Ana Maria Alves Machado Gonçalves da Silva
EVALUATION OF ANTIMICROBIAL STRATEGIES
AGAINST BIOMATERIAL-ASSOCIATED INFECTIONS
– IMPACT OF THE SURROUNDING ENVIRONMENTAL
CONDITIONS
Escola de Engenharia
Ana Maria Alves Machado Gonçalves da Silva
Evaluation of antimicrobial strategies against
biomaterial-associated infections
– impact of the surrounding
environmental conditions
Trabalho realizado sob a orientação da Professora Doutora Maria Olívia Pereira e da
Professora Doutora Anália Lourenço
DECLARAÇÃO
Nome: Ana Maria Alves Machado Gonçalves da Silva
Título dissertação: Evaluation of antimicrobial strategies against biomaterial-associated infections - impact of the surrounding environmental conditions
Orientador: Doutora Maria Olívia Pereira Co-orientador: Doutora Anália Lourenço Ano de conclusão: 2015
Designação do Mestrado: Mestrado em Bioengenharia Escola: de Engenharia
Departamento: de Engenharia Biológica
DE ACORDO COM A LEGISLAÇÃO EM VIGOR, NÂO É PERMITIDA A REPRODUÇÃO DE QUALQUER PARTE DESTA DISSERTAÇÃO
Universidade do Minho, 31/01/2015
Assinatura:
Acknowledgements
First I would like to thank to my supervisors Prof. Dr. Olívia Pereira and Prof. Dr. Anália Lourenço for all the support and guidance throughout the course of the work. A special thanks to Prof. Dr. Olívia Pereira for her patient in answering all my doubts and always being willing to help in any situation.
A huge thanks to Diana for teaching me all the laboratory techniques, for all the guidance and knowledge transmitted, for her patient and support.
I would like to thank to Ana Margarida for taking my work further, teaching me all about colony morphology and for her willingness to support my work at any time.
I would also like to thank to the MOP group and to the Biofilm Group colleagues, namely to Maria João, Marta Leite, Daniela Machado, Joana Castro and Daniela Correia. A especially thanks to Tânia and Sofia for their support, company and friendship during all our working days. I would also like to thank to Marisa and Vânia for their friendship support and company and for making my work days more fun.
A special thanks to Prof. Dr. Luísa Peixe and Dr. Angela Novais from the Faculty of Pharmacy of the University of Porto for their gentle cooperation in my work.
Finally, a special thanks to Pedro, to my family and friends for always being present and for accompanying this thesis with full support.
Abstract
Bacterial colonisation of biomedical devices frequently leads to biofilm formation on its surface, originating biomaterial-associated infections (BAI). Antibiotherapy is the most common BAI treatment, however with development of antibiotic resistance, antimicrobial peptides (AMP) are emerging as potential alternatives. However, salinity, an intrinsic condition of the human body, may impair AMP action. Moreover, the hypoxic conditions characteristic of BAI can modulate the establishment and control of the infection. Thus, the main goals of this work were to understand how different oxygen and salt concentrations can affect single and polymicrobial biofilm establishment and the efficiency of the AMP colistin in a prophylactic approach. The microbial composition of the mixed biofilms and the potential development of resistance phenomena were also assessed.
The experimental work was conducted using Klebsiella pneumoniae, Proteus mirabilis and Pseudomonas aeruginosa as models of infection, testing different NaCl and oxygen conditions. Colistin minimum inhibitory and minimum bactericidal concentrations against single and polymicrobial planktonic cultures were determined. Biofilm formation and susceptibility to colistin was also evaluated through the determination of biomass production and biofilm-cells viability. Relative distributions of microorganisms within polymicrobial biofilms were assessed and colony morphology was observed. A population analysis profile was conducted in planktonic and biofilm cultures of K. pneumoniae in order to access the occurrence of heteroresistance.
Sodium chloride seemed to impair colistin action in P. aeruginosa planktonic cultures. In biofilms, it seemed to promote biomass production namely in P. aeruginosa, however the effect of NaCl on colistin action was highly dependent on the microorganism. Prevalence of P. aeruginosa in polymicrobial biofilms was significantly reduced in the presence of NaCl. In general, oxygen deficiency impaired P. aeruginosa biofilm formation and the antibacterial action of colistin both in planktonic and biofilm lifestyles, which was more notorious in presence of NaCl. K. pneumoniae revealed to possess a sub-population resistant to colistin exclusively associated with biofilms and characterized by small colony morphologies.
In conclusion, colistin appeared to be an antimicrobial with a questionable efficacy in the treatment of BAI. It was not possible to establish a relationship between the presence of NaCl and the action of colistin, however NaCl seemed to modulate P. aeruginosa biofilm formation exerting a bactericidal effect or by interfering with its motility. In hypoxic conditions, colistin action is impaired and biofilm formation is significantly lower. K. pneumoniae was found to be a strain heteroresistant to colistin and this phenomenon seemed to be closely linked to biofilm formation. To our knowledge this is the first report linking heteroresistance to biofilm formation and to a morphological distinct sub-population.
Keywords
Resumo
A colonização bacteriana de dispositivos biomédicos conduz frequentemente à formação de biofilmes na sua superfície originando as infecções associadas aos biomateriais (IAB). A antibioterapia é o tratamento mais comum, no entanto, com o desenvolvimento da resistência aos antibióticos, os péptidos antimicrobianos (PAM) estão a emergir como potenciais alternativas. No entanto, a salinidade, uma condição intrínseca do corpo humano, pode prejudicar a acção dos PAM. Além disso, a hipoxia, característica das IAB, pode modular o estabelecimento e o controlo da infecção. Os principais objectivos deste trabalho foram compreender como diferentes concentrações de oxigénio e sal podem afectar a formação de biofilmes simples e mistos, e a eficiência do PAM colistina, numa abordagem profiláctica. A composição microbiana dos biofilmes mistos e o potencial desenvolvimento de fenómenos de resistência foram também estudados. O trabalho experimental foi realizado utilizando Klebsiella pneumoniae, Proteus mirabilis, e Pseudomonas aeruginosa como modelos de infecção testando diferentes condições de NaCl e de oxigénio. A concentração mínima inibitória e bactericida da colistina em culturas planctónicas simples e mistas foi determinada. A formação de biofilme e a sua susceptibilidade à colistina foi também avaliada recorrendo à determinação da produção de biomassa e da análise da viabilidade celular do biofilme. As distribuições relativas de microorganismos dentro de biofilmes mistos foram determinadas e a morfologia das colónias foi observada. Uma análise ao perfil populacional (APP) foi realizada em culturas planctónicas e de biofilme de K. pneumoniae de modo a detectar a ocorrência de heterorresistencia.
O NaCl pareceu prejudicar a acção da colistina nas culturas planctónicas de P. aeruginosa. Em biofilmes, pareceu promover a acumulação de biomassa nomeadamente em P. aeruginosa, no entanto o efeito do NaCl sobre a acção da colistina foi altamente dependente do microrganismo. A prevalência de P. aeruginosa em biofilmes mistos foi significativamente reduzida em presença de NaCl. No geral, a deficiência de oxigénio inibiu a formação de biofilme em P. aeruginosa e a acção antibacteriana da colistina tanto nos ensaios planctónicos como nos de biofilme, o que foi potenciado na presença de NaCl. K. pneumoniae revelou possuir uma sub-população resistente à colistina exclusivamente associada a biofilmes e caracterizada por morfologias de colónia pequena. Em conclusão, a colistina parece ser um agente antimicrobiano com uma eficácia discutível no tratamento de IAB. Não foi possível estabelecer uma relação entre a presença de NaCl e a acção da colistina, no entanto, o NaCl parece modular a formação de biofilmes em P. aeruginosa, exercendo um efeito bactericida ou por interferir com a sua motilidade. Em condições de hipoxia, a acção da colistina é prejudicada e a formação e biofilme é significativamente menor. K. pneumoniae revelou-se uma estirpe heterorresistente à colistina e este fenómeno parece estar intimamente ligado à formação de biofilme. Ao nosso conhecimento, este é o primeiro estudo a associar a heterorresistencia à formação de biofilme e a uma sub-população morfologicamente distinta.
Palavras-chave
List of Contents
ACKNOWLEDGEMENTS ... v
ABSTRACT ... vii
RESUMO ... ix
LIST OF CONTENTS ... xi
LIST OF ABBREVIATIONS ... xiii
LIST OF TABLES ... xv
LIST OF FIGURES ... xvii
1. Introduction ... 1 1.1 Background ... 3 1.2 Main objectives ... 3 1.3 Thesis outline ... 3 2. Literature review ... 5 2.1 Biomaterial-associated infections ... 7
2.1.1 Some biomaterials and their applications ... 7
2.1.2 The origin of infection ... 9
2.1.3 Infection on primary and secondary implant ... 9
2.1.4 Biofilm formation and microbial adhesion... 10
2.2 Antimicrobial strategies ... 15
2.2.1 Preventive/prophylactic strategies ... 15
2.2.2 Therapeutic strategies ... 17
2.2.3 Biofilm disruption agents ... 21
2.3 Environmental conditions surrounding BAI ... 22
2.3.1 The importance of reducing oxygen conditions ... 22
2.3.2 The importance of salinity ... 24
3. Materials and Methods ... 27
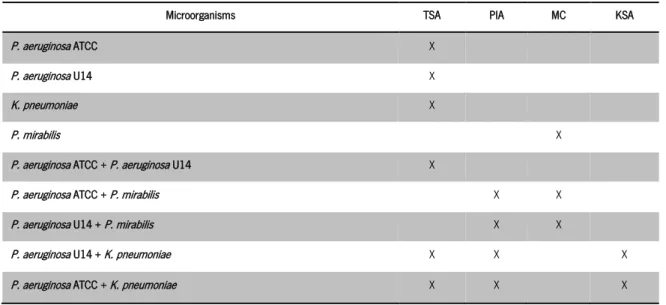
3.1 Microorganisms ... 29
3.2 Culture media ... 29
3.3 Microorganisms preservation... 29
3.4 Antibacterial agent ... 30
3.6 Inocula preparation ... 30
3.7 Adjustment of cell concentration ... 30
3.8 Determination of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration ... 30
3.9 Biofilm formation ... 31
3.11 Determination of biofilm adhered biomass ... 32
3.12 Determination of biofilm-cell viability and cultivability ... 32
3.13 Assessment of the impact of NaCl on colistin action ... 33
3.14 Evaluation of the impact of oxygen deficiency on colistin action ... 33
3.15 Population analysis profile (PAP) ... 33
3.16 Observation of colony morphology ... 34
1.17 Statistical analysis ... 34
4. Results and Discussion ... 35
4.1 Effect of colistin on planktonic bacterial cultures - impact of NaCl and oxygen deficiency ... 37
4.2 Impact of NaCl on biofilm formation ... 40
4.3 Impact of NaCl on biofilm susceptibility to Colistin ... 42
4.3.1 Mono-species biofilms... 42
4.3.2 Polymicrobial biofilms ... 45
4.4 Impact of oxygen deficiency on biofilm formation ... 51
4.5 Impact of oxygen deficiency on biofilm susceptibility to colistin ... 53
4.6 Colony morphology ... 55
4.7 Phenotypic heterogeneity in K. pneumoniae biofilms as a strategy to survive to colistin ... 57
4.7.1 Population analysis profile... 57
4.7.2 Small morphotype analysis ... 60
5. Conclusions and future work ... 63
5.1 Conclusions ... 65
5.3 Future work ... 66
List of Abbreviations
AHL’s Acyl homoserine lactones AMP Antimicrobial peptide
BAI Biomaterial-associated infection
CAUTI Catheter-associated urinary tract infection CF Cystic fibrosis
CFTR Cystic fibrosis transmembrane regulator CFU Colony Former Units
CLSI Clinical and laboratory standards institute CMS Colistimethate sodium
CO2 Carbon dioxide
CRKP Carbapenem resistant Klebsiella pneumoniae DNA Deoxyribonucleic acid
EPS Extracellular polymeric substance ESBL Extended spectrum beta lactamases
EUCAST European Committee on Antimicrobial Susceptibility Testing IFN-ᵞ Interferon gamma
KSA Klebsiella Selective Agar LPS Lipopolysaccharide
MBC Minimum bactericidal concentration MC MacConkey Agar
MDR Multi drug resistant MHA Muller Hinton Agar MHB Muller Hinton Broth
MIC Minimum inhibitory concentration MRSA Multi resistant Staphylococcus aureus NaCl Sodium chloride
NCCLSI National Committee for Clinical and Laboratory Standards OD Optical density
PAP Population analysis profile PIA Pseudomonas Isolation Agar PMMA Polymethylmethacrylate PQS Pseudomonas quinolone signal QS Quorum sensing
SCV Small colony variants SM Small morphotype TSA Tryptic Soy Agar TSB Tryptic Soy Broth
UHMWPE Ultra-high-molecular-weight polyethylene UTI Urinary tract infection
VAP Ventilator associated pneumonia WTM Wild type morphotype
List of Tables
Table 1 Examples of biomaterials and their main applications, advantages and disadvantages, and some alternatives to avoid infection. ... 8 Table 2 Some of the most common biomedical devices, the material composing them and their typical colonizer
microorganisms. ... 10 Table 3 Different strategies for biomaterial functionalization and their applications. Adapted from Busscher et al.,
(2012) ... 16 Table 4 Colistin spectrum of action. (Biswas et al., 2012; Landman et al., 2008; García-Rodríguez et al., 1989;
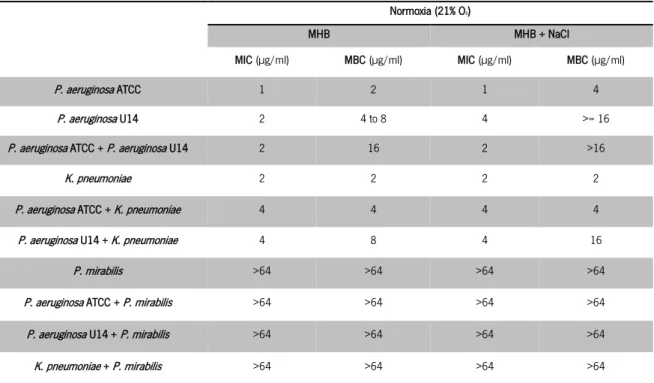
Giamarellou & Poulakou, 2009; Li et al., 2005; Kosakai & Oguri, 1976; Gales et al., 2001; Niks et al., 2004). ... 21 Table 5 Media used for determination of MBC of colistin for the different microorganisms and polymicrobial cultures. 31 Table 6 Media used for determination of CFU of single- and dual-species biofilms. ... 32 Table 7 Media used for observation of colony morphology of single- and dual-species biofilms. ... 34 Table 8 MIC and MBC of colistin determined under normal oxygen conditions in MHB and MHB supplemented with
NaCl... 37 Table 9 MIC and MBC of colistin determined under oxygen deficiency in MHB and MHB supplemented with NaCl. ... 38 Table 10 MBC of colistin determined under normoxia and hypoxia against planktonic dual- species cultures of P.
aeruginosa with other species in MHB and MHB with NaCl. ... 39 Table 11 Resistant fractions of the population calculated for each concentration of colistin based on the PAP. ... 58 Table 12 MIC and MBC of colistin determined in MHB for the wild type morphotype and for the small morphotype
List of Figures
Figure 1 Biofilm life-cycle. Adapted from Bazaka et al. (2012) and Garnett & Matthews (2012). ... 11 Figure 2 Model for the spatial distribution of polymicrobial biofilms regarding the three different forms: (a) Separate microcolonies; (b) Coaggregation and (c) Layering. (Elias & Banin, 2012) ... 12 Figure 3 Some of AMP mechanisms of action. Adapted from Sang & Blecha, (2008). ... 19 Figure 4 Chemical structure of Colistin sulphate. (Chemicalland21, 2013) ... 20 Figure 5 Biomass (OD 570nm) (A) and number of cultivable biofilm-cells (B) obtained for single- and dual-species biofilms
developed in TSB or in TSB with NaCl. ** *** and *** indicate differences between biofilms developed in presence of NaCl and their respective controls. ... 41
Figure 6 Biomass (OD 570nm) and number of cultivable cells obtained for single-species biofilms of P. aeruginosa ATCC
10145 (A); P. aeruginosa U147016 (B); K. pneumoniae (C) and P. mirabilis (D) developed in TSB or in TSB with NaCl supplemented with increasing concentrations of colistin. *; **; *** and **** indicate differences between biofilms developed in presence of NaCl and their respective controls. ... 43 Figure 7 Biomass (OD 570nm) and number of cultivable cells obtained for dual-strain biofilms encompassing P. aeruginosa
ATCC 10145 and P. aeruginosa U147016-1 developed in TSB or in TSB with NaCl supplemented with increasing concentrations of colistin. ... 46 Figure 8 Biomass (OD 570nm) and number of cultivable cells obtained for dual-species biofilms encompassing K.
pneumoniae and P. aeruginosa ATCC 10145 (A) and K. pneumoniae and P. aeruginosa U147016 (B) developed in TSB or in TSB with NaCl supplemented with increasing concentrations of colistin. *; ** and **** indicate differences between biofilms developed in presence of NaCl and their respective controls. ... 47 Figure 9 Relative distributions of microorganisms within dual-species biofilms encompassing K. pneumoniae and P. aeruginosa developed in TSB or in TSB with NaCl supplemented with increasing concentrations of colistin. ... 48 Figure 10 Biomass (OD 570nm) and number of cultivable cells obtained for dual-species biofilms encompassing K.
pneumoniae and P. aeruginosa ATCC 10145 (A) and K. pneumoniae and P. aeruginosa U147016 (B) developed in TSB or in TSB with NaCl supplemented with increasing concentrations of colistin. **; *** and **** indicate differences between biofilms developed in presence of NaCl and their respective controls. ... 49 Figure 11 Relative distributions of microorganisms within dual-species biofilms encompassing P. mirabilis and P. aeruginosa developed in TSB or in TSB with NaCl supplemented with increasing concentrations of Colistin. ... 50
Figure 12 Biomass (OD 570nm) (A) and number of cultivable cells (B) obtained for single- and dual-species biofilms
developed in TSB or in TSB with NaCl. *; *** and **** indicate differences between biofilms developed in presence of NaCl and their respective controls. ... 52 Figure 13 Biomass (OD 570nm) and number of cultivable cells obtained under oxygen deficiency for single-species biofilms
of P. aeruginosa ATCC (A) and P. aeruginosa U147016-1 (B) developed in TSB or in TSB with NaCl supplemented with increasing concentrations of colistin. *; ** and *** indicate differences between biofilms developed in presence of NaCl and their respective controls. ... 53 Figure 14 Colony morphology observed for simple and mixed biofilms formed in TSB and TSB with NaCl. Colonies were grown for 48 h at 37ºC in TSA, PIA, KSA or MC medium. Each black bar represents 1 mm. ... 55 Figure 15 Colony morphology observed for dual-species biofilms encompassing K. pneumoniae and P.aeruginosa
U147016 formed in TSB and TSB with NaCl. Colonies were grown for 48 h at 37ºC in TSA, PIA and KSA medium. Each black bar represents 1 mm. ... 56 Figure 16 K. pneumoniae cultured in presence of various concentrations of colistin exhibiting a heterogeneous behaviour. ... 57 Figure 17 PAP of K. pneumoniae regarding cultivable cells in MHA plates supplemented with increasing colistin concentrations: (A) planktonic cultures grown in MHB and MHB with NaCl and (B) biofilms formed in TSB and TSB with NaCl. ... 58 Figure 18 Colony morphology observed in the PAP of biofilms formed in TSB and TSB with NaCl. Colonies were grown for 48 h at 37ºC in MHA plates. Each black bar represents 1 mm. ... 59
Figure 19 K. pneumoniae exhibiting WTM and SM in MC (left) and in MHA (right) plates. ... 59
Figure 20 Number of cultivable cells obtained for biofilms of K. pneumoniae WTM and SM developed in TSB supplemented with increasing concentrations of colistin. *; ** and *** indicate differences between biofilms developed in presence of NaCl and their respective controls. ... 60 Figure 21 Colony morphology observed for biofilms of K. pneumoniae SM formed in TSB supplemented with increasing Colistin concentrations. Colonies were grown for 48 h at 37ºC in TSA plates. Each black bar represents 1 mm. . 61
1.1 Background
Microbial infections as a result of bacterial adhesion to biomaterial surfaces remain a major cause of morbidity and mortality in modern healthcare. Just in the United States, the costs associated with nosocomial infections were estimated to range from 28 to 45 billion dollars annually. More than half of these infections are attributed to biomaterials. Antibiotherapy is the most common treatment, however with development of antibiotic resistance, antimicrobial peptides (AMP) are emerging as potential alternatives less likely to be affected by bacterial resistance. Moreover, the environmental conditions surrounding BAI must be taken into consideration as they can impact the establishment of the infection. In effect, it is a well-established fact that multiple sites in the body lack of oxygen and that biofilms formed therein live under hypoxia conditions. Furthermore, salinity is one of the obstacles associated with the establishment of AMP as new candidates to treat BAI as it showed to influence their action. Therefore, AMP efficacy can be disturbed by conditions naturally occurring in the human body like variable oxygen and salt concentrations. This carries a necessity to develop studies about AMP performance under different oxygen conditions as well as strategies to increase salt resistance of antimicrobial peptides and to discover new ones intrinsically resistant.
1.2 Main objectives
The main goal of this work was to understand how different oxygen and salt concentrations can affect single and polymicrobial biofilm formation and the efficiency of the AMP colistin when used in a prophylactic approach. The microbial composition of the mixed biofilms and the potential development of resistance phenomena were also assessed.
1.3 Thesis outline
This thesis is structured into five chapters. In chapter 1, the motivation for the present work, the main objectives as well as the thesis outline are addressed. Chapter 2 provides the state of the art about the clinical impact of biomaterial-associated infections, some prophylactic and therapeutic strategies to fight them as well as the role played by the surrounding environmental conditions on the success of those strategies. Chapter 3 reports the microorganisms, materials and techniques used throughout this work. Chapter 4 describes the results obtained in this work as well as their discussion. Finally, in chapter 5 the main conclusions are presented and some future work is proposed.
2.1 Biomaterial-associated infections
Biomaterials have led to great improvements in modern medicine as they are increasingly used to support or restore human body functions and thus to add quality of life to patients. Biomaterials, as devices and implants, can be defined as man-made materials designed to interact with living tissue or with a body fluid. They may be utilized for a short-time period, months or even for a life-time. Often, bacteria and fungi adhere to a biomaterial surface, colonizing it and impeding their normal functions (Liu et al., 2004; Stobie et al., 2008; Locci et al., 1981) reducing its usage time.
Biomedical devices are normally used for surveillance, diagnosis or aesthetic functions but they also play a fundamental role in disease as they carry the risk of developing an infection (Van Vlierberghe et al, 2011; Ansari and Husain, 2012). A biomaterial-associated infection can be defined as the one occurring in patients with a device for at least 48 hours before the beginning of the infection (Garner et al., 1996). BAI are usually associated with microorganisms capable to form biofilms and very common in patients subjected to an implant surgery (Raad, 1998). Devices like contact lenses, feeding tubes, urinary and vascular catheters, which constitute temporary devices, are also susceptible to infections and should not be considered less important (Busscher et al., 2012).
2.1.1 Some biomaterials and their applications
It has been noticed a significant growth in diversity, function and number of materials used to manufacture implanted or temporary devices (Bazaka et al., 2012). The type of material is closely associated with the risk of developing an infection, acting on factors like bacterial adhesion, immune activation and phagocytosis (Rochford et al., 2012). However, it has been shown that once implanted biomaterials are infected, the amount of biofilm that can be formed at the coating is not related to the material composition (Gómez-Barrena et al., 2012). Table 1 gathers some of the most common biomaterials, their applications, as well as advantages and disadvantages related to their use and some alternatives to avoid infection. These biomaterials have been presenting some problems related with low biocompatibility leading to inflammation, non-haemocompatibility and lack of tissue integration, as well as deterioration of the biomaterial properties (Elbert, 2011; Sun et al., 2011).
Table 1 Examples of biomaterials and their main applications, advantages and disadvantages, and some alternatives to avoid infection.
Material Application Advantages Disadvantages Alternatives to avoid infection
Titanium and alloys
Hard tissue replacement and reconstruction of soft tissue (Niinomi et al.,
2012)
Highest biocompatibility among metallic materials;
corrosion resistance (Niinomi et al., 2012)
Bone atrophy; poor bone remodelling (Niinomi et al., 2002)
Implantation of Ca+ on surface (Hanawa et al., 1997)
Stainless steel
Higher ductility and cyclic twist strength (Niinomi et
al., 2012)
Surface modification with vancomycin or gentamicin linked to
self-assembled monolayers (Kruszewski et al., 2013)
Cobalt-chromium
Highest wear resistance and strength (Niinomi et al.,
2012)
______
UHMWPE
Hip and knee replacement (Gómez-Barrena et al., 2011)
Wear resistance and good biocompatibility (Brach Del
Prever et al., 2009)
Material oxidation leading to osteolysis (Brach Del Prever et al., 2009)
Covering with alphatocopherol reduce attachment of some Staphylococcus epidermidis and
Staphylococcus aureus strains (Gómez-Barrena et al., 2011)
Silicone
Catheters (Tran & Webster, 2013)
Reduce the risk of encrustation in long-term catheterized patients (Gould
et al., 2010)
Excessive activation of the complement system Desensitization of leucocytes (Anderson & R. E. Marchant, 2000)
Use polyurethane or polyvinylchloride (Sherertz et al.,
1995; Marosok et al., 1996) Graft the surface with P(PEGDMA) and P(DMAPS) (M. Li et al., 2012) Graft the surface with selenium nanoparticles (Tran & Webster,
2013)
Hydroxyapatite
Biomaterial coatings, and low-loaded implants (Suchanek &
Yoshimura, 2011)
Among ceramics is the most biocompatible (Suchanek & Yoshimura,
2011)
Poor mechanical properties (Suchanek & Yoshimura, 2011)
Graft the surface with Silver particles (Akiyama et al., 2013)
PMMA
Bone cement of artificial joints (Arora et
al., 2013) Rigid contact lenses
(Tyagi et al., 2012)
Cement can act as a drug delivery system as a local carrier of antibiotics (Breusch & Kühn, 2003)
When polymerized in vivo there exists a high incidence of post-operative infections due to heat created during
polymerization (Schlegel & Perren, 2006; Arens et al., 1999)
Use tobramycin + vancomycin as additives (Penner et al., 1996; González Della Valle et al., 2001) Surface implantation of COO-/(COO
-+SO3-) (Anagnostou et al., 2006)
Add chitosan or silver nanoparticles (Arora et al., 2013)
As an attempt to overcome these problems, some bioresorbable materials are recently emerging as alternatives to overcome the aforementioned problems associated with the usage of biomaterials for tissue replacement (Bendrea et al., 2011; Naderi et al., 2011).
2.1.2 The origin of infection
BAI are typically caused by bacteria (Costerton, 1999) very difficult to kill by normal antimicrobial strategies. The disruption of the body by invasive procedures, e.g. the insertion of a catheter or a scalpel (Dobbins et al., 2002; Broekhuizen et al., 2010; Lorente et al., 2005) may lead to the transport of natural flora to different tissues and start an infection. Further, nosocomial pathogens may also trigger a BAI and it has been estimated that 50 % of the cases of nosocomial infections are associated with medical devices (Guggenbichler et al., 2011). In fact, multi-resistant nosocomial bacteria are the most common microorganisms colonizing catheters (Chambless et al., 2006). Another cause of severe infections, closely related to intravascular devices (Darouiche, 2004), is the haematogenous spread of bacteria which may occur several years after implantation. Bacteria infecting other place of the body may disseminate in the bloodstream, colonizing the biomaterial, what is very common, for instance, after dental treatments (Busscher et al., 2012). Focusing implantable biomaterials, the contamination may occur during the surgery, when the pathogen is attached to the implant or enters by the wound site. In this situation, contamination is normally named as perioperative (Busscher et al., 2012). After implantation, the surface of a biomaterial is rapidly covered with a conditioning film of host proteins. This film, composed by fibrinogen, fibronectin and vitronectin, may differ between implants in accordance with the topography and chemistry of the material composition and also with the exposure time. Therefore, the posterior colonization of an implanted device is dependent on the ability of bacteria to adhere on this film, being their attachment to the surface significantly affected by the film characteristics as well as the immune response and tissue integration (Patel et al., 2007). This kind of contamination is named postoperative and may occur during hospitalization before the wound is closed (Busscher et al., 2012).
2.1.3 Infection on primary and secondary implant
When an implant surgery is done for the first time (primary implant), surrounding tissues are not yet infected or inflamed and the surgery involves a low number of microbial contaminants (Neut et al., 2011). However, a primary implant surgery is not always well succeeded, and an infection may appear several years later (Busscher et al., 2012), and replacement surgeries become necessary. Secondary implants are subject to higher infection rates as tissues are already infected, inflamed and possibly damaged by the first surgery (Wilson and Costerton, 2012). In this situation, bacteria are strongly present on peri-implant tissue and replicate very fast (Neut et al., 2003). It has also
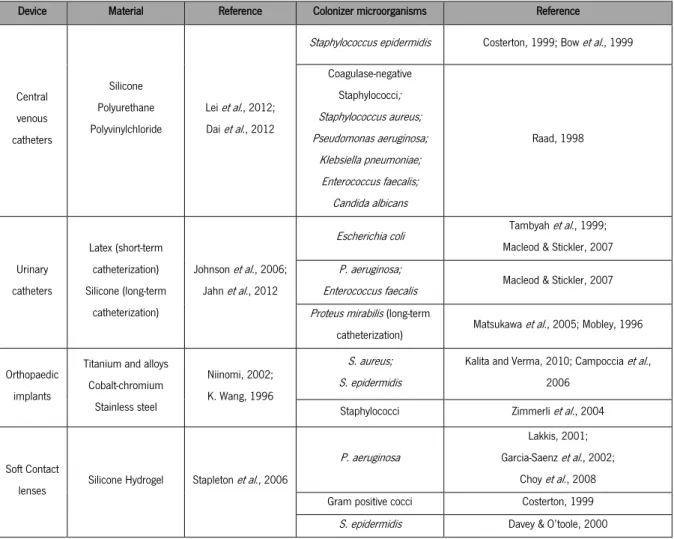
been noticed that infections caused by revision surgeries are associated with higher resistances provoked by bacterial exposure to antibiotics for long periods of time, and possibly with acquired drug resistances or changes in adhesiveness (Del Pozo and Patel, 2007; Campoccia, 2006). Consequently, the risk of developing an infection is higher and bacteria can easily proliferate if affected tissue is not correctly eliminated during the surgery (Neut et al., 2003). On table 2 some of the most common biomedical devices are presented as well as the material composing them and their typical colonizer microorganisms.
Table 2 Some of the most common biomedical devices, the material composing them and their typical colonizer microorganisms.
Device Material Reference Colonizer microorganisms Reference
Central venous catheters Silicone Polyurethane Polyvinylchloride Lei et al., 2012; Dai et al., 2012
Staphylococcus epidermidis Costerton, 1999; Bow et al., 1999 Coagulase-negative Staphylococci; Staphylococcus aureus; Pseudomonas aeruginosa; Klebsiella pneumoniae; Enterococcus faecalis; Candida albicans Raad, 1998 Urinary catheters Latex (short-term catheterization) Silicone (long-term catheterization) Johnson et al., 2006; Jahn et al., 2012
Escherichia coli Tambyah et al., 1999; Macleod & Stickler, 2007 P. aeruginosa;
Enterococcus faecalis Macleod & Stickler, 2007 Proteus mirabilis (long-term
catheterization) Matsukawa et al., 2005; Mobley, 1996 Orthopaedic
implants
Titanium and alloys Cobalt-chromium Stainless steel Niinomi, 2002; K. Wang, 1996 S. aureus; S. epidermidis
Kalita and Verma, 2010; Campoccia et al., 2006
Staphylococci Zimmerli et al., 2004
Soft Contact
lenses Silicone Hydrogel Stapleton et al., 2006
P. aeruginosa
Lakkis, 2001; Garcia-Saenz et al., 2002;
Choy et al., 2008 Gram positive cocci Costerton, 1999
S. epidermidis Davey & O’toole, 2000
2.1.4 Biofilm formation and microbial adhesion
In the actual scenario, most microorganisms reside predominantly in biofilms. Biofilms are communities of microorganisms, living on a reversible sessile state, attached to a surface or to each other, and surrounded by a self-produced polymeric matrix (Dunne, 2002). When compared to planktonic cells, they have a different phenotype with an altered gene transcription and growth
rate (Costerton, 1999; Donlan and Costerton, 2002) and is well established that their virulence can cause a lot of problems in humans (Costerton, 1999; Garnett & Matthews, 2012). In effect, it is estimated that biofilms are present at 65 % of all bacterial infections (Lewis, 2001).
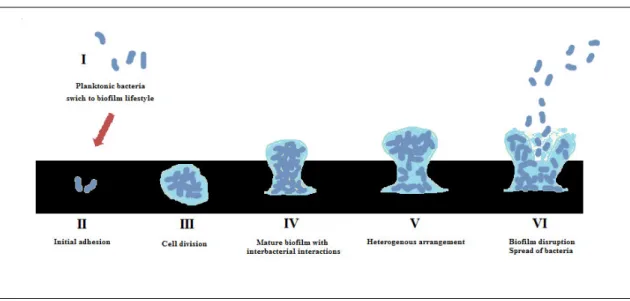
Biofilms play a fundamental role in BAI since they are capable to growth on biomaterial surfaces prejudicing their performance (Liu et al., 2004; Stobie et al., 2008). According to Rochford et al. (2012), the infection risk in a biomaterial is linked to an immunological deficit present at its surface since the normal immune function is absent on the interface between the implant and host tissue. The concept “race for the surface” was first introduced by Anthony Gristina (1987), who suggested that host tissue integration competes with microbial cell adhesion on the surface of a biomaterial, being the success of an implant directly dependent on the competition be won by the host tissue. Therefore, tissue integration on permanent implants, as the host cell adhesion to the surface, has a fundamental role on preventing infections, since it protects biomaterial from invading microorganisms establishing a normal immune function at the implant site (Busscher et al., 2012). Due to the high number of patients who develop BAI, there have been several attempts to develop strategies for their control and prevention. Understanding the different stages in the development of a biofilm is of great importance when trying to develop those strategies (Francolini & Donelli, 2010). It is quite unanimous that the typical biofilm life-cycle comprises six steps (figure 1).
On the first, cells are in a planktonic lifestyle (I), and the transition to the second stage happens when they adhere to the surface using cell-surface adhesins, limiting some of the communication with the exterior, being this process highly reversible (II) (An et al., 2000). On the next stage, cells
begin to divide (III) and change their phenotype, in response to signs from the surrounding cells, stimulating a stronger attachment and providing inter-bacterial interactions. In this stage, the matrix of polymeric extracellular substances is largely secreted giving protection and stability to the biofilm (IV) (Flemming & Wingender, 2010). Then, the microbial consortium achieves a heterogeneous arrangement (V) starting to lose some cells to the exterior, and dispersins, present on the environment, may disrupt inter-bacterial interactions, leading to the spread of bacteria that are capable to colonize another environment and form a new biofilm (VI) (Davies, 1998; McDougald et al., 2012).
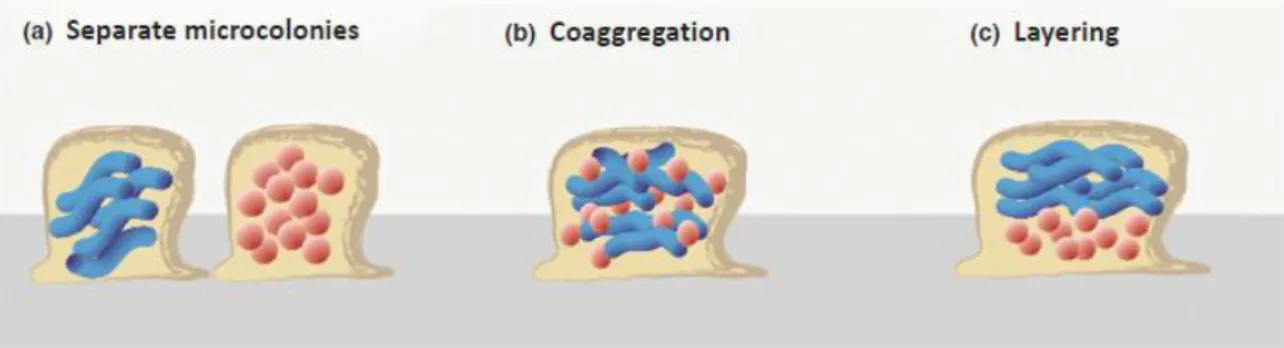
According to the surrounding conditions, biofilms can be composed of single-species populations or instead contain different species living in a community (Costerton, 1999). In nature, microorganisms are rarely found as mono-species, being the majority of them composed by two or more different microorganisms, forming polymicrobial biofilms. The predominance of multi-species biofilms in nature indicates some evolutionary advantage over the single-species like the occurrence of synergistic interactions in the community (Periasamy & Kolenbrander, 2009). According to Elias & Banin (2012) synergistic interactions between microorganisms play an important role in antimicrobial resistance as they cooperate to protect the entire community from the antimicrobial. In vitro studies are usually conducted in single species biofilms, so it is important to implement further studies in mixed biofilms in order to understand these interactions. Various infectious diseases are caused by multispecies biofilms, including otitis, diabetic wounds and oral cavity infections. In respect to BAI, polymicrobial biofilms are also very common and can be found in intravenous and urinary catheters, contact lens, endotracheal tubes, and orthopaedic devices between others (Peters et al., 2012). In 2012, Elias & Banin proposed a model for the spatial distribution of mixed biofilms based on three different forms.
Figure 2 Model for the spatial distribution of polymicrobial biofilms regarding the three different forms: (a) Separate microcolonies; (b) Coaggregation and (c) Layering. (Elias & Banin, 2012)
In the first (figure 2a), biofilms are organized in microcolonies, in which each species forms individual structures. This represents the characteristic form of biofilms lacking commensal interactions. The second form (figure 2b) represents a biofilm where microorganisms are equally dispersed and co-aggregated throughout the biofilm. The third (figure 2c) is the layering form, where microorganisms are strategically organized in layers and may compete with each other or instead, act synergistically.
As already mentioned, biomaterial infections are provoked by several different microorganisms such as P. aeruginosa and P. mirabilis, however other species can be found colonizing these surfaces, although less commonly, such as K. pneumoniae.
Proteus mirabilis
Proteus mirabilis is a motile gram-negative bacillus commonly found in water, soil and in the human gut (Jacobsen et al., 2008; Chow et al., 1979). This pathogen is among the principal causers of nosocomial infections, including those affecting the respiratory tract, skin and also wounds (Rózalski et al., 1997). Despite this, a particular attention should be given to the urinary tract infections (UTI) provoked by P. mirabilis. These are frequent among patients with anatomic or physiological defects (Rózalski et al., 1997) as well as on catheterized patients (Warren et al., 1982; Warren et al., 1987). UTI related to catheterization are commonly named Catheter Associated Urinary Tract Infections (CAUTI) and are known to be extremely hard to handle and potentially fatal (Jacobsen et al., 2008). In fact, CAUTI is one of the most common nosocomial infections and represents a serious concern in hospital management as treatments account for extremely high costs. Previous studies about microbial adhesion already revealed the strongest attachment capacity of Proteus mirabilis in various biomaterials and its implication in biofilm formation (Hawthorn & Reid, 1990; Roberts et al., 1990).
Pseudomonas aeruginosa
P. aeruginosa is an ubiquitous facultative anaerobic (Van Hartingsveldt & Stouthamer, 1973) responsible for serious nosocomial infections namely in immunocompromised patients. This gram-negative pathogen is commonly related to pneumonia, urinary tract infections, postoperative wound infections and also bacteremias (Wisplinghoff et al., 2004; Lister et al., 2009) resulting in high mortality rates (Morita et al., 2014). P. aeruginosa has developed resistance to various classes of antimicrobials, which led to the emergence of several multi-drug resistant (MDR) strains, many of
them currently found in intensive care units (Hancock & Speert, 2000; Tam et al., 2010). Despite these resistances, many MDR strains remain susceptible to polymyxins (Martis et al., 2014). P. aeruginosa is also responsible for several BAI (Mishra et al., 2014), and is among the principal causers of Ventilator Associated Pneumonia (VAP), mainly in children and newborns due to its ability to colonize endotracheal tubes and consequently form biofilm on its surface (Foglia et al., 2007; Weber et al., 2007). This microorganism has a wide metabolic diversity and a great ability to adapt to different environments, giving sometimes rise to phenotypically distinct sub-populations (Häussler, 2004). Of particular interest are the small variant colonies (SVC) of P. aeruginosa, a form of slow-growing bacteria that present unusual colony morphology and different biochemical properties. SVC are closely linked to biofilm formation and responsible for persistent infections in various species (Proctor et al., 2006; Häussler et al., 2003). According to Sousa et al., (2012), it is possible to detect phenotypic switching by observing colony morphology and also to establish a relationship between colony morphotypes and antimicrobial resistance.
Klebsiella pneumoniae
K. pneumoniae, a natural inhabitant of the human body, is a gram-negative pathogen that commonly infects immunocompromised patients on various tissues and organs and easily develops antibiotic resistance (Nordmann et al., 2011; Queenan & Bush, 2007). It is able to cause serious infections in the urinary tract, wounds and also to provoke intra-abdominal infections, pneumonia and bacteremia. This opportunistic behaviour makes K. pneumonia responsible for many nosocomial infections (Tzouvelekis et al., 2012). Treatment normally involves administration of penicillins, however the emergence of strains producing extended-spectrum β-lactamases (ESBLs) led to a progressively ineffectiveness of this antibiotic class (Pitout & Laupland, 2008). Thus, carbapenems were introduced for treating ESBL-producing strains and since then carbapenem-resistant K. pneumoniae (CRKP) appeared and have become disseminated (van Duin et al., 2013). Colistin, remains now, one of the last resort options that is still effective in MDR strains (Ah et al., 2014). Despite not being a major cause of BAI, studies have already demonstrated that K. pneumoniae is capable to infect catheters and provoke ventilator associated pneumonia (Pontikis et al., 2014; Antoniadou et al., 2007; Falagas et al., 2008).
2.2 Antimicrobial strategies
The first step in preventing any infection should be the adoption of good practices by health professionals. In fact, sources of contamination are various and difficult to control. Regardless of the implementation of good practices, most implantable devices are commonly susceptible to perioperative contaminations within operation theatres (Knobben et al., 2006). As a result, different prophylactic strategies are commonly applied and will be briefly revised herein.
2.2.1 Preventive/prophylactic strategies
Biomaterial surfaces have a massive contact with the biological medium, being the inflammatory response and rejection of a biomaterial dependent on it. Thus, it is important to have knowledge about surface physicochemical properties, aiming to understand their relation with the medium in order to develop modifications and functionalization of biomaterials capable to favour the host tissue integration and to avoid microbial adhesion (Endrino et al., 2010). Surface modification is, at the moment, the most preventive used strategy to minimize the incidence of BAI (Wang et al., 2011; Khoo et al., 2009; Jen et al., 2012).
In the absence of a conditioning film, surface roughness has a fundamental role in controlling bacterial adhesion (Hosoya et al., 2003; Verheyen et al., 1993). Differences between a smooth polished surface and a microporous coating were studied in mice and results showed that it was necessary a significant smaller amount of bacteria to cause bone infection in the microporous coat. This might be due to the probability of bacteria inside the pore escape from the antibiotics/immune defenses and suggests that biomaterial roughness is directly associated with the probability of developing an infection (Cordero et al., 1994). Furthermore, other authors have reported benefits in using smoother coatings on biomaterials (Harris et al., 2007, Kinnari et al., 2010; Whitehead & Verran, 2006; Wu et al., 2011). The addition of hydrophilic polymers to the surface of a biomaterial gives it anti-adhesive properties against microorganisms since the microbial surface becomes hydrophobic (van der Mei et al., 1988; Darouiche, 2001). The grafting of hyaluronic acid already demonstrated to have ability to reduce the adhesion of S. epidermidis. Surfaces grafted with silver ions present a strong bactericidal effect against several biomaterial infective microorganisms like S. epidermidis, P. aeruginosa and E. coli (Birla et al., 2009; Rai et al., 2009) being innocuous to eukaryotic cells (Silver, 2003; Agarwal et al., 2010). Explanation lies in
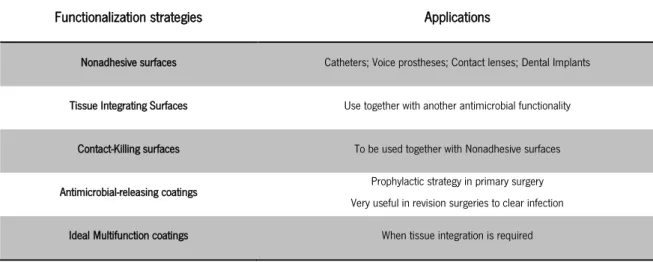
the interaction of Ag+ ions with cytoplasmic membranes, changing their permeability (Lok et al., 2007) and trough an inhibition of enzymes involved in the respiratory chain (Holt & Bard, 2005). Antimicrobial-releasing coatings are emerging as they can provide a localized treatment of tissue near the implant, preventing biofilm formation on its surface and also on surrounding tissues. These coatings act localized, enabling a large release of drug at the beginning of treatment without risk of systemic effects. This becomes particularly favourable to the elimination of biofilms, as they require higher antimicrobial doses to be successfully eradicated (Hetrick & Schoenfisch, 2006). On the other hand, microorganisms may release endotoxins in response to high drug concentrations and induce an inflammatory response, also provoking toxicity to the host cells (Sanders & Sanders, 1979). This antimicrobial approach presents a variable clinical success, dependent on the location of the device, concentration and degree of depuration of the antimicrobial (Darouiche, 2001). Vancomycin, tobramycin and gentamicin are examples of antibiotics already used for incorporating coatings (Hetrick & Schoenfisch, 2006). It should be emphasised that antimicrobial-releasing coatings should never release the drug at levels below the minimal inhibitory concentration (MIC) since it is likely to induce development of resistance (Lin et al., 2001). Busscher and colleagues (2012) suggested different strategies for biomaterial functionalization as described on table 3.
According to the same authors, monofunctional coatings are, at reality, ineffective. In fact, coatings promoting host tissue integration also promote adhesion of microorganisms, once the various mechanisms of adhesion are the same for eukaryotic and prokaryotic cells (Johansson et al., 1997). This may also occur when using coatings that prevent adhesion of microorganisms. In effect, these devices prevent prokaryotic adhesion but at the same time they impair host tissue
Functionalization strategies Applications
Nonadhesive surfaces Catheters; Voice prostheses; Contact lenses; Dental Implants
Tissue Integrating Surfaces Use together with another antimicrobial functionality
Contact-Killing surfaces To be used together with Nonadhesive surfaces
Antimicrobial-releasing coatings Prophylactic strategy in primary surgery Very useful in revision surgeries to clear infection Ideal Multifunction coatings When tissue integration is required Table 3 Different strategies for biomaterial functionalization and their applications. Adapted from Busscher et al., (2012)
integration (Busscher et al., 2012). For instance, fibronectin is a protein found in the extracellular matrix that adsorbs to the surfaces and promotes both host tissue and Staphylococci adhesion (Fowler et al., 2000). These limitations can be overcome by creating multifunctional coatings that allow host tissue integration and avoid microbial adhesion at the same time (Wang et al., 2013). They consist of a combination of two or more strategies in a single surface (Brohede et al., 2009; Bruellhoff et al., 2010).
2.2.2 Therapeutic strategies
It is has been reported an increasing bacterial resistance to antibiotics (Hawkey, 2008; Livermore, 2003). Moreover, the development of new antimicrobials is not keeping pace with the development of resistance (McDevitt & Rosenberg, 2001). The continuous exposure to antibiotics associated to inappropriate usage is the primary cause of the problem (Kaufmann & Hung, 2010). Bacteria found several strategies to acquire resistance to antimicrobials, like the extrusion of the drug from the cell and changes of the target protein. This may be achieved by bacterial mutations or by transferring genetic material between cells, like plasmids and transposons (Baltzer & Brown, 2011). Resistance to antibiotics is enlarged in biofilm state, which seriously complicates therapy (Aslam, 2008; Ramage et al., 2009). Several mechanisms specifically involved in biofilm resistance have been already reported. The slow and low diffusion of compounds into the matrix, the heterogeneous ambient existing on lower layers with oxygen and nutrients depletion as well as the accumulation of toxic residues from metabolism occur normally in biofilms (Borriello et al., 2004). Biofilms constitute an excellent site for plasmid transfer between cells, since bacterial conjugation is higher in sessile than in planktonic form. Thus, genes codifying for drug resistance can be easily dispersed into various bacteria increasing resistance of microorganisms (Francolini & Donelli, 2010). As already mentioned, inhibition of microorganisms in a complex biofilm requires a higher antimicrobial dose, when compared to planktonic bacteria, that can reach 1000-times more (Smith, 2005). Moreover, antibiotics are only effective on metabolic active bacteria, which often makes them ineffective against the inner layers of biofilms (Lewis, 2001). Bacteria reside not only on the implant but also on peri-implant tissue and intracellularly and these locations are not always possible to achieve with antibiotics. Vancomycin and gentamicin are examples of two ineffective agents against intracellular bacteria. To overcome this limitation, it is possible to use combinations of antibiotics like vancomycin-rifampicin, being the last active against intracellular bacteria (Busscher et al., 2012).
Antibiotic resistances are extensively studied as they represent a worldwide concern. Consequently, they are routinely screened in the clinical practice and used as treatment guiders. Despite this, there is another type of resistance which is far from being fully understood and may compromise the effectiveness of antimicrobials and slip into routine antibiograms (Falagas et al., 2008). To this phenomenon is given the name of heteroresistance and it can be defined as a heterogeneous resistance to an antimicrobial within a single clinical isolate, in which only a sub-population is resistant (Rinder, 2001; Wang et al., 2014). As aforementioned, heteroresistant strains are usually classified as susceptible in routine antibiograms which can be easily explained by the extremely low fraction of the resistant sub-population that usually goes unnoticed (Falagas et al., 2008). Nevertheless, this small fraction has an important clinical significance since in the presence of the antimicrobial it is selected and dominates the infection. It is therefore easy to conclude that the failure of antimicrobial-based treatments may be closely linked to this phenomenon. Furthermore, some authors have proposed heteroresistance as an evolutionary process that leads to the appearance of fully resistant strains (Folkvardsen et al., 2013). Heteroresistance has been widely reported in various microorganisms, namely S. aureus (Moore et al., 2003; Cafiso et al., 2010; Deresinski, 2013), Mycobacterium tuberculosis (Zhang et al., 2014; Folkvardsen et al., 2013; Zhang et al., 2012) and Acinetobacter baumannii (Hung et al., 2012; Lee et al., 2011; Hawley et al., 2008), whereas fewer reports are found about K. pneumoniae (Poudyal et al., 2008; Meletis et al., 2011).
Antimicrobial peptides
As antibiotic resistance is developing among diverse microorganisms, it is necessary to develop alternatives, like new anti-microbial peptides. AMP are short-length peptides composed by less than 50 aminoacids, natural produced by organisms from Plantae and Animal kingdoms, responsible to act on innate non-specific defenses (Brown and Columbia, 2007; Hancock, 2001). They are positively charged and capable to gain an amphipathic conformation that disrupts bacteria membranes (Powers & Hancock, 2003).
Despite its long existence, peptides remain effective in immune defense (Zasloff, 2002), suggesting that they might evolved along with associated mechanisms of resistance (Peschel & Sahl, 2006). Unlike the conventional antimicrobials which cover mainly fungi or bacteria, the activity of AMP covers fungi, resistant bacteria, parasites, viruses, and even cancer cells (Sang & Blecha, 2008) Furthermore, these agents act in a more rapid and selective way (Bradshaw, 2003) distinguishing
between host and non-host cells, based on the difference between the membrane of a eukaryotic and a prokaryotic cell (Gottler & Ramamoorthy, 2010). They are capable of acting on less metabolic active bacteria due to their mode of action based on pore formation and permeabilization of membranes (Jorge et al., 2012). In sum, they constitute potential substitutes for traditional antibiotics (Zasloff, 2002). The applications of peptides are closely related to their mechanisms of action (figure 3), thus a detailed knowledge of those mechanisms is crucial upon the choice thereof. Their use on surface coatings is already been established and it was found that AMP able to target the membrane are the best choice (Bagheri et al., 2012). They may also be used as a prophylactic strategy due to their anti-adhesive properties (Peschel & Sahl, 2006). One of the most interesting aspects of AMP is that they are less likely to develop bacterial resistance (Bradshaw, 2003; Zasloff, 2002; Brogden, 2005) as they have reduced target specificity and different mechanisms of action (Zasloff, 2002; Beckloff et al., 2007). Resistance to an antimicrobial peptide would also imply changes in the membrane configuration, which is unlikely to occur (Costa et al., 2011). Contrariwise, some resistances have been described, like mutations altering membrane structure and charge, modifications on lipopolysaccharides of Gram-negative bacteria, and drug extrusion by active transport (Altman et al., 2006). In fact, establishment of AMP as therapeutic drugs has been impaired in some ways. Many peptides are active in vitro, however when tested in in vivo animal models, require higher doses to be effective, often considered toxic (Hancock & Sahl, 2006). Costs associated with the production of antimicrobial peptides are very high which makes it unfeasible to be used by many health systems. This, in turn causes a lack of economic interest by the pharmaceutical industry in research and production of these compounds (Marr et al., 2006).
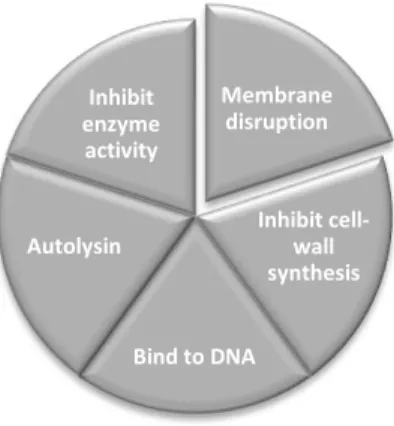
Figure 3 Some of AMP mechanisms of action. Adapted from Sang & Blecha, (2008).
Membrane disruption Inhibit cell-wall synthesis Bind to DNA Autolysin Inhibit enzyme activity
Colistin
In the year of 1947, a group of antimicrobial peptides was discovered to be produced by Bacillus polymyxa. These AMP, named Polymyxins consist of cationic cyclic lipodecapeptides (Martti Vaara, 2013) and comprise five chemically different compounds (A, B, C, D and E) (Storm et al., 1977; Livermore, 2011; Theuretzbacher & Mouton, 2011). In fact, only B and E reached the clinical stage (Matthew et al., 2005) and their use has been strongly impaired by its toxicity, mainly in terms of nefrologic and neurologic effects. Efforts to create derivate compounds with lower toxicity were made, however, despite their lower toxicity, this compounds had a lower antibacterial effect (Kurihara et al., 1974; Danner et al., 1989; Vaara et al., 2008; Vaara & Vaara, 1983).
Polymyxin E, also known as colistin (figure 4), has been used since 1959 for treating Gram-negative bacterial infections (Reed et al., 2001). In the early 1970’s, it was largely abandoned due to the advent of safer alternatives for treatment, such as aminoglycosides and anti-pseudomonal agents (Li et al., 2005). However, the emergence of multidrug-resistant microorganisms and the lack of effective antimicrobials led to its the re-introduction as a salvage therapy (Falagas & Kasiakou, 2005). Colistin is composed of a cyclic heptapeptide ring with a tripeptide side-chain which is, in turn, acylated in the N-terminus by a fatty acid (Li et al., 2005) and presents two major components: colistin A (Polymyxin E1) and B (Polymyxin E2) (Orwa et al., 2001). Commercially, it
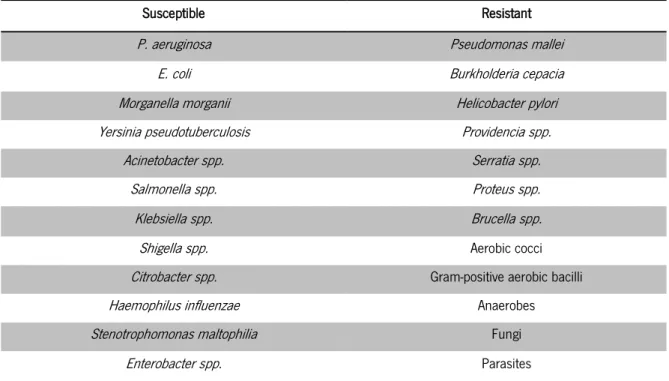
exists as colistin sulphate and as colistimethate sodium (CMS), being the last produced by the reaction of colistin sulphate with formaldehyde and sodium bisulphite (Barnett et al., 1964). While colistin sulphate is mainly oral and topically used, CMS is commonly parenterally administered and presents lower toxicity (Li et al., 2005; Schwartz et al., 1992). Its mechanism of action is based on the electrostatic interaction between the cationic polymyxin and the anionic outer membrane of Gram-negative bacteria. Divalent cations (calcium and magnesium), responsible for stabilizing lipopolysaccharide (LPS), are competitively displaced by colistin decreasing the membrane stability, which leads to its permeabilization and consequently cell death (Storm et al., 1977; Davis et al., 1971; Newton, 1956). Its spectrum of action can be found in detail in table 4.
2.2.3 Biofilm disruption agents
The phenomenon of Quorum Sensing
Within a biofilm, bacteria communicate through small signalling molecules responsible to regulate various physiological activities, like gene expression, their virulence, and antibiotic production (Fernandes, 2006; Pearson et al., 1999).This phenomenon is named quorum sensing (QS) and comprises a large range of signals (Dong & Zhang, 2005; McDougald et al., 2007), being the most studied the acyl homoserine lactones (AHL’s) and peptide-based quorum sensing systems. AHL’s are produced by various species of gram-negative bacteria and diffuse through the membrane to bind to intracellular regulatory proteins. Peptide-based quorum sensing occurs in gram-positive bacteria and operates binding to histidine kinases membrane receptors (Amara et al., 2011; Waters & Bassler, 2005). QS phenomena may be inhibited by different mechanisms: (1) decreasing the activity of receptors and syntheses of ALH’s; (2) degrading it; (3) inhibiting the production of signal molecules; and (4) using analogues of the signal molecules. The most applied strategy has been the enzymatic degradation of QS signal molecules (Kalia & Purohit, 2011). In fact, implants and catheters can be impregnated with QS inhibiters as they have demonstrated to impede the fouling caused by P. aeruginosa biofilms (Choudhary & Schmidt-Dannert, 2010).
Susceptible Resistant
P. aeruginosa Pseudomonas mallei
E. coli Burkholderia cepacia
Morganella morganii Helicobacter pylori Yersinia pseudotuberculosis Providencia spp.
Acinetobacter spp. Serratia spp.
Salmonella spp. Proteus spp.
Klebsiella spp. Brucella spp.
Shigella spp. Aerobic cocci
Citrobacter spp. Gram-positive aerobic bacilli
Haemophilus influenzae Anaerobes
Stenotrophomonas maltophilia Fungi
Enterobacter spp. Parasites
Table 4 Colistin spectrum of action. (Biswas et al., 2012; Landman et al., 2008; García-Rodríguez et al., 1989; Giamarellou & Poulakou, 2009; Li et al., 2005; Kosakai & Oguri, 1976; Gales et al., 2001; Niks et al., 2004).
Enzymes
Antimicrobial enzymes are generally dispersed in the environment (Augustin et al., 2004) and constitute an emerging target of study in the search for new antimicrobial strategies (Stickler, 2008). They can be very useful on treating BAI once they can be incorporated on biomaterial coatings, preventing microbial colonization (Augustin et al., 2004). By degrading the extracellular matrix, enzymes make possible to attack the biofilm structure leading to dispersal of bacteria, which become more sensible to antibiotics. There are some matrix components, like DNA, polysaccharides and proteins, that constitute targets to several enzymes (Kiedrowski & Horswill, 2011). They act by different ways, such as attacking the microorganism, interfering with biofilm formation and by destroying them, for example, intervening in reactions involved in antimicrobial synthesis (Augustin et al., 2004). This kind of antimicrobials can be grouped on three classes based on their mode of action: the proteolytic class; the polysaccharide degrading class; and the oxidative enzymes class (Thallinger et al., 2013). Proteolytic enzymes are hydrolyser proteins that constitute a diverse group, differing in their structure and targets (Hedstrom, 2002). Lysostaphin is a wide studied proteolytic enzyme that cleaves staphylococci walls, consequently is a potential agent on treating MRSA (Miao et al., 2011) and several other staphylococcal infections like those provoked by Staphylococcus epidermidis (Mierau et al., 2005). Its ranges of MIC values are significantly lower than certain antibiotics and can be incorporated on biomaterials like catheters to prevent microbial colonization (Augustin et al., 2004; Pangule et al., 2010).
2.3 Environmental conditions surrounding BAI
2.3.1 The importance of reducing oxygen conditions
Multiple sites in the body lack of oxygen and biofilms formed therein live under reduced oxygen conditions (hypoxia). Also, the exacerbated oxygen consumption, characteristic of the infectious process, leads to a depletion of oxygen concentration in surrounding tissues (Schaible et al., 2013). Moreover, the internal layers of mature biofilms are usually subjected to the absence of oxygen (anoxia) (Borriello et al., 2006). In vitro studies of biofilm formation are usually implemented under aerobic conditions (normoxia), therefore it is easily to notice a lack of knowledge about the behaviour of biofilms under different oxygen conditions (Hess et al., 2013). Hypoxia influences gene expression both in host cells and microorganisms, thus it has a significant influence on infection development being a phenomenon of numerous chronic infectious diseases
(Schaible et al., 2010; Taylor, 2008). Furthermore, it has already been proved that hypoxia can modulate the population density within a biofilm and change the community structure present therein (Cheung et al., 2014). In addition, hypoxic environments inhibit the activity of some classes of antibiotics, another reason for the implementation of studies under such conditions (King et al., 2010). In a recent study, the antibiotic inhibitory concentrations for biofilms cultivated under different oxygen conditions were determined (Hess et al., 2013). It was concluded that biofilm development was less accentuated under anoxia when compared to normoxia and that oxygen concentration have a toughly effect on antibiotic efficacy. Extracellular Polymeric Substance (EPS) production was found to be inhibited by anoxia and several biochemical differences were reported between aerobic and anaerobic biofilms.
Infections of Chlamydia trachomatis are often related to a deficient oxygen environment (Juul et al., 2007) Recently, a model of infection in HeLa cells was used to access the impact of Gamma Interferon (IFN-y) on the antibiotic activity of azithromycin and doxycycline both in normoxia (20 % O2) and hypoxia (2 % O2). Results clearly revealed a reduced antimicrobial efficacy under hypoxia for
both antibiotics. Conversely, the same authors also found that a previously treatment of host cells with IFN-y was able to potentiate doxycycline action under hypoxia, even surpassing its activity in normal oxygen conditions (Shima et al., 2013).
Cryptococcus neoformans is an obligate aerobe yeast that normally infects the lungs of immunocompromised patients (Odds et al., 1995). In 2009, Moranova and colleagues prosed a biofilm model for the adaptation of this pathogen to the hypoxic environment. According to the authors, in the presence of low oxygen concentrations, C. neoformans adapts to a dormant state characterized by low proliferation and intense phenotypic and transcriptomic changes. This dormancy may be responsible for subsequent outbreaks of the infection in previously treated patients. The same authors emphasized the limitations of aerobic in vitro experiments for study C. neoformans pathogenesis and the importance of the implementation of studies in reduced oxygen environments.
In recent years, P. aeruginosa biofilms grown under different oxygen conditions have been extensively studied (McCaughey et al., 2012; King et al., 2010; Schertzer et al., 2010; Field et al., 2005). P. aeruginosa is a major responsible for infecting the lungs of patients with cystic fibrosis (CF) (Hassett et al., 2009), a genetic disorder associated with the accumulation of a thick expectoration within lungs (Riordan et al., 1989; Govan & Deretic, 1996). This mucus, ideal as a medium for growth of multiple opportunistic bacteria, (Hassett et al., 2009) has recently been
proven to contain multiple anaerobic environments and P. aeruginosa widely grows under these conditions (Tunney et al., 2008; Tunney et al., 2011). Higher MIC values for various classes of antibiotics were observed for hypoxia cultures when compared to normoxia. Moreover, this oxygen deprivation had an effect on the multidrug efflux pumps and by adding pump inhibitors the induced antibiotic resistance was reverted (Schaible et al., 2012). In another study, MIC values for levofloxacin appear to be unaffected by oxygen concentration, whereas amikacin, tobramycin, aztreonam presented higher MIC values when tested under anaerobic conditions (King et al., 2010). However, it should be stressed that biofilm formation in the airways of CF patients presents a different physiology and morphology to that of biofilm formation on biomaterials (Hassett et al., 2009), suggesting that experiments on biomaterial surfaces are needed.
2.3.2 The importance of salinity
Salinity is one of the obstacles associated with the development of new AMP as it showed to influence their efficacy (Park et al., 2004; Goldman et al., 1997). The use of AMP as therapeutic strategies is therefore limited by conditions naturally occurring in the human body like variable salt concentrations (Chu et al., 2013). In 1996, Smith and colleagues proposed a model in which the cystic fibrosis transmembrane regulator (CFTR) is responsible for an accumulation of NaCl in the airway surface fluid of these patients. The authors concluded that the high levels of NaCl result in an inhibition of antimicrobial molecules. The identity of such salt-sensitive molecules remained unknown until 1997, when it was discovered that it was the Human β-Defensin-1, an important antibacterial peptide, that was inactivated in CF lungs due to its higher NaCl content (Goldman et al., 1997). Nisin is an antimicrobial peptide commonly used to control Listeria monocytogenes (Franklin et al., 2004), an important pathogen that colonizes food and is able to develop in extremely salty environments (Gandhi & Chikindas, 2007). Previous experiments have shown that the exposure of L. monocytogenes to salt stress caused an increased expression of genes involved in nisin resistance, namely the liaR gene (Bergholz et al., 2012). Recently, a study was conducted to access whether exposure to salt actually affects L. monocytogenes response to nisin. It was concluded that salinity induces the expression of genes regulated by liaR and protects L. monocytogenes against nisin antibacterial effect by increasing its resistance (Bergholz et al., 2013).
Consequently, some experiments are being carried out in order to develop strategies to increase salt resistance in these peptides and to discover new ones intrinsically resistant. Clavanins were