ContentslistsavailableatScienceDirect
Behavioural
Brain
Research
j o ur na l h o me p a g e :w w w . e l s e v i e r . c o m / l o c a t e / b b r
Research
report
Neuroinflammatory
response
to
experimental
stroke
is
inhibited
by
eriodictyol
Emerson
de
Oliveira
Ferreira
a,
Mara
Yone
Soares
Dias
Fernandes
b,
Neila
Maria
Rocha
de
Lima
a,
Kelly
Rose
Tavares
Neves
b,
Marta
Regina
Santos
do
Carmo
b,
Francisco
Arnaldo
Viana
Lima
b,
Analu
Aragão
Fonteles
b,
Ana
Paula
Fontenele
Menezes
a,
Geanne
Matos
de
Andrade
a,b,c,∗aPost-GraduateProgrammeinMedicalSciences,DepartmentofClinicalMedicine,FacultyofMedicine,FederalUniversityofCeará,Brazil bPost-GraduateProgrammeinPharmacology,DepartmentofPhysiologyandPharmacology,FacultyofMedicine,FederalUniversityofCeará,Brazil cInstituteofBiomedicineofBrazilianSemi-arid,Brazil
h
i
g
h
l
i
g
h
t
s
•Neuroprotectiveeffectoferiodictyol inbrainischemia.
•Eriodictyol ameliorates memory impairmentinmice.
•Anti-inflammatoryactivityof eriod-ictyol.
g
r
a
p
h
i
c
a
l
a
b
s
t
r
a
c
t
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received27March2016
Receivedinrevisedform21June2016 Accepted24June2016
Availableonline25June2016
a
b
s
t
r
a
c
t
Background:Cerebralischemiaisacommondiseaseandoneofthemostcommoncausesofdeathand disabilityworldwide.Thelackofglucoseandoxygeninneuronaltissueleadstoaseriesof inflamma-toryevents,culminatinginneuronaldeath.EriodictyolisaflavonoidisolatedfromtheChineseherb Dracocephalumrupestrethathasbeenproventohaveanti-inflammatoryproperties.
Abbreviations:BDNF,brain-derivedneurotrophicfactor;CREB,cAMP-response-element-bindingprotein;DAB,3,3′-diaminobenzidine;ERK,extracellular-signal-regulated kinase;Etyol,eriodictyol;GFAP,glialfibrillaryacidicprotein;HTAB,hexadecyltrimethylammoniumbromide;iNOS,induciblenitricoxidesynthase;MAPK,mitogen-activated proteinkinases;MPO,myeloperoxidase;NF-kB,nuclearfactorkappaB;NO,nitricoxide;Nrf2,nuclearfactor(erythroid-derived2)-like2;PBS,phosphatebufferedsaline; PKB,proteinkinaseB;pMCAO,permanentmiddlecerebralarteryocclusion;RT,roomtemperature;SO,sham-operated;TNF␣,tumornecrosisfactoralpha;t-PA,tissue plasminogenactivator;TTC,2,3,5-triphenyltetrazolium.
∗ Correspondingauthorat:DepartmentofPhysiologyandPharmacology,FacultyofMedicine,Federal,UniversityofCeará,RuaCel.NunesdeMelo1127,Porangabussu, Fortaleza,CE,60430-270,Brazil.
E-mailaddresses:emersonoliveira.shalom@hotmail.com(E.d.O.Ferreira),maraiony@hotmail.com(M.Y.S.D.Fernandes),neilamrl@hotmail.com(N.M.R.d.Lima), kelly.rose@hotmail.com(K.R.T.Neves),martacarmo@yahoo.com.br(M.R.S.d.Carmo),arnviana@hotmail.com(F.A.V.Lima),analufonteless@gmail.com(A.A.Fonteles), apfontenele@yahoo.com.br(A.P.F.Menezes),gmatos@ufc.br,geannecunha@yahoo.com.brr(G.M.d.Andrade).
Keywords: Eriodictyol Flavonoids Memory Neuroprotection pMCAO
Neuroinflammation
Hypothesis/Purpose:Thus,thepresentstudywasdesignedtoexplorewhethereriodictyolhas neuropro-tectiveeffectsagainsttheneuronaldamage,motorandmemorydeficitsinducedbypermanentmiddle cerebralarteryocclusion(pMCAO)inmice.
StudyDesign:Animalswereorallytreatedwitheriodictyol(1,2and4mg/kg)orvehicle(saline)30min beforepMCAO,2hafter,andthenoncedailyforthefollowingfivedays.
Methods:Theparametersstudiedwereneuronalviability,braininfarctedarea;sensorimotordeficits; exploratoryactivity;workingandaversivememory;myeloperoxidase(MPO)activity;TNF␣,iNOSand GFAPimmunoreactivity.
Results:Thetreatmentwitheriodictyolpreventedneuronaldeath,reducedinfarctareaandimproved neurologicalandmemorydeficitsinducedbybrainischemia.TheincreaseofMPOactivityandTNF-␣, iNOSandGFAPexpressionwerealsoreducedbyeriodictyoltreatment.
Conclusion:Thesefindingsdemonstratethateriodictyolexhibitpromisingneuroprotectioneffectsagainst thepermanentfocalischemiacerebralinjuryinthemiceexperimentalmodelandtheunderlying mech-anismsmightbemediatedthroughinhibitionofneuroinflammation.
©2016PublishedbyElsevierB.V.
1. Introduction
Strokeisadebilitatingdiseasethataccountsforbehavioraland cognitivedisturbances,especiallythoseinvolvinglearning,motor andmemorydeficits[1,2].Strokeoccursafterasuddenblockof bloodflowtothebrain,deprivingthetissueofoxygenandglucose
[3,4].Clearly,glutamate excitotoxicity,calciumoverload, oxida-tivestress,inflammationandapoptosisareimportantcontributing factorsinthepathophysiologyofcerebralischemia[5]. Inflamma-tionisresponsibleformuchofcerebralischemictissueinjury[6,7]. Togetherwithmicroglia,astrocytescontributetotheproductionof inflammatorymediators,suchasTNF-␣andiNOS,whichare trig-geredbypro-inflammatorygenes,andNF-kB,whichisdrivenby astrogliosisandmicrogliosis[8].Anti-inflammatoriesconstitutean importanttherapeuticstrategyforstroke[2,9]becausecurrently, theonlyavailabletreatmenttoreducebraindamageafterstrokeis the“clot-buster,”tissueplasminogenactivator(t-PA).Thus,thereis agreatneedtodevelopnoveltherapiesforcerebrovasculardiseases
[10].
A diet rich in vegetables and fruits with polyphenols is knowntopromotefunctionalbenefitsforhealth[11].Amongthe polyphenols,flavonoidsarecompoundsthatarecommonin nat-urally occurring plants and vegetables [12]. Eriodictyol (Etyol) isaflavonoid,“flavanone”,(3′,4′,5,7-tetrahydroxyflavanone) iso-latedfromtheChineseherb(Dracocephalumrupestre)andcitrus fruits.Thereisevidenceforitsanti-inflammatory,anti-allergenic, antimicrobial,anti-cancer[13]andantioxidantproperties[14,15]. Eriodictyol reduced nitric oxide (NO) and pro-inflammatory cytokines in LPS-stimulated Raw 264.7 cells and suppressed the phagocytic activity of activated macrophages. This anti-inflammatory effect of eriodictyol wasrelated to the blockade ofnuclear factorkappa B(NF-B)[13].In a rat modelof tran-sientfocalcerebralischemia,eriodictyolreducedbraindamageand neurologicaldeficits[9].However,theeffectsoferiodictyolon neu-roinflammationandmemorydeficitsafterfocalcerebralischemia havenotbeenreported.Thus,theobjectiveofthepresentstudy wastoinvestigatetheeffectsoferiodictyolonthememorydeficits inducedbypermanentmiddlecerebralarteryocclusion(pMCAO) inmiceandtheprobablemechanismsofactioninvolved.
2. Materialandmethods
2.1. Drugs
Eriodictyol (Sigma, USA), xylazine (2%, Kensol®
, König, Argentina)andketamine(5%,Vetanarcol®
,König,Argentina).All otherreagentswereofanalyticalgrade.
2.2. Animals
MaleSwissmiceweighing25–30gobtainedfromtheCentral AnimalHouseofthePhysiologyandPharmacologyDepartmentof theFederalUniversityofCearáwereused.Animalswerehoused undera12-hlight,12h-darkcycleandallowedaccesstofoodand wateradlibitum.Allproceduresinthisstudywereinagreement withtheGuidefortheCareandUseofLaboratoryAnimalsfrom theUSHealthandHumanServicesDepartmentandwereapproved bytheethicscommitteeonanimalexperimentationoftheFederal UniversityofCeará,undertheregistrationnumber90/2013.
2.3. Inductionofpermanentmiddlecarteryocclusion(pMCAO)
pMCAOwasproducedbyelectrocoagulation oftheleft mid-dlecerebralartery asreported previously[16].Briefly, animals wereanaesthetizedwithxylazine(10mg/kg, i.p.)andketamine (90mg/kg,i.p.),anincisionwasmadeonthelefttemporo-parietal region,andthetemporalismusclewaspartiallyremoved.Aburr holewasdrilledintotheskulloverthemiddlecerebralarteryand thevesselwasoccludeddirectlyproximaltothelateral lenticu-lostriatebranchesusingelectrocoagulationwithamicro-unipolar coagulator.Thecompleteinterruptionofbloodflowwasconfirmed byvisualinspection.Bodytemperaturewaskeptnear37◦C. Sham-operatedanimals(SOgroup)underwentthesameprocedurewith theexceptionofcauterizationofthemiddlecerebralartery.
2.4. Experimentalprotocols
The animals were divided in groups and submitted to oral administration of 5% Tween 80 and saline (pMCAO group) or eriodictyoland saline(1,2, and 4mg/kg,by mouth,orallyp.o.) thirtyminutesbeforepMCAOandtwohoursafter;thetreatment continued oncedaily for the following five days. At 24h post-ischemia, a sub-group of animals (n=6/group) were tested for neurologicdeficitsand euthanizedforischemicdamage evalua-tion.At72and96hpost-ischemia,anothersub-groupofanimals (n=8/group)weretestedforlocomotoractivityandworkingand aversivememories,immunohistochemistryandhistology.At24h post-ischemia,athirdsub-group,animalswereevaluatedforthe MPO(n=6/group).
2.5. Neurologicalevaluation
describedbyGarciaetal.[17].Sixitemsweremeasured,andthe totalscorerangedfrom3to18:thehigherthescore,thebetter themotorperformance.Items1–4(spontaneousactivity, symme-tryofmovements,symmetryofforelimbsandclimbingthewall ofthewirecage)measuredmotorperformance;items5–6 (reac-tiontotouchonandresponsetovibrissaetouch)measuredsensory function(Table1).
2.6. QuantificationofcerebralinfarctsizethroughTTCstaining
Animals were euthanized 24h after ischemia. Brains were removed,and 2mm coronalsectionsweremade fromthe pre-frontal cortex to the midbrain. Slices were immersed in a 2% solutionof TTC (2,3,5-triphenyltetrazolium)in normal salineat 37±◦Cfor20min.Thissaltacceptsaprotonfromsuccinate dehy-drogenase in the inner membrane of the mitochondria, which reducesittoitsredinsolubleformknownasformazan[18].Thus, anareawithinactiveenzymesandtheinfarctionisnotstainedand appearspale.TheunstainedareasweremeasuredbytheOsirisTM software(UniversityofGeneva,Switzerland)andcalculatedasthe percentofthewholecoronalsection.
2.7. Openfieldtest
Allanimalsweretestedforlocomotoractivityusinganopen field apparatus, which consisted of a black acrylic chamber (30×30cm)withthefloordividedinto9squaresofequalareas
[19].Seventy-twohoursaftersurgery,theanimalwaspositioned inthecenterofthearenaandallowedtoexplorefreely.Thenumber ofcrossings(horizontalexploration)andrearings(vertical explo-ration)werescoredfor5min.Thearenawascleanedwith20%ethyl alcoholtoremoveanyodorsbeforethenexttest.
2.8. Y-Mazetest
Onthethirddayaftersurgery,spatialworkingmemorywas assessedbyrecordingspontaneousalternationbehaviorinthe Y-maze[20].Themazewasconstructedofwhitewoodwiththree identicalarms(40×15×4.5cm)positionedatequalangles. Ani-malswereplacedattheendofonearmandallowedtomovefreely throughthemazeduringan8-minsession.Theseriesofarmentries wasrecordedvisually,andarmentrywasconsideredtobe com-pletewhenthehindpawsofthemicewerecompletelyplacedin thearm.Theabilitytoalternaterequiresthemicetoremember whicharmshavealreadybeenvisited.Eachexperimentwasscored;
thepercentageofspontaneousalternationwascalculatedusingthe followingformula:
Spontaneous alternations(%)= alternationbehavior
maximumalternations×100
Alternationbehaviorisdefinedasthenumberofconsecutive entriesintoeach of thethree armswithoutrepetition,and the maximumalternationsarethetotalnumberofarmentriesminus two.
2.9. Passiveavoidancetest
Aversivememorywasassessedat72haftersurgeryasdescribed byGold[21]usingatwo-compartmentapparatus(48×22×22cm; Insight,Brazil).Onecompartmenthadanelectrifiedgridfloorand theotheraplatform.Eachanimalwasplacedontheplatformand allowedtoexploretheapparatusfor1min.Thirtysecondslater it wasplaced oncemore ontheplatformand whentheanimal steppeddownafootshockof0.5mAwasdeliveredfor1sthrough thegridfloor.Thelatencytimetosteponthegridcompartment wasmeasureduptoacut-offtimeof5min(training).Theanimal wasthenremovedfromtheapparatus,andthetrialwasrepeated 15minlater(earlymemory).Theretrievaltrialwasperformedin thesamemanner,24hlater,butnoanimalwasshocked(late mem-ory).
2.10. Enzymaticactivityofmyeloperoxidase(MPO)
Myeloperoxidase(MPO)isanenzymepresentinthegranules ofneutrophils.Thisenzymeisusedasanindicatorof inflamma-tion,morespecifically,asneutrophilmigrationoftissuemarker. Twenty-fourhoursafterinductionofischemia,theanimalswere sacrificed,theirbrainsrapidlyremovedandsamplesofthecortex andstriatumweredissectedandweighed.Theareaswere homog-enized(50mg/ml)inasolutionofhexadecyltrimethylammonium bromide(0.5%HTAB)in50mMphosphatebuffer,pH6.0.Thenthe sampleswerecentrifugedat14,000rpmat4◦Cfor2min.30Lwas addedto200Lofsamplesupernatantandthesolutioncontaining 0.167mg/mlo-dianisidine hydrochlorideand 0.0005%hydrogen peroxide.Theabsorbancewasmeasuredat0.1timesand3min atwavelength460nm.TheresultswerereportedasunitsofMPO permgoftissue.AndanMPOunitistheamountofenzymewhich degrades1mol/minofhydrogenperoxide.
Table1
Usedscoreforneurologicalevaluation(Garciaetal.,1995).
Test Score
0 1 2 3
Spontaneousactivity Nomovement Norearingand barelymoves
Movesbutdoesnot approachatleast3 sidesofthecage
Movesandapproaches atleastthreesidesof thecage
Symmetryof movements(4limbs)
Contraleteral side:no movement Contraleteralside: slightmovement Contraleteralside: slowmovement Bothsides: symmetrical movement Symmetryofforelimbs
(outstretchingwhile heldbytail)
Contraleteral side:no movement Contraleteralside: slightmovement Contraleteralside: slowmovement Bothsides: symmetrical movement Climbingwallofwire
cage
... Failstoclimbor
tendstomovein circles
Contralateralsideis weakerwhengriping
Normalclimbingand gripping
Bodyproprioception ... Noresponseon contralateralside
Weakerresponseon contralateralside
Symmetricalresponse
Responsetovibrissae touch
... Noresponseon
contralateralside
Weakerresponseon contralateralside
2.11. ImunohistochemistrystainingTNF-˛,iNOSandGFAP
Briefly,theanimalswereanesthetizedwithsodium thiopen-talandtranscardiallyperfusedwithice-coldPhosphateBuffered Saline(PBS)followedby4%paraformaldehydeinPBS.Thebrains wereremoved,post-fixed in4%paraformaldehydefor 24h, and cryoprotectedin30%sucrosefor48hat4◦C.Coronalsectionsfrom
cortexandstriatumwerecollectedinaone-in-sixseriesof50m (300mofinterval)usingacryostat(LeicaCM3050S,Heidelberg, Germany)at−21◦C.
Thesectionswere washed3 timesfor 10minwithPBS and incubatedwithPBSsupplementedwith10%methanoland1.05% hydrogenperoxidefor40minatroomtemperature(RT),toblock endogenousperoxidase-likeactivities.Afterwashing3timesfor 10minwithPBSandblockingendogenousproteinswith10% nor-malgoatseruminPBSsupplementedwithTritonX-100(blocking solution)fortwohoursatRT,thesectionswereincubatedwith theprimaryantibodies(anti-TNF-␣,1:250 oranti-iNOS,1:400, SantaCruzBiotechnology)dilutedinblockingsolutionat4◦Cfor
48h.Thesectionswerethenwashedthreetimesfor10mininPBS andsubsequentlyincubatedwithavidin–biotin–horseradish per-oxidaseconjugate(ABCStainingSystem,SantaCruzBiotechnology) for30min.Afterwashing,theslideswereincubatedwith biotiny-latedgoatanti-rabbitsecondaryantibody,diluted1:500inblocking solution.The color was developed using DAB as a chromogen. ThesectionsweremountedinEntellan(Merck,Germany),cover slippedandvisualizedunderamicroscope(NikonElipseE200)at 40×zoom.Cellswereconsideredpositivelylabeledwhenabrown colorationwasundoubtlyobservedoverahematoxiline counter-coloration.Threefieldsof each sectionwereselectedrandomly alongthelesionareaandquantificationofimageswascarriedout usingImageJsoftware(NIH,Bethesda,MD,USA).
ForGFAPimmunostaining,sectionswerewashed3timesfor 10minwithPBSandsimultaneouslypermeabilizedandblocked with0.2% Triton-X and 10% horse serum in PBS for 1hat RT. Thesectionswereincubatedfree-floatingwiththeprimary anti-body (anti-GFAP, 1:500, rabbit polyclonal, Sigma-Aldrich, USA) dilutedin blocking solutionat 4◦C for 48h. The sectionswere
thenwashedthreetimesfor10mininPBSwith0.25%Triton-X, followedbyincubationwiththesecondaryantibody(AlexaFluor 594donkeyanti-rabbit,1:500,Invitrogen,Portugal).Thesections werewashed3timesinPBSandmountedinFluoromount(Sigma), cover slippedand visualized undera microscope(Nikon Elipse E200)at 40× zoom.The meanfluorescence intensitywasused asasemi-quantitativemeasureoftheimmunoreactivityandwas estimatedusingtheprogramImageJsoftware(NIH,Bethesda,MD, USA).Thevaluesofmeanfluorescenceintensityobtainedforthe controlgroup(SO)wereaveragedandallofthemeanfluorescence intensityvalueswerecalculatedasapercentageofthatmean.
2.12. Cellviabilityevaluation
The cresyl-violet staining is used to show the Nissl bodies presentin thecytoplasm of viable neurons[22]. Brainsections (50mthickand300mapart)weremountedonslideswith5% gelatin,lefttodryandthenincubatedinacetate-buffered0.5% cre-sylvioletsolutionfor10min.Then,theslidesweredehydratedin alcohol(50,70and100%).Theywerethenimmersedinxyleneand mountedwithEntellan(Merck,Germany).Theslideswereviewed underamicroscope(NikonE200Ellipse)with40×magnification. Threeslicesfromeachanimalwererandomlyselectedalongthe lesionareaandquantifyingthecellswasperformedusingImageJ software(NIH,Bethesda,MD,USA)withagride100.Cellswere consideredpositivewhentheyshowedcresylvioletstaininginthe cytoplasmofcells, aswellasnormalmorphology(cellroundor ovalnucleiwithcentralized).Cellswereconsiderednegativewhen
Etyol 4 Etyol 1 Etyol 2 Etyol 4
12 14 16 18 20
*
#
SO
pM CAO
s
er
o
c
S
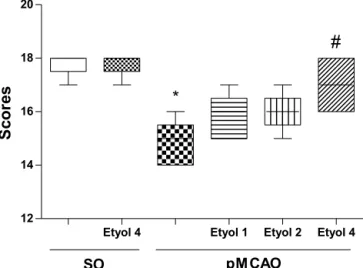
Fig.1.Eriodictyolintakereducestheischemia-inducedneurologicaldeficits.Swiss malemicereceivedeithervehicle(Tween80+saline)oreriodictyol (1,2,and 4mg/kgp.o.,n=6)at30minbeforeandtwohoursaftertopMCAOorasham operation(SO).Theneurologicalscore,calculatedasacompositeofsixmeasured itemsandmeasured24hafter ischemia,rangedfrom3to18andthehigher thescore,thebetterthemotorperformance.Dataareexpressedasthemedian (interquartileranges).*p<0.05vs.SOgroupand#p<0.05vs.pMCAOgroup,using
aKruskall–WallisandMann–Whitneytests.
noviolet colorationassociated withmorphologicalchanges, i.e. shrunkencellnucleiaccompaniedbypyknoticnuclei,waspresent. Theresultswereexpressedaspercentage ofcresylpositive and cresylnegativecells.
2.13. Statisticalanalysis
Dataareexpressedasmean±S.E.M.orasmean(interquartile range)and statistical differenceswereestimated usingeither a one wayANOVA, Tukeypost hoctest or a Kruskall-Wallis and Mann-Whitneytests. Analyseswere performedwiththeuseof GraphPad Prism software (GraphPad Software Inc., La Jolla,CA, USA).P-values<0.05wereconsideredsignificant.
3. Results
3.1. EriodictyolimprovedneurologicaldeficitsinpMCAOmice
Theneurologicaldeficitsscoreswereassessed24hpoststroke induction.AsshowinFig.1,thepMCAOanimalsshowedsignificant neurologicaldeficits(reducedmotorabilityanddecreasedability torespondtostimulionthesideofthebodycontralateraltothe ischemia)comparedtoshamoperatedanimals(SO:17.8(17–18); SO+Etyol4:17.8(17–18);pMCAO:14.8(14–16),p<0.05). Eriod-ictyol(Etyol)treatmentat4mg/kgdoseimprovedtheneurological deficitsinducedbypMCAO(pMCAO+Etyol4:17(16–18),p<0.05).
3.2. EriodictyolreducedcerebralinfarctsizeinpMCAOmice
Fig.2. Eriodictyolintakereducestheischemia-inducedinfarctarea.Swissmalemicereceivedeithervehicle(Tween80+saline)oreriodictyol(1,2,and4mg/kgp.o.,n=6) at30minbeforeandtwohoursafterpMCAOorashamoperation(SO).Thepercentageofinfarctareaat24hafterpMCAO(B),wascalculatedfromcoronalsectionsstained with2%2,3,5-triphenyltetrazolium,asshownintherepresentativesetofimages(A).Thepale-coloredregionshowstheinfarctregion,whereasthereddishcoloredregion showstheviabletissue.Dataareexpressedasmean+SEM.*p<0.05vs.SOgroupand#p<0.05vs.pMCAOgroup,usingaKruskall–WallisandMann–Whitneytests.
Etyol 4 Etyol 1 Etyol 2 Etyol 4 0
20 40 60 80 100
Crossings Rearings
*
# #
SO pM CAO
st
n
e
v
e
f
o
r
e
b
m
u
N
Fig.3.Eriodictyolattenuatestheischemia-inducedlocomotormodifications.Swiss malemicereceivedeithervehicle(Tween80+saline)oreriodictyol (1,2,and 4mg/kgp.o.,n=8)at30minbeforeandtwohoursafterpMCAOorasham oper-ation(SO)andonceadayinthefollowingdays.Miceweretestedforhorizontal explorationandverticalexplorationinanopenfieldat72haftersurgery.Dataare expressedasmean+SEM.*p<0.05vs.SOgroupand#p<0.05vs.pMCAOgroup,
usingaKruskall–WallisandMann–Whitneytests.
3.3. EriodictyolimprovedlocomotoractivitydeficitsinpMCAO mice
The locomotor activity was evaluated 72h after ischemia. Nosignificantdifferencewasfoundin thenumber ofcrossings between groups. However, the number of rearings was lower in the pMCAOgroup compared to thesham (SO: 11.88±1.76; SO+Etyol4:13.38±1.63;pMCAO:5.25±0.88,p<0.05). Eriodic-tyoltreatmentatdosesof2and4mg/kg,significantlypreventedthe reductioninthenumberofrearings(pMCAO+Etyol1:8.87±2.33; pMCAO+Etyol 2: 18.75±2.34; pMCAO+Etyol 4: 19.00±2.08, p<0.05)(Fig.3).
3.4. Eriodictyolimprovedworkingandaversivememorydeficits inpMCAO
TheanimalssubjectedtopMCAOshowedsignificantdeficitsin workingmemoryat72hafterischemia;thiseffectwasprevented by eriodictyol (spontaneous alternations−SO: 79.73±2.47; SO+Etyol 4:78.75±1.04; pMCAO: 50.88±3.39; pMCAO+Etyol 1:73.50±2.67;pMCAO+Etyol2:76.13±0.95;pMCAO+Etyol4: 77.75±4.29,p<0.05)(Fig.4A).
The step-downlatency of animalssubjected topMCAO was significantly shorter compared to the sham-operated group 72 and96haftertheinductionofischemia(Latency–earlymemory: SO:244±18.81;SO+Etyol4:265±16.90;pMCAO:31.75±5.15; lateMemory:SO:270±16.28;SO+Etyol4:261±14.89;pMCAO: 26.63±9.45, p<0.05). These findings demonstrated significant deficitsinearlyand late memories.Eriodictyol atdose4mg/kg showedprotectionagainstdeficits(pMCAO+Etyol4:212.6±26.64, p<0.05)(Fig.4B).
3.5. Eriodictyoladministrationdecreasedmyeloperoxidase activityandpro-inflammatorymediatorsinpMCAOmice
pMCAOincreasedenzymaticactivityofmyeloperoxidase(MPO) intheipsilateralcortexbutnotinstriatum24haftersurgery com-pared to sham-operated animals (SO: 5.35±1.57; SO+Etyol 4: 7.24±2.50;pMCAO:43.45±2.73u/mgtissue,p<0.05).Treatment witheriodictyol at doseof 4mg/kg significantly decreased this effect(pMCAO+Etyol4:5.00±0.97u/mgtissue,p<0.05)(Fig.5). OurfindingsalsodemonstratedthatthenumberofTNF-␣-positive (Fig.6AandB)andiNOS-positivecells(Fig.7AandB)were sig-nificantlyloweronischemicpenumbraincortexandstriatumof animalstreatedwitheriodictyolcomparedwithpMCAOgroup.
3.6. EriodictyolattenuatedastroglialcellsactivationinpMCAO mice
Theischemicbraindamagepromotedtheactivationof astro-cytesdemonstratedbyanincreasedglialfibrillaryacidicprotein (GFAP). Astrogliosiswasevidenced incortex andstriatum from pMCAOanimals.GFAPimunoreactivitywassignificantlydecreased with the highest eriodictyol dosage (cortex: pMCAO+Etyol 4: 200.2±28.9;striatum:pMCAO+Etyol4:232.1±42.82)(Fig.8Aand B).
3.7. Eriodictyolincreasedthenumberofviablecellsincortexand striatumfrompMCAOmice
Etyol 4 Etyol 1 Etyol 2 Etyol 4 0 20 40 60 80 100
*
# # #SO pM CAO
) %( s n oi t a nr et l a s u o e n at n o p S
Etyol 4 Etyol 1 Etyol 2 Etyol 4 0 100 200 300 Training Early Memory Late Memory
* *
# # SO pMCAO La te n cy (s )A
B
Fig.4.Eriodictyolattenuatestheischemia-inducedmemorydeficits.Swissmalemicereceivedeithervehicle(Tween80+saline)oreriodictyol(1,2,and4mg/kgp.o.,n=8) at30minbeforeandtwohoursafterpMCAOorashamoperation(SO)andonceadayinthefollowingdays.Miceweretested72haftersurgeryforworkingmemoryina Y-mazetest[A,wherethepercentageofalternationwascalculatedaccordingtotheformula:totalofalternations/(totalarmentries−2)×100]andforaversivememory[B, estimatedattwodifferenttimeintervals(15minand24h)].Dataareexpressedasmean+SEM.*p<0.05vs.SOgroup,#P<0.05vs.pMCAOgroup,usingaKruskall–Wallis
andMann–Whitneytests.
Etyol 4 Etyol 4 0 10 20 30 40 50
Cortex
Striatum
*
#SO pM CAO
) e u s si t g m/ U( yti vi t c a O P M
Fig.5. Eriodictyol(4mg/kg)preventstheincreaseofMPOactivityinthecortexafter pMCAO.Swissmalemicereceivedeithervehicle(Tween80+saline)oreriodictyol (4mg/kgp.o.,n=6)at30minbeforeandtwohoursafterpMCAOorashamoperation (SO).MPOactivity,asaneutrophilinfiltratemarker,wasevaluatedincorticaland striataltissue.Dataareexpressedasmean+SEM.*p<0.05vs.groupand#p<0.05
vs.pMCAOgroup,usingaKruskall–WallisandMann–Whitneytests.
cellswithcentralizednuclei.Inanimalstreatedwitheriodictyol, thenumber and cellular morfology wassignificantly preserved (Fig.9A–C).
4. Discussion
Thisstudyprovidedthefirstreportofpreclinicalevidenceofthe neuroprotectiveeffectoftheflavonoideriodictyol(Etyol)on mem-orydeficitsandneuroinflammationinmicesubjectedtopermanent middle cerebralartery occlusion (pMCAO). Etyol is a flavonoid isolatedfromtheChineseherb Dracocephalumrupestrethathas anti-inflammatory[13]andantioxidantproperties[14,15].
The permanent middle cerebral artery occlusion (pMCAO) modelby electrocoagulationin miceprovedextremely reliable, inducingneuronaldamage[23]andcognitivedeficits[24,25].Thus, thismodelwaschosenforthestudyofprotectiveeffectonmemory impairmentfromeriodictyolduringtheischemicevent.
Aspreviouslyreported[26,27]infarctsmodeledbythedistal middlecerebralarteryocclusionmodelinmiceencompassabout 10–15%ofthehemisphere,therebymimickingamajorityofhuman strokelesionswhichare locatedinthecorticalmiddlecerebral arteryterritory[28,29].Ourresultsshowed24hafterthepMCAO
a lesionsize around12%that wasconsistentwitha previously publishedreport[26].Wefoundthattheoraladministrationof Etyolimprovedneurologicalfunctionandreducedinfarctareain theischemicanimals.ThesefindingsareconsistentwithJingetal.
[9],which showedthateriodictyol-7-O-glucoside(E7G) ina rat modeloffocalcerebralischemia,significantlyreducedbrain dam-ageandamelioratedneurologicaldeficits.Otherflavonoidssuch as(−)-epicatechin[30],luteolin[31]andquercetin[32]havealso shown neuroprotective effects against ischemia-induced injury. Themechanismsofflavonoidsneuroprotectionmayinvolvetheir interactionwithneuronalandglialsignalingcascadesinthebrain leadingto apromotion of neuronal survivaland differentiation
[33,34],theincreaseofperipheralandcerebralvascularbloodflow thatmayleadtotheinductionofangiogenesis[35],andby react-ingdirectlywithandscavengespeciesandproinflammatoryagents
[36].
Evidencehasshownthat thereissignificantlossofneuronal viabilityinanimalssubjectedtopMCAOandthatthemostaffected areasarethecortexandthestriatum[37,38].Inthepresentstudy, weobservedsignificantlossofneuronalviabilitybothincortexand striatumofischemicanimals5daysafterpMCAO.Etyoltreatment significantlyprevented this celldeathin both structures.These resultsareconsistentwithSunil etal. [39]study thatreported theneuroprotectiveactionoftheflavonoidsobtainedfrom Cype-rusrotundusonthecelldeathinducedbyexperimentalcerebral ischemiainrodents.
Humandeficitsafterstrokeoccurwithdamagetospecific cir-cuits,suchasinmotorcortexmaps[40]and recoveryfollowsa reproduciblydeterminedfunctionalreorganization inthebrain. Motor,sensory,andlanguagerecoveryinvolvesaprogressive reor-ganizationandrecoveryofactivationinperi-infarctandipsilateral connectedcorticalsitesafterstroke[41,42].Inthis study,Etyol treatmentprotectedanimalsfrommotorimpairment,probablyby reducingtheinfarctedarealocatedinthemotorcortexand stria-tum.Flavonoislikerutin [43],andsilymarin[44]alsoprotected animalsfrommotorimpairmentinducedbybrainischemia.
The cortex, striatum and hippocampus are brain structures related tocognition and memory functions[45]. These regions are themost strongly affectedby MCAO [46].Previous studies using pMCAO-induced neurological deficits have demonstrated mnemonicdeficitsinrodents[24,47–49].
Fig.6. Eriodictyol(4mg/kg)attenuatestheincreaseofTNF-␣expressioninthecortexandstriatumofmicesubmittedtopMCAO.Swissmalemicereceivedeithervehicle (Tween80+saline)oreriodictyol(4mg/kgp.o.,n=4)at30minbeforeandtwohoursafterpMCAOorashamoperation(SO)andonceadayinthefollowingdays.TNF-␣ immunostainingwasperformedinseriesofcoronalsections(50mthickand300mapart)representativeoftheipsilateralcortexandstriatum(A).Thebargraphs(B) showthequantificationofthenumberofimmune-positivecellsforTNF-␣.Dataareexpressedasmean+SEM.*p<0.05vs.SOgroup,#p<0.05vs.pMCAOgroup,usinga
Kruskall–WallisandMann–Whitneytests.
information.Thedorsolateralprefrontalcortexhasbeenknownto participateinworkingmemory[50].Wecanassesstheworking memoryusingtheYmazetest.Thistestisbasedonthetendency of animalstoexplore newenvironments withouttheinfluence of emotional or motivational tricks [51]. Additionally, this test providesdissociation betweenlearning and memorybecauseit doesnotrequirerules tobelearned[52].Inanimals,the explo-rationofanewenvironmentdependsontheintegrityoflimbic andnon-limbicsystems,suchasthehippocampus,basalforebrain, prefrontalcortex,thalamus,dorsalstriatumbeyondthe vestibu-larsystemand thecerebellum [51].Carmoet al.[49] observed deficitsinworkingmemoryinmiceafterpMCAO.Inthepresent study, the animals that underwent pMCAO showed deficits in workingmemory72haftertheinductionofischemia,andEtyol treatmentpreventedthesedeficits. Therearereports inthe lit-eraturethat showthat phenolic compoundssuchas flavonoids are abletoimprove performance of animalssubjected to tran-sientcerebralhypoperfusion-inducedontheY-maze;thishasbeen
demonstratedfororoxylinA,aflavonoidderivedfromthe Scutel-lariabaicalensisGeorgiplant[53].
Step-down-type passiveavoidanceparadigmis basedonthe naturalbehavior ofanimalstoexplorebeyondtheplatformbut alsotodeviatefromaversivesituationgeneratedbytheshockin theelectrifiedpartoftheapparatus.Therefore,thismodelcanbe usedtostudyaversivememory[54].AnimalssubjectedtoMCAO havedeficitsinrecentandlatememory,asevaluatedinthe pas-siveavoidancetest[55].Inthisstudy,weobservedthatthepMCAO groupshoweddeficitsinearlyandlateaversivememory,and treat-mentwithEtyolprotectedtheanimalsfromthisdeficit.Thisresult corroboratesthefindings of Wuet al. [56],which showedthat theisoflavonepuerarincouldimprovethelearning-memoryability afterglobalcerebralischemiaandreperfusioninratsevaluatedby thepassiveavoidancetest.
Fig.7.Eriodictyol(4mg/kg)attenuatestheincreaseofiNOSexpressioninthecortexandstriatumofmicesubmittedtopMCAO.Swissmalemicereceivedeithervehicle (Tween80+saline)oreriodictyol(4mg/kgp.o.,n=4)at30minbeforeandtwohoursafterpMCAOorashamoperation(SO)andonceadayinthefollowingdays.iNOS immunostainingwasperformedinseriesofcoronalsections(50mthickand300mapart)representativeoftheipsilateralcortexandstriatum(A).Thebargraphs(B) showthequantificationofthenumberofcellsimmune-positiveforiNOS.Dataareexpressedasmean+SEM.*p<0.05vs.SOgroup,#p<0.05vs.pMCAOgroup,usinga
Kruskall–WallisandMann–Whitneytests.
exertpharmacologicalactivityonreceptors,kinasesand transcrip-tionfactors[36].Flavonoidsmayinteractthroughthemodulation ofkinaseactivity,includingMAPKkinase,MAPKkinaseorMAPKs; through ATP-binding enzymes and receptors; by changing the roleof phosphatasesthat actin opposition tokinases;through activationoftheconnectionbetweentranscriptionfactorsand pro-motersequences;bymaintainingthehomeostasisof Ca++,thus preventingthekinase-dependentinfluxofCa++intoneurons[36]. Whenthesepathwaysare activated, thesynthesis of new pro-teinsisinducedinneurons,promotingneuroplasticityandcausing morphologicalchangesthatdirectlyinfluencetheacquisition, con-solidationandstorageofmemory[34].Thus,itispossiblethatEtyol, beinga flavonoid,haspreventedworkingandaversivememory deficitsbyactingonsomeofthesepathways,butthemechanism hasnotbeeninvestigatedinthiswork.
Studieshave shownthatacuteneuroinflammatory processes areresponsibleformuchofcerebralischemictissueinjury[6,7]. Neutrophilsaretheearliestleukocytestomigratetotheischemic
focus.Myeloperoxidase(MPO)isanenzymepresentinthegranules ofneutrophils[59].HighMPOactivitywasestablishedasa quanti-tativeindicatorofleukocyteinfiltrationduetoneuroinflammation inischemicanimals[60].
In ourstudy, a significantincrease in the MPOactivity was observed24hafterpMCAOinthetemporalcortexbutnotinthe striatumofischemicanimals.InagreementtothisresultTuetal.
Fig.8.Eriodictyol(4mg/kg)attenuatestheincreaseofGFAPexpressioninthecortexandstriatumofmicesubmittedtopMCAO.Swissmalemicereceivedeithervehicle (Tween80+saline)oreriodictyol(4mg/kgp.o.,n=4)at30minbeforeandtwohoursafterpMCAOorashamoperation(SO)andonceadayinthefollowingdays.The immunoreactivityofGFAPwasperformedinseriesofcoronalsections(50mthickand300mapart)representativeoftheipsilateralcortexandstriatum(A).Thebar graphs(B)showthequantificationofthemeanfluorescenceintensity(MFI)expressedasapercentageofthemeanMFIoftheSOgroup.Dataareexpressedasmean+SEM. *p<0.05vs.SOgroup,#p<0.05vs.pMCAOgroup,usingaKruskall–WallisandMann–Whitneytests.
theactivation of microglia and astrocytes after brainischemia, whichcontributestotheproductionofvariouspro-inflammatory mediators[65]andanti-inflammatorytherapieshaveshown neu-roprotectiveeffectsinanimalssubjectedtopMCAO[66].Astrocytes playafundamentalroleinmaintainingbrainhomeostasis.They expressglialfibrillaryacidicprotein(GFAP),a astrocyticprotein thatcanbeusedtodistinguishastrocytesfromothercelltypes[67]. Inanytypeofbraininjury,suchasinflammation,surviving astro-cytesintheaffectedareasareactivated.Theybegintoproliferate andtohypertrophy;thiseventiscalledreactiveastrogliosis[68].
In the present study, the animals that underwent pMCAO showedsignificantastrogliosisinthetemporalcortexandstriatum fivedaysaftertheischemicinduction,corroboratingtheresultsof Yangetal.[69]whoobservedGFAP-positiveastrocytesinpMCAO model.WehaveshownthatEtyoltreatmentsignificantlyprevented theincreaseinGFAPimmunoreactivityinthecortexandstriatum ofischemicanimals.
Jingetal.[9]havedemonstratedthateriodictyol-7-O-glucoside (E7G)significantlyprotectsagainst astrocyte-inducedcelldeath
Fig.9.Eriodictyol(4mg/kg)attenuatestheincreasecelldeathinthecortexandstriatumofmicesubmittedtopMCAO.Swissmalemicereceivedeithervehicle(Tween 80+saline)oreriodictyol(4mg/kgp.o.,n=4)at30minbeforeandtwohoursafterpMCAOorashamoperation(SO)andonceadayinthefollowingdays.Thecresylviolet stainingwasperformedinseriesofcoronalsections(50mthickand300mapart)representativeoftheipsilateralcortexandstriatum(A).Thebargraphs(BandC)showthe quantificationofthenumberofcellsstainedwithcresylviolet.Dataareexpressedasmean+SEM.*p<0.05vs.SOgroup,#p<0.05vs.pMCAOgroup,usingaKruskall–Wallis
andMann–Whitneytests.(Forinterpretationofthereferencestocolourinthisfigurelegend,thereaderisreferredtothewebversionofthisarticle.).
Inthisstudy,iNOSandTNF-␣immunoreactivityinthecortex andstriatumwassignificantlyhigherintheanimalssubjectedto pMCAOthanintheanimalsoftheSOgroupatfivedaysafterthe ischemicinduction.OtherstudiesreportedanincreaseinTNF-␣ expressionand iNOSinanimalssubjectedtopMCAO[74].Etyol treatmentpreventedtheincreasesinTNF-␣andiNOSexpression onischemicanimals,corroboratingpreviousstudiesthatshowed theability of Etyol tosuppress TNF-␣ and iNOS production in macrophagesstimulatedwithLPS,throughToll-likereceptortype 4blockade[75].Furthermore,Etyolreducedthecytokines(TNF-␣, IL-1andIL-6)andNOproductioninRAW264.7cellsstimulated withLPS,bydirectblockadeofNF-kB[13].
Vafeiadouetal.[76]havebeenshownthathesperetinand narin-genin,twoflavonoidsofthesameflavanonegroupthatEtyol,and theflavanols(+)-catechinand(−)-epicatechin,attenuated LPS/IFN-gamma-inducedTNF-alpha productionin glial cells. Naringenin alsoinhibitedLPS/IFN-gamma-inducediNOSexpressionandnitric oxideproduction,andalsoinhibitedLPS/IFN-gamma-inducedp38 mitogen-activated protein kinase (MAPK) phosphorylation and downstream signal transducer and activator of transcription-1 (STAT-1)inLPS/IFN-gammastimulatedglialcells.Itislikelythat theeriodictyol,tobeofthesamechemical groupofhesperetin
and naringenin, has its anti-inflammatory action acting in the p38/MAPKsignalingpathway,butthisroutehasnotbeen inves-tigatedinthiswork.
5. Conclusions
In conclusion, the present study, for the first time, shows neuroprotective potential of eriodictyol in experimental brain ischemia.Eriodictyolmarkedly reducedcerebralinfarction, neu-ronal damage, sensorimotor and memory deficits. We propose thatanti-inflammatory property,at leastinpart,couldaccount forneuroprotectiveactionoferiodictyol.Althoughpriortreatment oferiodictyolamelioratedneuronaldamageinducedbyischemic injury,anassessmentofearly-treatmentefficacyandwindowof opportunityneedtobestudiedinfuturetoidentifyclinical rele-vanceoferiodictyolinstroketreatment.
Conflictofinterests
financialsupportforthisworkthatcouldhaveinfluencedits out-come.
Acknowledgements
WewishtothanktheBrazilianNationalResearchand Develop-mentCouncil(CNPq),INCT-IBISAB-Capes,andtheResearchSupport FoundationofCeará(FUNCAP)forfinancialsupportintheformof grantsandfellowshipawards.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,athttp://dx.doi.org/10.1016/j.bbr.2016.06.046.
References
[1]R.Wang,J.Tu,Q.Zhang,X.Zhang,Y.Zhu,W.Ma,C.Cheng,D.W.Brann,F. Yang,Genisteinattenuatesischemicoxidativedamageandbehavioraldeficits viaeNOS/Nrf2/HO-1signaling,Hippocampus23(2013)634–647.
[2]Y.Gu,J.Chen,J.Shen,Herbalmedicinesforischemicstroke:combating inflammationastherapeutictargets,J.NeuroimmunePharmacol.9(2014) 313–339.
[3]J.B.Dietrich,TheadhesionmoleculeICAM-1anditsregulationinrelation withtheblood-brainbarrier,J.Neuroimmunol.128(2002)58–68. [4]N.Reglero-Real,B.Marcos-Ramiro,J.Millán,Endothelialmembrane
reorganizationduringleukocyteextravasation,Cell.Mol.LifeSci.69(2012) 3079–3099.
[5]E.N.S.Pires,R.L.Frozza,J.B.Hoppe,B.M.Menezes,C.G.Salbego,Berberinewas neuroprotectiveagainstaninvitromodelofbrainischemia:survivaland apoptosispathwaysinvolved,BrainRes.1557(2014)26–33.
[6]S.Z.Hou,Y.Li,X.L.Zhu,Z.Y.Wang,X.Wang,Y.Xu,Ameliorativeeffectsof diammoniumglycyrrhizinateoninflammationinfocalcerebral ischemic-reperfusioninjury,BrainRes.1447(2012)20–27.
[7]L.Wu,K.Zhang,G.Hu,H.Yan,C.Xie,X.Wu,Inflammatoryresponseand neuronalnecrosisinratswithcerebralischemia,NeuralRegener.Res.9 (2014)1753–1762.
[8]A.M.Blanco,M.Pascual,S.L.Valles,C.Guerri,Ethanol-inducediNOSand COX-2expressioninculturedastrocytesviaNF-kappaB,Neuroreport15 (2004)681–685.
[9]X.Jing,D.Ren,X.Wei,H.Shi,X.Zhang,R.G.Perez,H.Lou,H.Lou, Eriodictyol-7-O-glucosideactivatesNrf2andprotectsagainstcerebral ischemicinjury,Toxicol.Appl.Pharmacol.273(2013)672–679.
[10]B.R.Broughton,R.Lim,T.V.Arumugam,G.R.Drummond,E.M.Wallace,C.G. Sobey,Post-strokeinflammationandthepotentialefficacyofnovelstemcell therapies:focusonamnionepithelialcells,Front.Cell.Neurosci.6(2013)66. [11]L.Dauchet,J.Ferrières,D.Arveiler,J.W.Yarnell,F.Gey,P.Ducimetière,J.B.
Ruidavets,B.Haas,A.Evans,A.Bingham,P.Amouyel,J.Dallongeville, Frequencyoffruitandvegetableconsumptionandcoronaryheartdiseasein FranceandNorthernIreland:thePRIMEstudy,Br.J.Nutr.92(2004)963–972. [12]G.DiCarlo,N.Mascolo,A.A.Izzo,F.Cappaso,Flavonoids:oldandnewaspects
ofaclassofnaturaltherapeuticdrugs,LifeSci.65(1999)337–353. [13]J.K.Lee,Anti-inflammatoryeffectsoferiodictyolin
lipopolysaccharide-stimulatedraw264.7murinemacrophages,Arch.Pharm. Res.34(2011)671–679.
[14]Q.Hu,D.D.Zhang,L.Wang,H.Lou,D.Ren,Eriodictyol-7-O-glucosideanovel Nrf2activator,confersprotectionagainstcisplatin-inducedtoxicity,Food Chem.Toxicol.50(2012)1927–1932.
[15]H.Lou,X.Jing,D.Ren,X.Wei,X.Zhang,Eriodictyolprotectsagainst H(2)O(2)-inducedneuron-likePC12celldeaththroughactivationofNrf2/ARE signalingpathway,Neurochem.Int.61(2012)251–257.
[16]M.Bernaudin,H.H.Marti,S.Roussel,D.Divoux,A.Nouvelot,E.T.MacKenzie,E. Petit,Apotentialroleforerythropoietininfocalpermanentcerebralischemia inmice,J.Cereb.BloodFlowMetab.19(1999)643–651.
[17]J.H.Garcia,S.Wagner,K.F.Liu,X.J.Hu,Neurologicaldeficitandextentof neuronalnecrosisattributabletomiddlecerebralarteryocclusioninrats. Statisticalvalidation,Stroke26(1995)627–634.
[18]J.B.Bederson,L.H.Pitts,S.M.Germano,M.C.Nishimura,R.L.Davis,H.M. Bartkowski,Evaluationof23,5-triphenyltetrazoliumchlorideasastainfor detectionandquantificationofexperimentalcerebralinfarctioninrats, Stroke17(1986)1304–1308.
[19]R.N.Walsh,R.A.Cummins,Theopen-fieldtest:acriticalreview,Psychol.Bull. 83(1976)482–504.
[20]M.Sarter,G.Bodewitz,D.N.Stephens,Attenuationofscopolamineinduced impairmentofspontaneousalterationbehaviourbyantagonistbutnot inverseagonistandagonistbeta-carbolines,Psychopharmacology94(1988) 491–495.
[21]P.E.Gold,Theuseofavoidancetraininginstudiesofmodulationofmemory storage,Behav.NeuralBiol.46(1986)87–98.
[22]F.A.Scorza,R.M.Arida,R.M.Cysneiros,C.A.Scorza,M.deAlbuquerque,E.A. Cavalheiro,Qualitativestudyofhippocampalformationinhypertensiverats withepilepsy,Arq.Neuropsiquiatr.63(2A)(2005)283–288.
[23]K.Türeyen,R.Vemuganti,K.A.Sailor,R.J.Dempsey,Infarctvolume quantificationinmousefocalcerebralischemia:acomparisonof triphenyltetrazoliumchlorideandcresylvioletstainingtechniques,J. Neurosci.Methods139(2004)203–207.
[24]F.D.P.Fernandes,A.P.F.Menezes,J.C.S.Neves,A.A.Fonteles,A.T.Silva,P.A. Rodrigues,M.R.S.Carmo,C.M.Souza,G.M.Andrade,Caffeicacidprotectsmice frommemorydeficitsinducedbyfocalcerebralischemia,Behav.Pharmacol. 25(2014)637–647.
[25]A.A.Fonteles,C.M.Souza,J.C.S.Neves,A.P.Menezes,M.R.S.Carmo,F.D. Fernandes,P.R.Araújo,G.M.Andrade,Rosmarinicacidpreventsagainst memorydeficitsinischemicmice,Behav.BrainRes.297(2016)91–103. [26]D.Tsuchiya,S.Hong,T.Kayama,S.S.Panter,P.R.Weinstein,Effectofsuture
sizeandcarotidclipapplicationuponbloodflowandinfarctvolumeafter permanentandtemporarymiddlecerebralarteryocclusioninmice,Brain Res.970(2003)131–139.
[27]D.W.Howells,M.J.Porritt,S.S.Rewell,V.O’Collins,E.S.Sena,H.B.Vander Worp,R.J.Traystman,M.R.Macleod,Differentstrokesfordifferentfolks:the richdiversityofanimalmodelsoffocalcerebralischemia,J.Cereb.BloodFlow Metab.30(2010)1412–1431.
[28]T.Brott,J.R.Marler,C.P.Olinger,H.P.AdamsJr.,T.Tomsick,W.G.Barsan,J. Biller,R.Eberle,V.Hertzberg,M.Walker,Measurementsofacutecerebral infarction:lesionsizebycomputedtomography,Stroke20(1989)871–875. [29]A.Lindgren,A.Roijer,O.Rudling,B.Norrving,E.M.Larsson,J.Eskilsson,L.
Wallin,B.Olsson,B.B.Johansson,Cerebrallesionsonmagneticresonance imaging,heartdisease,andvascularriskfactorsinsubjectswithoutstroke.A population-basedstudy,Stroke25(1994)929–934.
[30]C.C.Leonardo,M.Agrawal,N.Singh,J.R.Moore,S.Biswal,S.Doré,Oral administrationoftheflavanol(−)-epicatechinbolstersendogenous protectionagainstfocalischemiathroughtheNrf2cytoprotectivepathway, Eur.J.Neurosci.38(2013)3659–3668.
[31]Y.C.Zhang,F.F.Gan,S.B.Shelar,K.Y.Ng,E.H.Chew,AntioxidantandNrf2 inducingactivitiesofluteolinaflavonoidconstituentinIxerissonchifolia Hance,provideneuroprotectiveeffectsagainstischemia-inducedcellular injury,FoodChem.Toxicol.59(2013)272–280.
[32]R.Q.Yao,D.S.Qi,H.L.Yu,J.Liu,L.H.Yang,X.X.Wu,Quercetinattenuatescell apoptosisinfocalcerebralischemiaratbrainviaactivationof
BDNF-TrkB-PI3K/Aktsignalingpathway,Neurochem.Res.37(2012) 2777–2786.
[33]S.J.Spencer,M.A.Galic,M.Tsutsui,Q.J.Pittman,A.Mouihate,Effectsofglobal cerebralischemiainthepregnantrat,Stroke39(2008)975–982.
[34]J.P.E.Spencer,Conferenceon‘Overandundernutrition:challengesand approaches’NutritionSocietySilverMedalLectureBeyondantioxidants:the cellularandmolecularinteractionsofflavonoidsandhowtheseunderpin theiractionsonthebrain,Proc.Nutr.Soc.69(2010)244–260.
[35]D.Vauzour,K.Vafeiadou,A.Rodriguez-Mateos,C.Rendeiro,J.P.Spencer,The neuroprotectivepotentialofflavonoids:amultiplicityofeffects,GeneNutr.4 (2008)115–126.
[36]J.P.Spencer,Theimpactofflavonoidsonmemory:physiologicaland molecularconsiderations,Chem.Soc.Rev.38(2009)1152–1161. [37]J.Cho,H.K.Lee,Wogonininhibitsischemicbraininjuryinaratmodelof
permanentmiddlecerebralarteryocclusion,Biol.Pharm.Bull.27(2004) 1561–1564.
[38]F.Rivera,G.Costa,A.Abin,J.Urbanavicius,C.Arruti,G.Casanova,F.Dajas, Reductionofischemicbraindamageandincreaseofglutathionebya liposomalpreparationofquercetininpermanentfocalischemiainrats, Neurotoxic.Res.13(2008)105–114.
[39]A.G.Sunil,K.S.Kesavanarayanan,P.Kalaivani,S.Sathiya,V.Ranju,R.J.Priya,B. Pramila,F.D.Paul,J.Venkhatesh,C.S.Babu,Totaloligomericflavonoidsof Cyperusrotundusamelioratesneurologicaldeficits,excitotoxicityand behavioralalterationsinducedbycerebralischemic-reperfusioninjuryin rats,BrainRes.Bull.84(2011)394–405.
[40]K.R.Crafton,A.N.Mark,S.C.Cramer,Improvedunderstandingofcortical injurybyincorporatingmeasuresoffunctionalanatomy,Brain126(2003) 1650–1659.
[41]R.Traversa,P.Cicinelli,A.Bassi,P.M.Rossini,G.Bernardi,Mappingofmotor corticalreorganizationafterstroke.Abrainstimulationstudywithfocal magneticpulses,Stroke28(1997)110–117.
[42]C.Calautti,F.Leroy,J.Y.Guincestre,R.M.Marié,J.C.Baron,Sequential activationbrainmappingaftersubcorticalstroke:changesinhemispheric balanceandrecovery,Neuroreport12(2001)3883–3886.
[43]R.Gupta,M.Singh,A.Sharma,Neuroprotectiveeffectofantioxidantson ischaemiaandreperfusion-inducedcerebralinjury,Pharmacol.Res.48(2003) 209–215.
[44]M.M.Muley,V.N.Thakare,R.R.Patil,A.D.Kshirsagar,S.R.Naik,Silymarin improvesthebehavioural,biochemicalandhistoarchitecturealterationsin focalischemicrats:acomparativeevaluationwithpiracetamand protocatachuicacid,Pharmacol.Biochem.Behav.102(2012)286–293. [45]Y.Gu,C.S.Huang,T.Inoue,T.Yamashita,T.Ishida,K.M.Kang,A.Nakao,
Drinkinghydrogenwateramelioratedcognitiveimpairmentin senescence-acceleratedmice,J.Clin.Biochem.Nutr.46(2010)269–276. [46]J.E.Rice,R.C.Vannucci,J.B.Brierley,Theinfluenceofimmaturityon
[47]M.Tamura,Y.Aoki,T.Seto,Y.Itoh,Y.Ukai,Cerebroprotectiveactionofa Na+/Ca2+channelblockerNS-7.II.Effectonthecerebralinfarction,behavioral andcognitiveimpairmentsatthechronicstageofpermanentmiddlecerebral arteryocclusioninrats,BrainRes.890(2001)170–176.
[48]A.E.Willing,J.Lixian,M.Milliken,S.Poulos,T.Zigova,S.Song,C.Hart,J. Sanchez-Ramos,P.R.Sanberg,Intravenousversusintrastriatalcordblood administrationinarodentmodelofstroke,J.Neurosci.Res.73(2003) 296–307.
[49]M.R.Carmo,A.P.Simões,A.A.Fonteles,C.M.Souza,R.A.Cunha,G.M.Andrade, ATPP2Y1receptorscontrolcognitivedeficitsandneurotoxicitybutnotglial modificationsinducedbybrainischemiainmice,Eur.J.Neurosci.39(2014) 614–622.
[50]M.D’Esposito,J.A.Detre,D.C.Alsop,R.K.Shin,S.Atlas,M.Grossman,The neuralbasisofthecentralexecutivesystemofworkingmemory,Nature378 (1995)279–281.
[51]R.Lalonde,Theneurobiologicalbasisofspontaneousalternation,Neurosci. Biobehav.Rev.26(2002)91–104.
[52]F.Dellu,W.Mayo,M.Vallée,M.LeMoal,H.Simon,Reactivitytonovelty duringyouthasapredictivefactorofcognitiveimpairmentintheelderly:a longitudinalstudyinrats,BrainRes.653(1994)51–56.
[53]D.H.Kim,S.J.Jeon,K.H.Son,J.W.Jung,S.Lee,B.H.Yoon,J.W.Choi,J.H.Cheong, K.H.Ko,J.H.Ryu,Effectoftheflavonoid,oroxylinA,ontransientcerebral hypoperfusion-inducedmemoryimpairmentinmice,Pharmacol.Biochem. Behav.85(3)(2006)658–668.
[54]I.Izquierdo,J.H.Medina,Memoryformation:thesequenceofbiochemical eventsinthehippocampusanditsconnectiontoactivityinotherbrain structures,Neurobiol.Learn.Mem.68(1997)285–316.
[55]F.Wahl,M.Allix,M.Plotkine,R.G.Boulu,Neurologicalandbehavioral outcomesoffocalcerebralischemiainrats,Stroke23(1992)267–272. [56]H.Q.Wu,H.N.Guo,H.Q.Wang,M.Z.Chang,G.L.Zhang,Y.X.Zhao,Protective
effectsandmechanismofpuerarinonlearning-memorydisorderafterglobal cerebralischemia-reperfusioninjuryinrats,Chin.J.Integr.Med.15(2009) 54–59.
[57]B.Shukitt-Hale,A.Carey,L.Simon,D.A.Mark,J.A.Joseph,EffectsofConcord grapejuiceoncognitiveandmotordeficitsinaging,Nutrition22(2006) 295–302.
[58]J.Joseph,G.Cole,E.Head,D.Ingram,Nutritionbrainaging,and neurodegeneration,J.Neurosci.29(2009)12795–12801.
[59]Y.F.Wang,Y.T.Gu,G.H.Qin,L.Zhong,Y.N.Meng,Curcuminamelioratesthe permeabilityoftheblood-brainbarrierduringhypoxiabyupregulatingheme oxygenase-1expressioninbrainmicrovascularendothelialcells,J.Mol. Neurosci.51(2013)344–351.
[60]S.S.Shi,W.Z.Yang,Y.Chen,J.P.Chen,X.K.Tu,Propofolreducesinflammatory reactionandischemicbraindamageincerebralischemiainrats,Neurochem. Res.39(2014)793–799.
[61]X.K.Tu,W.Z.Yang,C.H.Wang,S.S.Shi,Y.L.Zhang,C.M.Chen,Y.K.Yang,C.D.Jin, S.Wen,Zileutonreducesinflammatoryreactionandbraindamagefollowing permanentcerebralischemiainrats,Inflammation33(2010)344–352. [62]Q.Wang,X.N.Tang,M.A.Yenari,Theinflammatoryresponseinstroke,J.
Neuroimmune184(2007)53–68.
[63]A.Annapurna,M.A.Ansari,P.M.Manjunath,Partialroleofmultiplepathways ininfarctsizelimitingeffectofquercetinandrutinagainstcerebral ischemia-reperfusioninjuryinrats,Eur.Rev.Med.Pharmacol.Sci.17(2013) 491–500.
[64]C.Iadecola,J.Anrather,Theimmunologyofstroke:frommechanismsto translation,Nat.Med.17(2011)796–808.
[65]L.Yu,C.Chen,L.F.Wang,X.Kuang,K.Liu,H.Zhang,J.R.Du,Neuroprotective effectofkaempferolglycosidesagainstbraininjuryandneuroinflammation byinhibitingtheactivationofNF-BandSTAT3intransientfocalstroke,PLoS One8(2)(2013)55839.
[66]H.Huang,R.Zhong,Z.Xia,J.Song,L.Feng,Neuroprotectiveeffectsof rhynchophyllineagainstischemicbraininjuryviaregulationoftheAkt/mTOR andTLRssignalingpathways,Molecules19(2014)11196–11210.
[67]J.P.O’Callaghan,K.Sriram,Glialfibrillaryacidicproteinandrelatedglial proteinsasbiomarkersofneurotoxicity,Exp.Opin.DrugSaf.4(2005) 433–442.
[68]R.A.Swanson,W.Ying,T.M.Kauppinen,Astrocyteinfluencesonischemic neuronaldeath,Curr.Mol.Med.4(2004)193–205.
[69]M.Yang,F.Gao,H.Liu,W.H.Yu,S.Q.Sun,Temporalchangesinexpressionof aquaporin-3−4,−5and−8inratbrainsafterpermanentfocalcerebral ischemia,BrainRes.1290(2009)121–132.
[70]S.S.Raza,M.M.Khan,A.Ahmad,M.Ashafaq,G.Khuwaja,R.Tabassum,H. Javed,M.S.Siddiqui,M.M.Safhi,F.Islam,Hesperidinamelioratesfunctional andhistologicaloutcomeandreducesneuroinflammationinexperimental stroke,BrainRes.1420(2011)93–105.
[71]M.Ashafaq,S.S.Raza,M.M.Khan,A.Ahmad,H.Javed,M.E.Ahmad,R. Tabassum,F.Islam,M.S.Siddiqui,M.M.Safhi,F.Islam,Catechinhydrate amelioratesredoximbalanceandlimitsinflammatoryresponseinfocal cerebralischemia,Neurochem.Rev.37(2012)1747–1760.
[72]C.Y.Cheng,J.G.Lin,N.Y.Tang,S.T.Kao,C.L.Hsieh,Electroacupuncture-like stimulationattheBaihui(GV20)andDazhui(GV14)acupointsprotectsrats againstsubacute-phasecerebralischemia-reperfusioninjuriesbyreducing S100B-mediatedneurotoxicity,PLoSOne9(2014)91426.
[73]L.Stevenson,N.Matesanz,Reducednitro-oxidativestressandneuralcell deathsuggestsaprotectiveroleformicroglialcellsinTNFalpha-/-micein ischemicretinopathy,Invest.Ophthalmol.VisualSci.51(2010)3291–3299. [74]F.Li,Q.Gong,L.Wang,J.Shi,Ostholeattenuatesfocalinflammatoryreaction
followingpermanentmiddlecerebralarteryocclusioninrats,Biol.Pharm. Bull.35(2012)1686–1690.
[75]E.Lee,K.W.Jeong,A.Shin,B.Jin,H.N.Jnawali,B.H.Jun,J.Y.Lee,Y.S.Heo,Y. Kim,BindingmodelforeriodictyoltoJun-Nterminalkinaseand itsanti-inflammatorysignalingpathway,BMBRep.46(2013)594–599. [76]K.Vafeiadou,D.Vauzour,H.Y.Lee,A.Rodriguez-Mateos,R.J.Williams,J.P.