dysfunction and cardiovascular risk
José Carlos de Lima-Júnior1* Alexandre Moura-Assis2,3* Riobaldo M. Cintra1
Thiago Quinaglia1 Lício A. Velloso2,3 Andrei C. Sposito1
1. Laboratory of Vascular Biology and Atherosclerosis, Department of Internal Medicine, State University of Campinas (Unicamp), Campinas, SP, Brasil 2. Laboratory of Cell Signaling, Department of Internal Medicine, State University of Campinas (Unicamp), Campinas, SP, Brasil 3. Center for Research on Obesity and Comorbidities, OCRC, State University of Campinas (Unicamp), Campinas, SP, Brasil * JCLJ and AMA contributed equally to this review.
http://dx.doi.org/10.1590/1806-9282.65.1.87
SUMMARY
Atherosclerosis is the leading cause of mortality in the contemporary world. The critical role of the endothelial cells (EC) in vascular homeostasis, the metabolic changes that take place when the cell is activated, and the elements involved in these processes have been widely explored over the past years. Obesity and its impact, promoting a rise in blood levels of free fatty acids (FAs) are often asso-ciated with atherosclerosis and cardiovascular mortality. However, the mechanisms that promote cardiovascular structural changes and adaptive changes in the ECs, particularly in the context of obesity, are little known. Here, we reviewed studies that assessed the metabolic adaptations of healthy and dysfunctional ECs during exposure to FAs, as well as the epidemiological perspectives of cardio-vascular structural changes in obesity. Finally, we explored the role of new agents – sphingolipids, dietary unsaturated fatty acids and sodium-glucose cotransporter-2 inhibitors (iSGLT2) – in atherosclerosis and their relationship with obesity.
KEYWORDS: Obesity. Risk factors. Atherosclerosis. Endothelium.
DATE OF SUBMISSION: 12-Oct-2018 DATE OF ACCEPTANCE: 26-Oct-2018 CORRESPONDING AUTHOR: Andrei C Sposito
Cardiology Division, State University of Campinas – 13084-971, Campinas São Paulo, Brasil –Phone: +55 19 35219590 – Fax: (19) 35218771
E-mail: andreisposito@gmail.com
INTRODUCTION
Atherosclerosis is the leading cause of mortality worldwide and is associated with obesity. This dis-ease has a physiopathological component key to the dysfunction of ECs, the cells that make up the luminal surface of blood vessels1. The activation of the
endo-thelial cell by different biochemical or biomechanical stimuli results in an inflammatory phenotype, with loss of the homeostatic function as a micro-barrier, expression of adhesion molecules and prothrombotic
molecules on its surface, as well as the generation of reactive oxygen species (ROS), which results in the progression of the atherosclerotic injury in the vessel wall2. This review focused on epidemiological
insights and identifying potential therapeutic targets for the obesity/atherosclerosis binomial. In the final part of the review, we will discuss the roles of mol-ecules or other substances identified more recently or fewer studies, sphingolipids, dietary unsaturated fatty acids and sodium-glucose cotransporter-2 in-hibitors (iSGLT2), and their potential involvement in the physiopathology or therapy of the cardiovascular changes that accompany obesity.
OBESITY AND RISK OF CARDIOVASCULAR DISEASE
The rapid increase in the prevalence of obesity in Brasil and in the world over the last few decades was accompanied by a parallel increase in other co-morbidities associated with obesity3. The
epidemio-logical evidence establishes an association between obesity and mortality; however, there are controver-sies in the relationship between obesity and cardio-vascular risk, and that seems to be associated with the pattern of body fat distribution and metabolic factors that involve insulin sensitivity, the profile of hormone secretion of the adipocytes and cardiorespi-ratory fitness4.
The heterogeneity of obesity and the concept of “obesity paradox,” in which individuals with over-weight and obesity present an improved cardiomet-abolic prognosis in comparison with eutrophic in-dividuals, are fertile ground for an investigation of the mechanistic and physiological fundamentals of the diversity of effects of obesity on cardiovascular health5,6.
Although widely used in epidemiological studies and the clinical routine, the body mass index (BMI) is unable to distinguish areas of concentration of white adipose tissue. The local distribution of white adipose tissue and its impact on the cardiometabol-ic risk have been described since the 1940s, and the deposits of ectopic fat – deposits in visceral organs – and in the abdominal cavity are significantly cor-related with cardiometabolic abnormalities7.
The deposits of visceral fat are more resistant to the insulin action and release free FAs in a high-er proportion in comparison to the subcutaneous adipose tissue. The excess of free FAs in the blood-stream is closely related with the onset of inflamma-tion, notedly observed through the increase in the serum levels of C-reactive protein, interleukin-6, and tumor necrosis factor- α (TNF-α) in peripheral
tis-sues4. Additionally, the excess of circulating FAs and
their influx into the hepatic portal system triggers a higher production of VLDL by the liver. In this con-text, although many obese individuals present stan-dard levels of low-density lipoproteins (LDL-c), most of the LDL-c produced are small and dense particles, classically more atherogenic8. In rodents,
perivascu-lar fat and the role of the resistance to the effect of insulin on vascular cells during atherosclerosis are well documented. These studies demonstrate that the resistance to the effect of insulin on vascular cells reduces the bioavailability of nitric oxide and favors the adhesion and infiltration of immune cells, com-promising the vasodilation properties and favoring the endothelial dysfunction9,10.
Taken together, the mechanisms mentioned above constitute some of the pillars of the heterogeneity of obesity and the physiopathology of cardiovascular diseases associated with obesity. The following top-ics discuss these interfaces in greater detail.
METABOLIC ADAPTATIONS IN THE ENDOTHELIAL CELL IN OBESITY – BIOCHEMICAL PERSPECTIVES
In healthy conditions, the endothelium coordi-nates the formation of blood vessels and keeps the vascular homeostasis and its structure. In patholog-ical conditions, however, the metabolic changes that take place in the ECs can promote dysfunction and be a trigger event for atherosclerosis1. ECs support
different cellular processes through several meta-bolic pathways, which can have different signatures according to bad metabolic adaptations, of which the understanding might help us prevent injuries and identify new therapeutic targets11. Initially, we
present the fundamental aspects of the endotheli-al metabolism of blood glucose and Fas,12, both the
most critical energy-generating pathways in healthy ECs, before exploring which fundamental changes in these mechanisms are present in the endothelial cells of obese individuals.
for example, in which the glycolytic flux also prevails in relation to the oxidation of FAs and blood glucose (> 200x). Thus, even though the ATP generation through anaerobic glycolysis is less efficient than Oxphos, in a cellular environment where there is abundant availability of glucose, glycolysis becomes a efficient option12,13. Besides, that non-oxidative
cel-lular approach decreases the generation of reactive oxygen species associated with Oxphos, as well as reduces the use of oxygen, making it available, pri-marily, for perivascular cells. Furthermore, this pro-cess allows the ECs to use the lactate protectively, as a molecule for controlling the angiogenesis12,14. In
parallel, an intermediary of the glycolytic flux feeds the pentose phosphate pathway, which results in the generation of glutathione (GSH), a molecule with an-ti-oxidant potential (ROS scavenging)1,15.
FAs represent another energetic pathway for the production of energy in the ECs (approximately 5%); however, the primary use of the FAs that enter an EC is not generating ATP12. However, in the absence
of glucose, the metabolic flow is diverted to the oxi-dation of the FAs (FAO) in a process regulated by the AMPK (adenosine monophosphate-activated protein kinase), a cellular metabolic sensor16. The plasma
FAs enter the cell through passive diffusion or FAs translocase, which also acts as acyl-CoA synthetase, leading to the formation of fatty acyl-CoA. The fatty acyl-CoA is conjugated to carnitine through carnitine palmitoyltransferase 1A (CPTIA) before being trans-ported to the mitochondria by the carnitine/acyl-carnitine shuttle for β-oxidation. After entering the mitochondrial matrix, enzymes of the CPT2 family catalyze the formation of acylcarnitines back to the fatty acyl-CoAs, which enter the β-oxidation path-way17. Thus, one of the regulators that play an
im-portant role in FAO is the CPT1A, limiting the FA flux destined to β-oxidation. For example, the EC-specific gene silencing of CPT1A causes defects in cell pro-liferation (though it does not cause changes in other homeostatic elements, such as cell migration) and EC angiogenesis18,19. Such changes occur because, in
ECs, FAO is crucial for the synthesis of deoxyribonu-cleotide, since the FAs are a source of carbon as crit-ical as glucose and glutamine to the citric acid cycle in the ECs19. In this context, occurs the generation of
aspartate and glutamate, precursors of nucleotides, which, in turn, are critical for the DNA (deoxyribonu-cleic acid) synthesis during cell proliferation1.
Obesity is associated with a high level of
circulat-ing FAs, and that increase in supply is a metabolic challenge for the ECs since this process is also asso-ciated with the generation of reactive oxygen species and is deleterious to the cells (ROS)20. Most
endothe-lial cells in adults are in quiescent form (QEC), un-like the cells in proliferation (PECs). The QECs are continuously exposed to an environment rich in ox-ygen in the peripheral blood, and, consequently, to the oxidative stress promoted by the ROs, capable of promoting endothelial dysfunction through the decoupling of the vasoprotective function of the en-dothelial nitric oxide synthase (eNOS)21. Studies have
sought evidence of the mechanisms through which these cells protect themselves to keep the vascular homeostasis, which could lead to the identification of new therapeutic targets that promote vascular pro-tection in a FA-rich environment, like obese individu-als18. According to what has been stated above, PECs
use the β-oxidation of FAs as a source of carbon for the synthesis of nucleotides during the proliferative stage of the angiogenesis19. Recently, Kalucka et al.22
demonstrated that QECs are not hypometabolic, as was believed.
On the contrary, QECs increase the FAO flux when they become quiescent. Surprisingly, they use the β-oxidation of FAs not for the synthesis of nucle-otides, such as in PECs, but to keep the redox homeo-stasis through the regeneration of antioxidant mol-ecules 22. That understanding certainly contributes
to the view that EC adopts protective mechanisms during stress situations and the use of FAs to main-tain redox homeostasis is a key mechanism.
This view that there are adaptive mechanisms that protect against the excess of FAs has been re-cently described. In general, it is known that highly acute levels of free FAs induce a bad adaptation and a consequent endothelial dysfunction in vivo in hu-mans due to a worsen vasodilation mediated by ni-tric oxide23, as well as promotes the activation of the
inflammasome and the of inflammatory signaling cascade controlled by the factor nuclear kappa B (NF-kB)24. Similarly, obesity/insulin resistance also
pro-motes in vivo endothelial dysfunction in humans25.
In the same way, FAs promote apoptosis through a mechanism dependent on the generation of ROS us-ing the NAD(P)H oxidase26, as well as the membrane
saturation of the endoplasmic reticulum and the con-sequent endoplasmic reticulum stress, which also activates inflammatory pathways27.
in-duction of the CPT1A upregulation or the promotion of other metabolic sensors upregulation, such as the peroxisome-proliferator-activator-receptor (PPAR), is capable of reducing endothelial dysfunction and the EC apoptosis, therefore, being a possible thera-peutic target for endothelial protection in situations in which there is an increased supply of FAs in the plasma24,28. Besides, bariatric surgery can reduce
markers of systemic inflammation and endothelial activation29 significantly. This improvement in the
endothelial dysfunction might be associated to bet-ter management of fatty acids by lean tissue afbet-ter the surgery since there is a decrease of the systemic lipolysis during the intravenous overload of lipids, as well as better disposal of triglycerides and produc-tion of acylcarnitine after the bariatric surgery30.
CARDIOVASCULAR STRUCTURAL ADJUSTMENTS IN OBESITY
Obesity is a well-known risk factor; however, part of its effect on the cardiovascular structure is caused by the concomitance of other risk factors. The isolat-ed expression of the excess of white fat, thus, would happen only in so-called metabolic-healthy obese individuals. The analysis of cohorts featuring indi-viduals with these characteristics would allow us to understand which cardiovascular phenotype is relat-ed to obesity. The largest cohort, so far, in number of patients (n = 3,500,000), suggests that this action leads to an adjusted risk of heart failure two times greater (Risk ratio: 1.96; confidence interval of 95%: 1.86–2.06) and 50% higher chance of coronary ar-tery disease (Risk ratio: 1.49; confidence interval of 95%: 1.45–1.54).0.46-1.81). This same study revealed a lower risk, but not insignificant, of cerebrovascu-lar disease (Risk ratio: 1.07; confidence interval of 95%: 1.04–1.11) also linked to obesity. The average fol-low-up time for these outcomes to occur was 5.431.
Another meta-analysis confirms these findings in different populations that differ only on whether or not there is an increase in the overall mortality of these patients in comparison with the general pop-ulation32,34.
These adverse cardiovascular outcomes are me-diated by structural changes that precede them. The most important of these outcomes seems to be heart failure. The association of obesity with diastolic dys-function and left ventricular hypertrophy is well es-tablished35,36, and both factors precede this outcome.
The increase in mass and the deficit of left ventricle relaxation seem to be related with an increase in the total peripheral vascular resistance determined by a chronic increase of blood volume, already suggest-ed by the Framingham study37. Also associated with
heart failure, the left ventricular systolic dysfunc-tion, as the diastolic, is more frequent in obese indi-viduals. There is a clear linear correlation between BMI and the ejection fraction of the left ventricle, as well as between BMI and the E/e’ ratio (marker of the diastolic dysfunction), and these relationships follow the increase or reduction of body weight in a individual, thus showing the strength of the associ-ation between these parameters38. The reduction in
the ejection fraction, however, seems to be slightly more connected to hyperglycemia and insulin resis-tance concomitant with the increase in adiposity. Hy-perglycemia can increase the intra and extracellular glycation of proteins, increasing the stress oxidation, inflammation, and injuries to the myocardium, cul-minating in the rigidity and reduction of contractili-ty39. However, there are also descriptions of
ventric-ular systolic dysfunction in obese individuals with standard blood glucose and glycated hemoglobin, in-dicating there must be other mediating mechanisms. In fact, obese individuals with no insulin resis-tance (Homa-IR < 2.5 and blood glucose < 100 mg/dl) or other components of metabolic syndrome present heart disease more often than non-obese patients. Subclinical atherosclerosis assessed by the coro-nary calcium score is two times more frequent in this group (Prevalence ratio: 2.26; confidence inter-val of 95%: 1.48–3.43), as shown by a cross-sectional study40. This same study, however, suggests that the
diagnosis parameters for metabolic syndrome are linked to a higher calcium score, even though they are below the cutoff values, especially when consid-ered with the values for LDL-cholesterol (also within the normal range in the study). This set of evidence suggests that the connection of the metabolic param-eters of obese patients and coronary disease might not have a threshold below which the risk would be similar to the general healthy population.
The association between obesity and cerebrovas-cular disease, however, seems less evident. Large cohort studies have shown relationships31, but
oth-ers were not able to confirm them41, in very distinct
individuals, since there is a clear correlation between cerebrovascular accident and adiposity when consid-ering the parameters of systolic blood pressure ≥ 130 mmHg and/or diastolic ≥ 85 mmHg, or hypertension treatment, fasting glucose > 100 mg/dl, or diabetes mellitus treatment, and total cholesterol > 240 mg/ dl or dyslipidemia treatment41. The set of data
indi-cates, thus, that the metabolic changes are necessary for the outcome of cerebrovascular disease to occur, unlike what happens with heart failure and, possibly, coronary artery disease.
NEW AND OLD MOLECULES IN THE OBESITY AND ATHEROSCLEROSIS INTERFACE
iSGLT2, obesity, and atherosclerosis
The iSGLT2 are a class of anti-diabetic drugs that inhibit the absorption of sodium and glucose in the proximal convoluted tubule, promoting osmotic di-uresis and the renal glucose excretion42. Among
the main effects of these drugs, are the reduction of blood glucose, arterial pressure (AP), and weight. All these effects can contribute to the reduction of ath-erosclerosis.
From the studies on safety requested by the American Food and Drug Administration (FDA) since 2009, interesting effects of anti-diabetic drugs have been observed in DM2 patients with high cardio-vascular risk43. The use of empagliflozin in the
Em-pla-REG study was associated with a 14% reduction (Risk ratio of 0.86; CI 95%: 0.74–0.99; p = 0.04 in the incidence of major cardiovascular events (Mace) af-ter an average of 3.1 years, an effect induced, mostly, by the 38% reduction in mortality due to cardiovas-cular diseases (risk ratio of 0.62; CI 95%: 0.49–0.77; p < 0.001) and of 35% in admissions due to heart fail-ure (risk ratio 0.65; CI 95%: 0.50–0.85; p=0.002)44.
A similar result was observed in the Canvas study, using canagliflozin with a 14% reduction (risk ratio 0.86; CI 95%: 0.75–0.97; p = 0.02) of Mace incidence after 3.6 years45. In uncontrolled studies conducted
from databases, the so-called real-world studies, the lower incidence of events was observed with more intensity, with a 51% reduction of mortality due to all causes (risk ratio: 0.49; CI 95%: 0.41–0.57; p < 0.001)46. The reasons behind this reduction remain
unclear; however, it might be associated with the ef-fects the iSGLT2 have on cardiovascular risk factors. Gliflozins are associated with dose/response weight loss47, with an average reduction of 2.1 kg (CI
95%: –2.3 – –2.0; p < 0.01) in comparison with the pla-cebo after12 weeks of use48 and an average reduction
of 2.9 kg (CI 95%: –3.72 – –2.07; p < 0.001) after two years, in comparison with other medications49. Still,
the weight loss is predominantly of adipose tissue (in a 2:1 ratio)50, particularly of visceral adipose tissue.
The weight reduction is due, mostly, to glycosuria, with a estimated calorie deficit of 50 kcal/day51,
de-spite the possible contribution of other mechanisms, such as the activation of brown adipose tissue and insulin reduction52.
The gliflozins promote a reduction of 3-5 mmHg in the systolic AP and 1-3 mmHg in the diastolic53.
Mechanistically, the osmotic diuresis induced by the glycosuria contributes to the blood pressure reduc-tion; however, other effects, such as the improve-ment of endothelial function54, arterial stiffness55,
sympathetic tone56, and increase in natriuresis might
be associated57.
Some studies have linked treatments with gli-flozin to lower levels of inflammatory factors asso-ciated with atherosclerosis, such as the TNF-alpha58,
interleukin 6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1)59. In animal models, the treatment
with iSGLT2 was capable of reducing the formation of atheromatous plaques in the aortic arch60, as well
as the formation of foam cells in the atheromatous plaque61. However, the iSGLT2 are associated with
an increase in LDL, perhaps due to the reduction of the hepatic metabolism of these proteins62, and have
a neutral effect on HDL. Still, no alterations were observed in the functions of HDL and the enzymes associated with this protein47,63. Studies on obese
pa-tients with no DM2 were not conducted. It is possi-ble, however, that these effects are found mainly on DM2 patients, but not on those with regular blood glucose levels.
Sphingolipids, lipid profile, and atherosclerosis
The increase in FA levels results, also, from a re-shaping of the synthesis of bioactive lipids, which might be involved in the physiopathology of condi-tions associated with obesity, such as diabetes and cardiovascular diseases. Among these bioactive lip-ids, the sphingolipids have been noted as an import-ant structural membrane element and as molecules with cell signaling functions64. The sphingolipid de
availability of palmitoyl-CoA, derived from a higher concentration of palmitate. Thus, a higher enzyme activity of the serine palmitoyltransferase results in a greater formation of several sphingolipids. Among them, the most involved in cellular processes are the ceramide and the sphingosine-1-phosphate, which implies directly in a larger pool fo these molecules in conditions of a higher supply of FAs. Such reshaping of sphingolipids can affect several tissues64,65.
Recently, plasma lipidomics data in the investi-gation of such bioactive lipids as biomarkers for un-stable coronary artery disease in the retrospective cohort Lipid identified several lipid classes positively associated with future cardiovascular events, such as ceramides and sphingolipids, while lysophosphati-dylcholine and diacylglycerol were negatively associ-ated66, which awakens an interest in other bioactive
molecules as possible effectors of lipid signaling. In total, 53 lipid species were associated with cardio-vascular events. Atherogenic lipoproteins, such as LDL and VLDL, are enriched in sphingolipids, which might also be associated with cardiovascular risk. However, the sphingolipids that are also associated with risk in the Lipid cohort had already been posi-tively associated previously with unfavorable meta-bolic conditions, including dihydroceramide and dif-ferent kinds of ceramides. These results suggest that the regulation might occur in a ceramide-synthase level, which has a distinct expression according to the tissue66.
Even though there are few divergent data, overall, the studies on lipidomic profile have shown a positive association between ceramides and several variables associated with metabolic disorders, such as athero-sclerosis and cardiomyopathy due to lipotoxicity. Mechanisms of action of the ceramides intracellular-ly involve, classicalintracellular-ly, the induction of apoptosis, in-sulin resistance, endoplasmic reticulum stress, and opening of the permeability transition pore in the mi-tochondria. On the contrary, the inhibition of the bio-synthesis of ceramides results in an improvement of several metabolic disorders in animal models, such as the decrease in the formation of atherosclerotic plaque67.
The effector signaling of the sphingolipids sphin-gosine-1-phosphate deserves attention for its sophis-ticated and still not fully understood behavior (Figure 1). Initially, as the class of sphingolipids in general, during obesity in animal models, there is a positive regulation of the enzyme apparatus for the synthesis
of ceramides with an increase of the sphingolipids and sphingosine-1-phosphate both in the plasma of obese mice as well as in adipocytes cultured in vitro. This increase culminates in the positive regulation of the cytokines involved in the inflammatory and pro-thrombotic status of obesity, possibly mediating atherosclerotic complications associated with weight gain70. Such findings are corroborated by the positive
association of the sphingosine-1-phosphate (S1P) with obesity in humans71 and by the experimental
finding that it positively regulates the inflammato-ry pathway triggered by the TNF-α receptor, a vital element of the meta-inflammation triggered by obe-sity72. Despite that, the S1P has a dual role73, since
the signaling cascade triggered by the S1P in the vas-cular endothelium and in the cardiomyocyte has a protective role, regulating the vascular development, migration, angiogenesis, vasodilation signaling, and microvascular barrier function, especially when the S1P é chaperoned by the HDL or the apolipoprotein M74,75, a condition in which the HDL/S1P induces a
complex with the β-arrestin in order to suppress the NF-κB activation induced by cytokines. Besides, the signaling of the S1P1 receptor is vessel-protective, since its EC-specific super expression protects from the formation of plaques in atherosclerosis models in a diet rich in fat75. Unfortunately, there is still a
gap concerning the specific reshaping of these sphin-golipids in obesity and its role in the vascular endo-thelium during obesity, although some light has been shed on the protective role of the S1P1 and one of its ligands (S1P carried by the HDL or ApoM). However, it is also reasonable to extend this atherogenic envi-ronment to the changes in the content of the dysfunc-tional HDL S1P of both diabetic and coronary artery disease patients76,77, who have a decreased capacity
to carry S1P. Indeed, since the biosynthesis pathway of sphingolipids is interconnected, the therapeutic modulation of any element would be complicated, and so far unpredictable, and the ceramide-synthase would have a pathogenic role in a given context64, or
even on how the entire system would behave in the endothelium of an obese individual.
Unsaturated fatty acids, diet, and atheroscle-rosis
for unsaturated FAs since the 1960s. The debates around the recommendations for the ingestion of FAs occupy a prominent position on the agenda of most dietary guides and, according to the American guide, the ingestion of saturated FAs should no ex-ceed the daily limit of 10% of the total energetic value. The dietary guidelines for the Brazilian population, in turn, was structured to stimulate dietary patterns and healthy behavior to the detriment of
recommen-dations of individual nutrients. Such action is neces-sary since nutrients per se seem to not represent a
sine qua non-condition for the development of diseas-es. However, even within the perspective of the Bra-zilian dietary guide, there is an instruction towards the reduction of saturated fats, substituting them for unsaturated ones.
Even though there are multiple associations be-tween lipid consumption and the development of
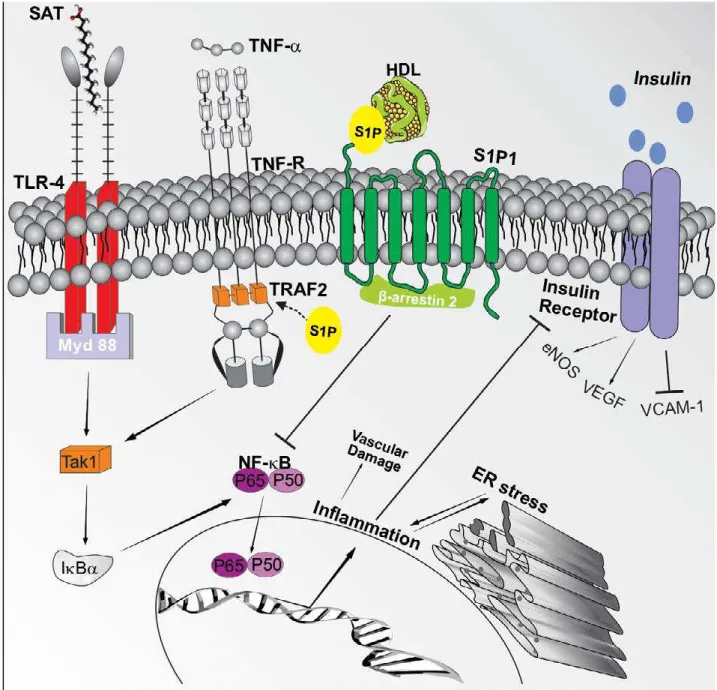
FIGURE 1 (adapted from Moura-Assis et al.68). Activation of inflammatory signaling by saturated fatty acids in conditions
asso-ciated with obesity and their interaction with the S1P The TAK1 (Transforming growth factor b-activated kinase 1) is activated by several stimuli, including cytokines, TLRs, and factors associated with the TNF receptor (Traf), which culminates in the activa-tion of the central pathway IkB kinase (IKK)–nuclear factor-kB (NF-kB), responsible for the activation of transcription of several inflammatory genes69 and the blocking of the insulin receptor. This scheme also demonstrates that the S1Pmolecule, when
generated intracellularly, acts as a secondary lipidic messenger, connecting itself to the Traf2 and promoting changes in the complex with Traf2 that are crucial to the inflammatory activation of the IKK-NF-kB70. When S1P is carried by HDL or ApoM,
when it connects to the endothelial cell, it promotes the formation of a S1P1-β-arrestin 2 complex that inhibits the activation of
cardiovascular diseases, so far, no evidence has been established through well-controlled clinical trials. Besides, the massive divergence between dietary pat-terns between countries makes it substantially more challenging to create guidelines.
Some studies designed to assess the effect of the replacement of saturated FAs by carbohydrates indi-cate there are no overall differences in the markers for cardiovascular risk prediction such as LDL-c e HDL-c78. However, some studies that emphasized the
replacement of saturated FAs by carbohydrates with low glycemic indexes found a reduction of LDL-c and increase in HDL-c79. These opposite effects emphasize
that specifying the source of foods that make up the replacement list is mandatory and the use of carbo-hydrates with low glycemic index as replacements for saturated FAs confers cardiovascular benefits. A recent analysis of the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) es-timated that the isocaloric replacement of 5% of the saturated FAs by polyunsaturated, monounsaturated Fas, and carbohydrates with low glycemic index rep-resented a reduction of 25%, 15%, and 9% of cardiovas-cular risk, respectively80.
Since the epidemiological findings on the low in-cidence of cardiovascular mortality among the Inuit of Greenland, the consumption of fish and the ome-ga-3 FAs have been extensively studied, In general, prospective studies suggest a lower risk of coronary disease in individuals with no previous cardiovascular disease who have a higher intake of polyunsaturated or fish FAs81. The randomized clinical trials who did
find benefits in the supplementation with fish oil in individuals with cardiovascular disease present certain limitations and need to be interpreted with caution. Individuals with cardiovascular disease al-ready receive, in general, pharmacological treatment (statins, for example), and the supplementation with fish oil hardly potentializes the action of these drugs. In addition, the benefits of the omega-3 FAs seem to have a threshold, and additional doses do not confer an increment to the cardiovascular protection in indi-viduals who already have an adequate intake of these FAs in their diet.
Some mechanistic studies in rodents have dis-sected with greater precision the effects of different types of FAs in the metabolism and in their effect as signaling molecules. Polyunsaturated FAs can in-crease the expression of genes involved in the oxida-tion of lipids (PPARα) and decrease the expression of
those involved in the hepatic lipogenesis (SREBP-1c), decreasing atherosclerotic injuries82,83. Additionally,
the partial and isocaloric replacement of saturat-ed FAs by unsaturatsaturat-ed FAs decreases inflammation and the endoplasmic reticulum stress in the aorta of obese and insulin-resistant mice68. On the other
hand, saturated FAs have been associated with an increase of endothelial inflammation and greater atherosclerotic injury in diet-induced obese mice82.
Such effects seem to be related with the activation of the pattern recognition toll-like receptor 4 (TLR4) of the innate immune system and its inflammatory cascade84.
Beyond the consensual difficulties, adherence to the traditional Mediterranean diet has been asso-ciated with lower cardiovascular risk and is widely encouraged for the high-risk population. Monoun-saturated FAs, especially the oleic acid, represent be-tween 16-29% of the total energetic value85 and their
inclusion in diet, as a replacement for simple carbo-hydrates, is significantly associated with a reduction in mortality81. Additionally, the Predimed study
(Pre-vención con Dieta Mediterránea) found a reduction in cardiovascular events in groups that received sup-plementation with extra-virgin olive oil or oilseeds in comparison with the control group in a five-year follow-up with men and women with no cardiovas-cular disease but high-risk. The individuals placed in the supplement group were instructed to consume at least 50 grams of olive oil or 30 grams of oilseeds, in-cluding nuts, almonds, and hazelnuts86. Finally, the
impact of this diet on effector mechanisms for the protection of the activated endothelial cells in obese individuals is still not clear.
CONCLUSIONS
RESUMO
A aterosclerose é a causa líder de mortalidade no mundo contemporâneo. O papel central da célula endotelial (EC) na homeostase vascular, as alterações metabólicas que ocorrem quando a célula se torna ativada e os elementos envolvidos nesses processos vêm sendo bastante explorados nos últimos anos. A obesidade e o seu impacto, promovendo uma elevação dos níveis sanguíneos de ácidos graxos (FAs) livres, é bastante associada à aterosclerose e à mortalidade cardiovascular. Entretanto, os mecanismos que promovem alterações estruturais cardiovasculares e alterações adaptativas nas ECs, particularmente no contexto da obesidade, são pouco con-hecidos. Aqui, nós revisamos estudos que avaliaram as adaptações metabólicas das ECs normais e disfuncionais durante exposição a FAs, bem como as perspectivas epidemiológicas das alterações cardiovasculares estruturais na obesidade. Finalmente, exploramos o papel de novos atores — esfingolípides, ácidos graxos insaturados da dieta e inibidores do cotransportador de sódio-glucose 2 (iSGLT2) — na aterosclerose e sua relação com a obesidade.
PALAVRAS-CHAVE: Obesidade. Fatores de risco. Aterosclerose. Endotélio.
is due to the incorporation of several tools that allow for the study of lipids at a omic perspective, as well for the integration of such knowledge to a knowledge of cell signaling and population data, which has al-lowed for the progress concerning the identification
of new biomarkers and new therapeutic targets. Indeed, over the next years, there will probably be more pieces available to this network of interdisci-plinary knowledge that goes beyond the from bench to bedside limitations.
REFERENCES
1. Pircher A, Treps L, Bodrug N, Carmeliet P. Endothelial cell metabolism: a novel player in atherosclerosis? Basic principles and therapeutic opportu-nities. Atherosclerosis. 2016;253:247-57.
2. Gimbrone Jr MA, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620-36.
3. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627-42. 4. Neeland IJ, Poirier P, Després JP. Cardiovascular and metabolic hetero-geneity of obesity: clinical challenges and implications for management. Circulation. 2018;137(13):1391-406.
5. Kim SH, Després JP, Koh KK. Obesity and cardiovascular disease: friend or foe? Eur Heart J. 2016;37(48):3560-8.
6. Neeland IJ, Turer AT, Ayers CR, Berry JD, Rohatgi A, Das SR, et al. Body fat distribution and incident cardiovascular disease in obese adults. J Am Coll Cardiol. 2015;65(19):2150-1.
7. Després JP. Body fat distribution and risk of cardiovascular disease: an up-date. Circulation. 2012;126(10):1301-13.
8. Cepeda-Valery B, Pressman GS, Figueredo VM, Romero-Corral A. Impact of obesity on total and cardiovascular mortality: fat or fiction? Nat Rev Cardiol. 2011;8(4):233-7.
9. Rask-Madsen C, King GL. Vascular complications of diabetes: mecha-nisms of injury and protective factors. Cell Metab. 2013;17(1):20-33. 10. King GL, Park K, Li Q. Selective insulin resistance and the development of
cardiovascular diseases in diabetes: the 2015 Edwin Bierman Award Lec-ture. Diabetes. 2016;65(6):1462-71.
11. Bierhansl L, Conradi LC, Treps L, Dewerchin M, Carmeliet P. Central role of metabolism in endothelial cell function and vascular disease. Physiology (Bethesda). 2017;32(2):126-40.
12. Eelen G, de Zeeuw P, Simons M, Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ Res. 2015;116(7):1231-44. 13. Groschner LN, Waldeck-Weiermair M, Malli R, Graier WF.
Endothe-lial mitochondria: less respiration, more integration. Pflugers Arch. 2012;464(1):63-76.
14. Lee DC, Sohn HA, Park ZY, Oh S, Kang YK, Lee KM, et al. A lactate-in-duced response to hypoxia. Cell. 2015;161(3):595-609.
15. Krützfeldt A, Spahr R, Mertens S, Siegmund B, Piper HM. Metabolism of exogenous substrates by coronary endothelial cells in culture. J Mol Cell Cardiol. 1990;22(12):1393-404.
16. Dagher Z, Ruderman N, Tornheim K, Ido Y. Acute regulation of fatty acid
oxidation and amp-activated protein kinase in human umbilical vein en-dothelial cells. Circ Res. 2001;88(12):1276-82.
17. Bastin J. Regulation of mitochondrial fatty acid β-oxidation in human: what can we learn from inborn fatty acid β-oxidation deficiencies? Bio-chimie. 2014;96:113-20.
18. Eelen G, Zeeuw P, Treps L, Harjes U, Wong BW, Carmeliet P. Endothelial cell metabolism. Physiol Rev. 2018;98(1):3-58.
19. Schoors S, Bruning U, Missiaen R, Queiroz KC, Borgers G, Elia I, et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520(7542):192-7.
20. Goldberg IJ, Bornfeldt KE. Lipids and the endothelium: bidirectional inter-actions. Curr Atheroscler Rep. 2013;15(11):365.
21. Wilhelm K, Happel K, Eelen G, Schoors S, Oellerich MF, Lim R, et al. FOXO1 couples metabolic activity and growth state in the vascular endo-thelium. Nature. 2016;529(7585):216-20.
22. Kalucka J, Bierhansl L, Conchinha NV, Missiaen R, Elia I, Brüning U, et al. Quiescent endothelial cells upregulate fatty acid β-oxidation for vasculo-protection via redox homeostasis. Cell Metab. 2018;S1550-4131(18)30459-5.
23. Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100(5):1230-9.
24. Theodorou K, Boon RA. Endothelial cell metabolism in atherosclerosis. Front Cell Dev Biol. 2018;6:82.
25. Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunc-tion. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97(11):2601-10.
26. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, et al. High glucose level and free fatty acid stimulate reactive oxygen species produc-tion through protein kinase C: dependent activaproduc-tion of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49(11):1939-45.
27. Li X, Gonzalez O, Shen X, Barnhart S, Kramer F, Kanter JE, et al. Endothe-lial acyl-CoA synthetase 1 is not required for inflammatory and apoptotic effects of a saturated fatty acid-rich environment. Arterioscler Thromb Vasc Biol. 2013;33(2):232-40.
28. Won JC, Park JY, Kim YM, Koh EH, Seol S, Jeon BH, et al. Peroxisome pro-liferator-activated receptor-gamma coactivator 1-alpha overexpression prevents endothelial apoptosis by increasing ATP/ADP translocase activi-ty. Arterioscler Thromb Vasc Biol. 2010;30(2):290-7.
train-ing on inflammation and endothelial function: a randomized controlled trial. Atherosclerosis. 2018;273:37-44.
30. Grenier-Larouche T, Carreau AM, Geloën A, Frisch F, Biertho L, Marceau S, et al. Fatty acid metabolic remodeling during type 2 diabetes remission after bariatric surgery. Diabetes. 2017;66(11):2743-55.
31. Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, et al. Metabolically healthy obese and incident cardiovas-cular disease events among 3.5 million men and women. J Am Coll Cardiol. 2017;70(12):1429-37.
32. Zheng R, Zhou D, Zhu Y. The long-term prognosis of cardiovascular disease and all-cause mortality for metabolically healthy obesity: a sys-tematic review and meta-analysis. J Epidemiol Community Health. 2016;70(10):1024-31.
33. Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy over-weight and obesity benign conditions? A systematic review and me-ta-analysis. Ann Intern Med. 2013;159(11):758-69.
34. Fan J, Song Y, Chen Y, Hui R, Zhang W. Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: a me-ta-analysis of prospective cohort studies. Int J Cardiol. 2013;168(5):4761-8. 35. Cil H, Bulur S, Turker Y, Kaya A, Alemdar R, Karabacak A, et al. Impact of
body mass index on left ventricular diastolic dysfunction. Echocardiogra-phy. 2012;29(6):647-51.
36. Ng ACT, Prevedello F, Dolci G, Roos CJ, Djaberi R, Bertini M, et al. Impact of diabetes and increasing body mass index category on left ventricular systolic and diastolic function. J Am Soc Echocardiogr. 2018;31(8):916-25. 37. Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, et al. Cor-relates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122(6):570-8.
38. Blomstrand P, Sjoblom P, Nilsson M, Wijkman M, Engvall M, Länne T, et al. Overweight and obesity impair left ventricular systolic function as mea-sured by left ventricular ejection fraction and global longitudinal strain. Cardiovasc Diabetol. 2018;17:113.
39. Berg TJ, Snorgaard O, Faber J, Torjesen PA, Hildebrandt P, Mehlsen J, et al. Serum levels of advanced glycation end products are associated with left ventricular diastolic function in patients with type 1 diabetes. Diabetes Care. 1999;22(7):1186-90.
40. Chang Y, Kim BK, Yun KE, Cho J, Zhang Y, Rampal S, et al. Metabolical-ly-healthy obesity and coronary artery calcification. J Am Coll Cardiol. 2014;63(24):2679-86.
41. Lee HJ, Choi EK, Lee SH, Kim YJ, Han KD, Oh S. Risk of ischemic stroke in metabolically healthy obesity: a nationwide population-based study. PLoS One. 2018;13(3):e0195210.
42. Zelniker TA, Braunwald E. Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(15):1845-55.
43. Lim S, Eckel RH, Koh KK. Clinical implications of current cardiovascular outcome trials with sodium glucose cotransporter-2 (SGLT2) inhibitors. Atherosclerosis. 2018;272:33-40.
44. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-28.
45. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(21):2099. 46. Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, et al.
Lower risk of heart failure and death in patients initiated on sodium-glu-cose cotransporter-2 inhibitors versus other glusodium-glu-cose-lowering drugs: the CVD-REAL Study (Comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors). Circulation. 2017;136(3):249-59.
47. Fadini GP, Bonora BM, Mayur S, Rigato M, Avogaro A. Dipeptidyl pepti-dase-4 inhibitors moderate the risk of genitourinary tract infections as-sociated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2018;20(3):740-4.
48. Storgaard H, Gluud LL, Bennett C, Grøndahl MF, Christensen MB, Knop FK, et al. Benefits and harms of sodium-glucose co-transporter 2 inhibi-tors in patients with type 2 diabetes: a systematic review and meta-analy-sis. PLoS One. 2016;11(11):e0166125.
49. Zhang XL, Zhu QQ, Chen YH, Li XL, Chen F, Huang JA, et al. Cardiovascu-lar safety, long-term noncardiovascuCardiovascu-lar safety, and efficacy of sodium-glu-cose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus:
a systemic review and meta-analysis with trial sequential analysis. J Am Heart Assoc. 2018;7(2):pii: e007165.
50. Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020-31.
51. Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1730-5.
52. Xu L, Nagata N, Nagashimada M, Zhuge F, Ni Y, Chen G, et al. SGLT2 In-hibition by empagliflozin promotes fat utilization and browning and atten-uates inflammation and insulin resistance by polarizing m2 macrophages in diet-induced obese mice. EBioMedicine. 2017;20:137-49.
53. Sarafidis PA, Lazaridis AA, Ruiz-Hurtado G, Ruilope LM. Blood pressure reduction in diabetes: lessons from ACCORD, SPRINT and EMPA-REG OUTCOME. Nat Rev Endocrinol. 2017;13(6):365-74.
54. Shigiyama F, Kumashiro N, Miyagi M, Ikehara K, Kanda E, Uchino H, et al. Effectiveness of dapagliflozin on vascular endothelial function and gly-cemic control in patients with early-stage type 2 diabetes mellitus: DE-FENCE study. Cardiovasc Diabetol. 2017;16(1):84.
55. Pfeifer M, Townsend RR, Davies MJ, Vijapurkar U, Ren J. Effects of canagli-flozin, a sodium glucose co-transporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: a post hoc analysis. Cardiovasc Diabetol. 2017;16(1):29.
56. Wan N, Rahman A, Hitomi H, Nishiyama A. The effects of sodium-glu-cose cotransporter 2 inhibitors on sympathetic nervous activity. Front En-docrinol (Lausanne). 2018;9:421.
57. León Jiménez D, Cherney DZI, Bjornstad P, Castilla Guerra L, Miramon-tes González JP. Antihyperglycemic agents as novel natriuretic therapies in diabetic kidney disease. Am J Physiol Renal Physiol. 2018. doi: 10.1152/ ajprenal.00384.2017.
58. Birnbaum Y, Bajaj M, Yang HC, Ye Y. Combined SGLT2 and DPP4 inhibi-tion reduces the activainhibi-tion of the Nlrp3/asc inflammasome and attenu-ates the development of diabetic nephropathy in mice with type 2 diabe-tes. Cardiovasc Drugs Ther. 2018;32(2):135-45.
59. Mancini SJ, Boyd D, Katwan OJ, Strembitska A, Almabrouk TA, Kennedy S, et al. Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemo-kine secretion in vascular endothelial cells by AMP-activated protein ki-nase-dependent and -independent mechanisms. Sci Rep. 2018;8(1):5276. 60. Nasiri-Ansari Ν, Dimitriadis GK, Agrogiannis G, Perrea D, Kostakis ID,
Kaltsas G, et al. Canagliflozin attenuates the progression of atherosclerosis and inflammation process in APOE knockout mice. Cardiovasc Diabetol. 2018;17(1):106.
61. Terasaki M, Hiromura M, Mori Y, Kohashi K, Nagashima M, Kushima H, et al. Amelioration of hyperglycemia with a sodium-glucose cotransporter 2 inhibitor prevents macrophage-driven atherosclerosis through macro-phage foam cell formation suppression in type 1 and type 2 diabetic mice. PLoS One. 2015;10(11):e0143396.
62. Briand F, Mayoux E, Brousseau E, Burr N, Urbain I, Costard C, et al. Em-pagliflozin, via switching metabolism toward lipid utilization, moderately increases LDL cholesterol levels through reduced LDL catabolism. Diabe-tes. 2016;65(7):2032-8.
63. Fadini GP, Bonora BM, Zatti G, Vitturi N, Iori E, Marescotti MC, et al. Ef-fects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a random-ized placebo-controlled trial. Cardiovasc Diabetol. 2017;16(1):42. 64. Cowart LA. Sphingolipids: players in the pathology of metabolic disease.
Trends Endocrinol Metab. 2009;20(1):34-42.
65. Choi S, Snider AJ. Sphingolipids in high fat diet and obesity-related diseas-es. Mediators Inflamm. 2015;2015:520618.
66. Mundra PA, Barlow CK, Nestel PJ, Barnes EH, Kirby A, Thompson P, et al. Large-scale plasma lipidomic profiling identifies lipids that predict cardio-vascular events in secondary prevention. JCI Insight. 2018;3(17). 67. Chaurasia B, Summers SA. Ceramides: lipotoxic inducers of metabolic
dis-orders. Trends Endocrinol Metab. 2015;26(10):538-50.
innate immune signaling. Trends Immunol. 2013;34(7):307-16.
70. Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes. 2006;55(9):2579-87. 71. Kowalski GM, Carey AL, Selathurai A, Kingwell BA, Bruce CR.
Plas-ma sphingosine-1-phosphate is elevated in obesity. PLoS One. 2013;8(9):e72449.
72. Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465(7301):1084-8.
73. Lee JF, Zeng Q, Ozaki H, Wang L, Hand AR, Hla T, et al. Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phos-phate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem. 2006;281(39):29190-200.
74. Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest. 2015;125(4):1379-87.
75. Galvani S, Sanson M, Blaho VA, Swendeman SL, Obinata H, Conger H, et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci Signal. 2015;8(389):ra79.
76. Keul P, Polzin A, Kaiser K, Gräler M, Dannenberg L, Daum G, et al. Potent anti-inflammatory properties of HDL in vascular smooth muscle cells me-diated by HDL-S1P and their impairment in coronary artery disease due to lower HDL-S1P: a new aspect of HDL dysfunction and its therapy. FASEB J. 2018:fj201801245R.
77. Frej C, Mendez AJ, Ruiz M, Castillo M, Hughes TA, Dahlbäck B, et al. A shift in ApoM/S1P between HDL-particles in women with type 1 diabe-tes mellitus is associated with impaired anti-inflammatory effects of the
ApoM/S1P complex. Arterioscler Thromb Vasc Biol. 2017;37(6):1194-205. 78. Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids
and carbohydrates on the ratio of serum total to HDL cholesterol and se-rum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77(5):1146-55.
79. Levitan EB, Cook NR, Stampfer MJ, Ridker PM, Rexrode KM, Buring JE, et al. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reac-tive protein. Metabolism. 2008;57(3):437-43.
80. Li Y, Hruby A, Bernstein AM, Ley SH, Wang DD, Chiuve SE, et al. Satu-rated fats compared with unsatuSatu-rated fats and sources of carbohydrates concerning risk of coronary heart disease: a prospective cohort study. J Am Coll Cardiol. 2015;66(14):1538-48.
81. Wang DD, Li Y, Chiuve SE, Stampfer MJ, Manson JE, Rimm EB, et al. As-sociation of specific dietary fats with total and cause-specific mortality. JAMA Intern Med. 2016;176(8):1134-45.
82. Lottenberg AM, Afonso MS, Lavrador MS, Machado RM, Nakandakare ER. The role of dietary fatty acids in the pathology of metabolic syndrome. J Nutr Biochem. 2012;23(9):1027-40.
83. Afonso MS, Castilho G, Lavrador MS, Passarelli M, Nakandakare ER, Lot-tenberg SA, et al. The impact of dietary fatty acids on macrophage choles-terol homeostasis. J Nutr Biochem. 2014;25(2):95-103.
84. Velloso LA, Folli F, Saad MJ. TLR4 at the crossroads of nutrients, gut mi-crobiota, and metabolic inflammation. Endocr Rev. 2015;36(3):245-71. 85. Wang DD, Hu FB. Dietary fat and risk of cardiovascular disease: recent
controversies and advances. Annu Rev Nutr. 2017;37:423-46.