w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Ethanol
extract
of
Prunus
mume
fruit
attenuates
hydrogen
peroxide-induced
oxidative
stress
and
apoptosis
involving
Nrf2/HO-1
activation
in
C2C12
myoblasts
Ji
Sook
Kang
a,
Dong
Joo
Kim
b,
Gi-Young
Kim
c,
Hee-Jae
Cha
d,
Suhkmann
Kim
e,
Heui-Soo
Kim
f,
Cheol
Park
g,
Hye
Jin
Hwang
a,h,
Byung
Woo
Kim
a,i,
Cheol
Min
Kim
j,
Yung
Hyun
Choi
a,k,∗aBlue-BioIndustryRICandAnti-AgingResearchCenter,DongeuiUniversity,Busan,RepublicofKorea bInstituteofHukyongFood,Busan,RepublicofKorea
cLaboratoryofImmunobiology,DepartmentofMarineLifeSciences,JejuNationalUniversity,Jeju,RepublicofKorea dDepartmentofParasitologyandGenetics,KosinUniversityCollegeofMedicine,Busan,RepublicofKorea eDepartmentofChemistryandCenterforProteomBiophysics,PusanNationalUniversity,Busan,RepublicofKorea fDepartmentofBiologicalSciencesandGeneticEngineeringInstitute,PusanNationalUniversity,Busan,RepublicofKorea gDepartmentofMolecularBiology,CollegeofNaturalSciences&HumanEcology,DongeuiUniversity,RepublicofKorea hDepartmentofFoodandNutrition,CollegeofNaturalSciences&HumanEcology,DongeuiUniversity,Busan,RepublicofKorea iDepartmentofLifeScienceandBiotechnology,CollegeofNaturalSciences&HumanEcology,DongeuiUniversity,Busan,RepublicofKorea jDepartmentofBiochemistry,BusanNationalUniversityCollegeofMedicine,Yangsan,RepublicofKorea
kDepartmentofBiochemistry,DongeuiUniversityCollegeofKoreanMedicine,Busan,RepublicofKorea
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received5April2015 Accepted7June2015
Availableonline29January2016
Keywords: Prunusmume
Oxidative stress DNA damage Apoptosis Nrf2/HO-1
a
b
s
t
r
a
c
t
ThefruitofthePrunusmume(Siebold)Siebold&Zucc.,Rosaceae(Koreanname:Maesil)haslong
beenusedasahealthfoodorvaluablemedicinalmaterialintraditionalherbmedicineinSoutheastAsian countries.Inthisstudy,wedeterminedthepotentialtherapeuticefficacyoftheethanolextractofP.mume fruits(EEPM)againstH2O2-inducedoxidativestressandapoptosisinthemurineskeletalmusclemyoblast
celllineC2C12,andsoughttounderstandtheassociatedmolecularmechanisms.Theresultsindicatedthat exposureofC2C12cellstoH2O2causedareductionincellviabilitybyincreasingthegenerationof
intra-cellularreactiveoxygenspeciesandbydisruptingmitochondrialmembranepermeability,leadingtoDNA
damageandapoptosis.However,pretreatmentofthecellswithEEPMbeforeH2O2exposureeffectively
attenuatedthesechanges,suggestingthatEEPMpreventedH2O2-inducedmitochondria-dependent
apo-ptosis.Furthermore,theincreasedex-pressionandphosphorylationofnuclearfactorerythroid2-related
factor2(Nrf2)andup-regulationofhemeoxygenase-1(HO-1),aphaseIIantioxidantenzyme,were
detectedinEEPM-treatedC2C12cells.WealsofoundthatzincprotoporphyrinIX,anHO-1inhibitor,
attenuatedtheprotectiveeffectsofEEPMagainstH2O2-inducedreactiveoxygenspeciesaccumulation
andcytotoxicity.Therefore,theseresultsindicatethattheactivationoftheNrf2/HO-1pathwaymight
beinvolvedintheprotectionofEEPMagainstH2O2-inducedcellularoxidativedamage.Inconclusion,
theseresultsshowthatEEPMcontributestothepreventionofoxidativedamageandcouldbeusedasa
nutritionalagentforoxidativestress-relateddiseases.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Oxidativestressisimplicatedinnumerousdiseasescausedby theoverproductionofreactiveoxygenspecies(ROS).ROS, deriva-tivesof cellularmetabolicreactions,modulate thefundamental physiological functions of aerobic life. Excess amounts of ROS
∗ Correspondingauthor.
E-mail:choiyh@deu.ac.kr(Y.H.Choi).
damagevariouscellularmolecules,suchasproteins,lipids,nucleic acids,andothermacromolecularsubstances,resultingincellular dysfunctionandapoptosis(Wangetal.,2013;Wu etal.,2010). Therefore,supplementationwithantioxidants,includingsynthetic andnaturalantioxidants,couldreduceoxidativestressand ame-liorateoxidativestress-relateddiseasesthroughinductionofphase IIantioxidantenzymesaswellassuperoxidedismutase(SOD)and catalase(Wojciketal.,2010;Guerra-Araizaetal.,2013).
AmongphaseIIantioxidantenzymes,whichareregulatedby antioxidantresponsiveelements(AREs)atthetranscriptionlevel,
http://dx.doi.org/10.1016/j.bjp.2015.06.012
J.S.Kangetal./RevistaBrasileiradeFarmacognosia26(2016)184–190
hemeoxygenase-1(HO-1)is arate-limitingenzymeinvolvedin theconversionofhemetobiliverdinandcarbonmonoxide,among othercellularreactions(Martinet al., 2004;Chen etal., 2003). Bilirubinfunctionsasapotentantioxidantandcarbonmonoxide hasbeenreportedto mediatetheanti-apoptoticeffects of HO-1inresponsetoinflammatorycytokinestimulation(Surhetal., 2008;Sonetal.,2013).Nuclearfactorerythroid2-relatedfactor 2(Nrf2),aleucinezipperredox-sensitivetranscriptionfactor,isa keyregulatorofantioxidantanddetoxificationgeneexpression. Undernormalconditions,Nrf2bindstokelch-likeECH-associated protein1(Keap1)inthecytoplasmandislatersubjectedto protea-somaldegradation.However,Nrf2translocatesfromthecytoplasm tothenucleus following a varietyof stimuli, and subsequently bindstoAREpresentwithinthepromoterregionofgenes encod-ingforphaseIIenzymes,resultingintheup-regulationof their transcription(Terazawaetal.,2013;Nitureetal.,2014).Moreover, recentevidenceindicatesthatNrf2promotescellsurvivalby pre-ventinganincreaseinROSinvariousconditionsofoxidativestress (Hybertsonetal.,2011;Nitureetal.,2014).Therefore,theNrf2-ARE pathwayiscurrentlythemostimportantendogenousantioxidant signalingpathway.
Prunusmume(Siebold)Siebold&Zucc.(Koreanname:Maesil) isadeciduoustreeoftheFamilyRosaceae,whichisnowwidely cultivatedinSoutheastAsiacountries,includingKorea,China,and Japan(Matsudaetal.,2003;WenandShi,2012).Thevariousparts ofthisplanthavebeenusedashealthfoodsormedicinal mate-rialsintraditionalmedicineforgenerations(WenandShi,2012; Yanetal.,2014).Inparticular,itsfruithasbeeneatensinceancient timesinAsiancountriesasatraditionalherbalmedicineforrelief offatigue,diarrhea,fever,dyspepsia,andintestinalandskin disor-dersforthousandsofyears(Yanetal.,2014;WenandShi,2012; Zhanget al.,2011;Jeongetal.,2006).The fruitoftheP.mume
contains abundantphenolic compounds,suchasphenolic acids andflavonoids(Jeongetal.,2006;Kitaetal.,2007;Mitanietal., 2013),whichmaybeinvolvedinthebiologicaleffectsofanti-viral, anti-inflammatory,immunoenhancing,andantineoplastic activi-ties(Zhangetal.,2011;Yingsakmongkonetal.,2008;Parketal., 2011;Enomotoetal.,2010;Tsujietal.,2011;Jungetal.,2010;Tada etal.,2012;Leeetal.,2013;Jeongetal.,2006).Althoughthereare severalreportsontheantioxidantactivityandfreeradical scaveng-ingactivitiesofP.mume(Yanetal.,2014;Sangetal.,2002;Leeetal.,
2013),theexact molecularmechanism(s)of actionsofP.mume
extractagainstoxidativestressinvolvedintheNrf2/HO-1signaling pathwayareyettobedescribed.Therefore,inthepresentstudy,we examinedtheabilityoftheethanolextractofP.mumefruits(EEPM) toprotectcellsfromhydrogenperoxide(H2O2)-inducedcell dam-ageandelucidatedthemechanismunderlyingtheprotectiveaction inamouse-derivedC2C12myoblastmodel.
Materialsandmethods
PreparationofEEPM
DriedfruitsofPrunusmume(Siebold)Siebold&Zucc.,Rosaceae, wereobtainedfromInstituteofHukyongFood(Busan,Republic ofKorea),whichwereauthenticatedbyProfessorSuHyunHong, DepartmentofBiochemistry,DongeuiUniversityCollegeofKorean Medicine(Busan,RepublicofKorea).ForthepreparationofEEPM, freeze-driedfruitsofP.mumewereextractedwithethanol(100g
per 1l) at 60◦C for three days using a blender. The extract wascentrifuged at 10,000×g for 20min, and thesupernatants werethen collected and immediatelyfiltered througha What-manfilter(poresize,0.22m).Thefiltratewaslyophilizedand
stored at −70◦C. The yield (w/w) of the extract was ∼5.0%. The powder wasdissolved to a 100mg/ml concentration with
dimethylsulfoxide (DMSO,Sigma–AldrichChemicalCo., St.Paul, MN, USA). The voucher specimens (accession number DEU-36) havebeendepositedatapubliclyavailableNaturalResourceBank ofDongeuiUniversityCollegeofOrientalMedicine.
CellcultureandEEPMtreatment
TheC2C12myoblastcelllinewaspurchasedfromtheAmerican TypeCulture Collection(Manassa,VA,USA).Theywerecultured in Dulbecco’s modified Eagle’s medium (DMEM,WelGENE Inc., Daegu,RepublicofKorea)supplementedwith10%heat-inactivated fetal bovine serum (FBS, WelGENE Inc.) and 100g/ml
peni-cillin/streptomycinantibiotics(WelGENEInc.)inahumidified5% CO2atmosphereat37◦C.ThestocksolutionofEEPMwasdiluted withDMEMbeforeeveryexperiment.
Measurementofcellviability
Toinvestigatethecytotoxicity,cellswereseededinto6-well plates and exposed to various concentrations of EEPM in the absenceorpresenceofH2O2and/orzincprotoporphyrinIX,a HO-1 inhibitor(ZnPP, Sigma–Aldrich) forthe indicatedtimes. After completionofthetreatments,5mg/mlof 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide(MTT,Sigma–Aldrich)was addedtoeachwellcontinuouslyat37◦C.After3hincubation,the culturesupernatantwasremovedfromthewellsandtheformazan complexwasdissolvedinDMSO.Theabsorbanceofeachwellwas detectedat540nmwithamicroplatereader(MolecularDevices, PaloAlto,CA,USA).Cellviabilityisexpressedasapercentageof untreatedcells.
MeasurementofintracellularROSlevels
The production of intracellular ROS was quantified using the oxidation-sensitive fluorescent probes 2′,7′ -dichlorodihydrofluorescein diacetate (H2DCFDA, Molecular Probes, Eugene, OR, USA). After treatment, the cells were har-vested, suspendedin phosphate-bufferedsaline(PBS), andthen incubatedwith10MH2DCFDAfor20minatroomtemperature
inthedark.Thefluorescenceintensitywasmeasuredbyaflow cytometer(BectonDickinson,SanJose,CA,USA)atanexcitation wavelength of 488nm and an emission wavelength of 530nm (Kimetal.,2014).
Measurementofmitochondrialmembranepotential(MMP)
The mitochondrial transmembrane electrochemical gradi-ent was measured using the mitochondrial potential sensor 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanineiodide (JC-1,Sigma–Aldrich),acellpermeable,cationic,andlipophilicdye, whichisinternalizedandconcentratedbyrespiringmitochondria, reflectingchangesinMMPinlivecells.Briefly,cellswerecollected, resuspendedinPBS,andthenincubatedwith10MJC-1under
darkconditionsfor30minat37◦C.AftertheJC-1wasremoved, thecellswerewashedwithPBStoremoveunbounddye.The cellu-larfluorescenceintensitywasquantifiedusingaflowcytometryat anexcitationwavelengthof480nmandanemissionwavelength of530nm(Seoetal.,2014).
DAPInuclearstaining
J.S.Kangetal./RevistaBrasileiradeFarmacognosia26(2016)184–190
2.5g/mlDAPIsolutionfor10minatroomtemperature.Thecells
werewashedtwicewithPBS,and stainednucleiwereanalyzed using a fluorescence microscope (Carl Zeiss, Oberkochen, Ger-many).
Determinationofapoptoticcellsbyflowcytometry
To assess the induced cell apoptosis rate quantitatively, a fluorescein-conjugatedannexinV(annexinV-FITC)stainingassay wasperformedaccordingtothemanufacturer’sprotocol(BD Bio-sciences,SanJose,CA,USA). Thecells werewashed twicewith ice-coldPBSandstainedwithannexinV-FITCandpropidiumiodide (PI)for15minatroomtemperatureinthedark.Thedegreeof apo-ptosiswasquantifiedasapercentageoftheannexinV-positiveand PI-negative(annexinV+/PI−cells)cellsbyflowcytometry.
Cometassay(single-cellgelelectrophoresis)
ThedegreeofoxidativeDNAdamagewasassessedinacomet assay.Thecellsuspensionwasmixedwith0.5%lowmeltingagarose (LMA)at37◦C,andthemixturewasspreadonafullyfrosted micro-scopicslideprecoatedwith1%normalmeltingagarose(NMA).After thesolidificationoftheagarose,theslidewascoveredwith0.5% LMAandthenimmersedinalysissolution[2.5MNaCl,100mM Na-ethylenediaminetetraaceticacid(EDTA),10mMTris,1%Trion X-100,and10%DMSO,pH10]for1hat4◦C.Theslideswerethen placedinagelelectrophoresisapparatuscontaining300mMNaOH and10mMNa-EDTA(pH13)for40mintoallowforDNAunwinding andtheexpressionofalkali-labiledamage.Anelectricalfieldwas thenapplied(300mA,25V)for20minat25◦Ctodrawthe neg-ativelychargedDNAtowardtheanode.Theslideswerewashed threetimesfor5minat25◦Cinaneutralizingbuffer(0.4MTris, pH7.5),followedbystainingwith20g/mlpropidiumiodide(PI,
120.0
A
B
100.0 80.0 60.0 Relative cell viability (%)
Relativ
e cell viability (%)
40.0 20.0 0.0 0 * # # 250 500
EEMP (μg/ml)
750 1000 1500
120.0 100.0 80.0 60.0 40.0 20.0 0.0 – – –
EEMP (μg/ml)
+ +
0 250 500 0 250
+ 500
H2O2(1mM)
Fig.1.Effects of EEPM on H2O2-induced growth inhibition in C2C12 cells. Cells weretreatedwithvariousconcentrationsofEEPMfor24h(A)orpretreatedwith 250g/mland500g/mlofEEPMfor1handthenincubatedwithorwithout1mM H2O2for6h(B).CellviabilitywasassessedusinganMTTreductionassay.Theresults arethemean±SDvaluesobtainedinthreeindependentexperiments(*p<0.05 compared with control group;#p< 0.05 compared with H
2O2-treated group).
Sigma–Aldrich).Theslides wereexaminedunderafluorescence microscope.
Westernblotanalysis
Thetotalcellularproteinwasextractedwithlysisbuffer(20mM of sucrose,1mMof EDTA, 20Mof Tris–HCl,pH7.2, 1mM of
dithiothreitol,10mMofKCl,1.5mMofMgCl2and5g/mlof
apro-tinin)for30min.Theproteinconcentrationwasmeasuredusing aBio-Radproteinassay(Bio-RadLab.,Hercules,CA,USA) accord-ingto themanufacturer’sinstructions. Aliquotsof each sample wereloadedintodedicatedwellsofSDS-polyacrylamidegels, sep-aratedbyelectrophoresis,andthentransferredtopolyvinylidene difluoridemembranes(Schleicher&Schuell,Keene,NH,USA).After blockingnonspecificbindingwith5%nonfatdrymilkinTBST (Tris-bufferedsaline-Tween),themembraneswerethenprobedwiththe desiredprimaryantibodiespurchasedfromSantaCruz Biotechnol-ogy(SantaCruz,CA,USA)andCellSignalingTechnology(Danvers, MA,USA),andincubatedovernightat4◦C.Themembraneswere washedwithTris-bufferedsaline-0.01%(v/v)containing Tween-20 at room temperature for 15min and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Amersham Co., Arlington Heights, IL, USA) in TBST for 2h at roomtemperature.Proteinswerevisualizedbyusinganenhanced chemiluminescence(ECL, AmershamCo.) detectionmethod fol-lowedbyfilmexposure.
800
A
B
700 600 500 400 300 Relative fluoresence intensity
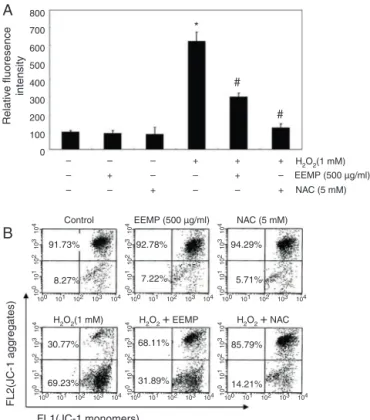
200 100 0 – – – – + – – – – + + – – + + – + # # * – +
H2O2(1 mM) EEMP (500 µg/ml)
EEMP (500 µg/ml)
NAC (5 mM)
H2O2(1 mM) H2O2+ EEMP H2O2+ NAC NAC (5 mM) Control FL2(JC-1 agg regates) FL1(JC-1 monomers) 91.73% 8.27% 30.77% 69.23% 68.11% 31.89% 85.79% 14.21% 92.78% 7.22% 94.29% 5.71% 10010 0 10 1 10 2 10 3 10 4 10 0 10 1 10 2 10 3 10 4 10 0 10 1 10 2 10 3 10 4 10 0 10 1 10 2 10 3 10 4 10 0 10 1 10 2 10 3 10 4 10 0 10 1 10 2 10 3 10 4
101 102 103 104 100 101 102 103 104 100 101 102 103 104
100 101 102 103 104 100 101 102 103 104 100 101 102 103 104
J.S.Kangetal./RevistaBrasileiradeFarmacognosia26(2016)184–190
Statisticalanalysis
Allmeasurementsweremadeintriplicateand allvaluesare presentedasmean±standarddeviation(SD).Theresultswere sub-jectedtoananalysisofvariance(ANOVA)usingtheTukeytestto analyzethedifference.p<0.05wasconsideredtobestatistically significant.
Results
EEPMprotectsC2C12cellsagainstH2O2-inducedcytotoxicity
ToexaminetheprotectiveeffectofEEPMonH2O2-induced cyto-toxicity,theeffectofEEPMontheviabilityofC2C12cellswasfirst performedforprimarydoseselection.AsshowninFig.1A,theEEPM treatmentdidnotresultin anycytotoxiceffects uptothe con-centrationof1500g/ml.Therefore,250and500g/mlEEPMwas
chosenastheoptimaldosesforstudyingthecytoprotectiveeffect ofEEPMagainstH2O2-inducedcytotoxicity.Inordertodetermine theeffectsofEEPM,thecellswerepre-treatedwiththeEEPMfor 1hpriortoH2O2administration.AsestimatedbyanMTTassay,cell viabilitywasmarkedlydecreased,toapproximately60%,aftera6h exposureto1mMH2O2.However,whencellswerepre-incubated withEEPM,thereductionofcellviabilitywassignificantly attenu-atedinadose-dependentmanner(Fig.2B).
EEPMdecreasesH2O2-inducedROSproductioninC2C12cells
To investigate whether EEPM could prevent H2O2-induced ROSgenerationandtheresultingoxidativestress,wenext mea-sured the ROS production in cells by using H2DCFDA reagent, a fluorescent dyethat visualizesROS. Asshown in Fig.2A, the intensityoftheDCF-liberatedfluorescentsignalfromtheH2O2 -treated cells was significantly increased, and the signal was markedly reduced in the presence of EEECas well as theROS scavengerN-acetyl-l-cysteine(NAC),whichwasusedasapositive
control.
EEPMattenuatesH2O2-inducedlossofMMPinC2C12cells
SinceMMPregulatesmitochondrialpermeability,which may becriticalforinducingorarrestingtheoxidativestress-induced apoptoticpathway,weevaluatedtheeffectofEEPMontheMMP ofC2C12cellsusingflowcytometry.Afterincubationwith1mM H2O2 for6h,theMMPlevelwasmarkedlyreducedascompared totheuntreatedcontrol(Fig.2B),indicatingmitochondrial dam-ageanddysfunction.Incontrast,pretreatmentwithEEPMorNAC effectivelypreventedthelossofMMPinducedbyH2O2.Thedata indicatedthatEEPMprotectedthecellsagainsttheH2O2-induced loweringofROSgenerationbyblockingmitochondrialdysfunction inC2C12cells.
EEMP (500 µg/ml)
A
C
B
D
PI fluorescence intensity
(log)
+ –
+ –
EEMP (500 µg/ml)
H
2O 2 (1 mM)
H2O2 (1 mM)
p-YH2AX
YH2AX +
+ –
EEMP (500 µg/ml)
Annexin V-FITC intensity
3.52% 4.42%
27.28% 8.76%
+ –
EEMP (500 µg/ml) +
Actin +
+
+ –
– –
–
–
H
2O 2 (1 mM)
+
–
H
2O 2 (1 mM)
+
–
10
4
10
3
10
2
10
1
10
0
100 101 102 103 104
10
4
10
3
10
2
10
1
10
0
100 101 102 103 104
10
4
10
3
10
2
10
1
10
0
100 101 102 103 104
10
4
10
3
10
2
10
1
10
0
100 101 102 103 104
J.S.Kangetal./RevistaBrasileiradeFarmacognosia26(2016)184–190
EEPMprotectsH2O2-inducedC2C12cellapoptosis
WealsoexaminedtheabilityofEEPMtoprotectagainstH2O2 -triggeredC2C12cell apoptosis usingthe DAPI and Annexin/PI-stainingassays. AsillustratedinFig.3A,C2C12cellsexposedto 1mMH2O2alonedemonstratedcharacteristicapoptoticfeatures, includingcell shrinkage and chromatincondensation and frag-mentationinthenucleusdetectedbyDAPIstaining.However,the pre-treatmentwithEEPMonH2O2-treatedC2C12cellssignificantly reducedthese morphologicalchanges. In addition,the percent-ageofapoptoticcellstreatedwith1mMH2O2wasapproximately 24.60%bytheflowcytometryassay(Fig.3B);however,500g/ml
EEPMeffectivelydecreasedthecellapoptosisinducedbyH2O2to 7.70%.TheseresultsshowthatpreconditioningbyEEPMprotects againstoxidativestress-inducedC2C12celldeath.
EEPMpreventsH2O2-inducedDNAdamageinC2C12cells
We next examined the effects of EEPM on H2O2-mediated damage to C2C12 cell DNA using the comet assay and West-ernblotting analysis. Asshown in Fig. 3C, exposureof cells to H2O2aloneincreasedthenumberofDNAbreaks,resultinginan increase influorescence intensityin thetails of thecomet-like structures;however,thisadverseeffectwasmarkedlyreducedby EEPMpretreatment.OurresultsalsoshowedthattreatingC2C12 cellswithH2O2 resultedintheup-regulationofthelevelofthe phosphorylatedhistonevariantH2A.Xatserine139(p-␥H2A.X),
asensitivemarkerforDNAdouble-strandbreaks(Rogakouetal., 1998)(Fig.2C);however,pretreatmentwithEEPMresultedina significantdecreasedp-␥H2A.Xexpression.
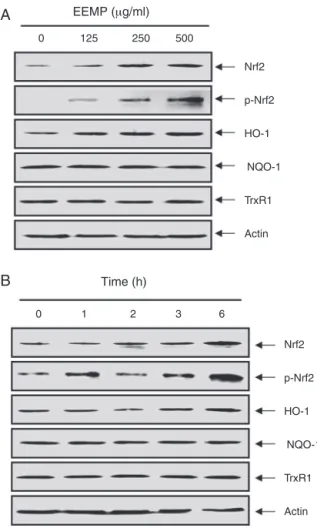
EEPMpromotestheexpressionofNrf2andHO-1inC2C12cells
ToexaminewhetherornotNrf2activationisassociatedwith EEPM-mediatedcytoprotection, we monitoredthelevelsof the HO-1protein.AsshowninFig.4,treatmentofC2C12cellswith EEPM induced the expression of the HO-1 protein in a dose-andtime-dependentmanner,butthatotherantioxidantenzymes, NADPH-quinoneoxidoreductase1(NQO1)andthioredoxin reduc-tase1(TrxR1),wereunaffectedbyEEPMtreatment, whichwas associatedwiththeinductionofNrf2.Sincethephosphorylation ofNrf2atSer40byseveralkinasesisalsoacriticalprocessinits stabilizationandnucleartranslocation(Surhetal.,2008;Niture etal.,2014),weexaminedthephosphorylationofNrf2underEEPM treatmenttofurtherconfirmtheNrf2-activatingpropertyofEEPM andobservedthattreatmentofcellswithEEPMcauseddose-and time-dependent increases in thelevels of phosphorylated Nrf2 expression.
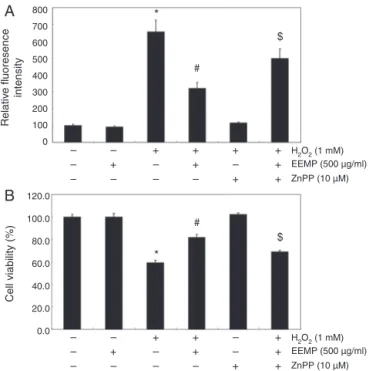
Nrf2/HO-1pathwayisinvolvedinEEPMprotectionagainstH2O2
treatmentinC2C12cells
In order to provide evidence for the involvement of Nrf2-mediated HO-1 induction in EEPM-mediated antioxidant and cytoprotectiveactivitiesagainstoxidativestress,weinhibited HO-1activitybyusingZnPP,aspecificinhibitorofHO-1.Asshownin Fig.5,inthepresenceofZnPP,theprotective effectofEEPMon H2O2-inducedproductionofROSandthereductionofcellviability weresignificantlyattenuated.Takentogether,theseresults sug-gestthatEEPMexertsitsprotectiveeffectsbyinducingthecellular defensemechanismagainstoxidativestressthroughHO-1 induc-tionviaNrf2activation,andthatHO-1playsacrucialroleinthis
protectioninC2C12cells.
EEMP (μg/ml)
0
0 1 2 3 6
125 250 500
Nrf2
p-Nrf2
HO-1
NQO-1
TrxR1
Actin
Nrf2
p-Nrf2
HO-1
NQO-1
TrxR1
Actin
Time (h)
B
A
Fig.4.Induction of Nrf2 and HO-1 expression by EEPM in C2C12 cells. Cells were incubated with the indicated concentrations of EEPM for 6 h (A) or 500 g/ml EEPM for the indicated time periods (B). Total cellular proteins were separated on SDS-polyacrylamidegelsandthentransferredontonitrocellulosemembranes.The membraneswereprobedwiththespecificantibodiesagainstNrf2,p-Nrf2,HO-1, NQO-1,andTrxR1.ProteinswerevisualizedusinganECLdetectionsystem.Actin wasusedasaninternalcontrol.
Discussion
Themitochondrialelectrontransportsysteminthe mitochon-drialmembraneisoneofthemajorsourcesofintracellularROS generation(Fleuryetal.,2002),wherebythemitochondriaplaya pivotalroleintheROS-mediatedcelldeathprocess.Excessive pro-ductionofROSbymitochondrialdysfunctionfollowingoxidative DNAdamageandexposuretoothergenotoxicfactorsultimately leadstothe activationof themitochondrial apoptotic pathway (alsotermedtheintrinsicapoptoticpathway)ratherthan necro-sis(Wangetal.,2013;Wuetal.,2010).Moreover,H2O2directly induces mitochondrial dysfunction followed by a rapid efflux of intracellular ROS, which increases the permeabilization and depolarizationofthemitochondrialmembrane.Thiseventcould facilitatearapiddisruptionofMMPandthereleaseof apoptosis-inducingfactors,whichactivatethecaspase-dependentsignaling cascades(Kimetal.,2006;Fleuryetal.,2002).Inthepresentstudy, treatmentofC2C12cellswithH2O2causedamarkeddecreasein cellsurvival,intracellularaccumulationofROS,andfurtherinduced thelossofMMP,leadingtoapoptosis.However,whenC2C12cells werepretreatedwithEEPM,theH2O2-inducedreductionofcell viability,accumulationofROS,lossofMMP,andapoptosiswere significantlyattenuated.
J.S.Kangetal./RevistaBrasileiradeFarmacognosia26(2016)184–190
800
A
B
*
#
– – –
– + –
+ – –
+ + –
+ – +
+ + + $
*
#
$
Relativ
e fluoresence intensity
Cell viability (%)
700
600
500
400
300
200
100
H2O2 (1 mM) EEMP (500 µg/ml) ZnPP (10 µM)
– – –
– + –
+ – –
+ + –
– – +
+ + +
H2O2 (1 mM) EEMP (500 µg/ml) ZnPP (10 µM) 0
120.0
100.0
80.0
60.0
40.0
20.0
0.0
Fig.5.Effects of an inhibitor of HO-1 on EEPM-mediated attenuation of ROS for-mation and growth inhibition by H2O2in C2C12 cells. Cells were pretreated for 1 h with 1500 g/ml EEPM and then treated for 6 h with or without 1 mM H2O2in the absenceorpresenceof10MZnPP.Then,ROSgeneration(A)andcellviability(B) wereestimated.Theresultsarethemean±SDvaluesobtainedinthree indepen-dentexperiments(*p<0.05comparedwithcontrolgroup;#p<0.05comparedwith H2O2-treatedgroup;$p<0.05comparedwithH2O2andEEPM-treatedgroup).
oxidative stress stemming from ROS production (Ayala-Pe˜na, 2013; Maynard et al., 2009), suggesting a direct link between ROS signaling and oxidative DNA damage. Severe ROS-induced oxidativestressleadstotheinductionofmitochondria-medicated apoptosis in affected cells due to high levels of DNA damage (Deavalletal.,2012;Chistiakovetal.,2014).Thus,wepresumed thatEEPMmightimprovemitochondrialfunctionthrough elim-inating the overproduction of ROS induced by H2O2, thereby reducingtheH2O2-inducedapoptosis.OurstudyshowedthatH2O2 treatmentincreasedDNAtaillengthinthecometassay,aswellas theexpressionofphospho-H2A.X,whicharewidely-used mark-ersforthedetectionofDNAdamage(Rogakouetal.,1998).Both wereattenuatedbyEEPMinthepresentstudy.Theseresults indi-catedthatEEPMprotectedagainsttheH2O2-inducedapoptosisof C2C12cellsbyreducingDNAdamagefromthedestructiveimpact ofoxidativestressinC2C12cells.
Nrf2, atranscription factor thatis partoftheredox homeo-staticgeneregulatorynetwork,isakeyregulatoroftheexpression of anexpansive setof ARE-mediated geneswhich remove ROS byinducingtheactionsofdetoxifyingenzymes(Hybertsonetal., 2011;Nitureetal.,2014).Increasingevidencesuggeststhatthe elevationofNrf2-mediatedtargetgenes,includingHO-1,also pro-motescellsurvivalinoxidizingenvironmentsviaenhancementof
freeradicalmetabolism,inhibitionofcytokine-mediated inflam-mation,andrecognitionofdamagedDNA(Surhetal.,2008;Son etal.,2013).Manyantioxidantagentshavebeenreportedtoreduce ROSproductionbytheactivationofNrf2/HO-1pathwaythrougha varietyofsignalingpathways(Terazawaetal.,2013;Nitureetal., 2014).Moreover,ithasbeenreportedthatNrf2phosphorylationby severalproteinkinasessuchasphosphatidylinositol-3kinase/Akt, mitogen-activatedproteinkinases,andproteinkinaseCfacilitates itstranslocationintothenucleus,whereuponit bindstoAREin thepromoter regions(Surhetal.,2008;Nitureetal.,2014;Lee etal.,2014).Therefore,wedeterminedthepotentialroleofNrf2 andHO-1inH2O2-inducedC2C12celldamageandEEPM-mediated
cytoprotection. The datafrom thepresent study indicated that treatmentofC2C12cellswithEEPMresultedinanincreaseofNrf2 expressionaswellasitsphosphorylatedformwithaconcomitant increaseinHO-1expression.Furthermore,treatmentwithan HO-1inhibitor,ZnPP,significantlyabrogatedtheprotectiveeffectsof EEPMonH2O2-inducedgrowthinhibitionandROSgeneration, pro-vidingevidencefortheinvolvementofHO-1inductionandNrf2 activationinEEPM-mediatedantioxidantpotential.
Insummary,ourresultsclearlyindicatedthatEEPMexhibiteda protectiveabilityagainstH2O2-inducedcytotoxicityandapoptosis inC2C12myoblasts.EEPMalsosuccessfullysuppressedthe forma-tionofintracellularROS,leadingtoasubstantialregainingofMMP through,atleastinpart,activationofNrf2signalingandinduction ofphaseIIantioxidantenzymes,suchasHO-1.Infuturestudies,this molecularmechanismneedstobevalidatedinvivoasapositive
result,ifconfirmed,wouldprovideessentialinformationtoward thedevelopmentofnewapproachesforeffectivestress-responsive antioxidantsagainstassaultstriggeredbyROS.
Authors’contributions
JSK,DJK,GYKandCPcontributedinrunningthelaboratorywork, analysisofthedataanddraftedthepaper.HJC,SKandHSK con-tributedtocriticalreadingofthemanuscript.HJK,BWK,CMKand YHCdesignedthestudy,supervisedthelaboratoryworkand con-tributedtocriticalreadingofthemanuscript.Alltheauthorshave readthefinalmanuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
ThisworkwassupportedbytheHighValue-addedFood Tech-nologyDevelopmentProgram(314043-3),MinistryofAgriculture, Food and Rural Affairs, and the R&D program of MOTIE/KEIT (10040391,DevelopmentofFunctionalFoodMaterialsandDevice forPreventionofAging-associatedMuscleFunctionDecrease).
References
Ayala-Pe˜na, S., 2013. Role of oxidative DNA damage in mitochondrial dysfunction and Huntington’s disease pathogenesis. Free Radic. Biol. Med. 62, 102–110. Chen, Y.H., Yet, S.F., Perrella, M.A., 2003. Role of heme oxygenase-1 in the regulation
ofbloodpressureandcardiacfunction.Exp.Biol.Med.(Maywood)228,447–453. Chistiakov,D.A.,Sobenin,I.A.,Revin,V.V.,Orekhov,A.N.,Bobryshev,Y.V.,2014. Mito-chondrialagingandage-relateddysfunctionofmitochondria.Biomed.Res.Int., http://dx.doi.org/10.1155/2014/238463.
Deavall,D.G.,Martin,E.A.,Horner,J.M.,Roberts,R.,2012.Drug-inducedoxidative stressandtoxicity.J.Toxicol.2012,645460.
Enomoto,S.,Yanaoka,K.,Utsunomiya,H.,Niwa,T.,Inada,K.,Deguchi,H.,Ueda,K., Mukoubayashi,C.,Inoue,I.,Maekita,T.,Nakazawa,K.,Iguchi,M.,Arii,K.,Tamai, H.,Yoshimura,N.,Fujishiro,M.,Oka,M.,Ichinose,M.,2010.Inhibitoryeffects ofJapaneseapricot(PrunusmumeSieboldetZucc;Ume)onHelicobacterpylori -relatedchronicgastritis.Eur.J.Clin.Nutr.64,714–719.
Fleury,C.,Mignotte,B.,Vayssière,J.L.,2002.Mitochondrialreactiveoxygenspecies incelldeathsignaling.Biochimie84,131–141.
Guerra-Araiza, C., Álvarez-Mejía, A.L., Sánchez-Torres, S., Farfan-García, E., Mondragón-Lozano, R., Pinto-Almazán, R., Salgado-Ceballos, H., 2013. Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free Radic. Res. 47, 451–462.
Hybertson, B.M., Gao, B., Bose, S.K., McCord, J.M., 2011. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol. Aspects Med. 32, 234–246.
Jeong, J.T., Moon, J.H., Park, K.H., Shin, C.S., 2006. Isolation and characterization of a new compound from Prunusmumefruit that inhibits cancer cells. J. Agric. Food Chem.54,2123–2128.
J.S.Kangetal./RevistaBrasileiradeFarmacognosia26(2016)184–190
Kim,B.H.,Oh,I.,Kim,J.H.,Jeon,J.E.,Jeon,B.,Shin,J.,Kim,T.Y.,2014.Anti-inflammatory activityofcompoundsisolatedfromAstragalussinicusL.incytokine-induced keratinocytesandskin.Exp.Mol.Med.21,e87.
Kim,R.,Emi,M.,Tanabe,K.,Murakami,S.,Uchida,Y.,Arihiro,K.,2006.Regulation andinterplayofapoptoticandnon-apoptoticcelldeath.J.Pathol.208,319–326. Kita,M.,Kato,M.,Ban,Y.,Honda,C.,Yaegaki,H.,Ikoma,Y.,Moriguchi,T.,2007. CarotenoidaccumulationinJapaneseapricot(PrunusmumeSiebold&Zucc.): molecularanalysisofcarotenogenicgeneexpressionandethyleneregulation.J. Agric.FoodChem.55,3414–3420.
Lee,H.S.,Lee,G.S.,Kim,S.H.,Kim,H.K.,Suk,D.H.,Lee,D.S.,2014.Anti-oxidizingeffect ofthedichloromethaneandhexanefractionsfromOrostachysjaponicusin LPS-stimulated RAW 264.7 cells viaupregulation of Nrf2 expression and activation of MAPK signaling pathway. BMB Rep. 47, 98–103.
Lee, J.A., Ko, J.H., Jung, B.G., Kim, T.H., Hong, J.I., Park, Y.S., Lee, B.J., 2013. Fermented Prunusmumewith probiotics inhibits 7,12-dimethylbenz[a]anthracene and 12-o-tetradecanoyl phorbol-13-acetate induced skin carcinogenesis through alleviation of oxidative stress. Asian Pac. J. Cancer Prev. 14, 2973–2978. Martin, D., Rojo, A.I., Salinas, M., Diaz, R., Gallardo, G., Alam, J., De Galarreta, C.M.,
Cuadrado, A., 2004. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor inresponsetotheantioxidantphytochemicalcarnosol.J. Biol.Chem.279, 8919–8929.
Matsuda,H., Morikawa,T., Ishiwada, T.,Managi, H., Kagawa, M., Higashi,Y., Yoshikawa,M.,2003.Medicinalflowers.VIII.Radicalscavengingconstituents fromtheflowersofPrunusmume:structureofprunoseIII.Chem.Pharm.Bull. (Tokyo)51,440–443.
Maynard,S.,Schurman,S.H.,Harboe,C.,deSouza-Pinto,N.C.,Bohr,V.A.,2009.Base excisionrepairofoxidativeDNAdamageandassociationwithcancerandaging. Carcinogenesis30,2–10.
Mitani,T.,Horinishi,A.,Kishida,K.,Kawabata,T.,Yano,F.,Mimura,H.,Inaba,N., Yamanishi,H.,Oe,T.,Negoro,K.,Mori,H.,Miyake,Y.,Hosoda,A.,Tanaka,Y., Mori,M.,Ozaki,Y.,2013.Phenolicsprofileofmume,Japaneseapricot(Prunus mumeSieb.etZucc.)fruit.Biosci.Biotechnol.Biochem.77,1623–1627. Niture, S.K., Khatri, R., Jaiswal, A.K., 2014. Regulation of Nrf2-an update. Free Radic.
Biol. Med. 66, 36–44.
Park, C., Jin, C.Y., Kim, G.Y., Jeong, Y.K., Kim, W.J., Choi, Y.H., 2011. Induction of apo-ptosis by ethanol extract of Prunusmumein U937 human leukemia cells through activation of caspases. Oncol. Rep. 26, 987–993.
Rogakou, E.P., Pilch, D.R., Orr, A.H., Ivanova, V.S., Bonner, W.M., 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868.
Sang, S., Lapsley, K., Jeong, W.S., Lachance, P.A., Ho, C.T., Rosen, R.T., 2002. Antioxida-tivephenoliccompoundsisolatedfromalmondskins(PrunusamygdalusBatsch). J.Agric.FoodChem.50,2459–2463.
Seo,K.,Ki,S.H.,Shin,S.M.,2014.Methylglyoxalinducesmitochondrialdysfunction andcelldeathinliver.Toxicol.Res.30,193–198.
Son,T.G.,Kawamoto,E.M.,Yu, Q.S.,Greig,N.H.,Mattson, M.P.,Camandola,S., 2013.Naphthazarinprotectsagainstglutamate-inducedneuronaldeathvia activationoftheNrf2/AREpathway.Biochem.Biophys.Res.Commun.433, 602–606.
Surh,Y.J.,Kundu,J.K.,Na,H.K.,2008.Nrf2asamasterredoxswitchinturningon thecellularsignalinginvolvedintheinductionofcytoprotectivegenesbysome chemopreventivephytochemicals.PlantaMed.74,1526–1539.
Tada,K.,Kawahara,K.,Matsushita,S.,Hashiguchi,T.,Maruyama,I.,Kanekura,T., 2012.MK615,aPrunusmumeSteb.EtZucc(‘Ume’)extract,attenuatesthegrowth of A375 melanoma cells by inhibiting the ERK1/2-Id-1 pathway. Phytother. Res. 26, 833–838.
Terazawa, R., Akimoto, N., Kato, T., Itoh, T., Fujita, Y., Hamada, N., Deguchi, T., Iinuma, M., Noda, M., Nozawa, Y., Ito, M., 2013. A kavalactone deriva-tive inhibits lipopolysaccharide-stimulated iNOS induction and NO production through activation of Nrf2 signaling in BV2 microglial cells. Pharmacol. Res. 71, 34–43.
Tsuji, R., Koizumi, H., Fujiwara, D., 2011. Effects of a plum (PrunusmumeSiebold and Zucc.) ethanol extract on the immune system invivoand invitro. Biosci. Biotechnol.Biochem.75,2011–2013.
Wang,C.H.,Wu,S.B.,Wu,Y.T.,Wei,Y.H.,2013.Oxidativestressresponseelicitedby mitochondrialdysfunction:implicationinthepathophysiologyofaging.Exp. Biol.Med.(Maywood)238,450–460.
Wen,J.,Shi,W.,2012.RevisionoftheMaddeniacladeofPrunus(Rosaceae).PhytoKeys 2012,39–59.
Wojcik,M.,Burzynska-Pedziwiatr,I.,Wozniak,L.A.,2010.Areviewofnaturaland syntheticantioxidantsimportantforhealthandlongevity.Curr.Med.Chem.17, 3262–3288.
Wu, Y.T., Wu, S.B., Lee, W.Y., Wei, Y.H., 2010. Mitochondrial respiratory dysfunction-elicitedoxidativestressandposttranslationalproteinmodification inmitochondrialdiseases.Ann.N.Y.Acad.Sci.1201,147–156.
Yan,X.T.,Lee,S.H.,Li,W.,Sun,Y.N.,Yang,S.Y.,Jang,H.D.,Kim,Y.H.,2014.Evaluation of the antioxidant and anti-osteoporosis activities of chemical constituents of the fruits of Prunusmume. Food Chem. 156, 408–415.
Yingsakmongkon, S., Miyamoto, D., Sriwilaijaroen, N., Fujita, K., Matsumoto, K., Jam-pangern, W., Hiramatsu, H., Guo, C.T., Sawada, T., Takahashi, T., Hidari, K., Suzuki, T., Ito, M., Ito, Y., Suzuki, Y., 2008. In vitro inhibition of human influenza A virus infection by fruit-juice concentrate of Japanese plum (PrunusmumeSIEB. et ZUCC). Biol. Pharm. Bull. 31, 511–515.