactors pro-ducedbymicroorganisms.Science.1965;147:747---8.
6.FoodandAgricultureOrganization(FAO),WorldHealth
Organi-zation(WHO). Report ofa JointFAO/WHOworking groupon
drafting guidelines for theevaluation of probiotics in food.
April30,May1.London,Ontario,Canada:FoodandAgriculture
Organization (FAO),World Health Organization(WHO); 2002.
Available from:ftp://ftp.fao.org/es/esn/food/wgreport2.pdf
[cited18.08.14].
7.RitchieML,RomanukTN.Ameta-analysisofprobioticefficacy forgastrointestinaldiseases.PLoSONE.2012;7:e34938. 8.BerinMC.Bugsversusbugs:probiotics,microbiomeandallergy.
IntArchAllergyImmunol.2014;163:165---7.
9.PatelS,GoyalA.Evolvingrolesofprobioticsincancer prophy-laxisandtherapy.ProbioticsAntimicrobProt.2013;5:59---67. 10.AbadCL,SafdarN.TheroleofLactobacillusprobioticsinthe
treatmentorpreventionofurogenitalinfections---asystematic review.JChemother.2009;21:243---52.
426 deAraujoGVetal.
12.KangE-J,KimSY,HwangI-H,JiY-J.Theeffectofprobiotics onpreventionofcommoncold:ameta-analysisofrandomized controlledtrialstudies.KoreanJFamMed.2013;34:2---10. 13.LiuS,HuPW,DuX,ZhouT,PeiX.LactobacillusrhamnosusGG
supplementationforpreventingrespiratoryinfectionsin chil-dren:ameta-analysisofrandomized,placebo-controlledtrials. IndianJPediatr.2013;50:377---81.
14.Vouloumanou EK, Makris GC, Karageorgopoulos DE, Fala-gas ME. Probiotics for the prevention of respiratory tract infections: a systematic review. Int J Antimicrob Agents. 2009;34(197):e1---10.
15.HaoQ,LuZ,DongBR,HuangCQ,WuT.Probioticsforpreventing acute upper respiratorytract infections.Cochrane Database Syst Rev. Available from:http://onlinelibrary.wiley.com/doi/ 10.1002/14651858[cited07.09.11].
16.SæterdalI,UnderlandV,NilsenES.Theeffectofprobioticsfor preventingacuteupperrespiratorytractinfections.GlobAdv HealthMed.2012;1:124---5.
17.OzenM,KocabasSG,DinleyiciEC.Probioticsforthe preven-tionofpediatricupperrespiratorytractinfections:asystematic review.ExpertOpinBiolTher.2015;15:9---20.
18.KingS,GlanvilleJ,SandersME,FitzgeraldA,VarleyD. Effective-nessofprobioticsonthedurationofillnessinhealthychildren and adultswhodevelopcommonacuterespiratoryinfectious conditions:asystematicreviewandmeta-analysis.BrJNutr. 2014;112:41---54.
19.HigginsJP,GreenS,editors.Cochranehandbookforsystematic reviewsofinterventionsversion5.1.0.TheCochrane Collabora-tion;2011,March.Availablefrom:http://www.cochrane.org/ training/cochrane-handbook[cited07.05.14].
20.Centre for Reviewsand Dissemination Systematic reviewsof clinicaltests.In:Centre forReviewsand Dissemination, edi-tor.Systematicreview:CRD’sguidanceforundertakingreviews inhealthcare.York:UniversityofYork;2009.
21.HigginsJP,AltmanDG,GotzschePC,JuniP,MoherD,Oxman AD,etal.TheCochraneCollaboration’stoolforassessingrisk ofbiasinrandomisedtrials.BrMedJ.2011;343:d5928. 22.SchünemannHJ,OxmanAD,BrozekJ,GlasziouP,JaeschkeR,
VistGE,etal.Gradingqualityofevidenceandstrengthof rec-ommendations for diagnostic testsand strategies.Br MedJ. 2008;336:1106---10.
23.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting sys-tematic reviewsand meta-analysesof studies that evaluate healthcareinterventions:explanationandelaboration.BrMed J.2009;339:b2700.
24.CohenR,MartinE,LaRocqueF,ThollotF,PecquetS,Werner A, et al. Probioticsand prebiotics in preventing episodesof acuteotitismediainhigh-riskchildren:arandomized, double-blind,placebo-controlledstudy.PediatrInfectDisJ.2013;32: 810---4.
25.HatakkaK,SavilahtiE,PönkäA,MeurmanJH,PoussaT,Näse L,etal.Effectoflongtermconsumptionofprobioticmilkon infectionsinchildrenattendingdaycarecentres:doubleblind, randomisedtrial.BrMedJ.2001;322:1---5.
26.Hatakkaa K, Blomgrenc K, Pohjavuoria S, Kaijalainene T, Poussaf T, Leinonene M, et al. Treatment of acute otitis media with probiotics in otitis-prone children --- a double-blind,placebo-controlledrandomisedstudy.BrJNutr.2007;26: 314---21.
27.Kumpu M, Kekkonen RA, Kautiainen H, Järvenpää S, Kristo A, Huovinen P,et al. Milk containing probiotic Lactobacillus rhamnosus GGand respiratoryillness inchildren: a random-ized, double-blind,placebo-controlled trial. EurJ ClinNutr. 2012;66:1020---3.
28.LeyerGJ,LiS,MubasherME,ReiferC,OuwehandAC. Probio-ticeffectsoncoldandinfluenza-likesymptom incidenceand durationinchildren.Pediatrics.2009;124:e172---9.
29.RautavaS,SalminenS,IsolauriE.Specificprobioticsin reduc-ing the risk of acute infections in infancy --- a randomised, double-blind, placebo-controlled study. Br J Nutr. 2009;101: 1722---6.
30.RoosK, HåkanssonEG,HolmS. Effectofrecolonisationwith interfering˛ streptococcionrecurrencesofacuteandsecretory otitismediainchildren:randomisedplacebocontrolledtrial.Br MedJ.2001;322:1---4.
31.SkovbjergS, Roos K, HolmSE,HåkanssonEG,Nowrouzian F, IvarssonM,etal.Spraybacteriotherapydecreasesmiddleear fluidin childrenwithsecretory otitismedia.Arch DisChild. 2009;94:92---8.
32.Taipale T, PienihäkkinenK, Isolauri E, Larsen C, Brockmann E,Alanen P,etal.Bifidobacteriumanimalissubsp.lactis BB-12 in reducing the risk of infections in infancy. Br J Nutr. 2011;105:409---16.
33.TanoK, HåkanssonEG, Holm SE,Hellström S.A nasal spray withalpha-haemolyticstreptococci aslong termprophylaxis againstrecurrentotitismedia.IntJPediatrOtorhinolaryngol. 2002;62:17---23.
34.Tapiovaara L, Lehtoranta L, Swanljung E, Mäkivuokko H, Laakso S, Roivainen M, et al. Lactobacillus rhamnosus GG in the middle ear after randomized, double-blind, placebo-controlledoraladministration.IntJPediatrOtorhinolaryngol. 2014;78:1637---41.
35.LaurentC,DugueAE,BrouardJ,NimalD,DinaJ,ParientiJ-J, etal.Viralepidemiologyandseverityofrespiratoryinfections in infantsin 2009 a prospective study.Pediatr Infect Dis J. 2012;31:827---31.
36.Venekamp RP, Sanders S, Glasziou PP, Del Mar CB, Rovers MM. Antibioticsfor acute otitismedia inchildren. Cochrane DatabaseSyst Ver.Available from:http://onlinelibrary.wiley. com/doi/10.1002/14651858[cited31.01.13].
37.Hernell O, West CE. Clinical effects of probiotics: scientific evidencefromapaediatricperspective.BrJNutr.2013;109: S70---5.
38.EspositoS,RiganteD,PrincipiN.Dochildren’supper respira-torytractinfectionsbenefitfrom probiotics?BMCInfect Dis. 2014;14:194.
39.MoraisMB,JacobCM.Theroleofprobioticsandprebioticsin pediatricpractice.JPediatr(RioJ).2006;82:S189---97. 40.VandenplasY,Veereman-WautersG,DeGreefE,PeetersS,
Cas-teelsA,MahlerT,etal.Probioticsandprebioticsinprevention andtreatmentofdiseasesininfantsandchildren.JPediatr(Rio J).2011;87:292---300.
41.Generally Recognized as Safe (GRAS). Ingredients, addi-tives, GRAS & Packaging Guidance Documents & Regulatory Information. EUA. U.S. Food and Drug Administration; 2006. Available from: http://www.fda.gov/food/ingredient spackaginglabeling/gras/default.htm[cited26.11.14]. 42.VandenplasY,HuysG,DaubeG.Probiotics:anupdate.JPediatr
(RioJ).2015;91:6---21.
43.VillenaJ,ChibaE,TomosadaY,SalvaS,MarranzinoG,Kitazawa H, et al. Orallyadministered Lactobacillusrhamnosus mod-ulates the respiratory immune response triggered by the viral pathogen-associated molecular pattern poly(I:C). BMC Immunol.2012;13:53.
44.Baba N, Samson S, Bourdet-Sicard R, Rubio M, Sarfati M. Selected commensal-related bacteria and Toll-like recep-tor 3 agonist combinatorial codes synergistically induce interleukin-12 production by dendritic cells to trigger a T helper type1 polarizing programme. Immunology. 2009;128: e523---31.
45.ForsytheP.Probioticsandlungdiseases.Chest.2011;139:901---8. 46.BeckJM,YoungVB,HuffnagleGB.Themicrobiomeofthelung.
TranslRes.2012;160:258---66.
Probioticsandrespiratoryinfectionsinchildren 427
probioticsand/orprebiotics:asystematicreviewandcomment bytheESPGHAN committeeonnutrition.JPediatr Gastroen-terolNutr.2011;52:238---50.
48.ThomasDW,GreerFR,AmericanAcademyofPediatrics Com-mitteeonNutrition. AmericanAcademyofPediatricsSection
onGastroenterology,Hepatology,andNutrition.Probioticsand prebioticsinpediatrics.Pediatrics.2010;126:1217---31. 49.JadadAR,MooreRA,CarollD.Assessingthequalityofreports