Vila Real, 2019
CONTRIBUTION TO THE CHARACTERIZATION OF
REINFORCED SILK FIBERS PULLED OUT OF
SILKWORMS DIRECTLY FED WITH
NANOPARTICLES
Dissertação de Mestrado em Engenharia Biomédica
Nuno Filipe Oliveira Ramos
Orientador:
Prof. Doutor Carlos Alberto Antunes Viegas Universidade de Trás-os-Montes e Alto Douro
Coorientadora:
Prof.ª Doutora Maria Manuela Estima Gomes
Instituto de Investigação em Biomateriais, Biodegradáveis e Biomiméticos (I3Bs), Universidade do Minho
Vila Real, 2019
CONTRIBUTION TO THE CHARACTERIZATION OF
REINFORCED SILK FIBERS PULLED OUT OF
SILKWORMS DIRECTLY FED WITH
NANOPARTICLES
Dissertação de Mestrado em Engenharia Biomédica
Nuno Filipe Oliveira Ramos
Orientador:
Prof. Doutor Carlos Alberto Antunes Viegas Universidade de Trás-os-Montes e Alto Douro
Coorientadora:
Prof.ª Doutora Maria Manuela Estima Gomes
Instituto de Investigação em Biomateriais, Biodegradáveis e Biomiméticos (I3Bs), Universidade do Minho
À minha avó Camila, e ao meu tio Alfredo
“If this book has a lesson, it is that we are awfully lucky to be here-and by 'we' I mean every living thing. To attain any kind of life in this universe of ours appears to be quite an achievement. As humans we are doubly lucky, of course: We enjoy not only the privilege of existence but also the singular ability to appreciate it and even, in a multitude of ways, to make it better. It is a talent we have only barely begun to grasp.”
i
AGRADECIMENTOS
Em primeiro lugar, quero agradecer ao meu orientador, o Prof. Doutor Carlos Viegas, e à minha coorientadora, a Prof.ª Doutora Manuela Gomes, pela oportunidade que me deram em integrar este projeto, por toda a orientação, apoio, confiança e disponibilidade. Agradecer-lhes também, e sobretudo, pelo privilégio que foi para mim poder trabalhar e aprender com pessoas e instituições tão reconhecidas, competentes e talentosas, tanto na Universidade de Trás-os-Montes e Alto Douro (UTAD), como no Instituto de Investigação em Biomateriais, Biodegradáveis e Biomiméticos (I3Bs).
Quero também agradecer, e de forma especial, ao Prof. Doutor Jorge Azevedo, pela aquisição dos ovos dos bichos da seda e por tudo o que me ensinou sobre estes seres, por todo o incentivo e disponibilidade, pelo tratamento estatístico dos dados, e principalmente pela forma como me tratou e recebeu. Tornou-se para mim uma referência, não só como profissional, mas sobretudo como pessoa. Aproveito, também, para agradecer a todos os funcionários e responsáveis do Laboratório de Nutrição e Alimentação Animal da UTAD, por toda a disponibilidade.
À Doutora Margarida Miranda, à Doutora Simone Silva, e à Doutora Albina Franco, quero agradecer toda a colaboração no projeto, tudo o que me ensinaram, todo o incentivo, apoio, disponibilidade e compreensão, e sobretudo por me terem permitido crescer e aprender com os meus próprios erros. A todos os colegas de laboratório no I3Bs, quero agradecer toda a ajuda, simpatia, boa disposição, e por me terem recebido tão bem.
À Prof.ª Doutora Maria dos Anjos Pires do Laboratório de Histologia e Anatomia Patológica da UTAD, quero agradecer o processamento e avaliação histológica dos bichos da seda, toda a disponibilidade e ensinamentos que me forneceu.
Ao Prof. Doutor João Coutinho do Laboratório de Solos e Plantas da UTAD, quero agradecer as análises feitas às amostras.
Quero também agradecer, de forma especial, à Prof.ª Doutora Isabel Dias, por toda a colaboração e envolvência no projeto, pela simpatia, disponibilidade e preocupação.
Por fim, quero agradecer a toda a minha família, especialmente à minha mãe, ao meu pai e ao meu irmão, aos meus amigos do peito e à Lara, por todo o apoio, motivação e paciência. Por caminharem juntos ao meu lado, pelo incentivo, e por nunca me deixarem desistir dos meus sonhos. Obrigado!
iii
RESUMO
Nos últimos anos, têm sido feitas várias tentativas de produzir fibras de seda (FS) com novas e melhoradas funcionalidades, alimentando os bichos da seda com nanopartículas (NP) orgânicas e inorgânicas. Este método “verde” evita o uso excessivo de recursos e mantém as propriedades intrínsecas das FS. As nanopartículas de óxido de ferro (NPOF) surgiram como uma ferramenta importante numa vasta variedade de aplicações biomédicas, como por exemplo como agente de contraste em imagiologia por ressonância magnética (IRM).
Este trabalho teve como objetivo produzir FS funcionalizadas a partir de bichos da seda Bombyx mori alimentados com dietas modificadas contendo diferentes concentrações de NPOF (0.3 wt%, 1.5 wt%, 3 wt%, 4.5 wt% e 9 wt% de NPOF). Para alcançar este objetivo, bichos da seda foram alimentados com dietas modificadas contendo 0.3 wt% e 3 wt% de NPOF no 5º instar, e 4.5 wt% e 9 wt% de NPOF a partir do 3º e 5º instares. A análise por espectroscopia de raios X por dispersão em energia (EDS), revelou a presença de ferro nos casulos do grupo alimentado no 5º instar com 3 wt% de NPOF entre 0-1.50%, dos grupos alimentados a partir do 3º e 5º instares com 4.5 wt% de NPOF entre 0-1.80%, e do grupo alimentado no 5º instar com 9 wt% de NPOF entre 0-3.70%. Nos casulos do grupo alimentado no 5º instar com 0.3 wt% de NPOF não foi detetado ferro. Nas fibras após o degumming do grupo alimentado no 5º instar com 3 wt% de NPOF foi detetado ferro entre 0-0.25%, nas do grupo alimentado com 4.5 wt% de NPOF a partir do 3º instar foi detetado entre 0-0.46%, e nas do grupo alimentado com 4.5 wt% no 5º instar foi detetado entre 0-0.95%. Nas fibras após o degumming do grupo alimentado no 5º instar com 9 wt% de NPOF não foi detetado ferro. Nos filmes de fibroína foi detetado ferro entre 0.09% no grupo alimentado no 5º instar com 3 wt% de NPOF, entre 0-1.48% no grupo alimentado a partir do 3º instar com 4.5 wt% de NPOF, e entre 0-0.62% no grupo alimentado no 5º instar com 4.5 wt% de NPOF. A morfologia das fibras dos casulos, fibras após o degumming e filmes de fibroína foi analisada por microscopia eletrónica de varrimento (MEV). As fibras dos casulos do grupo alimentado no 5º instar com 9 wt% de NPOF mostraram visíveis alterações, relativamente aos restantes grupos. As fibras após o degumming dos grupos alimentados a partir do 3º e 5º instares com 4.5 wt% de NPOF, e no 5º instar com 9 wt% de NPOF apresentaram detritos nas suas superfícies, enquanto que as do grupo alimentado no 5º instar com 3 wt% de NPOF apresentaram uma superfície lisa.
iv
O efeito toxicológico da concentração das NPOF nos bichos da seda B. mori também foi estudado. Para tal, os bichos da seda foram alimentados diariamente no 5º instar, com dietas modificadas contendo 0.3 wt%, 1.5 wt% e 3 wt% de NPOF. O crescimento larval (peso e comprimento) e sobrevivência dos bichos da seda foram monitorizados. Foi, também, avaliada a digestibilidade das dietas e realizada a análise histológica das larvas. Os bichos da seda dos grupos 1.5 wt% e 3 wt% de NPOF mostraram crescimento insuficiente e baixa sobrevivência, enquanto que o grupo 0.3 wt% de NPOF não foi afetado, assemelhando-se ao grupo controlo. O aumento da concentração de NPOF nas dietas levou a um aumento do conteúdo em ferro nas fezes dos bichos da seda. A análise histológica revelou que com o aumento de NPOF surgiram distúrbios celulares, aumento de espaços intercelulares no epitélio, e espaços no lúmen das glândulas sericígenas. O intestino médio mostrou distribuição irregular das células caliciformes e colunares, assim como alterações morfológicas e presença de células em apoptose. Foram observados aglomerados de NPOF no tubo digestivo das larvas, sugerindo que este tenha atuado como barreira, não permitindo que as NPOF fossem totalmente absorvidas nas glândulas sericígenas, e consequentemente incorporadas na seda.
Assim, o uso de surfactantes e o revestimento das NPOF com polímeros parece necessário para prevenir a aglomeração das mesmas e para que consigam ser absorvidas nas glândulas sericígenas, sem provocar lesões no tubo digestivo dos bichos da seda.
Palavras-chave: Fibra de seda, bicho da seda, nanopartículas de óxido de ferro, alimentação, fibroína, glândula sericígena.
v
ABSTRACT
In recent years several attempts have been made to produce silk fibers (SFs) with new and improved functionalities by feeding silkworms with inorganic and organic nanoparticles (NPs). This “green” method avoids the overuse of resources and maintains the intrinsic properties of SFs. Iron oxide nanoparticles (IONPs) emerged as an important tool in a wide range of biomedical applications, such as magnetic resonance imaging (MRI) contrast enhancement.
The present work aimed to produce functionalized SFs from Bombyx mori silkworms fed with modified diets containing different concentrations of IONPs (0.3 wt%, 1.5 wt%, 3 wt%, 4.5 wt% and 9 wt% IONPs). To achieve this purpose, silkworms were fed with modified diets with 0.3 wt% and 3 wt% IONPs at the 5th instar, and 4.5 wt% and 9 wt% IONPs from the 3rd and 5th instars. Energy dispersive X-ray spectroscopy (EDS) analysis revealed the presence of iron on cocoons in the range of 0-1.50% from the group fed with 3 wt% IONPs at the 5th instar, 0-1.80% from the groups fed with 4.5 wt% IONPs from the 3rd and 5th instars, and 0-3.70% from the group fed with 9 wt% IONPs at the 5th instar. No iron was detected on cocoons from the group fed with 0.3 wt% IONPs at the 5th instar. Iron was detected on degummed SFs in the range of 0-0.25% from the group fed with 3 wt% IONPs at the 5th instar, 0-0.46% from the group fed with 4.5 wt% IONPs from the 3rd instar, and 0-0.95% from the group fed with 4.5 wt% IONPs at the 5th instar. No iron was detected on degummed SFs from the group fed with 9 wt% IONPs at the 5th instar. On fibroin films iron was detected in the range of 0-0.09% from
the group fed with 3 wt% IONPs at the 5th instar, and 0-1.48% and 0-0.62% from the groups
fed with 4.5 wt% IONPs from the 3rd and 5th instars, respectively. Herein, the morphology of
the cocoons’ fibers, degummed SFs, as well as films were analyzed by scanning electron microscopy (SEM). The cocoons’ fibers from the group fed with 9 wt% IONPs at the 5th instar showed visible alterations when compared to the other groups. The degummed SFs from the groups fed with 4.5 wt% IONPs from the 3rd and 5th instars and 9 wt% IONPs at the 5th instar presented debris on their surfaces, whereas those from the group fed with 3 wt% IONPs at the 5th instar presented a smooth surface.
The toxicological effect of IONPs concentration on B. mori silkworms was also studied. Herein, the silkworms were fed daily with modified diets containing 0.3 wt%, 1.5 wt%, and 3 wt% IONPs at the 5th instar. The larval growth (weight and length) and viability of the silkworms were monitored. The digestibility of the diets was assessed, and histological analysis
vi
of silkworm larvae was performed. The silkworms from the 1.5 wt% and 3 wt% IONPs groups showed insufficient growth and low viability, whereas the 0.3 wt% IONPs group was not affected, presenting values that can be compared with the control group. The increasing content of IONPs in the diets led to an increase of the iron content in the silkworms' excrement. Histological analysis revealed that increasing content on IONPs led to cellular disturbances, increased intercellular spaces in the epithelium and spaces in the lumen of the silk glands. The midgut sections showed irregular distribution of the goblet and columnar cells, as well as morphological changes and the presence of apoptotic cells. IONPs agglomerates were observed in the alimentary canal of the silkworm larvae suggesting that the alimentary canal worked as a barrier to the IONPs, not allowing them to be fully absorbed into the silk glands and consequently incorporated in silk.
Thus, the addition of surfactants and polymer coatings to the IONPs seems necessary to prevent their agglomeration and to be able to reach the silk glands, without damaging the silkworms' alimentary canal.
vii
TABLE OF CONTENTS
AGRADECIMENTOS ... i
RESUMO ... iii
ABSTRACT ... v
TABLE OF CONTENTS ... vii
LIST OF FIGURES ... xi
LIST OF TABLES ... xvii
LIST OF ABBREVIATIONS, ACRONYMS, AND INITIALISMS ... xix
CHAPTER I. INTRODUCTION (STATE OF THE ART) ... 1
1.1 SILK AS A BIOMATERIAL ... 3
Source ... 3
Composition and structure ... 3
Processing of raw silk ... 5
Properties and biomedical applications ... 6
1.2 SILKWORM (B. mori) LIFE CYCLE ... 7
Larval stage ... 8
Pupal and moth stages ... 8
1.3 ANATOMY AND PHYSIOLOGY OF SILKWORM (B. mori) LARVA ... 9
Alimentary canal ... 9
Silk gland ... 11
Hemolymph ... 11
1.4 FUNCTIONALIZATION AND REINFORCEMENT OF SILK ... 12
Feeding silkworms with modified diets ... 13
Carbon-based nanomaterials ... 17
Multi-walled carbon nanotubes ... 17
Single-walled carbon nanotubes and graphene ... 17
Metal oxide nanoparticles... 18
Fe3O4 nanoparticles ... 18 TiO2 nanoparticles ... 19 Metal nanoparticles ... 22 Cu nanoparticles ... 22 Ag nanoparticles ... 23 Fe nanoparticles ... 24 Luminescent nanoparticles ... 24 Hydroxyapatite nanoparticles ... 26
viii
1.5 IRON OXIDE NANOPARTICLES (IONPs) ... 26
1.6 AIM OF THIS STUDY ... 27
CHAPTER II. MATERIALS AND METHODS ... 29
2.1 MATERIALS ... 31
2.2 FUNCTIONALIZATION OF IONPs ... 32
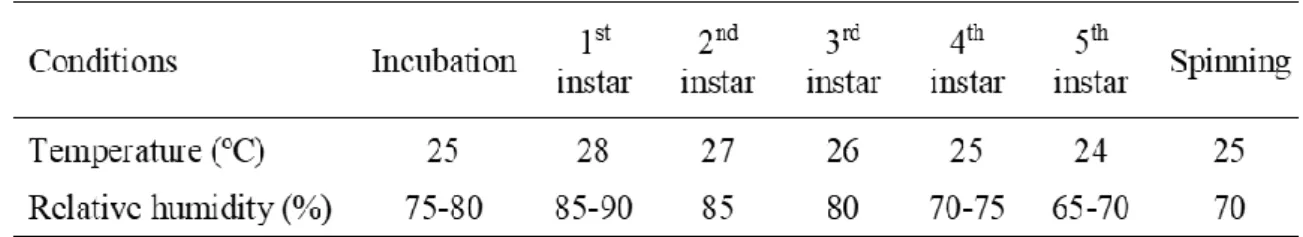
2.3 SILKWORMS REARING ... 33
Preparation of the diets ... 34
Rearing conditions ... 35
2.4 STUDY OF THE ASSESSMENT OF IONPs CONCENTRATION ON B. mori SILKWORMS’ VIABILITY ... 35
Degumming of raw silk ... 37
Processing of silk fibroin ... 37
Dissolution of silk fibroin and dialysis ... 38
Preparation of films ... 38
Morphological and elemental analyses of cocoons, degummed SFs, and films ... 39
SEM and EDS characterization ... 39
2.5 STUDY OF THE TOXICOLOGICAL EFFECT OF IONPs ON B. mori SILKWORMS ... 40
Larval growth ... 40
Digestibility of the diets ... 41
Iron and other element concentrations in silkworm excrement and mulberry leaf powder . 41 Silkworm larvae processing for histological analysis ... 41
Statistical analysis of data ... 43
CHAPTER III. RESULTS... 45
3.1 STUDY OF THE ASSESSMENT OF IONPs CONCENTRATION ON B. mori SILKWORMS’ VIABILITY ... 47
Silkworm survival rate ... 47
Morphological and elemental analyses ... 48
Cocoons ... 48
Degummed SFs ... 50
Films ... 52
3.2 STUDY OF THE TOXICOLOGICAL EFFECT OF IONPs ON B. mori SILKWORMS ... 53
Larval growth ... 53
Weights of the silkworm larvae ... 54
Lengths of the silkworm larvae ... 56
Mortality of the silkworm larvae ... 60
Digestibility of the diets ... 62
ix
Cocoons and pupae ... 66
Histological analysis of the silkworm larvae ... 67
Histological evaluation of the silk glands ... 68
Histological evaluation of the midgut ... 71
CHAPTER IV. DISCUSSION ... 75
CHAPTER V. CONCLUSIONS AND FUTURE PERSPECTIVES ... 83
REFERENCES ... 87
APPENDIX A – Digestibility of the diets ... 97
APPENDIX B – Histological micrographs of silk glands ... 101
xi
LIST OF FIGURES
Figure 1. The structure of B. mori silk. (Adapted from [3]). ... 4
Figure 2. B. mori silk in various formats. (a) The raw silk consists of two fibroin fibers held together with a layer of sericin on their surfaces. (b) After degumming to remove sericin, (c) the fibroin fibers are dissolved in lithium bromide solution followed by dialyzing against water to obtain regenerated fibroin solution. (d) Silk sponges, hydrogels, films, nanofibrous mats, microparticles, and microneedles constructed from the regenerated fibroin solution. (Adapted from [15]). ... 5
Figure 3. Life cycle of B. mori. (Adapted from [22]). ... 7
Figure 4. 5th instar silkworm larva, feeding on a fresh mulberry leaf... 8
Figure 5. Schematic representation of silkworm larva structures and organs. (Adapted from [23]). ... 9
Figure 6. Schematic representation of the B. mori midgut, at larval stage. The midgut is formed by columnar, goblet, and stem cells. (Adapted from [26]). ... 10
Figure 7. Schematic representation of silk gland of B. mori silkworm. (Adapted from [10]). ... 11
Figure 8. Three different approaches to produce functionalized silk: A) by processing the silk after it is produced, B) through genetic modification, and C) through modification of silkworms’ diet. ... 12
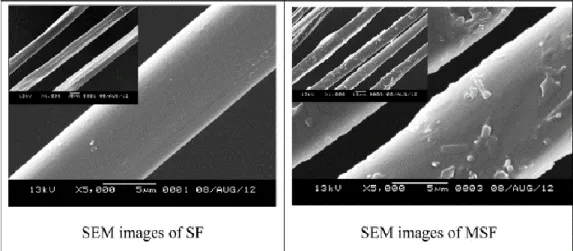
Figure 9. SEM micrographs of the normal silk fiber, SF (left), and magnetic silk fiber, MSF (right). (Adapted from [50]). ... 18
Figure 10. Stress-strain curve of (a) degummed SFs and (b) degummed SFs exposed to UV for 3 h. (Adapted from [51]). ... 19
Figure 11. (Top) Histophysiological examination of the posterior silk gland tissue at 72 h fed with TiO2 NPs. (left) Control; (right) TiO2 NPs. Yellow arrow indicates proteins in the lumen of the posterior silk gland. (Adapted from [63]). (Bottom) Histopathology of the midgut tissue in 5th instar larvae of B. mori under phoxim stress 48 h. (a) Control; (b) TiO2 NPs; (c) Phoxim; (d) TiO2 NPs + Phoxim. Green arrows indicate basal lamina, and yellow arrows indicated karyopyknosis. (Adapted from [66]). ... 21
Figure 12. (a) Silkworm fed with LNPs and (b) the corresponding cocoon under 365 nm UV illumination. (Adapted from [55]). ... 24
Figure 13. 3D confocal image of human colon fibroblast cells stained with fluorescein diacetate after culturing for ten days on a silk fibroin scaffold that was made of intrinsically luminescent silk containing rhodamine B (Adapted from [30]). ... 25
Figure 14. Calcium lignosulfonate molecular structure. ... 31
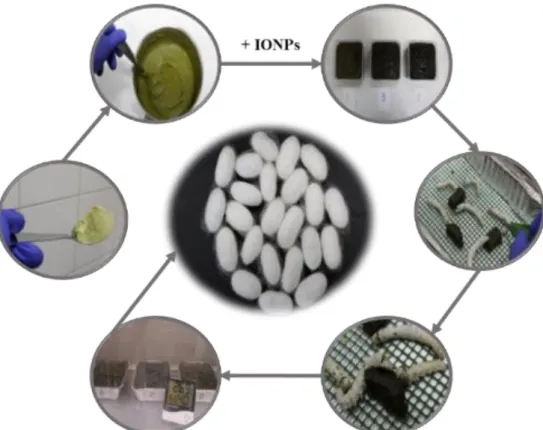
Figure 15. Silkworms hatching and rearing. (a) In a single laying there is from 250 to 1000 and more eggs; (b) silkworm hatching under microscope; (c) newborn silkworms; (d) silkworms feeding on an artificial diet; (e) silkworms in an aluminum foil box with breathable mesh; (f) silkworms reared under controlled conditions; (g) administration of a modified diet containing IONPs; (h) silkworms ingesting a modified diet containing IONPs. ... 33
Figure 16. Normal diet preparation. (a) Mulberry leaf powder; (b) mixing mulberry leaf powder with distilled water; (c) thick green paste obtained after heating the mixture for 6 min and allowed to cool. ... 34
Figure 17. Modified diets containing (a) 0.3 wt% IONPs, (b) 1.5 wt% IONPs and (c) 3 wt% IONPs. It is evident that the color of the modified diets became darker with increasing additive amount of IONPs. ... 34
Figure 18. Scheme of the work plan consisting in preparing modified diets with different amounts of IONPs and feeding them to silkworms at different stages to produce functionalized SFs. ... 36
Figure 19. Schematic representation of the degumming procedure of the cocoons. ... 37
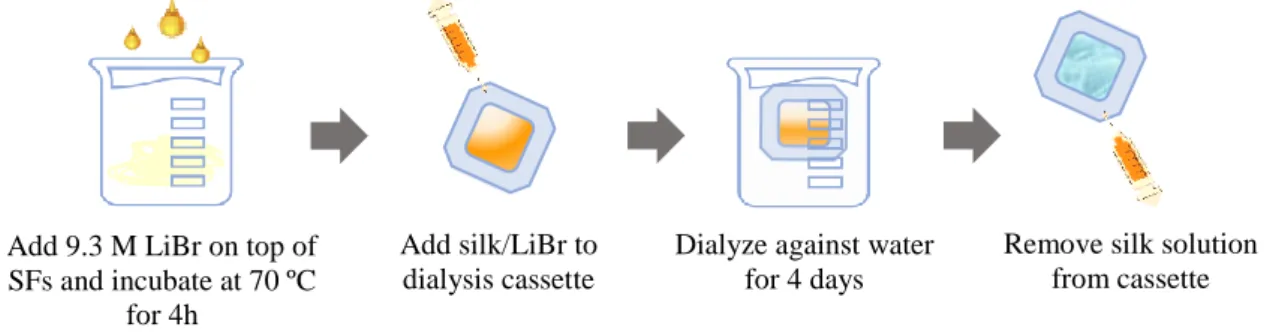
Figure 20. Schematic representation of the dissolution of silk fibroin in LiBr and dialysis. ... 38
Figure 21. Schematic representation of the preparation of films and crystallinity (β-sheet formation) induction. ... 38
xii
Figure 22. Larval growth monitoring. (a) Weighing a silkworm larva on a precision balance. (b)
Preparing to measure silkworm larvae of the group fed with 0.3 wt% IONPs on the 2nd day of their 5th
instar; caliper is visible on the right side of the image. ... 40
Figure 23. Three larvae from the group fed with 0.3 wt% IONPs preserved in 4% buffered formaldehyde
solution. The larvae showed in the image were euthanized on the 7th day of their 5th instar, while spinning
their cocoons. ... 42
Figure 24. SEM images of cocoons from B. mori silkworms from control and experimental groups. (a)
Control group fed with normal diet; (b) experimental group fed with 0.3 wt% IONPs from its 5th instar;
(c) experimental group fed with 4.5 wt% IONPs from its 5th instar; and (d) experimental group fed with
9 wt% IONPs from its 5th instar. Morphological alterations in image (d) are evident. Magnification of
1000 ×. ... 48
Figure 25. SEM images of degummed SFs obtained from silkworms of experimental groups. (a) Fed
with 3 wt% IONPs from its 5th instar; (b) fed with 4.5 wt% IONPs from its 3rd instar; (c) fed with 4.5
wt% IONPs from its 5th instar; and (d) fed with 9 wt% IONPs from its 5th instar. A smooth surface on
degummed SFs of image (a) is noted, while in images (b-d) debris on the surface of degummed SFs are evident. Green arrows - debris. Magnification of 3000 ×. ... 50
Figure 26. SEM images of films prepared from fibroin obtained from silkworms of control and
experimental groups. (a) Control group fed with normal diet; (b) experimental group fed with 3 wt% IONPs from the 5th instar; (c) experimental group fed with 4.5 wt% IONPs from the 3rd instar; and (d)
experimental group fed with 4.5 wt% IONPs from the 5th instar. No evident differences are noted in the
images. Magnification of 1500 ×. ... 52
Figure 27. Average silkworm larvae weights of control group and experimental groups over their 5th
instar. It is evident that the control group and the experimental group fed with 0.3 wt% IONPs maintained an identical and regular increase of weights, until the 7th day of their 5th instars, when they
initiated the formation of the cocoons. The experimental groups fed with 1.5 wt% and 3 wt% IONPs recorded lower weights, extended their 5th instar period and ultimately died, not forming cocoons. Data
are mean ± SD. ... 54
Figure 28. One-way analysis of weight by group. Comparisons for each pair using Student's t.
Comparison circles plot is a visual representation of group mean comparisons. Circles for means that are significantly different either do not intersect, or intersect slightly, so that the outside angle of intersection is less than 90 degrees. A – Experimental group fed with 0.3 wt% IONPs; B – experimental group fed with 1.5 wt% IONPs; C – experimental group fed with 3 wt% IONPs; D – control group fed with normal diet. ... 56
Figure 29. Average silkworm larvae lengths of control group and experimental groups over their 5th
instar. It is evident that the control group and the experimental group fed with 0.3 wt% IONPs maintained an identical and regular increase of lengths, until the 7th day of their 5th instars, when they
initiated the formation of the cocoons. The experimental groups fed with 1.5 wt% and 3 wt% IONPs recorded lower lengths, extended their 5th instar period and ultimately died, not forming cocoons. Data
are mean ± SD. ... 57
Figure 30. One-way analysis of length by group. Comparisons for each pair using Student's t.
Comparison circles plot is a visual representation of group mean comparisons. Circles for means that are significantly different either do not intersect, or intersect slightly, so that the outside angle of intersection is less than 90 degrees. A – Experimental group fed with 0.3 wt% IONPs; B – experimental group fed with 1.5 wt% IONPs; C – experimental group fed with 3 wt% IONPs; D – control group fed with normal diet. ... 59
Figure 31. Cumulative mortality of silkworms of control group and experimental groups recorded over
the days of their 5th instar. It is evident that the groups fed with 1.5 wt% and 3 wt% IONPs were the
xiii
Figure 32. Dead larva from experimental groups (a) fed with 1.5 wt% IONPs, and (b) fed with 3 wt%
IONPs. Black spots or dark color on their bodies are evident. ... 61
Figure 33. One-way analysis of daily mortality by group. Comparisons for each pair using Student's t.
Comparison circles plot is a visual representation of group mean comparisons. Circles for means that are significantly different either do not intersect, or intersect slightly, so that the outside angle of intersection is less than 90 degrees. A – Experimental group fed with 0.3 wt% IONPs; B – experimental group fed with 1.5 wt% IONPs; C – experimental group fed with 3 wt% IONPs; D – control group fed with normal diet. ... 62
Figure 34. Photos of cocoon and pupa from (a) experimental group fed with 0.3 wt% IONPs, and (b)
control group fed with normal diet. No evident differences are observable in the cocoons and pupae from the two groups. ... 66
Figure 35. One-way analysis of (a) empty cocoon weight by group and (b) pupa weight by group.
Comparisons for each pair using Student's t. Comparisoncircles plot is a visual representation of group mean comparisons. Circles for means that are significantly different either do not intersect, or intersect slightly, so that the outside angle of intersection is less than 90 degrees. A – Experimental group fed with 0.3 wt% IONPs; D – control group fed with normal diet. ... 67
Figure 36. Histology of the silk gland tissue stained with H&E in 5th instar larvae of B. mori from
control and experimental groups. (a) Control group fed with normal diet after 5 d; (b) experimental group fed with 0.3 wt% IONPs after 5 d; (c) experimental group fed with 1.5 wt% IONPs after 10 d; (d) experimental group fed with 3 wt% IONPs after 2 d. It is evident the normal architecture of the silk gland and the gland lumen fully filled with silk proteins in images (a, b). The gland structures in images (c, d) show cellular disturbances and increased gland membrane crevice. Red arrows - artefactual space in the lumen; yellow arrows - membrane crevice or intercellular spaces in the epithelium; L - lumen of the silk gland with proteinaceous content. Scale bar: 200 µm. ... 69
Figure 37. Histology of the gland epithelium and lumen of the silk gland tissue stained with H&E in 5th
instar larvae of B. mori from control and experimental groups. (a) Control group fed with normal diet after 5 d; (b) experimental group fed with 0.3 wt% IONPs after 5 d; (c) experimental group fed with 1.5 wt% IONPs after 2 d; (d) experimental group fed with 3 wt% IONPs after 6 d. It is evident the morphological alterations on the epithelium and lumen of the silk glands in images (b-d), where was recorded increased intracellular proteinaceous granular material and heterogeneous secretion in the glandular lumen, compared to the image (a) with normal epithelial cells and homogeneous luminal content. E - epithelium of the silk gland; L - lumen of the silk gland with proteinaceous content. Scale bar: 50 µm. ... 70
Figure 38. Histological images of the midgut tissue stained with H&E in 5th instar larvae of B. mori
from control and experimental groups. (a) Control group fed with normal diet after 5 d; (b) experimental group fed with 0.3 wt% IONPs after 5 d; (c) experimental group fed with 1.5 wt% IONPs after 10 d; (d) experimental group fed with 3 wt% IONPs after 2 d. Midgut consists of a monolayered epithelium (E) in which columnar (a, green arrowhead) and goblet (a, blue arrowhead) cells can be observed. Columnar cells show a thick luminal brush border (a, B), and goblet cells are characterized by a goblet cavity. Sparse stem cells (a, black arrowhead) are localized in the basal region of the epithelium. Morphological alterations were noted between the control and experimental groups. In the control group, the midgut epithelium presents regular columnar and goblet cells, and in the experimental groups it is morphologically altered (b), disorganized and pseudostratified (c, d). Red arrow - irregular distribution of the midgut epithelial cells with space in between; yellow arrows - apoptotic cells; L - lumen of the midgut. Scale bar: 50 µm. ... 71
Figure 39. Histological images of the midgut tissue stained with Prussian blue in 5th instar larvae of B.
mori from control and experimental groups. (a) Control group fed with normal diet after 2 d; (b) experimental group fed with 0.3 wt% IONPs after 5 d; (c) experimental group fed with 1.5 wt% IONPs after 10 d; (d) experimental group fed with 3 wt% IONPs after 2 d. It is visible the IONPs agglomerates
xiv
in the midgut lumen in images (b-d). Blue arrows - IONPs, positive to Prussian blue; E - epithelium of the midgut; L - lumen of the midgut; SG - silk gland. Scale bar: 200 µm. ... 73
Figure 40. Histological images of the midgut lumen stained with H&E (left) and Prussian blue (right)
in 5th instar larva of B. mori from experimental group fed with 3 wt% IONPs after 2 d. IONPs
agglomerates, found on the midgut lumen of silkworm larvae, have diameters up to approximately 50 µm, whereas the size of each nanoparticle is 50-100 nm. L - lumen of the midgut. ... 74
Figure 41. Histological micrographs of the silk gland tissue in 5th instar larvae of B. mori from control
group fed with normal diet: (a) after 2 d; (b) after 5 d; (c) during the spinning of the cocoon. (a1-c3) Hematoxylin and eosin staining. (a1’-c3’) Prussian blue staining. ... 101
Figure 42. Histological micrographs of the silk gland tissue in 5th instar larvae of B. mori from
experimental group fed with 0.3 wt% IONPs: (a) after 2 d; (b) after 5 d; (c) during the spinning of the cocoon. (a1-c3) Hematoxylin and eosin staining. (a1’-c3’) Prussian blue staining. ... 102
Figure 43. Histological micrographs of the silk gland tissue in 5th instar larvae of B. mori from
experimental group fed with 1.5 wt% IONPs: (a) after 2 d; (b) after 9 d; (c) after 10 d. (a1-c3) Hematoxylin and eosin staining. (a1’-c3’) Prussian blue staining. ... 103
Figure 44. Histological micrographs of the silk gland tissue in 5th instar larvae of B. mori from
experimental group fed with 3 wt% IONPs: (a) after 2 d; (b) after 6 d. (a1-b3) Hematoxylin and eosin staining. (a1’-b3’) Prussian blue staining. ... 104
Figure 45. Histological micrographs of the silk gland tissue in 5th instar larvae of B. mori from control
group fed with normal diet: (a) after 2 d; (b) during the spinning of the cocoon. (a1-b2) Alcian blue staining. (a1’-b2’) PAS staining. The blue content is related to its composition based on acid mucopolysaccharides... 105
Figure 46. Histological micrographs of the silk gland tissue in 5th instar larvae of B. mori from
experimental group fed with 0.3 wt% IONPs: (a) after 5 d; (b) during the spinning of the cocoon. (a-b2) Alcian blue staining. (a1’-b3’) PAS staining. Note the intense blue staining in the content of the gland. The PAS revealed different parts in the composition of the content in the b2’ and b3’ animals, more evident when compared with the control group. ... 106
Figure 47. (Top) Histological photomicrograph of the silk gland tissue in 5th instar larva of B. mori from
experimental group fed with 1.5 wt% IONPs after 2 d, stained with Alcian blue. (Bottom) Histological micrographs of the silk gland tissue in 5th instar larvae of B. mori from experimental group fed with 3
wt% IONPs after 6 d: (a) Alcian blue staining; (b) PAS staining. The content of the glands is notoriously different from that of the control group silkworms. ... 107
Figure 48. Histological micrographs of the midgut tissue in 5th instar larvae of B. mori from control
group fed with normal diet: (a) after 2 d; (b) after 5 d; (c) during the spinning of the cocoon. (a1-c3) Hematoxylin and eosin staining. (a1’-c3’) Prussian blue staining. The morphology of the midgut is different along the time, and it is notorious in these images. ... 109
Figure 49. Histological micrographs of the midgut tissue in 5th instar larvae of B. mori from
experimental group fed with 0.3 wt% IONPs: (a) after 2 d; (b) after 5 d; (c) during the spinning of the cocoon. (a1-c3) Hematoxylin and eosin staining. (a1’-c3’) Prussian blue staining. ... 110
Figure 50. Histological micrographs of the midgut tissue in 5th instar larvae of B. mori from
experimental group fed with 1.5 wt% IONPs: (a) after 2 d; (b) after 9 d; (c) after 10 d. (a1-c3) Hematoxylin and eosin staining. (a1’-c3’) Prussian blue staining. ... 110
Figure 51. Histological micrographs of the midgut tissue in 5th instar larvae of B. mori from
experimental group fed with 3 wt% IONPs: (a) after 2 d; (b) after 6 d. (a1-b3) Hematoxylin and eosin staining. (a1’-b3’) Prussian blue staining. The presence of IONPs in the diet led to morphological and functional differences, as observed in these pictures. ... 110
Figure 52. Histological micrographs of the midgut tissue in 5th instar larvae of B. mori from control
xv
staining. (a1’-b’) PAS staining. Note the intense Alcian blue staining in the lamina propria of the gut, and different content in the goblet cells. ... 110
Figure 53. Histological micrographs of the midgut tissue in 5th instar larvae of B. mori from
experimental group fed with 0.3 wt% IONPs: (a) after 5 d; (b) during the spinning of the cocoon. (a1-b) Alcian blue staining. (a’-b2’) PAS staining. Note the intense Alcian blue staining in the lamina propria of the gut, and different content in the goblet cells. ... 110
Figure 54. (Top) Histological photomicrograph of the midgut tissue in 5th instar larva of B. mori from
experimental group fed with 1.5 wt% IONPs after 2 d, stained with PAS. (Bottom) Histological micrographs of the midgut tissue in 5th instar larvae of B. mori from experimental group fed with 3 wt%
xvii
LIST OF TABLES
Table 1. Silk functionalization by feeding silkworms with nanomaterials. ... 15 Table 2. Preparation of four solutions with different amounts of IONPs and LGS and different mixing
times. Ultrasonication treatment is also represented. pH value was adjusted for solutions 2 and 3. .... 32
Table 3. Optimum temperature and humidity requirements of silkworm during various stages. (Adapted
from [78]). ... 35
Table 4. EDS analysis of cocoons from control and experimental groups. Iron was detected in cocoons’
samples of experimental groups fed with 3 wt% IONPs from its 5th instar, fed with 4.5 wt% IONPs from
their 3rd and 5th instars, and fed with 9 wt% IONPs from its 5th instar ... 49
Table 5. EDS analysis of degummed SFs from experimental groups fed with modified diets 3 wt% from
the 5th instar, 4.5 wt% from the 3rd and 5th instars, and 9 wt% from the 5th instar. ... 51
Table 6. EDS analysis of films from the control group and experimental groups fed with 3 wt% IONPs
from the 5th instar, and 4.5 wt% IONPs from the 3rd and 5th instars. Iron was detected in some regions of
the films from the experimental groups... 53
Table 7. Amounts of food and IONPs ingested by the silkworms, feces excreted, and the calculated
values of the digestibility of the diets fed to the control and experimental groups. The normal diet fed to the control group had the highest value of digestibility, whereas the modified diets containing 1.5 wt% and 3 wt% IONPs had the lowest values of digestibility. ... 62
Table 8. Iron and other element concentrations in silkworm feces obtained from the control group and
experimental groups fed with 0.3 wt%, 1.5 wt% and 3 wt% IONPs. ... 64
Table 9. Chemical composition of mulberry leaf powder obtained by ICP-OES analysis. ... 65 Table 10. Digestibility of the modified diet containing 0.3 wt% IONPs. Detailed values of dry matter
of food samples, food ingested, and feces excreted per day... 97
Table 11. Digestibility of the modified diet containing 1.5 wt% IONPs. Detailed values of dry matter
of food samples, food ingested, and feces excreted per day... 98
Table 12. Digestibility of the modified diet containing 3 wt% IONPs. Detailed values of dry matter of
food samples, food ingested, and feces excreted per day. ... 99
Table 13. Digestibility of the normal diet fed to the control group. Detailed values of dry matter of food
xix
LIST OF ABBREVIATIONS, ACRONYMS, AND INITIALISMS
ANOVA Analysis of variance
B. mori Bombyx mori
EDS Energy-dispersive X-ray spectroscopy
GR Graphene
H&E Hematoxylin and eosin
HA Hydroxyapatite
ICP-OES Inductively coupled plasma - optical emission spectrometry
IONPs Iron oxide nanoparticles
LGS Lignosulfonate
LNPs Luminescent nanoparticles
MRI Magnetic resonance imaging
MSF Magnetic silk fiber
MWCO Molecular weight cut-off
MWNTs Multi-walled carbon nanotubes
NPs Nanoparticles
PAS Periodic acid-Schiff
PMMA-co-MAA Poly (methyl methacrylate-co-methacrylic acid)
SD Standard deviation
SEM Scanning electron microscopy
SF Silk fiber
SWNTs Single-walled carbon nanotubes
UV Ultraviolet
v/v Volume per volume ratio (units)
1
CHAPTER I.
INTRODUCTION (STATE
OF THE ART)
3
SILK AS A BIOMATERIAL
Source
Silk is a natural protein-based material with outstanding mechanical properties which can be easily processed into different formats for biomedical applications [1]. Silk proteins are spun into fibers after the complete metamorphosis of silk-producing arthropods, e.g., silkworms, spiders, mites, bees, and scorpions [2]. However, the amino acid composition and structure of silk fibers (SFs) varies between the different species, leading to variation in their mechanical properties, processing steps, and cost-effectiveness between the SFs types [3,4]. SFs derived from silkworms are highly suitable for biomedical applications because of their impressive mechanical properties, stability, biocompatibility, and biodegradability [5].
The silkworms are classified as non-mulberry and mulberry. Non-mulberry silkworms, such as Antheraea species, are wild and heterogeneous in nature and have a relatively wide distribution throughout the world. Non-mulberry silk has recently gained some popularity as a novel biomaterial due to the presence of RGD (arginine-glycine-aspartic acid), a cell adhesion protein found within many matrix proteins [6,7]. Non-mulberry silk has been used in silk scaffolds for different tissues, such as bone and articular cartilage [6,8]. However, when compared to mulberry silk cocoons, non-mulberry silk cocoons are less soluble and have lower yield production of silk [6].
Mulberry silkworms, such as Bombyx mori (B. mori), are one of the first domesticated silk-producing arthropods and currently the leading producer of silk worldwide used in numerous biomedical applications, such as orthopedics, photonics and others [9].
Composition and structure
B. mori silk is made of two different proteins: fibroin (70-80%) and sericin (20-30%). Besides fibroin and sericin, raw silk also contains natural impurities namely, carbohydrates (1.2-1.6%), waxes (0.4-0.8%), inorganic matter (0.7%) and pigment (0.2%) [10].
4
B. mori silk consists of two filaments of fibroin covered and bound together by silk glue, sericin. Silk fibroin found in B. mori cocoons have two different subunits, a heavy chain (~ 325–350 kDa) and a light chain subunit (~ 25 kDa) connected by a single disulfide bond (Figure 1).
The heavy and light chains of silk fibroin differ in amino acid sequences. The heavy chain fibroin is dominated by repetitive sequences of glycine and alanine amino acids, mainly hydrophobic. Light chain fibroin has a more undifferentiated amino acid composition, with non-repetitive sequence, making it more hydrophilic and elastic [11].
Silk fibroin structure consists of two different configurations, silk I and silk II. Silk I is characterized by an α-helix formation and is the water-soluble structure found in the silkworm gland just before the silk is spun. Silk II has an anti-parallel β-sheet crystal conformation and is mainly found after silk is spun. About 55% of raw silk fibroin has the anti-parallel β-sheet structure of silk II, which is insoluble in water. That main secondary structure provides to fibroin enhanced stability, crystallinity and higher mechanical properties [3].
5
Processing of raw silk
Silk fibroin is extracted from raw silk by degumming (Figure 2a, b). Degumming is
performed by heating raw silk cocoons in an alkali aqueous solution, most commonly a dilute sodium carbonate solution, allowing the amide bonds of sericin to be cleaved, leaving only the fibroin fibers intact [3,12]. The removal of sericin is crucial due to its toxic effect [9,13].
The degummed fibroin fibers can be used as suture material [14], or dissolved in lithium bromide solution followed by dialyzing in order to render a soluble solution (Figure 2c) [13,15]. Regenerated silk fibroin solution is often used to produce gels, films, microspheres, tubes or sponges (Figure 2d) [13].
Figure 2. B. mori silk in various formats. (a) The raw silk consists of two fibroin fibers held together with a layer
of sericin on their surfaces. (b) After degumming to remove sericin, (c) the fibroin fibers are dissolved in lithium bromide solution followed by dialyzing against water to obtain regenerated fibroin solution. (d) Silk sponges, hydrogels, films, nanofibrous mats, microparticles, and microneedles constructed from the regenerated fibroin solution. (Adapted from [15]).
6 Once silk fibroin is processed into any format, the final step is often to convert silk I to the crystalline silk II conformation. Solvents, such as methanol or ethanol, eliminate water, forming hydrogen bonds in the amorphous phase of silk, increasing the number of β-sheets. This increases the crystallinity of the fiber as well as its strength and stability [3].
Properties and biomedical applications
SFs toughness is higher than the best synthetic materials, including Kevlar [16]. Regarding strength, silk fibroin is superior to generally used polymeric degradable biomaterials such as collagen and poly(l-lactic acid) (PLA), being thus an excellent polymer candidate for biomedical applications [13]. In fact, silk has been investigated as a biomaterial due to its successful use as a suture material for centuries. In addition to mechanical properties, biodegradability and biocompatibility mentioned above, other significant advantages of silk fibroin include water-based processing and the presence of functional groups, such as hydroxyl, amine and carboxyl, allowing facile chemical modifications [2].
The intrinsic properties of silk fibroin has stimulated its implementation in a great variety of applications, either as a textile material, exploiting its lustrous appearance, smooth texture, and ease of dyeing or as an attractive biomaterial in numerous biomedical fields, such as tissue engineering, drug delivery, optics, sensing or diagnostics, and also in medical surgery as a suture material [15]. Silk based tissue engineering systems cover vascular, neural, skin, bone, cartilage, ligament, tendon, cardiac, ocular, hepatic, spinal cord, inter-vertebral, gastrointestinal tract, bladder, tracheal and eardrum tissue regeneration [2,17]. For instance, silk films applied in full-thickness skin wounds in rats, healed faster with a lower inflammatory response than traditional porcine-based wound dressings [18]. Silk fibroin hydrogels also permitted the regeneration of critical size defects in the trabecular bone of rabbits and resulted in greater trabecular bone volume and thickness, significantly higher mineral and rate of bone formation when compared to poly(D,L lactide–glycolide) [19]. Silk fibroin scaffolds loaded with growth factors such as bone morphogenetic protein-2 (BMP-2) also successfully induced osteogenic differentiation both in vitro and in vivo [20].
In summary, the unique structure of silk fibroin, and its propitious features such as versatility in processing, biocompatibility and controllable degradation, make it a highly requested biomaterial for many clinical functions. Additionally, the exploration of biomedical applications for silk, aside from those already established, is continuously under intense
7 research which leaves a promising future for this family of structural proteins in order to fill clinical needs [5,9,21].
SILKWORM (B. mori) LIFE CYCLE
B. mori is used for sericulture and is one of the most economically important insects in the world. Also, it is a model for multiple biological studies and has contributed significantly to the advancement of research in several scientific fields [22].
During its life cycle (Figure 3), which can last six to eight weeks depending upon
subspecies characteristics and climatic conditions, the silkworm passes through four distinct stages, viz., egg, larva, pupa, and adult moth. Some silkworm subspecies are univoltine, producing only one generation a year, and the second-generation egg goes through a period of hibernation till the next year. Others are bivoltine (two generations a year) or multivoltine (three
8 or more generations a year). Usually, the silkworms distributed in the cold regions are univoltine, those adapted to warm regions are bivoltine, and those in tropical regions are multivoltine. In multivoltine subspecies, the life cycle is the shortest; adults of these silkworms lay nondiapausing eggs, and so, they may yield as many as seven to eight generations in a year in tropical countries [23].
Larval stage
Throughout the larval stage, the silkworm molts its skin four times to grow. The larval life is thus divided into five different stages or instars. Normally, in the uni and bivoltine subspecies, under standard rearing conditions the 1st instar duration is 3-4 days, the 2nd instar is for 2-3 days, the 3rd instar is for 3-4 days, the 4th instar is for 5-6 days, and the 5th instar is for 6-8 days (Figure 3) [22]. The 5th instar is the longest stage when the larvae show maximum consumption of food and high growth (Figure 4). At the end of this stage, the silkworm starts spinning a
cocoon that is composed of 600-1500 m of fiber [2]. Silkworms only eat in the larval stage. Their food source is fresh mulberry leaves, although they can also be fed with artificial diets based on mulberry leaf powder [15,24].
Pupal and moth stages
A silkworm larva takes about 3-4 days to complete a cocoon, and then turns to motionless (movable only abdomen) stage of development, the pupa. The pupal stage lasts for approximately 10 days. Molting to the adult occurs inside the cocoon. The moth emerges after softening the cocoon by orally excreting a particular enzyme, cocoonase. After emerging, the
9 only purpose of adult moth is to find a member of the opposite sex and mate. After which, the female moth lays abundant eggs and a new life cycle begins [22].
ANATOMY AND PHYSIOLOGY OF SILKWORM (B. mori)
LARVA
The silkworm has various structures and organs at the stage of larva and moth. In the case of the larva, some structures and organs more relevant within this thesis are represented in Figure 5.
Alimentary canal
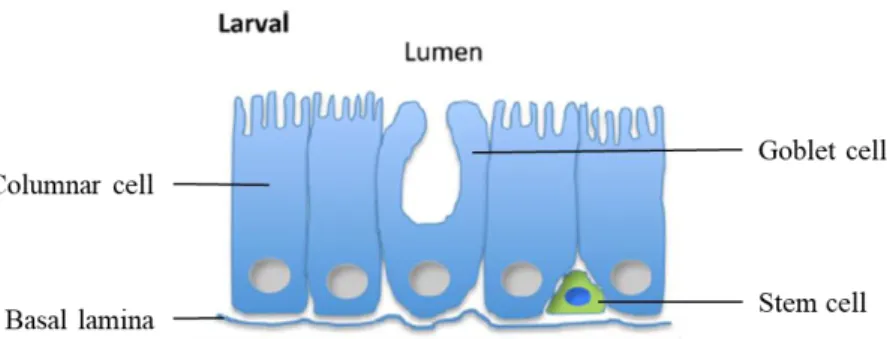
The alimentary canal of the silkworm larva is practically a straight tube from the mouth to the anus divided into three principal sections: the foregut, midgut, and hindgut (Figure 5). The foregut is the first region of the alimentary canal and it is responsible for the passage of food into the midgut [25]. The midgut (pH 9.4-9.8) is a long, wide cylindrical tube, narrow at the posterior end, occupies most of the larval body cavity and it is where digestion and absorption of food mainly take place [23]. It consists of a highly folded monolayered epithelium, supported by a basal lamina, and during the larval stage it is formed by three cell types that are morphologically and functionally distinct: columnar, goblet and stem cells (Figure 6).
10 Columnar and goblet cells are the prevalent cell types. Columnar cells are responsible for the production of digestive enzymes and absorption of nutrients, while goblet cells are involved in the control of ionic homeostasis of the midgut. Clusters of small stem cells are interspersed in the basal region of the epithelium, among the columnar and goblet cells. Stem cells are not only involved in midgut epithelial growth, but they are also responsible for repairing the tissue after damage, and at the pupal stage they are responsible for generating the midgut of the adult moth [26]. The final stages of the digestive process occur in the hindgut (pH 8.4), which is a passage for the absorption of a large portion of food moisture and elimination of digested food [23,25].
Figure 6. Schematic representation of the B. mori midgut, at larval stage. The midgut is formed by columnar,
11
Silk gland
The silk synthesized by the silkworm and spun in the form of a cocoon is initially synthesized in the silk gland. Silk gland of B. mori is a paired organ located at the two lateral sides under the alimentary canal (Figure 5) [10]. Each gland is basically a tube made of glandular epithelium surrounding the lumen. It may be divided into three different regions: anterior, middle and posterior (Figure 7).
Fibroin is secreted from the posterior region. The middle region acts as a reservoir for the maturation of fibroin and secretes sericin, a protein which serves as an adhesive to unite fibroin. The anterior region of the silk gland does not secrete any particular substance and only transports the assembled silk to the spinneret, where the silk is spun out in the form of a thin filament [10,23,27]. It is particularly interesting that the biosynthesis and secretion of fibroin on the gland are observed only during the 5th instar. From the 1st to the 3rd instar larvae, the
posterior silk gland is very rudimentary, and even in the 4th instar larvae it is minimal [27].
Hemolymph
The silkworm has an open circulatory system containing hemolymph (pH ~ 6.3-6.5), which surrounds all organs. Nutrients and oxygen are delivered to all parts of the silkworm body
12 through the hemolymph [28]. Hemolymph is not only a nutrient medium for tissues, but it also contains a stock of the nutritious materials spent during starvation, metamorphosis and at the stage of the moth [23].
FUNCTIONALIZATION AND REINFORCEMENT OF SILK
To expand the applicability of silk fibroin, we can introduce new functionalities through the incorporation of different functional components in the silk material. Some examples of functional components include dyes [29,30], drugs, peptides, proteins [31], metals/semiconductors, nanoparticles (NPs) [32–35] and conductive polymers [15]. The functionalized silk can effectively provide cellular response, antibacterial activity, stimuli-responsive ability, therapeutic effect, electrical conductivity, specific optical properties, and/or sensing functions [5,30,31,36–39]. There are three different approaches to produce functionalized silk, i.e. through post-functionalization of silk, genetically modified silkworms, and feeding silkworms with modified diets (Figure 8) [15,29].Post-functionalization involves the functionalization of SFs already produced or regenerated fibroin by functional component incorporation and/or morphological molding [15]. This strategy comprises physical techniques, such as ultraviolet (UV) or gas treatment, and chemical approaches, by using chemical agents and nanomaterials [40]. For instance, silver [41] and silver chloride [42] NPs were added to SFs externally to obtain antibacterial function through dip-coating technique. Also, Chang et al. [43] produced silk fibroin coated Mn2+-doped ZnSe quantum dots by a γ-radiation approach, exhibiting magnetic properties.
Figure 8. Three different approaches to produce functionalized silk: A) by processing the silk after it is produced,
13 Another strategy to produce functionalized silk is by using genetically modified silkworms [44–46]. Previous studies have inserted the genes of fluorescent proteins into silkworms in attempts to explore the use of these animals as bioreactors to produce recombinant proteins [44,45]. For instance, a vector containing a fluorescent gene was introduced into the silkworm genome to produce fluorescent colored silk to be used in the production of fabrics and biomaterials for medical applications [45]. Genetic engineering is a promising strategy for the production of massive recombinant proteins of biomedical and pharmaceutical interest [44]. However, more efforts are needed before it can be used to produce functionalized silk on a large scale because a stable transfer of transgene to the next generation remains a significant challenge [47]. Moreover, this alternative strategy for producing functionalized silk faces anti-GMO (genetically modified organism) consumer sentiment and market resistance [48].
Apart from the post-functionalization of silk and gene transfection strategies, the development of functionalized silk by feeding silkworms with modified diets containing functional substances (e.g., NPs [34,35,49–53] and dyes [30,48,54]) is currently not fully explored. This approach has proven to be a greener method of producing functionalized silk because it eliminates the need for overuse of resources such as water, energy or additional chemicals associated to the post-treatments of silk. Furthermore, it was shown that functionalization of silk via feeding can be achieved without undermining the intrinsic properties of SFs including the mechanical strength and elasticity [30]. With a detailed knowledge of the factors that allow the in vivo uptake of functional substances into silk fibroin, multiple functional components (e.g., growth factors, drugs, NPs, microbial and anti-inflammatory agents) can be designed/selected for favorable uptake into fibroin fibers for producing innovative biomaterials/devices with added functionalities (e.g., biocompatible optical devices, tissue engineering scaffolds, wound dressings, drug delivery carriers, microfluidics sensors, etc.) [15].
On section 1.4.1, a review of the literature regarding the production of functionalized/reinforced silk by feeding silkworms with modified diets containing inorganic and organic NPs is presented.
Feeding silkworms with modified diets
In recent years several attempts have been made to produce SFs with improved properties or new functionalities by feeding silkworms with inorganic and organic NPs. B. mori silkworms have been fed with different NPs (carbon-based nanomaterials, metal oxide, metal, luminescent
14 or hydroxyapatite NPs). This feeding strategy led to the enhancement of mechanical, electrical and thermal properties of SFs, as well as magnetic or luminescent functionalization. Table 1 summaries recent works on functionalization of silk through feeding silkworms with modified diets.
15
Table 1. Silk functionalization by feeding silkworms with nanomaterials.
Nanoparticles type Concentration Size Feeding period Feeding method Silk functionalization References
Multi-walled carbon nanotubes (MWNTs) MWNTs modified with LGS Diameter 10-30 nm + 35 nm (LGS coating) N/D Sprayed on mulberry leaves - Enhanced mechanical properties (stress and strain at break)
comparable with spider fiber - Enhanced electrical properties (conductivity) - Enhanced thermal stability [49] Single-walled carbon nanotubes (SWNTs) SWNTs modified with LGS solutions 0.2; 1.0 wt% Diameter 1-2 nm; length 10-30 m 3rd instar to spinning Sprayed on mulberry leaves - Enhanced mechanical properties (stress and strain at break and toughness modules) - Enhanced thermal stability - Enhanced electrical conductivity [52]
Graphene (GR) GR modified with LGS solutions 0.2; 2.0 wt% Nanoplates thickness 6-8 nm; width 5 m 3rd instar to spinning Sprayed on mulberry leaves - Enhanced mechanical properties (stress and strain at break and toughness modulus) - Enhanced thermal stability - Enhanced electrical conductivity [52]
Fe3O4 N/D (powder) 100 nm 1 month Sprayed on
mulberry leaves
- Magnetic properties - Enhanced thermal
stability
16
Nanoparticles type Concentration Size Feeding period Feeding method Silk functionalization References
- Enhanced mechanical properties (stress and strain at break) TiO2 TiO2 1%, 2%, 3% and 4% [51]; 100 mg in ultrapure water [34] 50-400 nm [51]; 30-50 nm [34] 2nd day of 5th instar to spinning [51]; 3rd to 7th day of 5th instar [34] Mixed in mulberry powder [51]; sprayed on mulberry leaves [34] - Enhanced mechanical properties (stress and strain at break) - Enhanced ultraviolet resistant property - Increased SF diameter [34,51] Cu 100 mg in ultrapure water [34];10 mg/mL [35] 30-50 nm [34]; 20 nm [35] 3rd to 7th day of 5th instar [34]; 1st day of 5th instar to spinning [35] Sprayed on mulberry leaves - Enhanced mechanical properties - Increased SF diameter - Enhanced thermal stability [34,35] Ag 10 mg/mL 20 nm 1st day of 5th instar to spinning Sprayed on mulberry leaves - Enhanced mechanical properties - Enhanced thermal stability [35] Fe 100 mg in ultrapure water 30-50 nm 3rd to 7th day of 5th instar Sprayed on mulberry leaves - Enhanced mechanical properties - Increased SF diameter [34] Alq3 encapsulated in PMMA-co-MAA nanoparticles N/D 100 nm N/D Sprayed on mulberry leaves - Luminescent silk - Enhanced mechanical properties [55]
HA N/D (powder) N/D 5th instar Sprayed on
mulberry leaves
- Enhanced mechanical properties
[53]
Abbreviations: MWNTs: multi-walled carbon nanotubes; SWNTs: single-walled carbon nanotubes; GR: graphene; HA: hydroxyapatite; Alq3 encapsulated in
17
Carbon-based nanomaterials Multi-walled carbon nanotubes
Silkworms were fed with multi-walled carbon nanotubes (MWNTs) by Wang et al. [49]. The authors reported the reinforcement of SFs by feeding silkworms with MWNTs modified with lignosulfonate (LGS) [49]. Comparing with normal SF by scanning electron microscopy (SEM), the MWNTs SF showed a sort of debris on the surface, suggesting that the presence of MWNTs in the silk protein mixture before spun influenced the silkworm spinning.
While normal SF presented a degradation temperature at about 170 ºC, the presence of MWNTs enhanced the degradation temperature to 217 ºC, indicating that the incorporation of the MWNTs can enhance the thermal stability of SF. The MWNTs SF also showed enhanced mechanical properties, being comparable with those of the spider fiber [56]. The stress and strain at break of MWNTs SF increased 2-fold and 1.4- fold, respectively, comparing to normal SF. The conductivity of MWNTs SF was also enhanced, as its electrical resistance decreased 0.8-fold, when compared to normal SF [49].
The MWNTs SF exhibited new functionalities and is certainly suitable for biomedical applications where both mechanical and electrical properties are required [57].
Single-walled carbon nanotubes and graphene
Other carbon nanomaterials, as single-walled carbon nanotubes (SWNTs) or graphene (GR), were also fed to silkworms to obtain reinforced SFs [52]. SWNTs and GR were dispersed in deionized water with calcium LGS and sprayed on mulberry leaves at different concentrations (0.2 and 1.0 wt% SWNTs, and 0.2 and 2.0 wt% GR). The cocoons obtained from the silkworms fed with the carbon nanomaterials exhibited similar colors and uniform sizes to the control [52].
The SFs obtained from silkworms fed with 0.2 wt% SWNTs and 0.2 wt% GR showed enhanced mechanical properties. The stress at break, strain at break and toughness modulus, which is defined as the area under the stress-strain curve and characterizes the toughness of the SF, of SWNTs SF increased 1.6-, 1.3- and 2.1-fold, respectively, and those of GR SF increased 1.6-, 1.1- and 1.7-fold, respectively, comparing to normal SF. The authors found that the SWNTs or GR incorporated into the SFs hindered the conformation transition from α-helix and random coils to β-sheet structures, which led to the increase of strain at break and toughness modulus due to the presence of more easily movable chains. However, for higher concentrations
18 of SWNTs (1.0 wt%) and GR (2.0 wt%) in the diets, these nanofillers may aggregate into the SFs and act as defects, resulting in low mechanical properties [52].
The modified SFs also showed enhanced electrical conductivity in comparison with the normal SF, and can thus be suitable for electrically induced drug release and other biomedical applications [57]. The researchers also found that although SWNTs or GR were successfully incorporated into the spun SFs, parts of the fed carbon nanomaterials went into the excrement of silkworms [52].
Metal oxide nanoparticles Fe3O4 nanoparticles
Researchers also fed silkworms with Fe3O4 NPs to obtain magnetic SF (MSF) [50]. SEM
micrographs (Figure 9) showed the surface of MSF presenting a sort of debris, in comparison
with normal SF. According to the authors, these findings proved that the Fe3O4 was embedded
in MSF. The MSF showed enhanced thermal stability and mechanical properties, compared with the normal SF [50].
The MSF presented effective magnetic properties when exposed to an external magnetic field, whereas the normal SF didn´t show any magnetic response, as expected. Although the MSF obtained through this feeding method showed lower saturation magnetization (0.3 emu/g) in comparison with literature reported values of Fe3O4 NPs/Ag NPs dual-coated SFs prepared
via electrostatic assembly (3.2 emu/g) [58] and magnetic silk fibroin scaffolds integrating
Figure 9. SEM micrographs of the normal silk fiber, SF (left), and magnetic silk fiber, MSF (right). (Adapted
19 magnetic NPs via dip-coating (2.7 emu/g) [59], the results in this study indicated that this greener method can be used to obtain SFs with magnetic responsiveness.
TiO2 nanoparticles
TiO2 NPs were mixed in mulberry powder and given to feed silkworms to improve the
mechanical properties of silk and to give UV resistance to silk by Cai et al. [51]. Although the silkworms of all groups did not exhibit any difference, the color of their excrement became shallow with increasing amount of TiO2. In fact, the authors found that a certain content of TiO2
were absorbed by the silkworms and entered the silkworm gland, while most of the TiO2 NPs
were excreted from the silkworms. It was also observed that the modified diets containing TiO2
did not cause any reduction of food intake, death of silkworms, or differences on the growth rate of silkworms [51].
The TiO2 modified SFs had higher α-helix and random coil content and lower β-sheet
content than control SF, which suggested that TiO2 hinders the conformation transition from
random coil/α-helix to β-sheet of silk fibroin. The stress-strain curves of the degummed SFs obtained are shown in Figure 10a. It is evident that TiO2 NPs had a remarkable influence on
the mechanical properties of SFs. TiO2-1% SF exhibitted a value of stress at break of 548 ± 33
MPa, exceeding 39% that of control SF, and although the stress at break of the TiO2-2% SF
was similar to the control SF, the strain at break increased 1.4-fold. However, when increasing the amount of TiO2 NPs to 3% and 4%, the modified SFs presented lower values of stress and
strain at break than control SF. The stress-strain curves of control, TiO2-1%, and TiO2-2% SFs Figure 10. Stress-strain curve of (a) degummed SFs and (b) degummed SFs exposed to UV for 3 h. (Adapted from
20 after exposed to UV light for 3 h are shown in Figure 10b. The stress and strain at break of the UV irradiated SFs were lower than those of the unexposed SFs, because the UV light may break protein chains. However, while the stress at break of TiO2-1% and TiO2-2% SFs only decreased
by 15.9% and 25.4%, respectively, the control SF showed a decrease of 52.9%. Therefore, the addition of TiO2 NPs improved the UV resistance of silkworm silk [51].
The functional SFs with exceptional mechanical properties and UV resistance obtained through this method can be used for non-conventional textiles as well as tissue engineering.
Wu et al. [34] also fed B. mori larvae with TiO2 NPs. The diameter, Young’s modulus,
stress and strain at break of TiO2 modified SF increased 1.3-, 1.2-, 1.5- and 1.2-fold,
respectively, in comparison with normal SF.
Whereas the normal SF had a smooth surface, the TiO2 modified SF exhibited debris on the
surface and, although no TiO2 NPs were observed directly on the surface of TiO2 modified SF,
those different morphologies indicated that the NPs might had an influence on the silkworm spinning process [34].
To better understand the distribution of TiO2 NPs in the SF and silk gland and the possible
transport mechanism of NPs in B. mori, the TiO2 contents in the SF, middle silk gland and
posterior silk gland were determined. The results confirmed that the feeding of TiO2 NPs
resulted in the increases of TiO2 content in the SF and silk gland. After the feeding, the TiO2
NPs entered the hemolymph from the alimentary canal of the silkworms, went into the silk gland, and subsequently ended up in the SFs, generating composite SFs that might be helpful in biotechnological and biomedical applications because of their excellent mechanical properties and biocompatibility.
21 Several other studies [60–68] have been carried out feeding silkworms with TiO2 NPs.
However, the purpose of these studies was to investigate the impact that TiO2 NPs had on B.
mori growth, feed efficiency, and cocoon quality, and not exactly the functionalization or reinforcement of the SFs. For instance, TiO2 NPs improves fibroin synthesis when added to
silkworms' diet (Figure 11, top) [63,64].
Figure 11. (Top) Histophysiological examination of the posterior silk gland tissue at 72 h fed with TiO2 NPs. (left)
Control; (right) TiO2 NPs. Yellow arrow indicates proteins in the lumen of the posterior silk gland. (Adapted from
[63]). (Bottom) Histopathology of the midgut tissue in 5th instar larvae of B. mori under phoxim stress 48 h. (a)
Control; (b) TiO2 NPs; (c) Phoxim; (d) TiO2 NPs + Phoxim. Green arrows indicate basal lamina, and yellow arrows

![Figure 5. Schematic representation of silkworm larva structures and organs. (Adapted from [23])](https://thumb-eu.123doks.com/thumbv2/123dok_br/15796950.1078807/37.892.164.710.422.615/figure-schematic-representation-silkworm-larva-structures-organs-adapted.webp)
![Figure 7. Schematic representation of silk gland of B. mori silkworm. (Adapted from [10])](https://thumb-eu.123doks.com/thumbv2/123dok_br/15796950.1078807/39.892.280.613.356.728/figure-schematic-representation-silk-gland-mori-silkworm-adapted.webp)