Universidade do Minho
Escola de Engenharia
Alberto Dimas Fernandes Leite de Carvalho
Development of Hyaluronic Acid, Dextrin
and Extracellular Matrix Hydrogels for
cell Expansion
Alberto Dimas Fernandes Leite de Carvalho
De
velopment of Hy
aluronic Acid, De
Dissertação de Mestrado
Mestrado Integrado em Engenharia Biomédica
Área de especialização em Engenharia Clínica
Trabalho efetuado sob a orientação do
Professor Doutor Francisco Miguel Gama
e do
Professor Doutor José Domingos Santos
Alberto Dimas Fernandes Leite de Carvalho
Development of Hyaluronic Acid, Dextrin
and Extracellular Matrix Hydrogels for
cell Expansion
Universidade do Minho
Escola de Engenharia
DE ACORDO COM A LEGISLAÇÃO EM VIGOR, NÃO É PERMITIDA A REPRODUÇÃO DE QUALQUER PARTE DESTA DISSERTAÇÃO.
Universidade do Minho, ___/___/______
Agradecimentos
"A verdadeira medida de um homem não é como ele se comporta em momentos de conforto e conveniência, mas como ele se mantém em tempos de controvérsia e desafio." Martin Luther King Jr.
Obrigado a todas as pessoas que contribuíram para meu sucesso e para meu crescimento como pessoa. Sou o resultado da confiança e da força de cada um de vocês.
Ao Prof. Dr. Miguel Gama e ao Prof. Dr. José Domingos Santos, orientador e co-orientador, respetivamente, agradeço o tema proposto, o apoio, a partilha do saber e as valiosas contribuições para o trabalho.
Um obrigado a todos os colegas do LTEB, Ana Cristina, Jorge, Alexandre, Paula, Sílvia, Karol, Tânia, João Pedro, Raquel, Isabel, Vera e Catarina pela vossa amizade, companheirismo e ajuda, fatores muito importantes na realização desta Tese e que me permitiram que cada dia fosse encarado com particular motivação. Também agradeço à Dina Silva e à Professora Colette Maurício pela disponibilidade e ajuda na preparação de mais uma etapa deste projeto. Uma referência especial à Paulinha e ao “States”, pela enorme amizade que criámos. Agradeço-lhes a partilha de bons momentos, a ajuda e os estímulos nas alturas de desânimo.
Um agradecimento a minha Família e Amigos por acreditarem sempre em mim e naquilo que faço e por todos os ensinamentos de vida. Um reconhecimento em especial para a minha mulher, pelo apoio e encorajamento, neste capítulo da minha vida e à minha filha pelas noites mal dormidas mas compensadas pela alegria de a ter na minha vida. Espero que esta etapa, que agora termino, possa, de alguma forma, retribuir e compensar todo o carinho, apoio e dedicação que, constantemente, me oferecem. A eles, dedico todo este trabalho.
Resumo
Diariamente, milhares de procedimentos cirúrgicos são realizados para substituir ou reparar tecido danificado resultante de um trauma ou doença. O campo em desenvolvimento de Engenharia de Tecidos (ET) ambiciona regenerar tecidos danificados através da combinação de células provenientes do corpo humano com matrizes altamente porosas provenientes de biomateriais, que irão atuar como modelos na regeneração dos tecidos, orientando o crescimento do tecido novo. As células, a matriz extracelular e os sinais de estimulação de crescimento são constantemente referenciados como a tríade da ET, considerando-se os principais componentes desta. As matrizes, normalmente construídas a partir de biomateriais poliméricos, permitem um suporte estrutural para a fixação das células e consequente desenvolvimento dos tecidos. No entanto, os investigadores frequentemente encontram uma enorme e variada oferta de opções quando selecionam matrizes na ET.
O objetivo deste projeto é desenvolver um novo hidrogel usando diferentes proporções de Dextrino, diferentes tipos de ácido hialurónico (AH) e Matriz Extracelular (MEC) proveniente da submucosa do intestino delgado. No presente estudo, AH e Dextrino foram oxidados com periodato de sódio para criar grupos funcionais de aldeído, o que pode ser reticulado por ácido adípico dihidrazida. A sua caracterização foi realizada com base no tempo de gelificação e taxa de degradação. Para além disso, os testes de viabilidade foram executados através de um ensaio de Resazurin, um ensaio de MTS e um ensaio de Live and Dead utilizando uma linha celular osteoblástica MC3T3 derivada de rato. Os resultados evidenciaram um tempo de gelificação reduzido para todos os hidrogéis e taxas de degradação baixas para hidrogéis misturados com elevados teores de AH de elevado peso molecular. Os testes de degradação comprovaram que o hidrogel escolhido poderia-se manter ao longo de 70 dias. As interações entre o hidrogel, as células MC3T3 e a MEC também foram avaliadas, tal como os efeitos dos hidrogéis no crescimento das células. As células em cultura não aderiram aos hidrogéis, mas sobreviveram e mantiveram a sua morfologia redonda, demonstrando que o hidrogel é biocompatível, mas não propicia o crescimento celular.
Abstract
Every day thousands of surgical procedures are performed to replace or repair tissue that has been damaged through disease or trauma. The developing field of Tissue Engineering (TE) aims to regenerate damaged tissues by combining cells from the body with highly porous scaffold biomaterials, which act as templates for tissue regeneration, to guide the growth of new tissue. Cells, scaffolds and growth-stimulating signals are generally referred to as the TE triad, the key components of engineered tissues. Scaffolds, typically made of polymeric biomaterials, provide the structural support for cell attachment and subsequent tissue development. However, researchers often encounter an enormous variety of choices when selecting scaffolds for Tissue Engineering.
The aim of this project is to develop a new hydrogel made of Dextrin (Dex), Hyaluronic acid (HA) and Extracellular matrix (ECM) from Small Intestine Submucosa (SIS), using different types of HA, mixed different proportions. In this study, HA and Dex were oxidized by sodium periodate to create aldehyde functional groups, which could be cross-linked by Adipic Acid Dihidrazide (ADH). Their characterization was performed based on gelation period and degradation rate. In addition, cell viability tests were performed through a Resazurin assay, a MTS assay and Live and Dead (LD) using osteoblastic cell line MC3T3 calvaria from mouse. Results showed a low gelation time for all the hydrogels and low degradation rates for mixed hydrogels with high contents of high molecular weight (MW) HA. The degradation tests demonstrated that the selected hydrogel could maintain the gel matrix over 70 days.
Interactions between the hydrogel, MC3T3 cells, and the extracellular matrix (ECM) were also evaluated, as were the effects of the hydrogels on MC3T3 cell growth. The hydrogels possess several clinical advantages, including sterilizability and rapid gelation time for injection. Cultured MC3T3 cells did not adhere to hydrogels, but survived and maintained their round morphology, evidencing that the hydrogel is biocompatible, but does not promote cell growth.
ab e o ontents
Content
Agradecimentos ...iv Resumo ...vi Abstract ...viii Table of Contents ...xList of Figures ...xii
List of Tables ...xv Abbreviations ...xvi 1. Introduction ... 2 1.1. Tissue Engineering ... 2 1.2. Scaffolds_Hydrogels ... 3 1.3. Hyaluronic acid ... 5 1.4. Dextrin ... 9 1.5. Extracellular Matrix ... 14
1.6. Mesenchymal stem cells ... 18
1.7. Maintenance of the undifferentiated state of mesenchymal stem cells ... 21
1.8. Objective ... 23
2. Materials and Methods ... 25
2.1. Hydrogel Production and Preparation ... 25
2.1.1. – Dextrin preparation ... 25
2.1.2 – Hyaluronic acid preparation ... 25
2.1.3 – Preparation of oxidized hyaluronic acid and oxidized dextrin-ADH and ECM hydrogels ... 26
2.2.2 – Degradation Assay ... 27
2.3. Biocompatibility Assays ... 27
2.3.1– MTS ... 27
2.3.2 – Resazurin Viability ... 27
2.3.3 – Live and Dead ... 28
2.3.4 – Histocompatibility ... 28
3. Results and Discussion ... 31
3.1. - Hydrogel Production and Preparation ... 31
3.1.1 - Oxidized Hyaluronic acid and Dextrin preparation ... 31
3.1.2 - Hydrogel conditions ... 31
3.2- Hydrogel Characterization ... 33
3.2.1 - Gelation time... 33
3.2.2 - Degradation Assays ... 35
3.2.2.1 -High and Average Molecular Weight oHA ... 35
3.3. Biocompatibility Assays ... 44
3.3.1 - MTS Assay ... 44
3.3.2 - Resazurin Viability Assay ... 46
3.3.3 - Live and Dead assay ... 47
3.3.4 Histocompatibility assays ... 52
4. Conclusion and Future Perspectives ... 56
Figure 1 - Tissue Engineering approaches with a combination of cells, biofactors and
scaffolds
Figure 2 - Chemical structure of HA
Figure 3 - Functionalization and crosslinking of HA with ADH
Figure 4 - A model for proposed protein-protein and protein-HA interactions
mediated by RHAMM
Figure 5 - Chemical structure of Dextrin
Figure 6 - Obtained Images by Cryo-SEM interior hydrogels ODex35% and odex
40% (30%, w / v) of 5% AAD (A) before and (B) after immersed in PBS (pH 7.4) at room temperature for 24 h; (C,D) odex-nanogel hydrogel. The arrows show the location of dextrin nanogel
Figure 7 - Microscopic analysis of the fibroblasts cultivated on Dex hydrogels
without recombinant proteins, coated with a starch-binding molecule (SBM), and coated with a starch-binding molecule and a RGD sequence (RGDSBM) with different incubation times
Figure 8 - (A) Periodate Oxidation of Dextrin, Yielding Two Aldehyde Groups at
Positions C2 and C3 of a D-Glucose Unit and (B) Polymerization Reaction of Oxidized Dextrin with ADH
Figure 9 - The extracellular matrix is composed of different macromolecules
Figure 10 Processing steps to achieve the various forms of decellularized
extracellular matrix scaffolds
Figure 11 Source of MSCs and multipotent differentiation capacity. MSCs can be
isolated from bone marrow, deciduous teeth, fat, hair follicles, peripheral blood, periodontal ligament, trabecular bone, scalp subcutaneous tissue, and skeletal muscle in ad adult tissues, and from bone marrow, liver, lung, placenta, spleen and umbilical cord
in fetal tissues. MSCs can generate multiple mesoderm-type cell lineages, such as osteocytes, chondrocytes, adipocytes, myocytes, tenocytes, and stromal cells
Figure 12 Progression of osteogenesis. (A) AP activity (units/ng DNA) and (B)
calcium deposition (¹g calcium/ng DNA) for MSC cultured on collagen films in static dishes, and collagen scaffolds either in static dishes or in spinner flasks. Data are shown as mean ± SD (n = 3–4). Asterisk (*) indicates significant difference from all other groups (p < 0.01; p < 0.05)
Figure 13 Hydrogels dispositions on rat for in vivo Assays
Figure 14 Diverse ratios oHA/oDex between different MW oHA and various
concentrations of ADH
Figure 15 100 % oDex Hydrogels with various concentrations of ADH
Figure 16 Degradation profile of 100% oHA1000 and oHA300 hydrogels with
different concentration of ADH
Figure 17 Degradation profile of 100% oDex hydrogels with different %
concentration of ADH
Figure 18 - Degradation profile of different ratios of oDex and oHA300 with a
concentration of ADH 4%
Figure 19 Degradation profile of different ratios of oDex and oHA300 with a
concentration of ADH 6%
Figure 20 Degradation profile of different ratios of oDex and oHA300 with a
concentration of ADH 8%
Figure 21 Degradation profile of different ratios of oDex and oHA1000 with a
concentration of ADH 4%
Figure 22 Degradation profile of different ratios of oDex and oHA1000 with a
concentration of ADH 6%
Figure 23 Degradation profile of different ratios of oDex and oHA1000 with a
Figure 24 Degradation profile of oHA20 hydrogels with different concentration of
ADH
Figure 25 Degradation profile of different ratios of oDex and oHA20 with a
concentration of ADH 4%
Figure 26 Degradation profile of different ratios of oDex and HA20 with a
concentration of ADH 6%
Figure 27 Degradation profile of different ratios of oDex and HA20 with a
concentration of ADH 8%
Figure 28 Microscopic photographs of the different hydrogels before the addition of
MTS (Amplification 40x)
Figure 29 Relative absorbance for the hydrogels with MC3T3 cells (n=3) hydrogels
without MC3T3 cells (n=3) and the cellular control
Figure 30 Relative intensity of the hydrogels with M3T3 cells (n=3), hydrogels
without M3T3 cells (n=3) and the cellular control, one, two, 7 days and 3 weeks after seeding using fluorescence at 530nm excitation wavelength and 590nm emission wavelength
Figure 31 Inverted Fluorescence Microscopy Images from different types of
samples: Cells, HG_cells, HG_cells_ECM, 2 days, 1 week and 3weeks after cultivation.
Figure 32 (A)-Confocal laser scanning microscopy images (n = 3) of MC3T3-E1
cells cultured on DMEM for 2 hours and 1 week. Green: live cells. Red: dead cells
Figure 33 (B) Confocal laser scanning microscopy images (n = 3) oHA/oDex
hydrogels for 2 days and 1 week
Figure 34 (C) Confocal laser scanning microscopy images (n = 3) of MC3T3-E1 cells
cultured on oHA/oDex hydrogels for 2 hours and 1 week. Green: live cells. Red: dead cells
Figure 35 (D)-Confocal laser scanning microscopy images (n = 3) HA/oDex/ECM
Figure 36 (E) Confocal laser scanning microscopy images (n = 3) of MC3T3-E1 cells
cultured on HA/oDex/ECM hydrogels for 2 hours and 1 week. Green: live cells. Red: dead cells
Figure 37 Subcutaneously implantation of oDex and oHA hydrogels in the lumbar
region of the rats
Figure 38 Macroscopic analysis of the subcutaneously implantation of oDex and
Table
Table 2.1 - Gelation periods estimated for Dex hydrogels with different ADH
concentrations. Gelation times correspond to more than 1h (+++), less than 30min (++) or less than 1min (+)
Table 2.2 - Hydrogel conditions to implantation in subcutaneously in the lumbar
region of rat
Table 2.3 - Efficiency of the process to different materials
Table 2.4 - Gelation time to determine optimal concentration of oHA
Table 2.5 - Gelation time for different ratios of oDex: HA and various concentration
Abbrev a
n
AADH Adipic Acid Dihydrazide
AH Ácido Hialurónico B BM Bone Marrow D Dex Dextrin DO Degree of Oxidation Dextrin-HEMA Dextrin-hydroxyethylmethacrylate Dextrin-VA Dextrin- vinyl acrylate
E
ECM Extracellular Matrix ETC Ethylcarbazate
EDC Carbodiimide hydrochloride ESCs Embryonic Stem Cells
ET Engenharia de Tecidos
F
FDA Food and Drug Administration
G
GAG Glucosaminoglycan
GMHA Glycidyl methacrylate–hyaluronic acid
H
HA Hyaluronic Acid HE Hematoxilin-Eosin HSC Hematopoietic Stem Cells
HESC Human Embryonic Stem Cells
L
LD Live and Dead
M
MSC Mesenchymal Stem Cell MESCs Mouse Embryonic Stem Cells MW Molecular Weight
MEC Matriz Extracelular
O
oDex Oxidized Dextrin
oHA Oxidized Hyaluronic Acid
R
RGD Arginine-Glycine-Aspartic Acid
S
SIS Small Intestine Submucosa
T
TE Tissue Engineering
U
UBM Urinary Bladder
UC Umbilical Cord
1 In r duc
n
1.1. Tissue Engineering
One of the most recurrent and costly problems in human healthcare nowadays is failure or loss of an organ. The most prevalent treatment for this problem is transplantation. Although organ transplantation shows remarkable results in saving lives, there are several limitations, the first and most important one is shortage of donors. Data from the U.S. Department of Health & Human Services shows that an average of 79 people receive organ transplants each day. None the less, every year, thousands of people die while waiting for transplants. The demand for organ transplantation increases dramatically each year, but the number of donors increases relatively slowly. A second challenge is the difficulty of finding a serotype matched donor. Furthermore, even after a successful transplantation, patients must take immune-suppression medicine for the rest of their lives to avoid transplant rejection. In addition to all this, due to complication and rejection responses, the percentage of recipients still living five years after transplant is not very high [1][2].
Tissue Engineering (TE) applies the principles of biology and engineering to the development of functional substitutes for damaged tissue. Bioengineers are in constant pursuit of solutions to problems facing the medical and pharmaceutical field by designing biomaterials that closely mimic the target natural systems. Tissues or organs can be potentially engineered with a number of different strategies, but a particularly appealing approach utilizes a combination of patient’s own cells combined with polymer scaffolds. In this approach, tissue-specific cells are isolated from a small tissue biopsy from the patient and harvested in vitro. The cells are subsequently incorporated into three-dimensional polymer scaffolds that act as analogues to the natural ECM found in tissues. Different materials can be used according to the cell type, expected duration and microenvironment, and they determine several physical properties such as biocompatibility, biodegradability and mechanical stability. The scaffold must also provide appropriate signals to direct cellular processes including cell adhesion and migration which can influence the survival of transplanted cells. The surface of the scaffolds can also be designed or functionalized with growth factors to control these processes [2][3][4][5].
1 – Introduction
The field of biomaterials has been undergoing a shift towards biologically active systems, i.e, with adequate answers to requests from the dynamic environment in which they are inserted. The studies are focused, more than ever, on the biological mechanisms that occur in the tissues and organs, as well as at the interface of biomaterials, both the molecular and cellular level or at the macroscopic level, in an attempt to map the key phenomena for understanding the fundamental processes that occur in living systems. Understanding these mechanisms is crucial to the development of surrogate entities capable of long-term cure.
1.2. Scaffolds_Hydrogels
Hydrogels are used in clinical practice and experimental medicine in diverse applications, including TE and as drug delivery systems. There are a number of different approaches to tissues or organs engineering, but a particularly appealing methodology uses a combination of a patient’s own cells combined with polymer scaffolds.
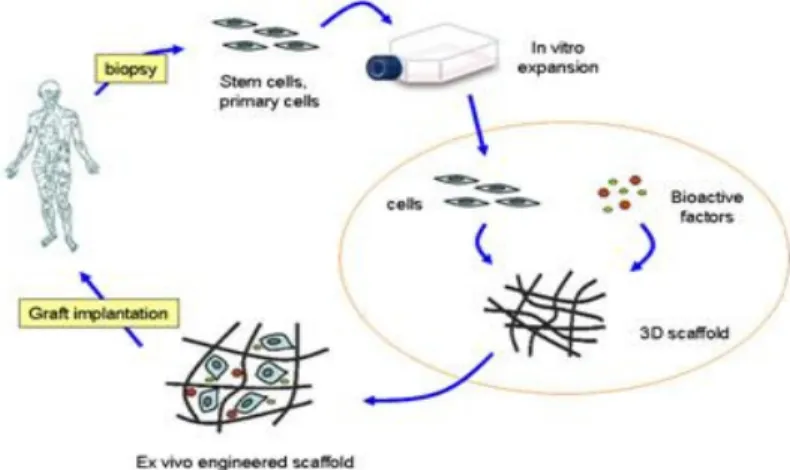
In this approach, tissue-specific cells are isolated from a small tissue biopsy from the patient and harvested in vitro. The cells are subsequently incorporated into three-dimensional polymer scaffolds that act as equivalents to the natural extracellular matrices found in tissues. These scaffolds deliver the cells to the desired site in the patient’s body, provide a space for new tissue formation, and potentially control the structure and function of the engineered tissue (Figure 1) [5].
Figure 1- Tissue Engineering approaches with a combination of cells, biofactors and scaffolds
1 – Introduction
Scaffold materials can be synthetic or biologic, degradable or non-degradable, depending on the intended use. They should promote attachment, migration and proliferation of cells, be easy to use, and retain its physical properties for the wanted time-period. It is obvious that both the chemistry and the topography of the material surface may directly influence the cells behaviour by adsorbing the ECM molecules and altering their conformation that, in turn, regulate cell-substrate interactions. Surface characteristics such as hydrophilicity, surface charge density, surface micromorphology, free energy, and specific chemical groups affect the cell adhesion, spreading, and signalling. Hence, surface characteristics regulate a wide variety of biological functions including cell growth, migration, differentiation, synthesis of ECM, and tissue morphogenesis. Synthetic polymer scaffolds are favoured by some as their properties (e.g. degradation time, porosity, and mechanical attributes) can be tailored for specific applications. Synthetic scaffolds can also be manufactured with a high degree of reproducibility and a long off-the-shelf storage possibility. Biologic materials are favoured by others as they are expected to mimic in vivo milieus better and as they are biologic [5].
Hydrogels are processed under mild conditions and have structural and mechanical properties similar to different tissues and ECM, and can be delivered in a non-invasive manner. Examples of synthetic hydrogels are poly (vinyl alcohol), poly (ethylene oxide), and polypeptides. Examples of biologic hydrogels are chitosan, collagen, gelatin, and HA. These materials are composed of hydrophilic polymer chains, which may be synthetic or natural in origin. Their chemical properties, such as hydrophilicity is determined by 1) the main polymer chain, 2) by the functional side chains in the monomer unit, and 3) the nature of the crosslinker. Since the physical properties, such as strength and swelling rate, are essentially governed by the density of the interconnections established between polymer chains via chemical and / or physical interactions links [5][7].
These materials are therefore typically degradable and processable under relatively mild conditions and have mechanical and structural properties similar to many tissues and in particular the ECM and allow the recurrence minimally invasive methods, upon implantation. Consequently, it is already known to use in numerous applications.
1 – Introduction
They can, for example, be conducted responding to various external stimuli, like pH, temperature, electric field, glucose, and many others [8][9].
However, in addition to the physicochemical and mechanical properties, one of the most important requirements for these biomaterials that may be useful in medical applications is biocompatibility, low toxicity along with its cellular degradation products. Much of cytotoxicity associated with hydrogels relates to unreacted monomers, oligomers and initiators which extend beyond the structure [5][10].
1.3. Hyaluronic acid
Hyaluronic acid (HA) was first discovered in the vitreous humour of the eye in the year 1934. It is a linear polysaccharide consisting of alternating 1,4-linked units of 1,3-linked glucoronic acid and N-acetylglucosamine (figure 2) [3].
Figure 2 Chemical structure of HA [11].
HA is dispersed throughout the human body, being especially abundant in connective tissue. It is one of the major components of the ECM and is involved in important biological processes such as cell mobility and proliferation, morphogenesis, wound repair, inflammation and it's found in all connective tissues [12]. HA may be obtained from different sources like certain strains of streptococcus bacteria but mostly by extraction from umbilical cord, rooster comb, synovial fluid, vitreous humour and is
1 – Introduction
biocompatible, biodegradable, anti-inflammatory, immunogenic, and non-thrombogenic. In cartilage, HA binds to form large aggregates within the collagenous framework, providing compressive resistance to the tissue. In mammals, there are identified three homologous synthetases responsible for segregation of the HA, they are: the AHS1, AHS2 and AHS3 [11].
The various properties and HA functions have been shown in continuous studies since 80’s. In present days, this biopolymer is recognized as a compound of high value with regard to numerous applications, notably in the biomedical and technological areas. The FDA (Food and Drug Administration) has approved numerous derivatives HA that have been employed, particularly in ophthalmology as a vitreous substitute in the eye; in rheumatology, in the treatment of osteoarthritis; in tissue repair of surgical wounds and others and as a matrix for the release of certain drugs. HA plays a key role in maintaining the mechanical integrity of tissues [12][13].
The wide applicability of HA is due to its excellent hygroscopic characteristics, its optimum rheological behaviour and viscous-elastic properties [14][15].
The catabolism of HA is extremely fast at skin level and its half-life is less than 24 hours. Dissolution of HA in aqueous media gives rise to a viscous liquid without any mechanical integrity that can be easily degraded by hyaluronidase. Hyaluronidase degradation of HA results in the cleavage of internal α-N-acetyl-D-glucosaminidic linkages, producing fragments with N-acetylglucosamine at the reducing terminus and glucuronic acid at the non-reducing end. This degradation is influenced by temperature conditions and enzymatic interactions [16][11].
To counter this fast degradation, researchers have made chemical modification and covalent crosslinking to help stabilize HA and improve its mechanical properties. It is necessary to resort to this modification of natural HA to make it a useful biomaterial. HA can be modified chemically at the hydroxyl groups on both the glucoronic and N-acetylglucosamine units or the carboxyl group on the glucoronic acid unit. Kim et al. modified hyaluronic acid with a methacrylate group of aminopropyl methacrylate and synthesized hyaluronic acid–poly(ethylene oxide) hydrogel via Michael-type addition reaction. Leach et al. modified hyaluronic acid with methacrylate groups and prepared glycidyl methacrylate–hyaluronic acid (GMHA) conjugates, which were subsequently photopolymerized to form crosslinked GMHA hydrogels [12][17].
1 – Introduction
Covalent crosslinking of native HA can require toxic reagents (such as EDC) and harsh conditions that are not suitable for cell and protein encapsulation. So, for TE applications, the synthesis of HA hydrogels should be chemo-selective and occur under physiological conditions without creating any toxic products. Prestwich et al. produced HA hydrogels using ADH as a crosslinking agent (Figure 3) and described them as possessing good biocompatibility. The hydrazide moieties are nucleophilic at low pH (3.0 to 4.75), leading to efficient coupling of the hydrazide to the carboxylic acids of the glucuronic acid units of HA. The hydrazide moieties are nucleophilic at low pH (3.0 to 4.75), leading to efficient coupling of the hydrazide to the carboxylic acids of the glucuronic acid units of HA. Linkers containing disulphide bridges can be introduced into the gel formation process to incorporate a way to unlink the gel. This type of gels can be reduced with thiols, hydrides, and phosphines back to sol form [11][18].
Figure 3 - Functionalization and crosslinking of HA with ADH [18].
The biological functions of HA depend on its MW and the result of their interaction with specific proteins binding and surface receptors. HA behaves as a lubricant at low shear rates (resting or walking) of joint fluid, under normal physiological conditions, and prevents mechanical damage acting as a shock absorber and reducing the friction of the moving bones at high shear rates. Low MW HA has high angiogenic
1 – Introduction
epithelial integrity and wound healing. High MW HA polymers (4・102 to 2・104 kDa) are space filling, antiangiogenic, immunosuppressive. The small fragments of degraded HA fragments are inflammatory, immunostimulatory and angiogenic [11][14][13][19].
There are at least two ways to HA interacts with cell surfaces. It can induce the transduction of a range of intracellular signals binding cell surface receptors, hyaladherins, such as CD44, LYVE-1, HARE, layilin, TLR4, and RHAMM, or directly or by activating other proteins. Through signal-transducing receptors, HA influences cell proliferation, survival, motility, and differentiation and might have roles in cancer pathogenesis. CD44 is a major cell surface receptor for HA and is one of the specific markers of MSCs. It is a cell surface transmembrane protein that mediates cellular interactions and cell-ECM interactions, and is expressed by several cells and tissues. CD44 has many functions depending on the cell type. For neuronal cells it is said to be involved in directed migration. Along with a receptor for HA mediated mobility (RHAMM) provide opportune sites for attachment of the crosslinked HA hydrogel to neuronal ends. HA has been documented as enhancing peripheral nerve regeneration. Rats with a sciatic nerve gap injury were implanted with a nerve guide consisting of ports to inject HA solutions [20]–[22].
Another important HA receptor is RHAMM (Figure 4) that is critical for cell motility and focal adhesion turnover. It is similar to CD44 in that it also has multiple isoforms. This receptor is activated during cell migration and its inhibition during embryogenesis causes loss of pluripotency and cell viability in human embryonic stem cells. Assman et al. reported that it is present in the cytoplasm where RHAMM interacts with microtubules and actin filaments. Further experiments revealed that in CD44 knockout mice, the RHAMM gene compensates for the loss of CD44 by supporting up-regulating genes associated with CD44. In addition, toll-like receptors for HA are involved in the signalling of bone marrow-derived mesenchymal stem cells (MSC) and are used to detect inflammation and initiate host defence response mechanisms. RHAMM is found primarily at protruding lamellae of migrating cells and its expression is regulated by motility promoting factors including serum and cytokines such as TGFß 1. Antibodies as well as peptides mimicking binding domain sequences of RHAMM block HA-induced cell [23][24].
1 – Introduction
Figure 4- A model for proposed protein-protein and protein-HA interactions mediated by RHAMM [25].
HA-based hydrogels may impart biological activity to cells, as evident by changes in cellular behaviour, including stem cell differentiation, when interaction with biomaterials based on HA compared to other polymers. It is likely that the coming years will see an expansion in this area through new material development with unique and interesting properties [26].
1.4. Dextrin
Dextrins are low-molecular-weight carbohydrates produced by partial hydrolysis of glycogen or starch. Is a polysaccharide obtained by partial hydrolysis of starch and exhibiting α(1 → 4)-Glc structure of amylose and the α(1 → 4)- and α(1 → 4,6)-Glc (Figure 5) branched structure of amylopectin, but with lower polymerization degree.
1 – Introduction
Figure 5- Chemical structure of Dextrin [27].
Depending on the source and on how it is digested, dextrin can exhibit different structural features and properties such as hygroscopicity, fermentability, sweetness, stability, gelation, solubility, bioavailability and molecular compositions. Dextrins can be linear, branched, or cyclic. Cyclodextrins, obtained through enzymatic degradation of starch, by certain bacteria such as Bacillus macerans, are of interest due to their capacity to improve drug bioavailability [28][29].
This biomaterial can be used in several ways in biomedical applications, food and the textile industry. Recent works describe dextrin hydrogels obtained by polymerization of dextrin-hydroxyethylmethacrylate (dextrin-HEMA) and dextrin- vinyl acrylate (dextrin-VA) showing that this hydrogel is biocompatible and that a controlled degradation rate may be obtained by grafting dextrin to HEMA and VA in different proportions. Silva et al. report the analysis of the encapsulation of cells on combined dextrin hydrogel consisting of a dextrin nanogel and porcine urinary bladder matrix without compromising the mechanical properties or microstructure, confirming the biocompatibility of the injectable hydrogels (figure 6). In a work by Carvalho et al. a dextrin self assembled nanogel was used to effectively incorporate a recombinant biologically active mutated murine IL-10 form (rIL-10) and shown to enable its release over time [29][30].
1 – Introduction
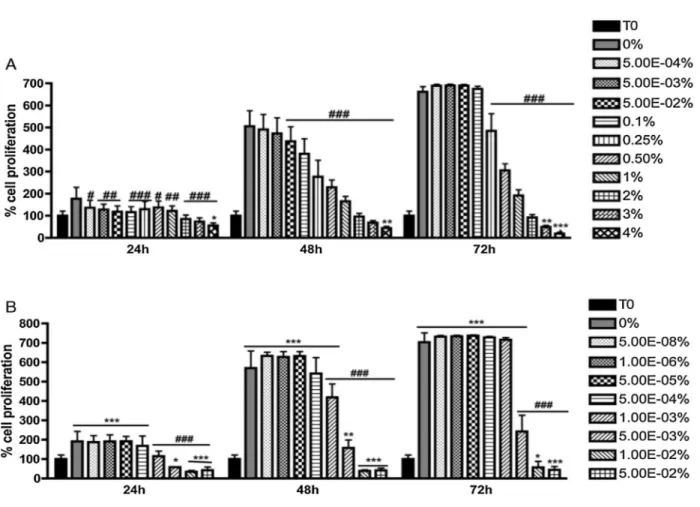
Figure 6- Cell proliferation of 3T3 cells in oDex hydrogels in the presence of different concentrations of adipic acid dihydrazide (A) and glutaraldehyde (B) for 24, 48 and 72 h, determined by sulforhodamin B assay. Results presented as mean ± SD (n = 3). Two-way Anova analysis was performed; statistical differences were reported as*P < 0.05;**P < 0.01 (when compared with the T0 control) and###P < 0.001 (when compared with the DMEM control—0%) [28].
Also in previous studies, cells spreading was evaluated on starch-based hydrogels and revealed differences in cell morphology. Cells appeared round and clustered but viable when grown in hydrogels. Moreira et al. developed a strategy to functionalize a Dex-based hydrogel using a RGD-sequence and results with fibroblasts showed that in this condition the cells spread and were uniformly distributed while exhibiting the characteristic fibroblast morphology (Figure 7) [32].
1 – Introduction
Figure 7 Microscopic analysis of the fibroblasts cultivated on Dex hydrogels without recombinant proteins, coated with a starch-binding molecule (SBM), and coated with a starch-binding molecule and a RGD sequence (RGDSBM) with different incubation times [32].
The homopolysaccharide chain of dextrin may be oxidized by using periodic acid, yielding reactive functional groups, aldehydes, which in turn may react with molecules such as ADH, which acts as a crosslinking agent of the so obtained hydrogels. The mechanical properties, such as gelation and degradation time of hydrogels can be changed by varying the amount of crosslinking agent and the degree of oxidation. On the other hand, the existence of excess reactive groups can compromise the final properties, so the degree of functionalization of the polymeric chain should be moderate.
1 – Introduction
oDex/ADH hydrogels are degradable in two different ways: by hydrolysis of its the hydrazone bonds involving the crosslinker, or by enzymatic cleavage of the glycosidic bonds by α-amylase. The degradation profile depends on the density of connections. oDex hydrogels have a pore diameter in the order of the hundreds of nanometers. After injected, the hydrogel will expand and cells can rapidly invade the hydrogel due to the rapid increase in porosity, along with a process of degradation, especially if chemoattractants displaying signs or adhesion peptides, which can be introduced, are present [27].
The oDex is characterized by their oxidation degree (DO) which consists on the oxidation of the sugar residues, characterized by the specific cleavage of the C2-C3 linkage of glucopyranoside rings, yielding two aldehyde groups per glucose unit (Figure 8).
Figure 8 (A) Periodate Oxidation of Dextrin, Yielding Two Aldehyde Groups at Positions C2 and C3 of a D-Glucose Unit and (B) Polymerization Reaction of Oxidized Dextrin with ADH [10].
The dextrin DO and the concentration of the crosslinker ADH, influence the gelation timeframe, as can be seen in table 2.1. The theoretical DO is calculated as the molar ratio of sodium periodate per initial glucose unit in Dex. Results show that gelation times decrease with increasing DO. It is reported that a DO above 40% yield very viscous solutions that react promptly with ADH, preventing good homogenization and resulting in mat and brittle hydrogels. This way, DO below 40% is the desired for the making of oDex hydrogels. Dex solutions can only be produced with concentrations lower than 30% (w/v). Above that value, the solution is extremely viscous and very difficult to homogenize. This way, this concentration is considered the threshold of Dex solubility in
1 – Introduction
Table 2.1- Gelation periods estimated for Dex hydrogels with different ADH concentrations. Gelation times
correspond to more than 1h (+++), less than 30min (++) or less than 1min (+) [27].
There are important advantages in using oDex hydrogels as opposed to other polymers since its production method is very simple and accessible, low molecular weight components which facilitates a good degradation and excretion, biocompatibility; possibility of inclusion / encapsulation of molecules / cells, economic raw material of natural origin and available with high degree of purity (medical degree), the potential to function as carrier in microparticle systems, including osteogenic and bioactive ceramic materials, high potential to function as a system for controlled release of drugs [27].
1.5. Extracellular Matrix
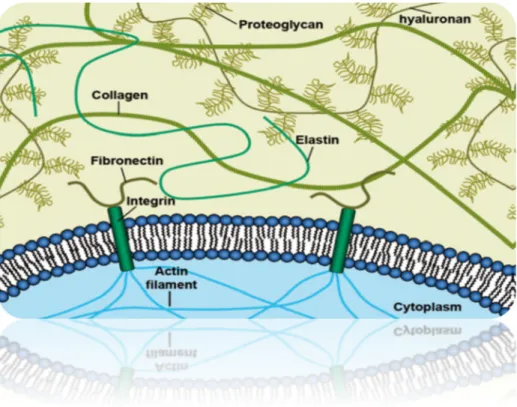
ECM is the major constituent of multicellular organisms. It is composed of large structural proteins, glycosaminoglycans and proteoglycans that are secreted and assembled locally into an organized network (Figure 9). It consists of structural and functional macromolecules, where collagen is the most abundant. Together with cells they define the structure and properties of different tissues in the body.The presence of certain biomolecules, as well as their proportion and arrangement in the array, gives rise to various types of ECM, with different functions. Connective tissues such as cartilage and bone consists of relatively few cells embedded in an extensive ECM, while parenchymal tissue like the liver almost exclusively consists of cells [33].
ADH (%)
DO (%) 5 15 30
25 + + +
32.5 ++ (~30 min) ++ (~10min) ++ (~5 min)
40 ++ (~2min) +++ +++
1 – Introduction
The ECM main functions are to confer resistance to tissues to compressive forces while maintaining its structural integrity, serve as a support for cells, serve as a reservoir for growth factors and other signalling molecules, which shape many physiological and pathological processes, and transmitting signals to the cell surface through receptors. ECM is by nature a natural scaffold for morphogenesis and maintenance of tissues and organs and regeneration / tissue repair.
Figure 9- The extracellular matrix is composed of different macromolecules
Collagen and elastin may be considered as the main contributors of the mechanical properties of ECM. There are more than 20 genetically different types of collagens identified so far. They are all based on the same structure; a right-handed triple helix made of three individual collagen chains wounded around each other [34]. Collagen type I is the primary structural protein in mammalian tissue and is ubiquitous across the animal and plant kingdom. Elastin is another structural protein, which can be found in tissues that have the ability of stretching such as skin, bladder and blood vessels. By permitting deformation and recoil of tissue, elastin is providing structural integrity in response to mechanical stress [35][36].
1 – Introduction
Collagens and elastin are supported in their biomechanical functions by the glycosaminoglycans (GAGs). GAGs are providing the gel-like properties of the ECM and in most cases they are components of proteoglycans. Proteoglycans consist of bottle brush structured GAGs grafted on a protein core. GAGs are strongly anionic polymers that absorb water, thus providing compressive strength to the tissue. GAGs are involved in regulation of water uptake, sequestering of growth factors and cell migration. HA is a special type of non-sulphated GAG which contributes to frictional resistance against interstitial fluid flow [37][38][39].
Proteins like fibronectin and laminin are responsible for cell adhesion, and can be regarded as the “ECM glue”. Laminin is found in the basement membrane, an ECM structure separating the epithelium from underlying layers of connective tissue and muscle. It is involved in cell differentiation, migration and proliferation and plays a critical role in angiogenesis. Many ECM proteins self-assemble outside the cell into structural scaffolds whose composition, supramolecular organization and biomechanical properties are adjusted to tissue functions. Their biosynthesis is often quite intricate and requires numerous and specific post-translational modifications which can occur both intra- and extracellular. This complexity is making it hard to mimic the ECM synthetically. Recombinant production of ECM proteins has been achieved, but the hierarchical order and multifunctionality of a complete ECM is still far from being realized [40][33][41].
This is why ECM has become an interesting material for the production of scaffolds for TE. Various forms of ECM have been used as scaffolds for promoting constructive remodelling of tissues and organs damaged. Is used for the production of these scaffolds derived from various tissues, including heart valves, vessel blood, skin, nerves, skeletal muscle, tendon, ligament, SIS, urinary bladder and liver. (Figure 10) These tissues can be collected from different mammals such as pork, beef, horse and man [42]. Although the scaffolds study is based on various types of ECMs, matrix of SIS and urinary bladder (UBM) are the most widely used.
1 – Introduction
Figure 10-Processing steps to achieve the various forms of decellularized extracellular matrix scaffolds [43]. The ECM degradation is one of the earliest events that occurs after tissue injury, and some studies have shown that the scaffold of ECM are completely degraded in vivo in less than 90 days [44][45][46].
On the other hand, when ECM scaffolds are used to become more resistant to degradation, such as scaffolds chemically crosslinked, the host remodelling process is a more prolonged and intense inflammatory response that results in deposition of fibrous connective tissue (scar tissue) in the damaged area, rather than tissue regeneration. While recognizing that the ECM scaffolds have regenerative properties, the mechanisms by which tissue regeneration is achieved are not yet fully understood. However, there is evidence that the degradation of the scaffold is required to conduct this process.
In summary, the ECM scaffolds are rapidly and completely degraded in vivo and induce a cell response in the host that supports tissue regeneration, rather than the healing process. These results suggest that during scaffold degradation there may be released molecules with biological activity. In addition to the ability to induce regeneration of injured tissue, the scaffold of ECM have also shown more resistance to microbial infections that synthetic scaffolds; interestingly, this antimicrobial activity is not a property of the intact ECM, but the scaffold degradation products [47][48][49].
1 – Introduction
1.6. Mesenchymal stem cells
Owen and Friedenstein et al. have demonstrated that culture-adherent cells present in the marrow stroma are capable of differentiating into bone and cartilage when placed into an appropriate environment in vivo. These experiments have led to the hypothesis that stroma contains a unique population of stem cells which are capable of differentiating along multiple mesenchymal cell lineages. Along the years, a series of experiments have been performed that demonstrate the existence of marrow-derived progenitors that give rise to different types of cells. These cells are referred to as Mesenchymal Stem Cells (MSCs) [50].
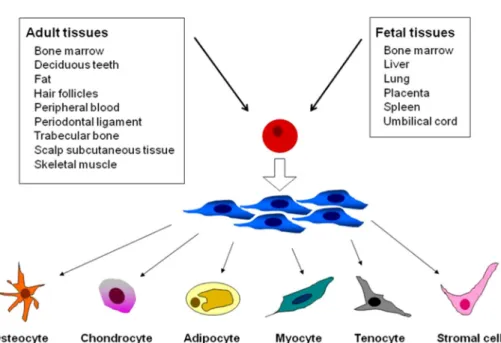
Stem cell is a specific cell population with the ability of self renewal and multipotent differentiation. According to the origin and potentiality of the cells, mammalian stem cells can be classified into two groups: one is embryonic stem cells (ESCs) the other is adult stem cells including MSCs which are found in adult tissues. MSCs have the ability to self-renew and can differentiate into a variety of cell types (Figure. 11), including: osteoblasts (bone cells) chondrocytes (cartilage cells), myocytes (muscle cells), cardiomyocytes (cardiac muscle cells ), adipocytes (fat cells) and neurons [51]. MSCs support hematopoiesis and enhance the engraftment of hematopoietic stem cells (HSC) after cotransplantation. Immunoregulatory functions that may contribute to reduced incidence of graft versus host disease following HSC transplantation were demonstrated by both experimental and clinical studies [4]. These data suggest that MSCs are a valuable tool for TE and cell based therapy. However, because of the invasive procedures for isolating MSCs from their common bone marrow (BM) sources and the significant age-related decreases in the frequency and differentiation potentials of BM-derived MSCs, alternative sources could be advantageous. Mesenchymal stem cells can be isolated from (Figure 11) periosteum, trabecular bone, adipose tissue, synovium, skeletal muscle, deciduous teeth, fatal pancreas, lung, liver, amniotic fluid, placenta, umbilical cord blood (UCB), and umbilical cord (UC). Of these, UCB and UC are particularly advantageous because of their availability, non-invasiveness, and potential for autologous cell-based therapy [51].
1 – Introduction
Figure 11- Source of MSCs and multipotent differentiation capacity. MSCs can be isolated from bone marrow, deciduous teeth, fat, hair follicles, peripheral blood, periodontal ligament, trabecular bone, scalp subcutaneous tissue, and skeletal muscle in ad adult tissues, and from bone marrow, liver, lung, placenta, spleen and umbilical cord in fetal tissues. MSCs can generate multiple mesoderm-type cell lineages, such as osteocytes, chondrocytes, adipocytes, myocytes, tenocytes, and stromal cells [52].
MSCs are naturally sensitive to their environment, responding to chemical, physical and mechanical features of their matrices or substrates, as well as the spatial/temporal presentation of biochemical cues. Solchaga et al. reports that FGF-2 enhances proliferation and differentiation of hMSCs adds to a growing body of evidence that cytokines modulate the differentiation potential and, perhaps, the multipotentiality of adult stem cells. With the generation of gene expression profiles of FGF-treated and control cells we have taken the first steps in the elucidation of the molecular mechanism behind these phenomena [52][53].
Cellular behaviours, such as adhesion, proliferation, differentiation and migration, can be influenced by custom-designed, synthetic scaffolds that essentially recapitulate the native stem cell niche [53]. Meinel et al. report studies of bone TE using human MSCs, a protein substrate (film or scaffold; fast degrading unmodified collagen, or slowly degrading cross-linked collagen and silk), and a bioreactor (static culture, spinner flask, or perfused cartridge). Results suggest that osteogenesis in cultured MSC can be modulated by scaffold properties and flow environment, and that the cell–scaffold–
1 – Introduction
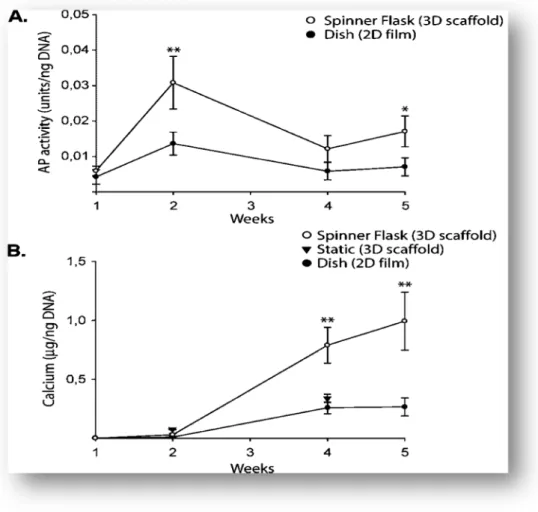
be utilized in controlled studies of cell function and tissue development (Figure 12). In summary, dynamic culture conditions enhanced the formation of mineralized bone in a manner dependent on scaffold degradation rate and the conditions of fluid flow. An envisioned scenario for bone TE involves the cultivation of MSC on slowly degrading, structurally stable silk scaffolds (to maintain construct size and DNA content), in perfusion cartridges [55].
Figure 12 --Progression of osteogenesis. (A) AP activity (units/ng DNA) and (B) calcium deposition (¹g calcium/ng DNA) for MSC cultured on collagen films in static dishes, and collagen scaffolds either in static dishes or in spinner flasks. Data are shown as Ave § SD (n = 3–4). Asterisk indicates significant difference from all other groups (0.01; < 0.05)
1 – Introduction
1.7. Maintenance of the undifferentiated state of mesenchymal
stem cells
Control of self-renewal and differentiation of human ESCs remains a challenge. Human and mouse embryonic stem cells (HESCs and MESCs, respectively) self-renew indefinitely while maintaining the ability to generate all three germ-layer derivatives. Despite the importance of ESCs in developmental biology and their potential impact on tissue replacement therapy, the molecular mechanism underlying ESC self-renewal is poorly understood [56].
One limitation to the use of MSCs in clinical applications is their tendency to lose potency for proliferation and differentiation when cultured in vitro which is influenced by donor age, plating density, serum composition, and passage time. This way, methods to maintain the long term multipotency of these cells would significantly improve their use in clinical settings [57]. MSCs must balance self-renewal and differentiation, and complex regulatory mechanisms are required to maintain stem cells undifferentiated and to control their subsequent differentiation and proliferation. It has been reported that the mesenchymal phenotype can be maintained under optimal culture conditions. In a study, Basciano et al. compared cultured human MSCs derived from bone marrow in normoxia (21% O2) versus hypoxia (5% O2) for up to passage 3. Cells under hypoxia tension were more undifferentiated than cells cultured in normoxia. In addition, hypoxia inhibited the expression of genes involved in DNA replication and cell division at passage 0. However, in later passages, cells expanded faster. In this study, cultured cells displayed a typical MSC profile with stable phenotype overtime and no significant phenotypic differences between hypoxic and normoxic conditions were found. Hypoxia also inhibited the biosynthesis of mitochondria in 50% to 75% of cells. The investigators also differentiated the MSCs in both conditions and observed that hypoxic cells were more prone to osteogenic differentiation than normoxic cells and that the expression of alkaline phosphatase was stronger in hypoxic MSCs. This study reflects the capacity of hematopoietic and stromal stem cells to adapt to hypoxia in culture. Hypoxic MSCs are
1 – Introduction
also more adhesive However, the degree and duration of hypoxia described in the literature vary greatly and may result in opposite effects on the proliferation and differentiation capacities of MSCs [58][59].
MSCs express Oct4 or Nanog, which can be increased under specific conditions such as serum deprivation. These are pluripotency genes that constitute the core regulatory network that suppresses differentiation-associated genes, thereby maintaining the pluripotency of the cells. Expression of these genes is restricted to plutipotent cells and is downregulated upon differentiation. Chih-Chien et al. reported that Oct4 and Nanog levels were higher in early passages, as expected, and in hypoxic cultures. In addition, the protein levels of Oct4, the isoform associated with pluripotency, were maintained over long-term cultures in hypoxia. Knockdown of Oct4 or Nanog induced an increase in the expression of Pax, Gata4, Gata6, Sox17, and FoxA2 at early passages in MSCs cultured in hypoxic conditions which indicates spontaneous differentiation. These results suggest that Oct4 and Nanog prevent spontaneous differentiation in MSCs. In addition, the overexpression of Oct4 and Nanog in MSCs enhances the proliferation rate and differentiation potential while inhibiting spontaneous differentiation during expansion under normal culture conditions [60].
In another study Zhang et al., demonstrated that by capturing MSCs to small islands restricted the inappropriate lineage commitment often observed in culture and promote the multipotent stem cell phenotype. This observation is in line with previous work where they demonstrated that restricting cell spreading decreases DNA synthesis. MSCs are routinely characterized by positive expression of markers such as CD31, CD44, CD90, Stro-1, endoglin, CD106, and CD166. They verified that MSCs confined to small 1000 mm2 islands express higher levels of endoglin and Stro-1 compared to cells cultured on nonpatterned surfaces. Expression of these markers persists even after the cells are removed from the islands after a week, and cultured on tissue culture plastic for up to 16 days. In addition, MSCs cultured without confinement show higher levels of osteogenesis markers suggesting that inappropriate differentiation occurs when they are allowed to proliferate under standard cell culture conditions. MSCs that were previously patterned retain their multipotency as assayed by in vitro differentiation in adipogenic and osteogenic media. They determined that restricting cell shape may be a
1 – Introduction
key factor in maintaining multipotency, and one can imagine that culture conditions restricting cell spreading either through substrate stiffness, chemistry, or topography may be used to prolong the MSC phenotype during ex vivo culture [57].
Similarly, these studies will serve to guide the design of 3D biomaterials where control of cell adhesion and spreading may prove important for the success of regenerative therapies and TE.
1.8. Objective
The aim of this project is to develop a new mixed oHA and oDex hydrogel, a biomaterial used in TE and study its interaction with mesenchymal stem cells, within the scope of a partnership between Minho University and FEUP.
This hydrogel is intended to work as an injectable formulation, to generate a three-dimensional structure to maintain the undifferentiated state of the mesenchymal stem cells and promote their growth. Several formulations will be tested in order to assess the best combination of ADH concentration, gelation and degradation times allied with the most appropriate swelling. The selected hydrogel formulation will then be tested for its biocompatibility, in regards to cell growth and interaction, with cells and additionally in
Dextrin (Dex) (2000 Da) was obtained from Roquette; HA with different MW (20kDa; 300 kDd and 1 MDa) were kindly provided by Soliance Naturellement Innovant (Pomacle, França). All reagents used were purchased from Sigma-Aldrich (Missouri, USA), unless stated otherwise.
2.1. Hydrogel Production and Preparation
2.1.1. – Dextrin preparation
Aqueous solutions of Dex (2% w/v) were oxidized with sodium m-periodate solution, in covered Erlenmayers and with stirring for 20h, in order to obtain a theoretical DO of 40%, at room temperature. The oxidizing reaction was stopped by adding an equimolar amount of diethyleneglycol to reduce any unreacted periodate. The resulting solution was dialyzed for 3 days against deionized water using a dialysis membrane with a MW cut off of 1000 Da and then lyophilized for 10 days using the freeze-dryer Scanlaf Scanvac 100-9 Pro Alpha 2-4.
2.1.2 – Hyaluronic acid preparation
Aqueous solutions of the different types of HA (0.5% w/v) were prepared and an equimolar amount of sodium m-periodate was added in order to obtain a theoretical DO of 100%, at room temperature in the dark and with stirring for 24h, to oxidize the solution. The oxidizing reaction was stopped by adding an equimolar amount of diethyleneglycol. The resulting solution was dialyzed for 3 days against deionized water using dialysis membranes with MW cut off of 2000 Da (for HA20) and of 12000 Da (for HA300 and HA1000), and then lyophilized for 10 days using the freeze-dryer Scanlaf Scanvac 100-9 Pro Alpha 2-4.
2 – Materials and Methods
2.1.3 – Preparation of oxidized hyaluronic acid and oxidized dextrin-ADH and ECM hydrogels
Hydrogels of oxidized Dex and oxidized HA were prepared as described in Molinos et al. [10] using different proportions of the polysaccharides: 100% oDex, 75:25oDex:oHA, 50:50oDex:oHA, 25:75oDex:oHA, and 100% oHA.
The preparation of the hydrogels for the degradation studies was done as follows: oHA and oDex were dissolved in PBS (30% w/v for oDex and 15% for oHA) buffer overnight at room temperature. Previously prepared ADH solution in PBS (4%; 6% and 8% w/v) was added to crosslink the polysaccharides in a 7:3 ratio (oDex/oHA:ADH)(v/v). The solution was placed in a Teflon mould and allowed to gel and crosslink for 1h in a thermal insulated box with humid paper so the hydrogels would not dry during that time. Hydrogels of oxidized Dex and oxidized HA were prepared as described in the previous assay. ECM was added to ADH solution (ADH- 4%; 6% and 8% w/v) and ECM (4%) was added to crosslink the solution in a 7:3 ratio (oDex/oHA:ADH)(v/v).
2.2. - Hydrogel Characterization
2.2.1. – Gelation Time
As to evaluate the gelation time, oHA and oDex solutions were prepared and mixed in Eppendorf tubes according to the compositions (oHA%: oDex%): 100:0, 75:25, 50:50, 25:75, 0:100. Then, to each eppendorf was added the previously prepared ADH (4%, 6%, 8%), in the ratio of 7:3 (oDex/oHA:ADH)(v/v). The preparations were quickly mixed and time count started. To determine the gelation time, the solution was pipetted at short time intervals until it could no longer be pipetted.
2 – Materials and Methods
2.2.2 – Degradation Assay
After being prepared and weighed (Wi), 300μl of the hydrogels with different proportions and from different types of HA (Low MW HA20 and High MW HA300, HA1000 MW) were immersed in PBS (pH 7.4) and incubated at 37°C. At regular intervals, the hydrogels were removed from the solution, blotted with filter paper, to remove excess of PBS, weighed (Wt) and returned to the same container with fresh PBS. The buffer solution was replaced at each measurement and the percentage of mass loss determined by the equation 1. For each condition, samples were analysed in triplicate.
Equation 1 - % = 100 − ∗ 100 .
2.3. Biocompatibility Assays
2.3.1– MTS
Cell viability was determined by MTS assay at the time points one, two and four days after seeding the cells (20000 cells) inside the (50:50 oDex:oHA:ADH8%) (100µl) (n=3) hydrogels displaying better degradation profiles. The medium was removed from the wells, the hydrogel was gently crushed with a syringe plunger. MTS solution (10%) was added in each well and samples were incubated for 2h30 at 37°C, 5% CO2. 100μl of the supernatant was transferred to a 96-well plate to read the optical density at 550 nm in a Power Wave XS2 spectrophotometer.
2.3.2 – Resazurin assay
Cell viability was also quantified by performing a Resazurin assay at the time points one, two, seven days and 3 weeks after seeding MCT3T cells (20000 cells) inside 50:50 Dex:HA1000:ADH8%:ECM4%(100µl) (n=3) hydrogels. The medium was removed from the wells, the hydrogel was gently crushed with a syringe plunger and 1ml of resazurin solution (10%) was added, all performed in the dark. The samples were incubated for 3h and then transferred to a 96-well plate to read the fluorescence in a Power Wave XS2 spectrophotometer at 530 nm excitation wavelength and 590 nm emission
2 – Materials and Methods
2.3.3 – Live and Dead assay
Viability and of the qualitative of the assessment of cells proliferation was also analysed by performing an Live and Dead assay on day one and three weeks after seeding the cells (20000 cells) inside the (50:50 Dex:HA:ADH8%:ECM4%) (100µl) (n=3) hydrogels. Assays for confocal microscopy were also performed on the samples collected after one day and one week. The medium was removed from the wells and the hydrogel was gently crushed with a syringe plunger. Live and Dead solution (10%) was added in each well and samples were incubated for 3h at 37oC, 5% CO2. Fluorescent images of the samples were captured on Fluorescence Inverted microscope Leica DMI 3000b to determine the percentage of live and dead cells.
2.3.4 – Subcutaneous implants
100μl hydrogels (ADH8%) (Table 2.2) were surgically implanted subcutaneously in the lumbar region of three male rats (Figure 13) (Sasco Sprague Dawley, Barcelona, Spain, weighing around 300 g). Three animals were housed per cage (Makrolon type 4, Tecniplast, VA, Italy), in a temperature and humidity controlled room with 12-12h light/dark cycles, and were allowed normal cage activities under standard laboratory conditions. The animals were fed with standard food and water ad libitum. Adequate measures were taken to minimize pain and discomfort taking into account human endpoints for animal suffering and distress.
All procedures were performed with the approval of the Veterinarian Authorities of Portugal, and in accordance with the European Communities Council Directive of November 24th 1986 (86/609/EEC). Anaesthesia was administered with an intraperitoneal injection of a pre-mixed solution consisting of ketamine (Imalgene 1000R), 100 mg/kg body weight, and xylazyne (RompunR), 200 mg/kg body weight. Blunt dissection towards the ventral aspect of the body, the two types of biomaterial were implanted subcutaneously. Skin and subcutaneous tissues were closed with a simple-interrupted suture of a non-absorbable filament (SynthofilR, Ethicon). The macroscopic aspect of the wound and the absence of inflammation and/or infection were
2 – Materials and Methods
clinically checked by a veterinarian before the surgical extraction of the implants and surrounding tissues. Two months later; and after performing the same anaesthetic protocol, skin and subcutaneous tissues from the implant area were collected and fixed in a container with 10% neutral buffered formalin (Panreac, Portugal) for posterior histological evaluation using ISO score as previously described. The rats were then euthanized, by lethal intracardiac injection of 5% sodium pentobarbital (EuthasilR). Samples were routinely processed, and 5 μm-thin sequential sections were stained with hematoxilin-eosin (HE).
Table 2.2- hydrogel conditions to implantation in subcutaneously in the lombar region of rat
HG n= HG(100%HA)+ECM+MSC 3 HG(50/50 HA/oDex)+ECM+MSC 3 HG(100%HA)+ECM 2 HG(100%HA) 2 HG(50/50 HA/oDex)+ECM 2 HG(50/50 HA/oDex) 2
Results
s uss
3.1. - Hydrogel Production and Preparation
3.1.1 - Oxidized Hyaluronic acid and Dextrin preparation
After the process of dissolution, oxidation and lyophilisation, oHA and oDex resulted in a soft and fluffy material, light, and white. The efficiency process was determined and the results presented in the table 2.3.
Table 2.3- Efficiency of the process to different materials
Possibly due to issues related to the lyophilisation, some of the HA1000 appeared different, resulting in a brittle and fragile material.
3.1.2 – Combinatorial production of oDEX:oHA Hydrogels
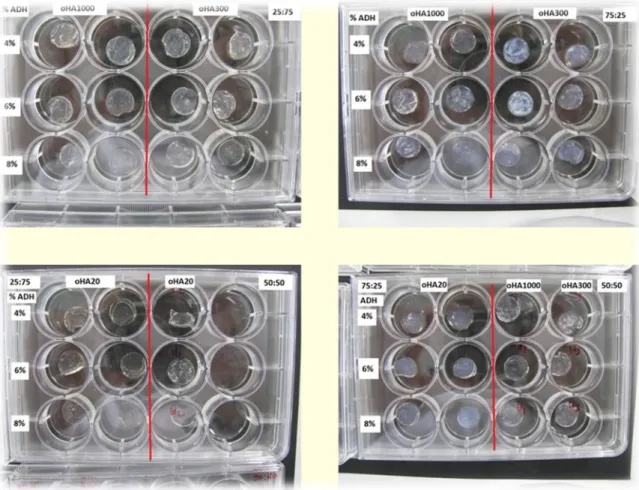
In this work we prepared hydrogels with different oHA:oDex ratios, and with different concentrations of ADH, in order to choose the best one for future trials. As can be seen in the images, oHA (Figure 14) hydrogels are transparent while oDex (Figure 15) hydrogels are white. There are no significant macroscopic differences in the hydrogels obtained varying the concentrations of ADH or the different types of oHA.
Material
EFFICIENCY
HA1000 70%
HA300 60%
HA20 60%
3. Results and Discussion
Figure 14- oHA/oDex hydrogels obtained using different rations of the components, different MW oHA and various concentrations of ADH
3. Results and Discussion
3.2- Hydrogel Characterization
3.2.1 - Gelation time
In previous work, Martins et al. [61] reported difficulties in the dissolution of oHA. Since high MW HA is highly hygroscopic and viscous [12], it was not possible to mix ADH properly, because oHA alone exhibited already gel like properties. In order to address this issue we first determined the optimal concentration of oHA to be used.
As can be seen in table 2.4, an optimal ratio of oHA/PBS solution (w/v), yielding a less viscous solution, was identified. According to Wen-Yu Su et al. [62] HA hydrogels with a concentration of 6% (w/v) dissolved in PBS should result in a fluid solution. However, after the addition of ADH we verified that the solution was not polymerizing; as such, it was necessary to determine an operational concentration, which we concluded to be 15% (w/v) HA/PBS.
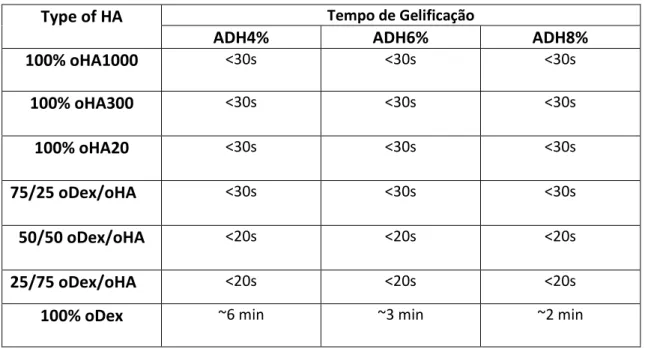
As can be seen in table 2.5, gelation times for different ratios of oHA/oDex hydrogels and concentrations of ADH were all under 30s. However, an increase in the ratio of oHA resulted in 5 to 10 second lower gelation times. Aurande et al.[63] states that, hydrogels with more than 1% HA polymerize in less than 30sec, which agrees with our results.
Table 2.4 Gelation time to determine optimal concentration of oHA
% (W/V) Gelation time ADH 4% Obs
30% Polymerized solution before
addition of ADH Polymerized
20% <10s Highly viscous
15% <30s Less viscous
3. Results and Discussion
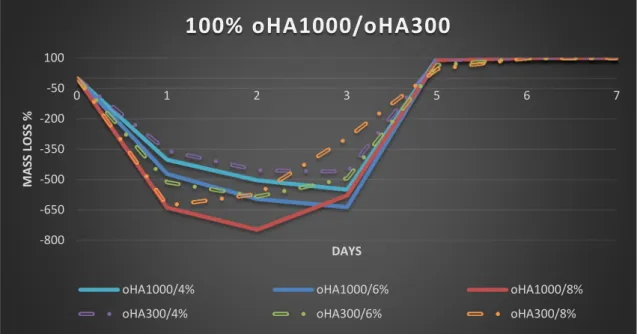
In regards to oDex, an increase in the concentration of ADH results in a reduction of gelation time, due to the increased number of possible crosslinks between ADH and the free aldehydes in oDex. Due to oDex being less viscous and having high solubility, 100% oDex gelation (6min), took longer than that of 100% oHA. According to Molinos
et al.[27] gelation time of this hydrogel, with 5% ADH, should be more than 2min, which is in accordance with our results.
Type of HA Tempo de Gelificação
ADH4% ADH6% ADH8%
100% oHA1000 <30s <30s <30s 100% oHA300 <30s <30s <30s 100% oHA20 <30s <30s <30s 75/25 oDex/oHA <30s <30s <30s 50/50 oDex/oHA <20s <20s <20s 25/75 oDex/oHA <20s <20s <20s
100% oDex ~6 min ~3 min ~2 min
An additional observation that could be made was a slight decrease in gelation time with an increase of ADH (data not shown). This slight increase was of 1 or 2 seconds and observational, therefore difficult to quantify precisely.
The oHA hydrogels can directly be applied to the tissue defects by injection, although the short gelation time [12].
![Figure 4- A model for proposed protein-protein and protein-HA interactions mediated by RHAMM [25]](https://thumb-eu.123doks.com/thumbv2/123dok_br/17612190.820386/29.892.182.749.165.617/figure-proposed-protein-protein-protein-interactions-mediated-rhamm.webp)